Abstract
Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) have been introduced as a possible therapy in hypoxic-ischemic encephalopathy (HIE). We report a 16-year-old boy who was treated with WJ-MSCs in the course of HIE due to post-cardiopulmonary resuscitation. He received a long period of mechanical ventilation and tracheostomy with spastic quadriparesis. He underwent the intrathecal (1×106/kg in 3 mL), intramuscular (1×106/kg in 20 mL) and intravenous (1×106/kg in 30 mL) administrations of WJ-MSCs for each application route (twice a month for 2 months). After stem cell infusions, progressive improvements were shown in his neurological examination, neuroradiological, and neurophysiological findings. To our best knowledge, this is a pioneer project to clinically study the neural repair effect of WJ-MSCs in a patient with HIE.
Keywords: hypoxic-ischemic encephalopathy, post-cardiopulmonary resuscitation, stem cell therapy, wharton’s jelly
Introduction
Hypoxic-ischemic brain injury (HIBI) results in various degrees of damage to the brain, and the immature brain is particularly fragile to oxygen deprivation which can be caused by extreme prematurity or perinatal asphyxia. For adolescents or adults, similar pathological changes are often caused by cardiac arrest/resuscitation or hypertension, termed ischemic stroke. These processes resulted in hypoxic-ischemic encephalopathy (HIE)1,2. To date, hypothermia is the only therapeutic intervention used clinically for infants with HIBI born at term or late preterm. Although it lowers infant mortality and morbidity, therapeutic hypothermia does not guarantee complete protection3. So far, treatment of HIBI consists largely of supportive care, which does little to prevent the ongoing injury that occurs in the hours immediately following the causative insult4,5. In addition, preclinical studies suggested that combined treatment with hypothermia and human umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) transplantation might be a novel therapeutic modality to improve the prognosis of severe HIE6,7. Thus, it is crucial to identify new therapeutic targets (e.g., stem cell therapy (SCT), etc.) for the performance of clinical trials to address the treatment of HIBI4,8,9.
Accumulating evidence has shown that SCT (embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), etc.) is a potential method in improving brain functions. Growing data have indicated that MSCs promote neurological functional recovery in HIBI8,9. MSCs are multipotent precursor cells with self-renewal ability and have characteristics that vary depending on their origin in many adult and fetal tissues. Ideally, for stem cells to be effective in regenerative medical applications, stem cells should be abundant and obtainable through a minimally invasive procedure to ensure donor safety10. Although bone marrow (BM) represents the major source of MSCs, the use of BM-derived MSCs is not always acceptable because of the small cell numbers, the decreasing proliferative capacity with age, and the high incidence of viral infection11,12. Perinatal tissues, for example umbilical cord blood (UCB), Wharton’s jelly (WJ), etc., are considered to be attractive sources of MSCs because they can be obtained by less invasive methods without harm to mothers or neonates. Among them, the results from isolations of UCB-MSCs have been inconsistent because of the low frequency and viability in UCB after the donor’s birth. Furthermore, a large UCB volume and a rapid isolation procedure after collection are needed to increase the success rate10. Unlike UCB cells, Wharton’s jelly mesenchymal stem cells (WJ-MSCs) have distinct advantages of being abundant, easy to obtain with minimal invasiveness, and readily cultured to a sufficient number for transplantation without ethical issues of allografting13.
To establish the optimal route for MSC transplantation is a critical issue that needs to be addressed for successful treatment in HIBI. MSCs have been transplanted via different routes (e.g., intravenous (i.v.), etc.). However, although systemically transplanted MSCs can cross the blood–brain barier in the injured brain, they can also be retained in other organs, such as the lungs, liver, spleen, and kidneys14.
Therefore, multiple routes may be more effective than a single route. In addition, the clinical trial registry and results database http://ClinicalTrial.gov lists three completed studies conducted worldwide using WJ-MSCs (e.g., autism, etc.). The i.v. and intrathecal (i.t.) cell applications were well tolerated with no adverse side effect in the 24 weeks post treatment13.
In our case study, we evaluated the clinical and functional outcome after i.t., intramuscular (i.m.), and i.v. transplantation of WJ-MSCs in a 16-year-old boy diagnosed with post-cardiopulmonary resuscitation (post-CPR) HIE.
Case Presentation
The presented pilot study was a prospective, longitudinal medical experiment. The study was performed at the University of Health Sciences, Gaziosmanpaşa Taksim Training and Research Hospital in Istanbul, Turkey. The MSC trial was approved by the Turkish Ministry of Health at 4 months of insult (date: March 2, 2017/ protocol number: 56733164 /203/E.643). The patient’s parents were informed about the procedure and a duly filled informed consent was obtained according to the Helsinki Declaration. The general data collected before the experimental therapy consisted of age, gender, the cause of HIE, the length of time since the HIE, previous medical treatment for HIE, and past medical history.
