Abstract
Purpose
Granulocyte-macrophage colony-stimulating factor (GM-CSF) cytokine stimulates growth, differentiation, and function of myeloid progenitors. We aimed to study the role of GM-CSF gene expression, its protein, and antibodies in patients with acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) and their correlation to disease behavior and treatment outcome. The study included 50 Egyptian patients with AML/MDS in addition to 20 healthy volunteers as control subjects.
Patients and Methods
Assessment of GM-CSF gene expression was performed by quantitative real-time polymerase chain reaction. GM-CSF proteins and antibodies were assessed by enzyme-linked immunosorbent assay.
Results
There was significant decrease in GM-CSF gene expression (P = .008), increase in serum level of GM-CSF protein (P = .0001), and increase in anti–GM-CSF antibodies (P = .001) in patients with AML/MDS compared with healthy control subjects. In addition, there was a significant negative correlation between serum levels of GM-CSF protein and initial peripheral blood blasts, percentage as well as response to therapy.
Conclusion
Any alteration in GM-CSF gene expression could have implications in leukemogenesis. In addition, GM-CSF protein serum levels could be used to predict outcome of therapy. GM-CSF antibodies may also play a role in the pathogenesis of AML/MDS. The use of these GM-CSF parameters for disease monitoring and as markers of disease activity needs further research.
INTRODUCTION
Acute myeloid leukemia (AML) is a heterogeneous group of leukemias that results from a genetic event or series of events occurring in an early hematopoietic precursor that both blocks differentiation and allows uncontrolled proliferation. The abnormally proliferating leukemic cells accumulate in the marrow space, eventually replacing normal marrow progenitors, with consequent diminished production of red cells, white cells, and platelets. This, in turn, leads to the common clinical manifestations of AML.1
The myelodysplastic syndromes (MDS) include a large spectrum of clonal hematopoietic stem cell disorders that are characterized by peripheral cytopenia(s), morphologic dysplasia, ineffective hematopoiesis, and a variable propensity to transform to AML.2
Granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulates multipotent progenitor cells depending on its concentration. First, it stimulates the proliferation of macrophage progenitors, which is followed by granulocyte, erythroid, eosinophil, megakaryocyte, and multipotent progenitors. It also stimulates the differentiation of myeloid leukemic cells and controls eosinophil function in some instances.3
The human GM-CSF gene is approximately 2.5 kilo-base pairs. The gene is located on the long arm of chromosome 5 (5q21–q32). It has four exons that are separated by three introns.4 In addition to GM-CSF, genes encoding various cytokines such as IL4, IL5, IL9, IL12, M-CSF, and the EGR1 gene are located at the 5q31.1 locus of chromosome 5.5 The 5q− syndrome has elucidated the role of these cytokines in development of clonal hematopoietic stem cells.6 The current study aimed to investigate and understand the role of GM-CSF in the pathogenesis, progression, and response to therapy in Egyptian patients with AML/MDS.
PATIENTS AND METHODS
Study Population
This study is an observational descriptive study and included 50 patients with AML/MDS. Patients were recruited from the outpatient clinic and the inpatient wards of Kasr Al Ainy Clinical Hematology Unit, Internal Medicine Department, and Clinical Oncology Department, Faculty of Medicine, Cairo University.
Patients in complete remission (CR) or those with a history of recombinant human GM-CSF intake were excluded. Twenty age- and sex-matched healthy volunteers were included in the study as a control group. The study was approved by the Research Ethical Committee of the Internal Medicine Department, Faculty of Medicine, Cairo University, and informed consent was obtained from all participants before enrollment in the study.
For the control subjects, a 4-mL EDTA blood sample was collected under completely aseptic conditions for molecular studies.
Patients’ samples at first presentation (either peripheral blood or bone marrow aspirated on EDTA and serum samples) were collected under completely aseptic conditions for molecular studies and enzyme-linked immunosorbent assay (ELISA) techniques, respectively.
Quantitative Assessment of GM-CSF Gene Expression
Extraction of total RNA was performed by QIAamp RNA Blood Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions. Total RNA was reverse transcribed using random primers with a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA).
GM-CSF gene expression was detected by real-time polymerase chain reaction on the basis of TaqMan technology using ABI Prism 7700 (Applied Biosystems.
