Abstract
Purpose
The management of central nervous system tumors is challenging in low- and middle-income countries. Little is known about applicability of twinning initiatives with high-income countries in neuro-oncology. In 2004, a monthly neuro-oncology video-teleconference program was started between King Hussein Cancer Center (Amman, Jordan) and the Hospital for Sick Children (Toronto, Ontario, Canada). More than 100 conferences were held and > 400 cases were discussed. The aim of this work was to assess the sustainability of such an initiative and the evolution of the impact over time.
Methods
We divided the duration in to three eras according to the initial 2 to 3 years of work of three consecutive oncologists in charge of the neuro-oncology program at King Hussein Cancer Center. We retrospectively reviewed the written minutes and compared the preconference suggested plans with the postconference recommendations. Impact of changes on the patient care was recorded.
Results
Thirty-three sets of written minutes (covering 161 cases) in the middle era and 32 sets of written minutes (covering 122 cases) in the last era were compared with the initial experience (20 meetings, 72 cases). Running costs of these conferences has dropped from $360/h to < $40/h. Important concepts were introduced, such as multidisciplinary teamwork, second-look surgery, and early referral. Suggestions for plan changes have decreased from 44% to 30% and 24% in the respective consecutive eras. Most recommendations involved alternative intervention modalities or pathology review. Most of these recommendations were followed.
Conclusion
Video-teleconferencing in neuro-oncology is feasible and sustainable. With time, team experience is built while the percentage and the type of treatment modifications change. Commitment and motivation helped maintain this initiative rather than availability of financial resources. Improvement in patients’ care was achieved, in particular, with the implementation of a multidisciplinary team and the continuous effort to implement recommendations.
INTRODUCTION
In pediatric oncology, video-teleconferencing is increasingly being used in the context of twinning between institutions in high-income countries (HICs) and low- and middle-income countries (LMICs) with the aim to improve the medical care delivered. Several twinning initiatives were successfully developed, mainly with a focus on childhood leukemia1-5. Different twinning initiatives focused on various aspects of health care according to the needs in targeted LMIC centers. The Nicaragua–Italy–Switzerland leukemia initiative showed how organizational and financial resources can be generated through a twinning program to provide a comprehensive care that includes supply of drugs and training of health professionals, in addition to care of children and their parents.2 The twinning between Indonesia and the Netherlands5 showed how beneficial it was to improve the whole team’s education and participation in research. The St Jude Children’s Research Hospital initiatives for retinoblastoma showed improving survival through awareness campaigns in Central America6 or local capacity building by Web site-based telemedicine experience.7 However, the use of teleconferencing in pediatric neuro-oncology has been limited.8-10 The management of childhood central nervous system (CNS) tumors faces numerous challenges in LMICs. Delayed diagnosis,11 insufficient numbers of specialists (eg, pediatric oncologists, pediatric neurosurgeons, and radiation oncologists with pediatric expertise),12 and lack of radiologic, neurosurgical, and/or radiation equipment are important obstacles.13 The treatment of pediatric CNS tumors is complex and requires coordinated multidisciplinary team work,14,15 which is usually absent in most LMICs.
King Hussein Cancer Center (KHCC) is the only cancer-dedicated hospital in Jordan and where most children with cancer in Jordan are treated. In 2011, 369 children younger than age 18 years with malignant tumors were evaluated at KHCC, including 18.7% with CNS tumors.16 KHCC is a reference radiotherapy center and the only institution with a multidisciplinary pediatric neuro-oncology-dedicated service in Jordan. This service has been developed through a twinning collaboration with the Hospital for Sick Children (Sickkids) in Toronto since 2004. Monthly video-teleconferences involving members of the multidisciplinary team on both sides were initiated to discuss the management of KHCC patients.
The aim of this paper is to update a previous report8 on this twinning experience and, in particular, to evaluate the knowledge gained and the impact on care delivered to children with CNS tumors treated at KHCC over this decade. Furthermore, we wanted to assess the feasibility and sustainability of this experience.