Medical History
No cardiac pathology was diagnosed in the medical history of the 16-year-old boy or his family. The patient was admitted to a private hospital having undergone a post-CPR of approximately 45 min. His cranial magnetic resonance imaging (MRI) including diffusion-weighted imaging (DWI) 4 days post-CPR showed increased signal intensity in the bilateral anterior/posterior watershed zone, basal ganglia, and occipital lobes (Fig. 1 A, B). His epicrisis and his family’s reports indicated that his eyes started to open at the end of 45 days and he had marked spasticity in the private hospital’s intensive care unit (ICU). After the 76th day of hospitalization, the patient was transferred to our hospital, a tertiary referral center, medical ICU, with tracheostomy and percutaneous endoscopic gastrostomy (PEG). On admission, the patient’s condition was moderate; regarding his neurological examination, his eyes were open spontaneously, pupils were equal and reactive to light; he had very limited cooperation, barely following one-step commands, and extensive contractures and spastic quadriparesis were present in his extremities. He was tracheotomized and still required intermittent mechanical ventilation. At almost 3 weeks of follow-up in ICU, he was hemodynamically stable without any vasoactive amine requirements, with ongoing i.v. antibiotherapy with normal kidney functions. Following infection control he underwent three unsuccessful weaning trials during his stay in our medical ICU. He was transferred to our Neurosurgery Department (ND) for SCT on the 20th day of hospitalization with home ventilator and feeding enterally via PEG.
Fig. 1.
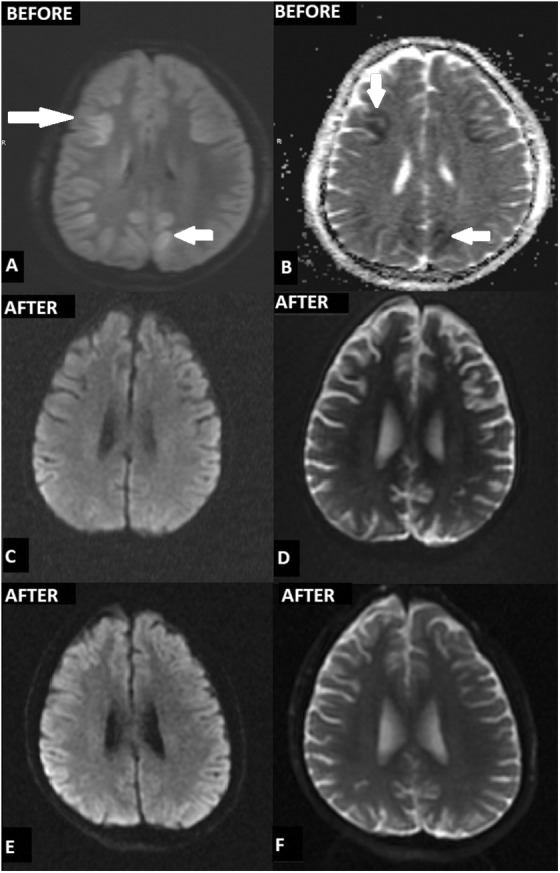
(A,B) cranial MRI including DWI sequences; after 4 days post-CPR demonstrated increased signal intensity in the bilateral anterior/ posterior watershed zone, basal ganglia, and occipital lobes; (C,D) after 6 months from the insult and 3 months a.f.i. showed no pathological signal; (E,F) after 12 months from the insult and 9 months a.f.i. showed no pathological signal.
Enrollment Criteria
The pilot study included the patient with HIE with cerebral infacts confirmed by imaging studies (MRI, DWI MRI) and with neurological examination and neurophysiological findings. Focal central nervous system lesions (e.g., neoplastic lesions) or chronic diseases (e.g., systemic diseases) that would require long-term pharmacotherapy were exclusion criteria. Prior to the treatment, the patient was examined by the doctors of anesthesia and reanimation, neurology, neurosurgery, and physical therapy and rehabilitation. The WJ-MSC implantation procedure was performed when the patient was stable, without contraindications for sedo-/general anesthesia from the view point of internal medicine and cardiology, and without any serious infectious diseases, including sepsis, immediately prior to the procedure.
Procedure
Ethics and Consent
WJ-MSCs were obtained from the Good Manufacturing Practice facility of LivMedCell (Istanbul, Turkey). The umbilical cord was obtained from one donor after informed consent as approved by an institutional regulatory board (LivMedCell). The postnatal umbilical cord was obtained from the donor of full-term pregnancy.
Umbilical Cord Processing and Quality Control
The cord was washed with phosphate-buffered saline (Invitrogen/Gibco, Paisley, UK). Tissue was cut into pieces (5–10 mm3) as explants after blood vessels were removed. Tissue explants were placed into the culture dishes and cultured at humanized culture conditions (5% CO2 and 37°C) until the cells migrate from the pieces. The cells were harvested after reaching 70–80% confluency and characterization tests were performed at passage 3. Quality control and quality assurance for the production of these cells were performed according to the standards of the Turkish Medicines and Medical Devices Agency.
