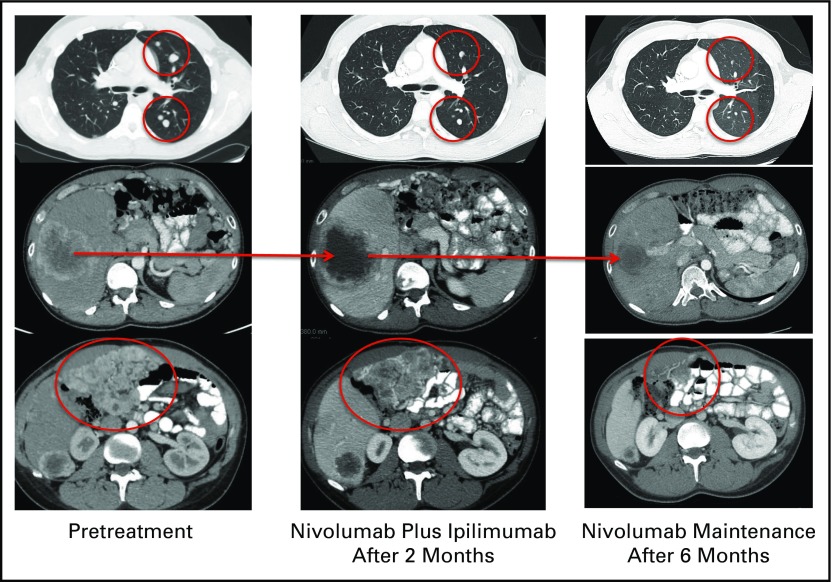
CASE PRESENTATION
A 29-year-old man was initially diagnosed with alveolar soft part sarcoma (ASPS) in May 2011 after presenting with acute abdominal pain requiring an exploratory laparotomy for an incarcerated internal jejunal hernia and an inflamed appendix. A 5.4-cm subdiaphragmatic mass was incidentally found during this procedure. The mass was removed. The patient had disease recurrence in May 2012 with distant metastases involving the lung, liver, pericardium, and omentum. The patient underwent surgical extirpation of liver and pericardial lesions. Further progression in lung metastases was noted by September 2012, and the patient underwent a right lung wedge resection. Between December 2012 and June 2015, he received multiple lines of vascular endothelial growth factor receptor (VEGFR)–targeted kinase inhibitors, including sunitinib, pazopanib, and axitinib, but the disease eventually progressed. Stable disease was the best response noted for each of these regimens before progression. Beginning in November 2015, he was treated with two cycles of liposomal doxorubicin with progression of disease.
The patient presented to our clinic in April 2016 for a second opinion. Despite intermittent abdominal pain, the patient still had a good performance status, with an Eastern Cooperative Oncology Group score of 0. PD-L1 immunohistochemical testing (clone RBT-PDL1; LifeSpan BioSciences) from archival tumor tissue revealed no expression of PD-L1. This tumor tissue was derived from a biopsy of the patient’s hepatic metastasis during his preceding treatment-free period. Using a next-generation sequencing platform to test the same liver metastasis, the patient was noted to have a single-nucleotide variant in the EGFR gene and a somatic deletion in the TP53 gene. No other alterations were detected from this panel. The patient was unable to be enrolled in an immediate clinical trial, and therefore commenced an ipilimumab plus nivolumab combination therapy off protocol at the beginning of June 2016. The patient received four cycles of intravenous nivolumab 1 mg/kg and ipilimumab 3 mg/kg on day 1 repeated every 21 days. After four cycles, ipilimumab was discontinued and maintenance therapy with nivolumab was continued at a dose of 3 mg/kg administered on day 1 of the 21-day cycle. Figure 1 presents an imaging assessment of ipilimumab plus nivolumab antitumor activity. After two cycles of combination ipilimumab and nivolumab, restaging studies demonstrated decrease in the size of bilateral metastatic pulmonary nodules. However, the liver metastasis and some of the extensive peritoneal implants slightly increased in size, with a decrease in intratumoral density. After four cycles of this combination therapy, the patient achieved a partial response (−51% from baseline), on the basis of Immune-Related Response Evaluation Criteria in Solid Tumors (irRECIST), with a substantial decrease in the size of multiple bilateral pulmonary metastases, liver metastases, and peritoneal implants. After three cycles of maintenance nivolumab, the tumor regression continued with a 69% decrease from baseline imaging. During the course of his treatment, the patient developed a grade 2 transaminitis after his third cycle of combination therapy. This was treated with a prednisone taper starting at 50 mg per day and temporary interruption of immunotherapy. The patient resumed therapy but continued on his taper of prednisone down to 2.5 mg per day. As the result of an increase in levels of ALT and AST, the patient’s dose of prednisone was increased to 15 mg per day. Interestingly, the patient has maintained response to therapy despite the low use of steroids.