The primers and probes for GM-CSF as well as the housekeeping gene GAPDH were provided by QIAGEN and were as follows. GM-CSF gene: forward primer 5′-CTGCTGAGATGAATGAAACAG-3′ and reverse primer 5′-TCCAAGATGACCATCCTGAG-3′; FAM (6-carboxy fluorescein) probe 5′-ACTCCCACCATGGCTGTGG-3′ (TaqMan GM-CSF, access no. M11220, Applied Biosystems). The thermocycler program conducted was initial denaturation at 50°C for 2 minutes followed by 40 cycles of denaturation at 95°C for 10 minutes, annealing at 95°C for 0.15 minute, and extension at 60°C for 1 minute. The relative quantification of gene expression was assessed by the 2−ΔΔCt method (ΔΔCt = {[Ct (GM-CSF sample) − Ct (GAPDH sample)] − [Ct (GM-CSF calibrator) − Ct (GAPDH calibrator)]}. The calibrator was the average ΔCt value of 20 controls.7
Assessment of GM-CSF Protein and Anti–GM-CSF Antibodies
The concentration of GM-CSF protein was measured in appropriately diluted sera from all patients with AML/MDS as well as healthy controls by using a specific ELISA assay (Quantikine human GM-CSF kit, catalog no. SGM00; R&D Systems, Minneapolis, MN). The minimum detectable dose of GM-CSF by this reagent is typically < 3 pg/mL.
The concentration of GM-CSF antibodies was measured in appropriately diluted sera from 42 patients with AML/MDS as well as healthy controls by using a specific ELISA assay (anti–GM-CSF Ab kit, catalog no. MBS162797; MyBioSource, San Diego, CA). The minimum detectable dose of GM-CSF antibodies by this reagent is typically < 0.52 ng/mL.
Treatment Regimen and Response to Therapy
All patients were treated according to the adopted protocol of the Internal Medicine Department, Faculty of Medicine, Cairo University. Patients with AML (except subtype M3 cases) were subjected to the 7-3 protocol for induction of remission. Subtype M3 patients were subjected to all-trans-retinoic acid. Treatment of AML depends on the fitness of the patient. Fit patients (< 60 years old) received intensive therapy. Treatment includes induction and postremission therapy (consolidation). Less fit patients (70 to 75 years and older, or younger patients with significant comorbidities) receive low-intensity therapy. For induction therapy, a combination of cytarabine and anthracycline or anthracenedione is recommended (cytarabine 100 to 200 mg/m2 continuous intravenous infusion for 7 days plus idarubicin 12 mg/m2/day for 3 days or daunorubicin 60 to 90 mg/m2/day for 3 days). Follow-up by bone marrow examination to assess remission is typically done 7 to 14 days after completion of induction chemotherapy. For postremission therapy, all patients should be assessed for risk of relapse. Specific drug regimens are recommended on the basis of a patient’s risk of relapse (eg, high-dose cytarabine 3 g/m2 intravenously over 3 hours every 12 hours on days 1, 3, and 5 for four cycles).
CR status is defined by normalization of the neutrophil count (≥ 1.5/μL) and platelet count (> 100 × 103/mm3), and marrow examination that demonstrates ≥ 20% cellularity, < 5% blasts and no Auer rods, as well as absence of extramedullary infiltration. Resistance to treatment is defined as > 25% blasts in the bone marrow, lack of regeneration of normal hematopoiesis, persistence of peripheral blood blasts, and/or extramedullary leukemia after induction. Relapse is defined as re-infiltration of the bone marrow by ≥ 5% leukemic blasts or proven leukemic blasts at any site. Death during induction is defined as death during or after the first course of therapy with aplastic or hypocellular marrow.8
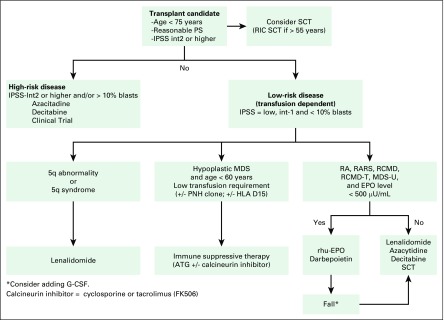
The only known curative modality for patients with MDS is stem-cell transplantation. Therefore, all appropriate candidates should be considered for stem-cell transplantation. They include patients younger than 70 years, with a reasonable performance status and no significant comorbidity (Fig 1).
Fig 1.