Characterization of WJ-MSCs by Flow Cytometry
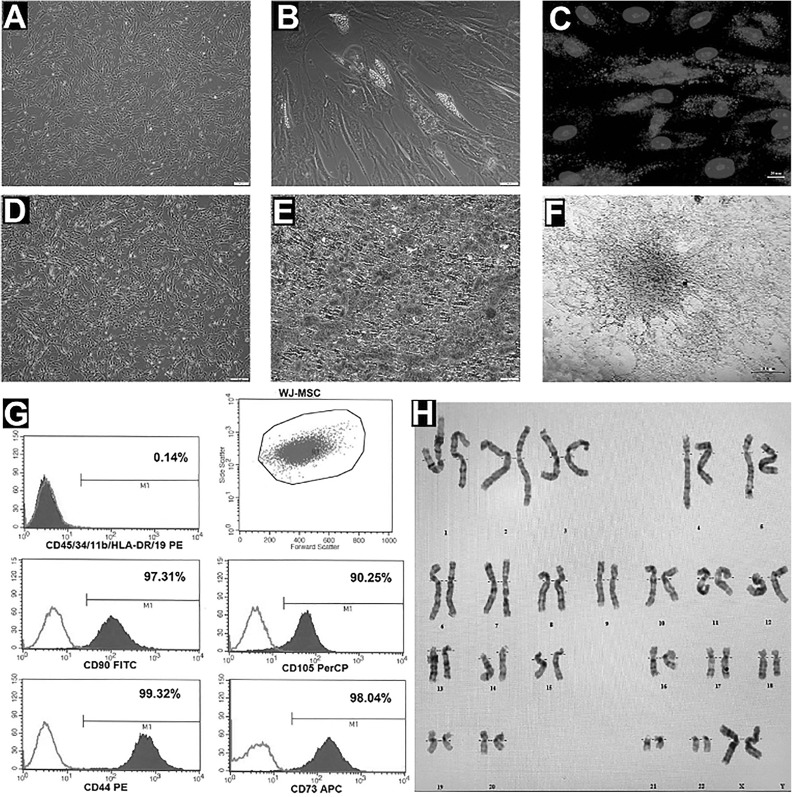
Flow cytometry analysis of expressed surface antigens showed that these cells were uniformly positive for CD44, CD73, CD105, and CD90, and negative for the hematopoietic lineage markers CD34, CD45, and HLA-DR (Fig. 2 G). It has been shown that the telomerase activities of WJ-MSCs stay stable during cell culture procedures, and they exhibited a large, flattened morphology (Fig. 2 A, D).
Fig. 2.
(A,D) Morphology of cultured WJ-MSC at P3, (B) morphology and (C) Oil Red O Staining of neutral lipid vacuole formation in WJ-MSC cultured in adipogenic differentiation medium, (E) mineral nodules that stained by Alizarin Red S, of WJ-MSCs cultured in osteogenic differentiation medium, (F) Alcian Blue Staining of WJ-MSCs cultured in chondrogenic differentiation medium, (G) flow cytometric analysis of cell surface markers of WJ-MSCs at P3, (H) chromosome karyotype analysis of the cultered WJ-MSCs.
Cell Differentiation and Karyotying
The expressions of some stem cell and differentiation markers such as TERT, POU5F1, SOX2, ZFP42, CD44, VCAM1, THY1, BMP2, RUNX-1, VIM, ICAM1, and NES were determined (data not shown). Differentiation experiments confirmed that these cells have trilineage (osteoblasts, adipocytes, and chondrocytes) differentiation capacity (Fig. 2 A–C, E, F). No structural or numerical chromosomal abnormalities were found in karyotype analyses of the cells (Fig. 2 H).
Pre-Transplantation Process
The final WJ-MSC preparations used in the infusion were harvested from cell culture passage 3 and suspended at final densities of 1×106/kg in 3 mL (i.t.), 1×106/kg in 20 mL (i.m.) and 1×106/kg in 30 mL (i.v.) in normal saline.
Surgical Procedure and WJ-MSC Transplantation Procedure
The procedure was performed in the operating theater by sedoanesthesia according to the protocol of the MSC treatment trial with i.t., i.m., and i.v. implantation of allogeneic WJ-MSCs as the standard by the same doctor (S.K.) (Table 1). WJ-MSCs were administered through i.t. injection (Spinocan® 0.7×88 mm, (22Gx3½”), Black, B.Braun, Germany) by lumbar puncture on the basis of previous studies15. The Clinical Cell Therapy Guidelines for Neurorestoration have recently suggested that the maximum injection volume of cell suspensions does not exceed 10 mL by i.t. injection into cerebrospinal fluid, and 10–100 mL by i.v. routes. In addition, it was stated that minimal suggested cell doses for MSCs derived from umbilical cord are: 0.5–0.8×106/kg body weight for i.v. infusion, and 5–8×106 for i.t. injection16. I.m. injection was applied by ultrasonography (USG) guidance for each muscle and i.v. infusion was applied slowly for 30 min. After the procedure, the patient was transferred for follow-up to ND ICU (1st level). Our patient showed only early complications, such as subfebrile fever, mild headache, or muscle pain due to i.m. injection, which were transient for 24 hours (Table 2). The next day, the patient was transferred to the ND service and physical therapy and rehabilitation was started. The exercise program was not performed on the days when the stem cell was applied. This procedure was repeated before and after each application.