Fig 1.
Imaging assessment of ipilimumab plus nivolumab antitumor activity.
DISCUSSION
ASPS is a rare soft tissue sarcoma (STS), accounting for < 1% of all STS cases.1 Molecularly, it is characterized by the ASPSCR1-TFE3 fusion gene, which is encoded by the unbalanced translocation der(17)t(X;17)(p11;q25).2 ASPS mostly affects young adults, with an age range at diagnosis of 19 to 35 years.3 Although considered a relatively indolent tumor, ASPS has high metastatic potential, commonly involving the lung, bone, and brain.1 The median overall survival for patients presenting with stage IV disease is approximately 40 months, with a 5-year overall survival rate of 20%.1 Unlike many other STSs, ASPS is resistant to traditional anthracycline-based chemotherapy.1 Recently, VEGFR-targeted small-molecule kinase inhibitors, such as sunitinib and cediranib, have demonstrated overall response rates of 35% to 50% in patients with metastatic ASPS.4,5 For ASPS refractory to VEGFR-targeted kinase inhibitors, there are no reliable salvage therapies; thus, there is an urgent need for new and effective treatments.
Checkpoint inhibitors are immuno-oncologic agents that potentiate T cell–mediated antitumor immunity. Ipilimumab, an anticytotoxic T-lymphocyte antigen 4, improves antitumor response through augmenting T-cell activation and proliferation.6 Nivolumab, a humanized immunoglobulin G4 monoclonal antibody against programmed cell death (PD-1), reverses T-cell exhaustion induced by the ligation of PD-1 receptor to its ligands, PD-L1 and PD-L2.6 With positive impact on overall survival, checkpoint inhibitors (such as ipilimumab, pembrolizumab, and nivolumab) have revolutionized the treatment approach to melanoma, non–small-cell lung cancer (NSCLC), and renal cell carcinoma.7-13 The number of emerging indications for checkpoint inhibitors is expected to grow to include many more human tumors. Furthermore, combined checkpoint blockade has been shown to produce substantially higher antitumor response and/or longer progression-free survival in patients with advanced melanoma or NSCLC when compared with monotherapy.13,14 In fact, ipilimumab plus nivolumab with nivolumab maintenance therapy has been FDA approved as front-line therapy for patients with unresectable or metastatic melanoma.
To date, several studies have evaluated the presence of immune infiltrate, including the expression of PD-1 and PD-L1, in various sarcoma subtypes.15-18 Expression of PD-1 and PD-L1 were noted on tumor and in the surrounding microenvironment, but the presence of these markers was variable among the diverse group of sarcomas, ranging from those with high PD-L1 expression (such as epithelioid sarcoma and undifferentiated pleomorphic sarcoma) to low PD-L1 expression in mesenchymal chondrosarcoma. In addition, several studies suggest an adverse prognosis associated with the presence of PD-L1 expression.15,18
The first signal of clinical activity of checkpoint inhibitors in sarcoma came from the SARC028 trial, a multicenter phase II study of pembrolizumab in patients with heavily pretreated advanced soft tissue and bone sarcomas.19 Four histologic subtypes (leiomyosarcoma, liposarcoma, undifferentiated pleomorphic sarcoma, and synovial sarcoma) were included in the STS arm. Among 37 evaluable patients, the overall response rate was 19%, with tumor response primarily observed in undifferentiated pleomorphic sarcoma and liposarcoma.19 Many clinical trials are ongoing to evaluate the safety and efficacy of checkpoint inhibitors, either as monotherapy or in combination, in patients with metastatic sarcoma.