Algorithm for management of a patient with myelodysplastic syndrome (MDS). ATG, antithymocyte globulin; EPO, erythropoietin; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HLA D15, human leukocyte antigen-D15; int 1, Intermediate-1; IPSS, International Prognostic Scoring System; MDS-U, myelodysplastic syndromes-unclassifiable; PNH, paroxysmal nocturnal hemoglobinuria; PS, performance status; RA, refractory anemia; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-T, refractory cytopenia with multilineage dysplasia in transformation; rhu-EPO, recombinant human erythropoietin; RIC, reduced-intensity conditioning regimen; SCT, stem-cell transplantation.
Data Analysis
Data were analyzed using IBM SPSS advanced statistics version 20 (SPSS, Chicago, IL). Numerical data of scores were expressed as the mean and standard deviation or the median and range, as appropriate. Qualitative data were expressed as number and percentage. Data distribution is not normal (the skewness and kurtosis SPSS was used). Statistical differences between groups were tested using the χ2 test for qualitative variables. The Spearman-ρ method was used to test correlations between numeric variables. Survival analysis was done using the Kaplan-Meier method and comparison between two survival curves was done using the log-rank test. A P value < .05 was considered significant.
RESULTS
Twenty-seven of 50 patients (54%) were men and 23 of 50 (46%) were women. Their ages ranged from 20 to 91 years, with a median of 45.5 years. According to disease subtypes, 44 patients (88%) had AML and six patients (12%) had MDS. According to the French-American-British classification of AML, 14 patients (28%) were subtype M1, nine (18%) were M2, three (6%) were M3, nine (18%) were M4, four (8%) were M5, and five (10%) were M7. According to the Revised International Prognostic Scoring System (IPSS-R) for MDS risk assessment, three patients (50%) had intermediate IPSS-R category, two patients (33.3%) had low IPSS-R category, and one patient (16.6%) had very low IPSS-R category. Seventeen patients did not receive chemotherapy as the result of bad general condition, poor performance, or refusal. Among patients with AML who received chemotherapy, the CR rate was 15 of 33 patients (45.4%), the failure of induction rate was six of 33 (18.1%), and the mortality rate was 12 of 33 (36.36%). Of 15 patients who achieved CR, relapse was reported in five of them (33.3%) after follow up of 24 months. Overall survival (OS) ranged from 1 to 24 months, with a mean of 4 ± 4.04 months. The OS for patients who achieved CR ranged from 2 to 12 months, with a mean of 7.6 ± 3.8 months.
GM-CSF Gene Expression
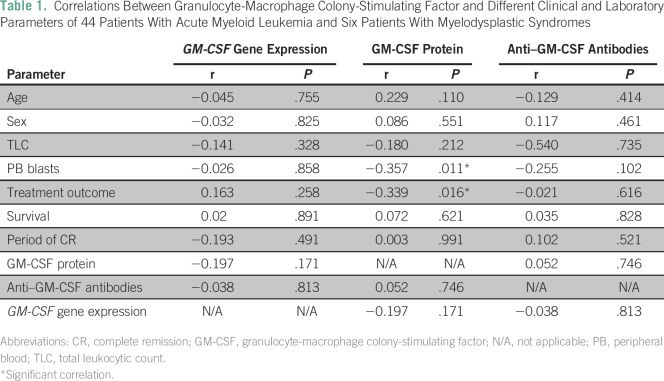
GM-CSF gene expression values in the 20 healthy controls ranged from 0.659 to 1.515, with a mean value of 0.62 ± 0.09 and a median value of 1.01. In the 50 patients with AML/MDS, GM-CSF gene expression values ranged from 0.0126 to 3.249, with a mean value of 0.629 ± 0.09 and a median value of 1.8. GM-CSF gene expression levels were significantly lower in patients with AML/MDS compared with controls (P = .008). We did not find significant correlations between GM-CSF gene expression and age, sex, clinical data, total leukocytic count, initial peripheral blood (PB) blasts percentage, treatment outcome, length of CR, and OS (Table 1).
Table 1.
Correlations Between Granulocyte-Macrophage Colony-Stimulating Factor and Different Clinical and Laboratory Parameters of 44 Patients With Acute Myeloid Leukemia and Six Patients With Myelodysplastic Syndromes
GM-CSF Protein and Anti–GM-CSF Antibodies
Serum GM-CSF protein concentrations in the healthy controls ranged from 7 to 8.6 pg/mL, with a mean of 7.6 ± 0.8 pg/mL and a median of 7.8 pg/mL. In the 50 patients with AML/MDS, serum GM-CSF protein concentrations ranged from 12 to 80 pg/mL, with a mean of 41.1 ± 18.4 pg/mL and a median of 40 pg/mL. We found higher GM-CSF protein concentrations in patients with AML/MDS compared with controls (P < . 001). We found a significant negative correlation between GM-CSF protein concentrations and initial PB blasts percentage (r = −0.357, P = .011) and treatment outcome (r = −0.339, P = .016). Otherwise, we did not find significant correlations between GM-CSF protein concentrations and age, sex, clinical data, other laboratory data, period of CR, and OS (Table 1).