Table 1.
Administration Schedule.
| Date | Route | WJ-MSC |
|---|---|---|
| Circle 1 | ||
| 02.03.2017 | IT | 1 ×106/kg in 3ml |
| 02.03.2017 | IV | 1 ×106/kg in 30ml |
| 02.03.2017 | IM | 1 ×106/kg in 20ml |
| Circle 2 | ||
| 17.03.2017 | IT | 1 ×106/kg in 3ml |
| 17.03.2017 | IV | 1 ×106/kg in 30ml |
| 17.03.2017 | IM | 1 ×106/kg in 20ml |
| Circle 3 | ||
| 03.04.2017 | IT | 1 ×106/kg in 3ml |
| 03.04.2017 | IV | 1 ×106/kg in 30ml |
| 03.04.2017 | IM | 1 ×106/kg in 20ml |
| Circle 4 | ||
| 18.04.2017 | IT | 1 ×106/kg in 3ml |
| 18.04.2017 | IV | 1 ×106/kg in 30ml |
| 18.04.2017 | IM | 1 ×106/kg in 20ml |
IT: Intrathecal, IV: Intravenosus, IM: Intramuscular
Table 2.
Early and Late Complications of the Procedures.
| Complications | 02.03.2017 | 17.03.2017 | 03.04.2017 | 18.04.2017 | |
|---|---|---|---|---|---|
| Early | |||||
| Infection | – | – | – | – | |
| Fever | + | + | – | – | |
| Pain | + | – | + | – | |
| Headache | + | + | – | – | |
| Increased level of C-reactive protein | – | – | – | – | |
| Leukocytosis | – | – | – | – | |
| Allergic reaction or shock | – | – | – | – | |
| Perioperative complications | – | – | – | – | |
| Late | – | – | – | – | |
| Secondary infections | – | – | – | – | |
| Urinary tract infections | – | – | – | – | |
| Deterioration of neurological status | – | – | – | – | |
| Neuropathic pain | – | – | – | – | |
| Carcinogenesis | – | – | – | – |
–: not present; +: present.
Pretreatment Neurological Examination
Pretreatment assessment included extensive evaluation by a team of medical and rehabilitation experts (Supp. Video 1). Detailed neurological and functional evaluation was documented in each step of the procedure. Spasticity was assessed using the Ashworth scale and quality of life was assessed on the basis of parental evaluation according to the Functional Independence Measure (FIM) scale17.
Safety Evaluation Criteria
The safety criteria for the transplantation procedure included the appearance of infection, fever, headache, pain, an increased level of C-reactive protein, increased leukocytosis, allergic reaction or shock, and perioperative complications (anesthesia- and analgesia-related complications, infections of the wound) for 7–14 days after the procedure. The safety criteria for using WJ-MSC included infection, neuropathic pain, cancer development, and deterioration of the neurological state, and were assessed for a 1-year follow-up period.
Follow-up Assessment of Treatment Success
The follow-up evaluation consisted of a neurological examination evaluating motor function according to the Medical Research Council (MRC) Muscle Strength Scale. Spasticity was assessed using the Ashworth scale and quality of life was assessed based on the functional recovery estimated by the FIM scale17,18. In addition, an evaluation of the development of neuropathic pain, secondary infections, urinary tract infections, or pressure ulcers of the skin was performed.
The Patient’s Clinical Progress
Starting from the first transplantation, the patient underwent intensive neurorehabilitation that included physiotherapy as a part of the treatment program. The patient was placed on a personalized exercise program that emphasized techniques to facilitate mobility and multiplication of the injected stem cells, thereby giving enhanced results. The personalized program was one session (50 min/day) 5 times a week, including posture, balance, range of motion (ROM), strength & stretch, and especially pulmonary exercise to defeat the ventilator-associated pneumonia’s sequela. A week after the SCT, the patient began to show marked improvements. After 1 month, trunk strength and upper limb control improved. The patient was walking with bilateral push knee splints and elbow crutches. He could wear his t-shirt on his own, independently, and eat by himself. Regarding his FIM, his motor score improved from 20 to 35 (maximum score 91) and his cognitive score from 7 to 19 (maximum score 35). His left knee contracture decreased from 15 to 5 degrees. Shoulder abduction ROM improved from 90 to 110 degrees.