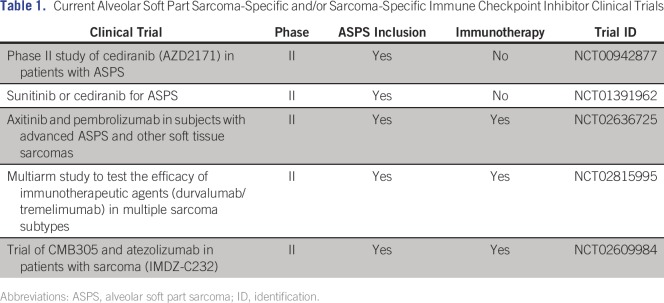
To the best of our knowledge, this is the first case report of clinical activity of combination checkpoint inhibitors in ASPS. Although rare cases of spontaneous regression in ASPS have been reported, it is unlikely that this is the explanation in the case of this patient, who achieved partial tumor response across multiple sites of metastatic disease after many years of continuous disease progression.20,21 On the other hand, spontaneous regression in ASPS, albeit unusual, suggests the presence of tumor immune surveillance in this disease, providing a rationale to evaluate the efficacy of immunotherapy in ASPS. A retrospective analysis of patients with sarcoma treated with nivolumab under a patient assistance program reported one patient with ASPS who concurrently received pazopanib and experienced stable disease followed by progression at month 10 of therapy.22 Considering that this study used RECIST version 1.1 for assessment of activity, it is unclear whether this patient’s stable disease indicated growth, shrinkage, or true stasis. Although SARC028 did not enroll patients with ASPS, several sarcoma-specific phase II studies of immune checkpoint inhibitors are ongoing and will shed light on the answer to this question (Table 1).
Table 1.
Current Alveolar Soft Part Sarcoma-Specific and/or Sarcoma-Specific Immune Checkpoint Inhibitor Clinical Trials
Several possibilities exist to explain the therapeutic effect of immune checkpoint blockade in ASPS. Because the ASPSCR1-TFE3 fusion is ubiquitous in ASPS, this fusion product would theoretically serve as a highly selective tumor-specific antigen. As previously described with other translocation-associated sarcomas, the breakpoint where both genes bind could serve as a neoantigen.23 We performed fusion antigen predictions using two fusion sequences: DPQQEQERER-LPVSGNLLDVYSSQG (type 1) and DPQQEQERER-IDDVIDEIISLESSY (type 2).24 We found at least one fusion peptide, which is potentially immunogenic: R-IDDVIDEI (R is from ASPS, IDDVIDEI is from TFE3). The NetMHC 4.0 server (http://www.cbs.dtu.dk/services/NetMHC-4.0) predicted a peptide–major histocompatibility complex class I (HLA-A*02:01) binding affinity of 114.6 nM. Clearly, this observation would need validation at least in an in vitro system, and it still does not address whether ASPS samples have normal expression of major histocompatibility complex class I molecules.
Interestingly, TFE3 may serve as a means to modulate immune activities in the surrounding microenvironment by several methods. First, TFE3 has been shown to upregulate genes of the transforming growth factor (TGF)–β pathway. TGF-β can promote and maintain the development of induced Trigs from naïve CD4+ T cells through unregulated expression of Foxp3.25 This, in turn, would affect CD8+ T-cell proliferation. Of note, TGF-β gene overexpression has been demonstrated from human ASPS samples.26 In addition, TFE3 plays an important role in the activation of CD40 ligand (CD40L) expression, an interaction that is critical for T cells, B cells, and antigen-presenting cells.27,28 Whether this interaction results in an inflamed state that favors expression of PD-1 and/or PD-L1 remains to be seen for this rare disease.
Although PD-L1 expression in his tumor was negative, the patient responded to the ipilimumab and nivolumab combination. This finding is consistent with prior experience with dual checkpoint blockade in melanoma, renal cell carcinoma, and NSCLC, where PD-L1 expression from pretreatment tumor specimens did not seem to correlate with response.29 A recent study that evaluated immune signatures from tumor samples obtained at multiple time points during the course of treatment with immune checkpoint inhibitors suggests that on-treatment changes in expression of various immune markers (including PD-1 and PD-L1) were of more value than a single assessment of these markers with pretreatment samples.30 Adding the inherent difficulties with evaluating PD-L1 expression in tumor tissues, which include the dynamic nature of expression, variable expression in different tissue sources (primary v metastatic lesions), heterogeneity of PD-L1 expression within the same lesion, discrepancy among various PD-L1 assays, and lack of standardized cutoff points,6 intratumoral PD-L1 expression status should not be used to select patients for checkpoint inhibitor therapies, especially in the case of dual checkpoint blockade.
In conclusion, ASPS is a fusion-driven, rare malignancy with a relatively quiet genome that is commonly treated with tyrosine kinase receptor inhibitors. Unfortunately, few options exist for patients with resistant disease. Although, to our knowledge, this case report represents the first documented response to combination immune checkpoint blockade in a patient with metastatic ASPS, clinical studies will be necessary to validate this observation. Lastly, a deeper understanding regarding the mechanism of action for this strong antitumoral immune response, with emphasis on the ASPSCR1-TFE3 fusion, may be necessary to improve therapeutic options for patients with ASPS.