METHODS
Twinning between Sickkids and KHCC was initiated after a visit from the head of the pediatric neuro-oncology service at Sickkids (E.B.) to KHCC to evaluate the needs and discuss the appropriateness of treatment protocols. Since then, the KHCC neuro-oncology service has a full-time and a part-time oncologist, lately N.A. and H.H., respectively. Over this 10-year experience, the three pediatric oncologists who sequentially lead the service (I.Q., N.R., and N.A.) had a 5- to 6-week observation period in the neuro-oncology division at Sickkids. In addition, the pediatric neurosurgeon (A.M.) trained for 1.5 years in neurosurgery at Sickkids. The head of pediatric neurosurgery at Sickkids (J.D.) visited KHCC and participated in surgical activities and in a few educational workshops. These personal interactions have strengthened the twinning initiative and allowed for a monthly video-teleconference to be organized to discuss optimal options for KHCC patients.
We retrospectively reviewed the written minutes of the conferences since 2004. We selected the minutes corresponding to the initial 2 to 3 years of work of the three consecutive leading pediatric oncologists at the KHCC neuro-oncology program. The results of the initial experience (December 2004 to April 2006) were previously reported.8 We included the minutes from the second (January 2007 to December 2009) and the third era (August 2011 to April 2014). For each set of minutes, we reviewed the preconference plans suggested by the KHCC team (written in the agenda sent before the conference) and the postconference recommendations suggested after the interaction with the Sickkids team (written in the minutes). Any discrepancy between these plans was considered as a change. Details on the change type were recorded. Molecular and genetic testing recommendations were considered as a suggestion rather than a change, because of the lack of appropriate laboratory facilities at KHCC.
The video-teleconference connection was started as a six-channel integrated services digital network telephone line with an approximate cost of $360 for each 1-hour conference.8 Almost 4 years later, we shifted to high-speed Internet connection. The cost of the Internet connection is difficult to calculate because it is part of the institution’s Internet connection; however, it is estimated to be < $40/h. These costs are covered by KHCC. The initial videoconference unit was established at KHCC for $85,000 (TANDBERG6000 model; Cisco Systems, San Jose, CA).8 However, with the improvement in technology, in addition to the high-speed Internet connection, running the conference only needs a videoconference codec (the cost is between $5,000 and $10,000 on the basis of the quality and brand) with a camera, a computer, and a screen in a regular room. Initially, we used hard copies of computed tomography or magnetic resonance imaging scans that were projected via the document camera during the discussion. With the new radiology picture archiving and communication system recently implemented at KHCC, images can be viewed easily during the meeting.
The teleconference is held monthly with few exceptions. It is prescheduled for the whole year at the same time (8:00 am in Toronto, 3:00 pm in Amman) allowing for the 6 to 7 hours’ difference between Amman and Toronto depending on the seasons. Attendance from KHCC includes pediatric oncologist(s), one radiologist, one pathologist, one neurosurgeon, fellows, and nurse-coordinators; from Sickkids, attendees are neuro-oncologist(s), one neurosurgeon, and fellows. Cases for discussion are chosen by the KHCC team on the basis of their difficulty or on specific management questions. “New” cases, patients receiving therapy or “old” (ie, previously discussed) cases may be discussed more than once depending on the challenges faced during their treatment course. However, occasionally, straightforward cases are presented to trigger discussions about latest developments in treatment or updates on clinical trials. Short descriptions of each case are sent ahead of time to a mailing list that includes, besides the aforementioned team members, other oncologists and neurosurgeons from Jordan and nearby countries. Written notes are taken during the conference and, recently, video-recording of the discussion has been introduced. Minutes of discussions are distributed within a week of the meeting with some full-text articles mentioned during the conference (usually provided by the Sickkids team), allowing for more education and feedback.
RESULTS
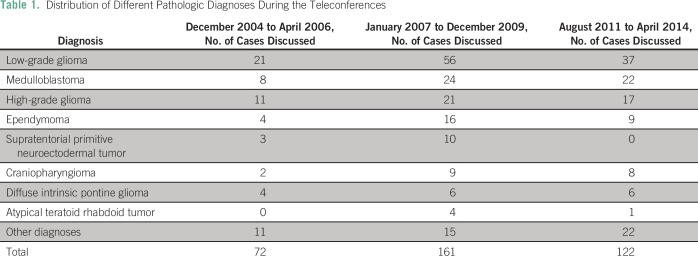
Since establishment of the teleconference, > 100 sessions were held and > 400 cases were discussed in these 10 years, with a median of four cases per teleconference (range, four to six cases). Management of different diagnoses was discussed, from the most common pathologies lke low-grade glioma (LGG) and medulloblastoma to more unusual entities such as papillary tumor of the pineal region or meningioma (Table1).