At the 2-month follow-up after the first intervention (a.f.i.), the patient kneel-walked for at least 30 min. Trunk balance and control had further improved. Transfers such as bed, sitting, and getting up from the floor were performed in a controlled manner, and were easier. Posture was more erect. Walking stability had improved (Supp. Video 2). Three months a.f.i., in activities of daily living, the patient could eat with better coordination, dress himself with minimal assistance, and achieved independent toileting activities. Hand functions improved, due to which his writing speed increased with a better handwriting. Four months a.f.i, on repeating the FIM, the motor score was increased from 35 to 89, cognitive from 19 to 31 and total sum was 120 of 126. On repeating MRI of the brain 3 and 9 months a.f.i., there was no pathological signal in the brain (Table 3) (Fig.1 E, F).
Table 3.
Summary of the Results of the Neuroradiological and Neurophysilogical Evaluations Before and After the Treatment Using MRI, EEG and EMG.
| MRI | EEG | EMG | |
|---|---|---|---|
| Pre-t.p. | Ischemia | Abnormal | Abnormal |
| Post-t.p. (3rd m) | Normal | Normal | Normal |
| Post-t.p.(9th m) | Normal | Normal | Normal |
MRI: Magnetic Resonance Imaging; EEG: electroencephalogram; EMG: electromyelography; transplantation: t.p.; week: w; month: m.
All clinical improvements have been almost sustained until the end of 4 months a.f.i. The patient has been followed every 6 months thereafter to further assess his progress. In the first-year clinical evaluation his FIM score was 125 (Table 4) (Supp.Video 3).
Table 4.
Quality-of-life Improvement, Spasticity and Muscle Strength Were Evaluated with the use of the FIM Scale, Modified Ashworth Grading and MRC Muscle Strength Scale Respectively.
| Quality-of-life Improvement, FIM Scale (Total:126) Motor Score Self-care/Sphincter Control/Transfers/Locomotion (TS:91) | Cognitive Score Communication/Social Cognition(TS:35) | Modified Ashworth Grading | MRC Muscle Strength Scale | |||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | |||
| Pre-t.p. | 20 | 7 | 4 | 4 | 1/5 | 1/5 |
| Post-t.p.(1st w) | 25 | 10 | 4 | 4 | 1-2/5 | 1-2/5 |
| Post-t.p. (1st m) | 35 | 19 | 2 | 2 | 4/5 | 4/5 |
| Post-t.p. (2nd m) | 45 | 25 | 2 | 2 | 4/5 | 4/5 |
| Post-t.p. (4th m) | 89 | 31 | 1+ | 1+ | 4/5 | 4/5 |
| Post-t.p.(12th m) | 91 | 35 | 0 | 0 | 5/5 | 5/5 |
FIM: Functional Independence Measure; MRC: Medical Research Council; TS: Total Score; t.p.: transplantation; w: week; m: month
Discussion
Many different therapies have been attempted in the treatment of HIE, but no specific therapy has demonstrated a clear effect in older children in the way that hypothermia has shown a clear effect in infants. Some of these therapies are being explored in isolation, while others are being combined with moderate hypothermia or other treatments in the hope that synergetic effects will improve outcomes18. On the other hand, cell transplantation is being actively explored as a treatment alternative for neurological disorders (NeDs) (e.g., stroke, etc.) as stem cells have shown regenerative and reparative potential. Notably, the presumptive goal in using cell-based therapies in HIBI would be to replace infarcted central nervous system tissue in an appropriate organotypic manner11,19,20. Stem cells promote angiogenesis, neurogenesis, reduce inflammation, and increase the oxygen supply to the brain. Sources for stem cells include neural stem/progenitor cells derived from fetal tissue, MSCs, ESCs, induced pluripotent stem cells (iPSCs), etc19. Accordingly, MSCs have been introduced as a possible therapy due to having the ability to differentiate into many different cell lineages and the capacity for self-renewal. However, subsequent studies have suggested that the most important therapeutic effect probably comes from the paracrine properties of MSCs. Although stem/progenitor cells have shown a promising role in HIBI in experimental studies as well as in initial clinical pilot studies, cellular therapy is still at an early stage in humans21. Among the studies, Min et al. suggested that UCB-MSC treatment ameliorated motor and cognitive dysfunction in children with cerebral palsy (CP) undergoing active rehabilitation, accompanied by structural and metabolic changes in the brain22. Furthermore, Miao et al. proposed that 1 year after the i.t. administration of UC-MSCs treatment, functional indices improved in 47 patients (47%): 12 patients with spinal cord injury (SCI), 11 patients with CP, nine patients with post-traumatic brain syndrome, nine patients with post-brain infarction syndrome, three patients with spinocerebellar ataxias, and three patients with motor neuron disease15. WJ represents a rich source of stem cells used in several animal models of NeDs23,24. WJ-MSCs became an alternative source to BM and adipose tissue-derived MSCs due to their non-invasive collection procedure, being produced to a sufficient number for transplantation, and their stem cell potency. It has been shown that WJ-MSCs have faster proliferation capability and reduced immunogenicity than BM-MSCs13,25,26. WJ-MSCs express higher levels of HLA-G, which has an immunosupressive effect on natural killer cells and T cells. This expression profile plays an important role in avoiding maternal immunity against the fetus during pregnancy and provides a better graft acceptance8,27. HLA-G secretion makes WJ-MSCs an ideal cell source for third-party/allogeneic applications. According to recent clinical trials, WJ-MSC treatment has promising effects on patients with NeDs such as stroke, SCI and cerebellar ataxia28–32.