Concentrations of serum anti–GM-CSF antibodies in the healthy controls ranged from 10 to 18 ng/mL, with a mean of 14 ± 4 ng/mL and a median of 14 ng/mL. In 42 of 50 patients with AML/MDS (84%), concentrations of serum anti–GM-CSF antibodies ranged from 37 to 240 ng/mL, with a mean of 64.4 ± 42.33 ng/mL and a median of 46 ng/mL. We found higher concentrations of serum anti–GM-CSF antibodies in patients with AML/MDS compared with controls (P = .001). We did not find significant correlations between concentrations of serum anti–GM-CSF antibodies and age, sex, clinical data, laboratory data, treatment outcome, period of CR, and OS (Table 1).
Response to Therapy in Patients With AML/MDS
After a 24-month observation period for the 38 patients who survived, the mean survival time was 4 ± 4.04 months (range, 1 to 24 months), with a median of 12 months. The mean CR was 7.6 ± 3.8 months (range, 2 to 12 months), with a median of 7 months.
In patients who did not receive chemotherapy, the mean survival time was 4.2 ± 2.4 months, with a median of 5 months. In patients who received chemotherapy, the mean survival time was 8.2 ± 4.5 months, with a median of 4 months. There was a highly significant association between mean survival time and receipt of chemotherapy (P = .006).
DISCUSSION
Growth and progression of leukemic cells are mediated by alterations in the microenvironment, which are often caused by an aberrant expression of growth factors and receptors.9 GM-CSF is an autocrine/paracrine cytokine that stimulates growth, differentiation, and function of normal and leukemic myeloid progenitors. Antibodies to GM-CSF are also implicated in the process of leukemogenesis.
This study aimed to investigate the roles of GM-CSF gene expression, serum GM-CSF protein concentrations, and levels of anti–GM-CSF antibodies in patients with AML/MDS in relation to treatment outcome and OS, to help understand their impact on the pathogenesis of the disease and hence predict prognosis, as well as response to treatment.
This study revealed that GM-CSF gene expression levels were significantly lower in patients with AML/MDS compared with controls. This is in accordance with previous studies,10,11 which reported that GM-CSF gene expression measured by real-time polymerase chain reaction was lower in patients with leukemia than in healthy volunteers. Also, it has been demonstrated that in vivo autocrine production of GM-CSF is not common in unperturbed AML, but allows the possibility that either autocrine or paracrine GM-CSF activity could be induced in leukemic cells under stress conditions.12 Furthermore, abnormalities in GM-CSF gene expression levels may contribute to the pathogenesis and abnormal proliferation of leukemia.13,14
In the current study, serum GM-CSF protein concentration was significantly higher in patients with AML/MDS compared with controls. This is in accordance with previous reports.15,16 Highly significant increases in serum levels of GM-CSF were previously reported in 14 Egyptian patients with AML compared with the reference control group.17 This was in contrast to results from another study, which reported comparable concentrations of serum GM-CSF in patients with AML and healthy controls.11
The current finding of a negative correlation between serum levels of GM-CSF and initial PB blasts percentage could be correlated to defects in the biologic functions of GM-CSF. These may be attributed to a functional alteration of the GM-CSF receptor or disturbances of signal transduction pathways, which need larger future studies. Furthermore, a lower serum level of GM-CSF was associated with better response to therapy, which suggests that GM-CSF protein concentration could be used as a biomarker to predict the outcome of therapy in AML/MDS cases.
In this study, higher levels of GM-CSF antibodies were found in patients with AML/MDS compared with controls. Our results are in agreement with a previous study that reported a high prevalence of anti–GM-CSF antibodies in patients with myeloid leukemia and MDS and a higher titer of GM-CSF antibodies in patients with active disease compared with patients in CR.11
In conclusion, our results suggest that GM-CSF expression levels may have implications in leukemogenesis. The use of GM-CSF protein levels and GM-CSF antibodies as prognostic markers of disease activity needs further investigation.