Table 1.
Distribution of Different Pathologic Diagnoses During the Teleconferences
Initial Era (2004 to 2006)
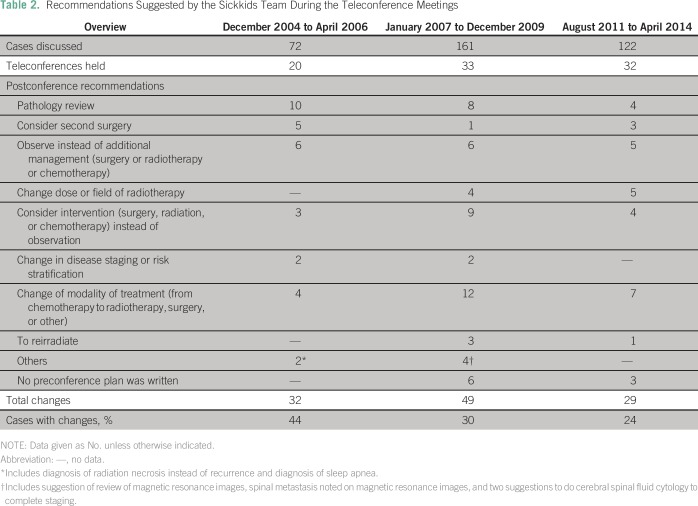
This experience was previously reported.8 During this period, 32 changes (44% of the total cases discussed) were suggested; all but two were followed. The most common recommendations were related to pathology review (31%) followed by observation instead of adjuvant treatment (19%).
Middle Era (2007 to 2009)
A total of 49 changes (30% of cases) were recommended; 12 (24%) involved suggestions to change the treatment modality. In nine cases, the Sickkids team thought an intervention would be better than observation and, in eight, they suggested pathology be reviewed. There was one suggestion to consider genetic testing and another one to test the tumor for BRAF fusion/ mutation.
Current Era (2011 to 2014)
A total of 29 changes (24% of cases) were suggested. Most (seven changes; 21%) involved preference of one treatment modality over another, whereas other recommendations were distributed among various items (Table 2). In seven cases, the recommendations were not followed; four were refused by the parents (a recommended biopsy for a suprasellar mass suspected to be germinoma rather than LGG, a recommended resection of a spinal LGG rather than observation, and, in two cases, debulking surgery for a meningioma and a chordoma was suggested before proceeding to radiotherapy). The three other cases included a nonmetastatic medulloblastoma with large residual for which repeated resection was recommended to downgrade risk staging before radiotherapy, a patient with spinal glioblastoma for whom the recommendation to use a wider field of radiation was not followed, and one patient who died before undergoing a biopsy for a suspected glioblastoma relapse.
Table 2.
Recommendations Suggested by the Sickkids Team During the Teleconference Meetings
Interestingly, during this more recent era, there were 16 suggestions for molecular testing, including BRAF fusion/mutation, medulloblastoma subgrouping, and genetic testing. In six cases (38%), the suggestions were followed (two BRAF mutation testing, two medulloblastoma subgrouping, one biallelic mismatch repair testing, and one 1p/19q testing). Until recently, however, these recommendations were not expected to change management, so they were considered as suggestions rather than real changes to the treatment plan.
Impact on Patients’ Care
Significant changes in treatment plans were suggested in a large proportion of patients (Table 2), ranging from 24% to 44% over this time. Personal interaction with the KHCC team facilitated implementation of most of these changes. The conferences introduced new concepts such as second-look surgery in ependymoma or the importance of medulloblastoma risk stratification, the role of chemotherapy over radiotherapy in treating pediatric LGG, the importance of avoiding radiotherapy delays, and the significance of pathology review in selected cases. In recent years, more discussions correlating molecular testing and pathology were held, with possible future implications. Lately, this facilitated the compassionate access to a BRAF inhibitor in a patient with disseminated recurrence of a pleomorphic xanthoastrocytoma after positive testing for BRAF mutation at the Sickkids laboratory. Several recent discussions as well emphasized the importance of suspecting cancer-predisposition syndromes. Such awareness led recently to early detection (with screening) and excision of a premalignant colonic polyp in a patient with glioblastoma suspected to have a cancer-predisposition syndrome.