Strategies of the Stem Cell Transplantation Protocol
Clinical cell transplantation approaches are i.v. implantation by artery using a catheter to a near lesion site/direct to the lesion or vein, i.t. implantation by lumbar puncture or cerebellar cistern puncture, intracerebral (i.c.) by stereotactic techniques, and i.m. implantation by USG28,33–36.
Commonly, a single kind of cell was administered by just one route. Studies have suggested that a combination of therapies (e.g., multiple routes) may enhance results and may be more effective than a single therapy34–37.
NeDs such as traumatic brain injuries and strokes can benefit enormously from neurorehabilitation. Thus, we can use a neurorehabilitation and physiotherapy program in the same manner to maximize these patients’ functional and cognitive abilities.
Based on the experimental and clinical studies summarized above, we suggested the strategy of SCT as multiple application and routes in combination with a neurorehabilitation and physiotherapy program. Thus, the key point of SCT should be focused on functional neurorestoration.
In a preclinical study, multiple injections of WJ-MSCs including intra-cisterna magna, intraarterial (i.a.), i.v., lumbar i.t., or i.c. suggested therapeutic benefit after stroke with similar application routes as in our case37. Our patient with HIE underwent 12 implantations and showed only early complications, such as subfebrile fever, mild headache, or muscle pain due to i.m. injection which were transient, and 1-year clinical follow-up demonstrated no long-term side effects (e.g., neuropathic pain, cancer, etc.). Our results expand on this safety and feasibility report by using WJ- MSC therapy for HIE with both triple routes and multiple implantations. Imaging findings in HIBI are highly variable and depend on a number of factors, including brain maturity, severity and duration of insult, and type and timing of imaging studies. Mild to moderate global ischemic insults to the brain usually result in watershed zone infarcts. During the first 24 hours, DWI may demonstrate increased signal intensity in the cerebellar hemispheres, basal ganglia, or cerebral cortex (in particular, the perirolandic and occipital cortices). The thalami, brainstem, or hippocampi may also be involved5,38,39. Our patient’s cranial MRI-DWI section 4 days post-CPR also showed increased signal intensity especially in the watershed zones, cerebral cortex, and basal ganglia, regardless of the severity of brain insult. In the chronic stage, T2-weighted images may demonstrate some residual hyperintensity in the basal ganglia, and T1-weighted images may show cortical necrosis. Our patient’s cranial MRI-DWI section after 6 months from the insult and 3 months a.f.i. showed no pathological signal. Furthermore, follow-up cranial MRI after 12 months from the insult showed no pathological signal. As well as therapeutic interventions enabling the prevention or reduction in HIBI, neurorehabilitation and physiotherapy also have an important therapeutic role for the patient. While performing cell transplantation, a well disciplined neurorehabilitation and physiotherapy program should be applied as soon as possible33,40.
The main reasons for our success in helping this patient’s progress by WJ-MSCs therapy are likely to be the following. First, he is of a young age (16 years) to respond to the SCT. Second, the time frame to apply the stem cell may be crucial. We performed the MSCs at 4 months from insult. A third possible reason concerns the dose (twice a month for 2 months), application routes, and the number of stem cells administered, which are parameters that still have no scientific consensus in WJ-MSCs treatment36. A fourth reason may relate to the application of the neurorehabilitation and physiotherapy program with stem cell transplantation simultaneously, and the patient’s adaptation to the whole process. As previously mentioned, we speculate that these clinical and neuroradiological improvements might be related to the paracrine properties of MSCs21. Notably, implantation of human MSCs, even though a limited number of the cells engrafted, can stimulate proliferation, migration, and differentiation of the host endogenous neural stem cells via paracrine beneficial growth factors36. On the other hand, regardless of the potentcy of MSCs’ paracrine effect, our application doses (1×106/kg for each application route) are consistent with the current literature as previously mentioned16,19,41.
The main limitations of this study are that is it a case report of only one patient, and the lack of a randomized, double-blinded, placebo-controlled design. Also, we did not track the WJ-MSCs in our patient after transplantation, which however remains technically difficult in clinical trials. Notably, there is currently no consensus regarding the cell culture condition, optimal dose, route, and duration of administration; these are parameters that need to be researched in depth in order to provide better guidance about the next step to follow in WJ-MSCs treatment16,36,42.