ACKNOWLEDGMENT
We thank Nashwa Medhat, MSc, Cairo University, for her technical efforts, sincere help, and interest in our work. Our work was performed in the Molecular Oncology Department of Kasr Al Ainy Centre of Clinical Oncology & Nuclear Medicine. Patients were recruited from the outpatient clinic and the inpatient wards of Kasr Al Ainy Clinical Hematology Unit, Internal Medicine Department, and Kasr Al Ainy Centre of Clinical Oncology Department, School of Medicine, Cairo University.
AUTHOR CONTRIBUTIONS
Conception and design: Neemat M. Kassem, Mervat M. Mattar
Financial support: Doaa M. El-Demerdash
Administrative support: Neemat M. Kassem, Alya M. Ayad, Mervat M. Mattar
Provision of study materials or patients: Noha M. El Husseiny, Doaa M. El-Demerdash, Hebatallah A. Kassem
Collection and assembly of data: Noha M. El Husseiny, Doaa M. El-Demerdash, Hebatallah A. Kassem
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Neemat M. Kassem
No relationship to disclose
Alya M. Ayad
No relationship to disclose
Noha M. El Husseiny
No relationship to disclose
Doaa M. El-Demerdash
No relationship to disclose
Hebatallah A. Kassem
No relationship to disclose
Mervat M. Mattar
No relationship to disclose
REFERENCES
- 1.Rubnitz JE, Gibson B, Smith FO: Acute myeloid leukemia. Hematol Oncol Clin North Am 24:35-63, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Brunning RD, Orazi A, Germing U: Myelodysplastic syndromes/neoplasms, overview, in Swerdlow SH, Campo E, Harris NL, et al (ed): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France, IARC Press, 2008, pp 88-107. [Google Scholar]
- 3.Nicola NA: Granulocyte colony-stimulating factor and differentiation-induction in myeloid leukemic cells. Int J Cell Cloning 5:1-15, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Huebner K, Isobe M, Croce CM, et al. : The human gene encoding GM-CSF is at 5q21-q32, the chromosome region deleted in the 5q- anomaly. Science 230:1282-1285, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Gasson JC: Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood 77:1131-1145, 1991 [PubMed] [Google Scholar]
- 6.Le Beau MM, Westbrook CA, Diaz MO, et al. : Evidence for the involvement of GM-CSF and FMS in the deletion (5q) in myeloid disorders. Science 231:984-987, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Wong ML, Medrano JF: Real-time PCR for mRNA quantitation. Biotechniques 39:75-85, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bacher U, Kern W, Schoch C, et al. : Evaluation of complete disease remission in acute myeloid leukemia: A prospective study based on cytomorphology, interphase fluorescence in situ hybridization, and immunophenotyping during follow-up in patients with acute myeloid leukemia. Cancer 106:839-847, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Witsch E, Sela M, Yarden Y: Roles for growth factors in cancer progression. Physiology (Bethesda) 25:85-101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young DC, Griffin JD: Autocrine secretion of GM-CSF in acute myeloblastic leukemia. Blood 68:1178-1181, 1986 [PubMed] [Google Scholar]
- 11.Sergeeva A, Ono Y, Rios R, et al. : High titer autoantibodies to GM-CSF in patients with AML, CML and MDS are associated with active disease. Leukemia 22:783-790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman DC, Baer MR, Gao XZ, et al. : Enhanced expression of the granulocyte-macrophage colony stimulating factor gene in acute myelocytic leukemia cells following in vitro blast cell enrichment. Blood 72:1329-1332, 1988 [PubMed] [Google Scholar]
- 13.Lang RA, Metcalf D, Gough NM, et al. : Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell 43:531-542, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Ymer S, Tucker WQ, Sanderson CJ, et al. : Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature 317:255-258, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Stauch M, Fritsche D, Höffken K: Cytokine serum levels during the course of acute myeloid leukemia, in Büchner T, Schellong G, Ritter J, et al (eds): Acute Leukemia VI. Haematology and Blood Transfusion / Hämatologie und Bluttransfusion, vol 38. Springer, Berlin, Heidelberg, 1997, pp 173-184. [Google Scholar]
- 16.Tao M, Li B, Nayini J, et al. : SCF, IL-1β, IL-1ra and GM-CSF in the bone marrow and serum of normal individuals and of AML and CML patients. Cytokine 12:699-707, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Elbaz O, Shaltout A: Implication of granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-3 (IL-3) in children with acute myeloid leukaemia (AML); malignancy. Hematology 5:383-388, 2001 [PubMed] [Google Scholar]