During this experience, we did not formally evaluate the parents’ perception regarding these conferences. However, the feedback from the clinical team is that parents tended to feel more comfortable when they knew that their child’s treatment plan was discussed with international experts. Families felt that their child was receiving state-of-the-art care without the need to travel abroad and they perceived such teleconference discussions as a unique opportunity to answer their queries. It was also particularly helpful for families when the consensus was that there was no curative option and that palliative care was in the best interest of the child. It also helped increase their trust in the local team by knowing that there was continuous international interaction with an institution from a HIC.
DISCUSSION
The number of new pediatric patients (age < 18 years) treated annually in the neuro-oncology service at KHCC has doubled since the implementation of this twining initiative, which has reached 50 to 70 new patients with an additional 10 to 20 second-opinion consultations yearly—a quarter being non-Jordanians.16 Patients are treated in the context of a multidisciplinary approach and receive holistic care, including palliation and management of tumor- or treatment-related early- and long-term morbidities.
To our knowledge, this report describes the longest reported experience of teleconferencing in pediatric neuro-oncology. Our 10 years’ experience proved that such interaction is feasible at affordable costs and does not require sophisticated equipment, making this technique an appealing twinning tool for institutions in LMICs. Commitment from both institutions, interpersonal relationships, and motivation of both teams have been more critical to maintain this initiative than the availability of resources. In our initial report, we were questioning the need to sustain such twinning initiatives and their optimal duration. With more than 10 years’ follow-up, we can now partially answer this question. For example, we did not anticipate a turnover of the oncologists leading the KHCC neuro-oncology service. The departure of physicians triggered, each time, the need to urgently train the next generation in charge of the service. The teleconferencing program was an integral part of this training and has allowed young neuro-oncologists to rely on the expertise and feedback of the physicians from the twinning program.
Our experience also shows that over the years of running the conferences, team knowledge has increased. Despite the loss of experienced neuro-oncologists, the level of care was maintained, as illustrated by the gradual decrease in the percentage of plan changes. Management ideas were transferred to neurosurgeons, pathologists, radiation oncologists, and radiologists; consequently, the whole team was able to take part in the revision of the patient’s plan of care. Comments on pathology diagnoses or radiology findings declined with time and even when a pathology review was done during the most recent era, the original diagnosis was unlikely to change. Supporting documents, such as results of clinical studies or review papers distributed with the minutes, helped increase the knowledge of the local team members who may have limited access to medical journals or lack protected time to remain updated with recent literature. Yet, there remains a certain proportion of suggested changes despite 10 years of collaboration. The turnover of pediatric neuro-oncologists over this time may have contributed to this need for continuous support. This highlights the importance of retaining key members to avoid loss of gained experience, which is, by itself, a challenge in LMICs. However, over the years, the Toronto team has also been able to continuously suggest new ideas and advice regarding management especially adapted to local circumstances. This is mostly owing to their large clinical experience and the cutting-edge research going on in this program. This is particularly obvious with their ongoing research regarding the biallelic mismatch repair mutation syndrome, a condition particularly prevalent in the Middle East.17
There are still challenges in applying some of the recommendations made during the conferences. In particular, there is some reluctance to proceed to repeated surgical interventions or deliver reirradiation, because of fear of eliciting long-term morbidities. This is especially important in countries that lack rehabilitation services and in communities that stigmatize physical and mental morbidities. The distinction between clinical care and research interest when it relates to sharing tumor samples for additional diagnostic testing is another challenge. Until recently, there were no clear local regulations for tissue transfer or participation in international collaborative research, in addition to the lack of experience in dealing with consequences of some research results.18
As we are now moving into an era of unprecedented changes, with the development of molecular targeted therapy, institutions in LMICs need to overcome more challenges than just having access to the new tests. Coordinated multidisciplinary team work is a critical part of management decisions when treating CNS tumors. People need to integrate in their daily life the principle of multidisciplinary care, to accept alternatives to their own opinion, and consider that a change in the plan of care is not a failure but rather a success of a multidisciplinary team approach. Physicians in LMICs need to feel comfortable in asking for a second opinion when they face challenges. Twinning with centers in HICs can facilitate this. They can also provide a modified treatment plan based on available resources and logistics while strengthening and emphasizing local teamwork spirit.