In conclusion, we report the safety and feasibility of both triple route and multiple WJ-MSC implantations, and of the introduced treatment strategy. Our preliminary results suggested the possibility of attaining neurological and quality-of-life improvement (motor and cognitive) in a patient with HIE with the use of both triple route and multiple WJ- MSC implantations. To our best knowledge, this study is the first reported controlled case clinical trial of WJ-MSCs therapy for HIE, and our study provides promising clinical evidence that supports SCT for the treatment of HIE. Any favorable results might provide new perspectives leading to the reduction of cerebral damage in HIE, and should be confirmed in a larger group of patients. Further randomized clinical trials are necessary to establish the efficacy of this procedure.
Acknowledgement
We would like to thank Olga Nehir Öztel, PhD; İrem Yılmaz, PhD; Buse Göçer, PhD; Cansu Subaşı, PhD; Eyüp Can Savrunlu, MD and Özlen Bahadır Kabataş, BA for their excellent technical assistance.
Footnotes
Ethical Approval: This study was approved by the Turkish Ministry of Health, Organ, Tissue Transplant and Dialysis Services Department Scientific Committee (date: March 2, 2017/protocol number: 56733164 /203/E.643).
Statement of Human and Animal Rights: This article contains human subject and does not contain any conflict with the Helsinki Declaration.
Statement of Informed Consent: There is human subject in this article and a informed consent was obtained from legally authorized representatives before the stem cell therapies.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chen A, Xiong LJ, Tong Y, Mao M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep. 2013;1(2):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meloni BP. Pathophysiology and neuroprotective strategies in hypoxic-ischemic brain injury and stroke. Brain Sci. 2017;7(8):E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joerger-Messerli MS, Oppliger B, Spinelli M, Thomi G, di Salvo I, Schneider P, Schoeberlein A. Extracellular vesicles derived from wharton’s jelly mesenchymal stem cells prevent and resolve programmed cell death mediated by perinatal hypoxia-ischemia in neuronal cells. Cell Transplant. 2018;27(1):168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dixon BJ, Reis C, Ho WM, Tang J, Zhang JH. Review: neuroprotective strategies after neonatal hypoxic ischemic encephalopathy. Int J Mol Sci. 2015;16(9):22368–22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28(2):417–439. [DOI] [PubMed] [Google Scholar]
- 6. Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park WS, Sung SI, Ahn SY, Yoo HS, Sung DK, Im GH, Choi SJ, Chang YS. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One. 2015;10(3):e0120893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dailey T, Metcalf C, Mosley YI, Sullivan R, Shinozuka K, Tajiri N, Pabon M, Acosta S, Kaneko Y, van Loveren H, Borlongan CV. An update on translating stem cell therapy for stroke from bench to bedside. J Clin Med. 2013;2(4):220–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dailey T, Mosley Y, Pabon M, Acosta S, Tajiri N, van Loveren H, Kaneko Y, Borlongan CV. Advancing critical care medicine with stem cell therapy and hypothermia for cerebral palsy. Neuroreport. 2013;24(18):1067–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee S, Park BJ, Kim JY, Jekarl D, Choi HY, Lee SY, Kim M, Kim Y, Park MS. The effect of fibroblast growth factor on distinct differentiation potential of cord blood-derived unrestricted somatic stem cells and Wharton’s jelly-derived mesenchymal stem/stromal cells. Cytotherapy. 2015;17(12):1723–1731. [DOI] [PubMed] [Google Scholar]
- 11. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Zhang Q, Li W, Nie D, Chen W, Xu C, Yi X, Shi J, Tian M, Qin J, Jin G, Tu W. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res. 2014;92(1):35–45. [DOI] [PubMed] [Google Scholar]
- 13. Joerger-Messerli MS, Marx C, Oppliger B, Mueller M, Surbek DV, Schoeberlein A. Mesenchymal stem cells from wharton’s jelly and amniotic fluid. Review. Best Pract Res Clin Obstet Gynaecol. 2016;31:30–44. [DOI] [PubMed] [Google Scholar]
- 14. Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Mesenchymal stem cells: the magic cure for intraventricular hemorrhage? Cell Transplant. 2017;26(3):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miao X, Wu X, Shi W. Umbilical cord mesenchymal stem cells in neurological disorders: a clinical study. Indian J Biochem Biophys. 2015;52(2):140–146. [PubMed] [Google Scholar]
- 16. Huang H, Young W, Chen L, et al. Clinical cell therapy guidelines for neurorestoration (IANR/CANR 2017). Cell Transplant. 2018;27(2):310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thorpe ER, Garrett KB, Smith AM, Reneker JC, Phillips RS. Outcome measure scores predict discharge destination in patients with acute and subacute stroke: a systematic review and series of meta-analyses. J Neurol Phys Ther. 2018;42(1):2–11. [DOI] [PubMed] [Google Scholar]