Our review has some limitations. We understand that the real impact of these recommendations depends on the ability to follow the new plan, which was not always easy to track in every discussed case. As a result of the lack of data on patients with CNS tumors before initiation of this program, we are unable to make comparisons and to measure the real impact of this program on survival. However, considering the importance of the changes suggested and knowing that most recommendations were followed, we would assume that better care was delivered to many patients. A recent report of children diagnosed with CNS tumors at KHCC between 2007 and 201319 seems to support this statement, showing favorable survival rates for different pediatric CNS tumors (mean ± standard deviation: 93.8% ± 3.6% for LGG, 60.2% ± 7% for medulloblastoma, and 53.2% ± 12.2% for ependymoma).
As the concept of twinning evolves, we hope that the knowledge gained by the local team will restrict discussions to the most complicated cases, and opportunities for collaborative research or cooperative clinical trials will be explored. We are also aware that the experience gained needs to be shared to other hospitals in Jordan and nearby countries, to emphasize the concept of multidisciplinary team work and the importance of early referrals, for example. This would be the first step to standardize the care of pediatric CNS tumors throughout the region. The possibility of involving other institutions from LMICs in such teleconferencing initiatives using Internet technology is being assessed. This may allow more interactive discussions in the case presentations and offer the opportunity to share knowledge with more partners. It is clear that without local and international collaborations, it would be difficult to improve cancer care in LMICs.
ACKNOWLEDGMENT
We thank the administration of the pediatric department (Dr Faris Madanat and Dr Iyad Sultan, the former and current pediatric department chairs, respectively) and the higher administration of King Hussein Cancer Center (KHCC; Dr Mahmoud Sarhan and Dr Asem Mansour, the former and current CEO, respectively); Dr Abhaya Kulkarni from the Hospital for Sick Children and Dr Normand Laperriere from Princess Margaret Hospital in Toronto, Ontario, Canada, for their continued clinical advice; the information technology personnel from KHCC (Ameen Harb and Ramzi Abu-khader) and from Sickkids (Margaret Horie and Agnes Cheng Tsallis); and KHCC neuro-oncology clinical nurse coordinators (Kawther Khaleifeh and Raed Ramlawi).
Footnotes
This work was presented partially as a poster at the 9th SIOP Asia Congress, Amman, Jordan, April 25-27, 2015.
AUTHOR CONTRIBUTIONS
Conception and design: Nisreen Amayiri, Najiyah Abuirmeileh, Eric Bouffet
Collection and assembly of data: Nisreen Amayiri, Maisa Swaidan, Najiyah Abuirmeileh, Maysa Al-Hussaini, Ibrahim Qaddoumi, Hadeel Halalsheh
Data analysis and interpretation: Nisreen Amayiri, Tarik Tihan, James Drake, Awni Musharbash, Uri Tabori, Ute Bartels, Eric Bouffet
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Nisreen Amayiri
No relationship to disclose
Maisa Swaidan
No relationship to disclose
Najiyah Abuirmeileh
No relationship to disclose
Maysa Al-Hussaini
No relationship to disclose
Tarik Tihan
No relationship to disclose
James Drake
Patents, Royalties, Other Intellectual Property: I have several patents filed related to surgical robotic tools, which are not relevant to this manuscript.
Awni Musharbash
No relationship to disclose
Ibrahim Qaddoumi
No relationship to disclose
Uri Tabori
No relationship to disclose
Hadeel Halalsheh
No relationship to disclose
Ute Bartels
No relationship to disclose
Eric Bouffet
No relationship to disclose
REFERENCES
- 1.Ribeiro RC, Pui CH. Saving the children--Improving childhood cancer treatment in developing countries. N Engl J Med. 2005;352:2158–2160. doi: 10.1056/NEJMp048313. [DOI] [PubMed] [Google Scholar]
- 2.Masera G, Baez F, Biondi A, et al. North-South twinning in paediatric haemato-oncology: The La Mascota programme, Nicaragua. Lancet. 1998;352:1923–1926. doi: 10.1016/s0140-6736(98)07077-9. [DOI] [PubMed] [Google Scholar]
- 3.Howard SC, Pedrosa M, Lins M, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA. 2004;291:2471–2475. doi: 10.1001/jama.291.20.2471. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Ribeiro RC. International collaboration on childhood leukemia. Int J Hematol. 2003;78:383–389. doi: 10.1007/BF02983810. [DOI] [PubMed] [Google Scholar]
- 5.Veerman AJ, Sutaryo, Sumadiono Twinning: a rewarding scenario for development of oncology services in transitional countries. Pediatr Blood Cancer. 2005;45:103–106. doi: 10.1002/pbc.20390. [DOI] [PubMed] [Google Scholar]
- 6.Leander C, Fu LC, Peña A, et al. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49:817–819. doi: 10.1002/pbc.21052. [DOI] [PubMed] [Google Scholar]
- 7.Qaddoumi I, Nawaiseh I, Mehyar M, et al. Team management, twinning, and telemedicine in retinoblastoma: A 3-tier approach implemented in the first eye salvage program in Jordan. Pediatr Blood Cancer. 2008;51:241–244. doi: 10.1002/pbc.21489. [DOI] [PubMed] [Google Scholar]
- 8.Qaddoumi I, Mansour A, Musharbash A, et al. Impact of telemedicine on pediatric neuro-oncology in a developing country: The Jordanian-Canadian experience. Pediatr Blood Cancer. 2007;48:39–43. doi: 10.1002/pbc.21085. [DOI] [PubMed] [Google Scholar]
- 9.Baskin JL, Lezcano E, Kim BS, et al. Management of children with brain tumors in Paraguay. Neuro-oncol. 2013;15:235–241. doi: 10.1093/neuonc/nos291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler E, Alexis C, Ali Z, et al. Bridging the distance in the Caribbean: Telemedicine as a means to build capacity for care in paediatric cancer and blood disorders. Stud Health Technol Inform. 2015;209:1–8. [PubMed] [Google Scholar]
- 11.Abdelkhalek E, Sherief L, Kamal N, et al. Factors associated with delayed cancer diagnosis in Egyptian children. Clin Med Insights Pediatr. 2014;8:39–44. doi: 10.4137/CMPed.S16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Fiki M. African neurosurgery, the 21st-century challenge. World Neurosurg. 2010;73:254–258. doi: 10.1016/j.wneu.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Galindo C, Friedrich P, Morrissey L, et al. Global challenges in pediatric oncology. Curr Opin Pediatr. 2013;25:3–15. doi: 10.1097/MOP.0b013e32835c1cbe. [DOI] [PubMed] [Google Scholar]
- 14. Messing-Junger AM, Janssen G, Pape H, et al: Interdisciplinary treatment in pediatric patients with malignant CNS tumors. Childs Nerv Syst 16:742-750, 2000. [DOI] [PubMed]
- 15.Pollack IF. Multidisciplinary management of childhood brain tumors: A review of outcomes, recent advances, and challenges. J Neurosurg Pediatr. 2011;8:135–148. doi: 10.3171/2011.5.PEDS1178. [DOI] [PubMed] [Google Scholar]
- 16. King Hussein Cancer Foundation. Cancer registry. http://www.khcc.jo/section/cancer-registry.
- 17.Amayiri N, Tabori U, Campbell B, et al. High frequency of mismatch repair deficiency among pediatric high grade gliomas in Jordan. Int J Cancer 138:380-385, 2016. [DOI] [PubMed]
- 18.Denburg AE, Joffe S, Gupta S, et al. Pediatric oncology research in low income countries: Ethical concepts and challenges. Pediatr Blood Cancer. 2012;58:492–497. doi: 10.1002/pbc.23419. [DOI] [PubMed] [Google Scholar]
- 19.Amayiri N, Jamal K, Swaidan M, et al. Profile and outcome of pediatric CNS tumors: Experience from a center in a lower-income country. Pediatr Blood Cancer. 2015;62(abstr p-202; suppl 4):S143–S418. [Google Scholar]