- 18. Allen KA, Brandon DH. Hypoxic ischemic encephalopathy: pathophysiology and experimental treatments. Newborn Infant Nurs Rev. 2011;11(3):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G, Chen J. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol. 2014;115:92–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teng YD, Kabatas S, Li J, Wakeman DR, Snyder EY, Sidman RL. Functional Multipotency of Neural Stem Cells and Its Therapeutic Implications In: Ulrich H, ed. Perspectives of Stem Cells. Springer Dordrecht Heidelberg London New York: © Springer Science+Business Media B.V; 2010:255–270. [Google Scholar]
- 21. Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in indian patients. a four-year follow up. J Stem Cells Regen Med. 2017;13(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, Jang SJ, Kim SH, Oh D, Kim MK, Kim SS, Kim M. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells. 2013;31(3):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiss M, Medicetty S, Bledsoe A, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24(3):781–792. [DOI] [PubMed] [Google Scholar]
- 24. Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton’s Jelly after complete transection of the rat spinal cord. PLoS One. 2008;3(10):e3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC, Schrepfer S. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20(5):655–667. [DOI] [PubMed] [Google Scholar]
- 26. Troyer DL, Weiss ML. Concise review: wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3),591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lila N, Carpentier A, Amrein C, Khalil-Daher I, Dausset J, Carosella ED.Implication of HLA-G molecule in heart-graft acceptance. Lancet. 2000;355(9221):2138. [DOI] [PubMed] [Google Scholar]
- 28. Jiang Y, Zhu W, Zhu J, Wu L, Xu G, Liu X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. 2013;22(12):2291–2298. [DOI] [PubMed] [Google Scholar]
- 29. Dongmei H, Jing L, Mei X, Ling Z, Hongmin Y, Zhidong W, Li D, Zikuan G, Hengxiang W. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy. 2011;13(8):913–917. [DOI] [PubMed] [Google Scholar]
- 30. Jin JL, Liu Z, Lu ZJ, Guan DN, Wang C, Chen ZB, Zhang J, Zhang WY, Wu JY, Xu Y. Safety and efficacy of umbilical cord mesenchymal stem cell therapy inhereditary spinocerebellar ataxia. Curr Neurovasc Res. 2013;10(1):11–20. [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Han D, Wang Z, Xue M, Zhu L, Yan H, Zheng X, Guo Z, Wang H. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15(2):185–191. [DOI] [PubMed] [Google Scholar]
- 32. Lv YT, Zhang Y, Liu M, Qiuwaxi JN, Ashwood P, Cho SC, Huan Y, Ge RC, Chen XW, Wang ZJ, Kim BJ, Hu X. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moviglia GA, Fernandez Viña R, Brizuela JA, et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8(3):202–209. [DOI] [PubMed] [Google Scholar]
- 34. Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, Montenegro X, Gonzalez R, Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant. 2008;17(12):1277–1293. [DOI] [PubMed] [Google Scholar]
- 35. Moviglia GA, Varela G, Brizuela JA, Moviglia Brandolino MT, Farina P, Etchegaray G, Piccone S, Hirsch J, Martinez G, Marino S, Deffain S, Coria N, Gonzáles A, Sztanko M, Salas-Zamora P, Previgliano I, Aingel V, Farias J, Gaeta CA, Saslavsky J, Blasseti N. Case report on the clinical results of a combined cellular therapy for chronic spinal cord injured patients. Spinal Cord. 2009;47(6):499–503. [DOI] [PubMed] [Google Scholar]
- 36. Xie B, Gu P, Wang W, Dong C, Zhang L, Zhang J, Liu H, Qiu F, Han R, Zhang Z, Yan B. Therapeutic effects of human umbilical cord mesenchymal stem cells transplantation on hypoxic ischemic encephalopathy. Am J Transl Res. 2016;8(7):3241–3250. [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang L, Li Y, Romanko M, Kramer BC, Gosiewska A, Chopp M, Hong K. Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res. 2012;1489:104–102. [DOI] [PubMed] [Google Scholar]
- 38. Glass HC, Ferriero DM.Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol. 2007;9(6):414–423. [DOI] [PubMed] [Google Scholar]
- 39. Scafidi J, Gallo V. New concepts in perinatal hypoxia ischemia encephalopathy. Curr Neurol Neurosci Rep. 2008;8(2):130–138. [DOI] [PubMed] [Google Scholar]
- 40. Jensen A, Hamelmann E. First autologous cell therapy of cerebral palsy caused by hypoxic-ischemic brain damage in a child after cardiac arrest—individual treatment with cord blood. Case Reports in Transplant. 2013;2013:951827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Misra V, Ritchie MM, Stone LL, Low WC, Janardhan V. Stem cell therapy in ischemic stroke: role of IV and intra-arterial therapy. Neurology. 2012;79(13 Suppl 1):S207–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: a systemic analysis of clinical trials. Cytotherapy. 2017;19(12):1351–1382. [DOI] [PubMed] [Google Scholar]