AUTHOR CONTRIBUTIONS
Provision of study materials or patients: Alexander J. Lazar
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Anthony P. Conley
Consulting or Advisory Role: Nektar, Novartis
Van Anh Trinh
No relationship to disclose
Chrystia M. Zobniw
No relationship to disclose
Kristi Posey
No relationship to disclose
Jaime D. Martinez
No relationship to disclose
Oscar G. Arrieta
Honoraria: Bristol-Myers Squibb
Expert Testimony: Bristol-Myers Squibb
Wei-Lien Wang
No relationship to disclose
Alexander J. Lazar
Stock or Other Ownership: ArcherDX
Honoraria: Novartis, Bristol-Myers Squibb, Genentech
Consulting or Advisory Role: Novartis, Bristol-Myers Squibb, ArcherDX
Neeta Somaiah
Consulting or Advisory Role: Bayer
Jason Roszik
No relationship to disclose
Shreyaskumar R. Patel
Consulting or Advisory Role: Novartis, Janssen Products, CytRx, Eli Lilly, Epizyme, Eisai, Bayer
Research Funding: Janssen Pharmaceuticals, Eisai, Morphotek
REFERENCES
- 1.Portera CA, Jr, Ho V, Patel SR, et al. : Alveolar soft part sarcoma: Clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer 91:585-591, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Jaber OI, Kirby PA: Alveolar soft part sarcoma. Arch Pathol Lab Med 139:1459-1462, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Herzog CE: Overview of sarcomas in the adolescent and young adult population. J Pediatr Hematol Oncol 27:215-218, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kummar S, Allen D, Monks A, et al. : Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 31:2296-2302, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stacchiotti S, Negri T, Zaffaroni N, et al. : Sunitinib in advanced alveolar soft part sarcoma: Evidence of a direct antitumor effect. Ann Oncol 22:1682-1690, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Taube JM, Anders RA, et al. : Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275-287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, O’Day SJ, McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711-723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Schachter J, Long GV, et al. : Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521-2532, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Brahmer J, Reckamp KL, Baas P, et al. : Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 373:123-135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst RS, Baas P, Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non–small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellman MD, Gettinger SN, Goldman JW, et al: CheckMate 012: Safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. J Clin Oncol 34, 2016 (suppl; abstr 3001)
- 15.Kim JR, Moon YJ, Kwon KS, et al. : Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One 8:e82870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Angelo SP, Shoushtari AN, Agaram NP, et al. : Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol 46:357-365, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paydas S, Bagir EK, Deveci MA, et al. : Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med Oncol 33:93, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Kim EK, Jung H, et al. : Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 16:434, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tawbi HA, Burgess MA, Crowley J, et al: Safety and efficacy of PD-1 blockade using pembrolizumab in patients with advanced soft tissue (STS) and bone sarcomas (BS): Results of SARC028—A multicenter phase II study. J Clin Oncol 34, 2016 (suppl; abstr 11006)
- 20.BaniHani MN, Al Manasra AR: Spontaneous regression in alveolar soft part sarcoma: Case report and literature review. World J Surg Oncol 7:53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reference deleted.
- 22.Paoluzzi L, Cacavio A, Ghesani M, et al. : Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res 6:24, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng WW, Somaiah N, Engleman EG: Potential for immunotherapy in soft tissue sarcoma. Hum Vaccin Immunother 10:3117-3124, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vistica DT, Krosky PM, Kenney S, et al. : Immunohistochemical discrimination between the ASPL-TFE3 fusion proteins of alveolar soft part sarcoma. J Pediatr Hematol Oncol 30:46-52, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Travis MA, Sheppard D: TGF-β activation and function in immunity. Annu Rev Immunol 32:51-82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazar AJ, Das P, Tuvin D, et al. : Angiogenesis-promoting gene patterns in alveolar soft part sarcoma. Clin Cancer Res 13:7314-7321, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Huan C, Kelly ML, Steele R, et al. : Transcription factors TFE3 and TFEB are critical for CD40 ligand expression and thymus-dependent humoral immunity. Nat Immunol 7:1082-1091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elgueta R, Benson MJ, de Vries VC, et al. : Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 229:152-172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callahan MK, Postow MA, Wolchok JD: CTLA-4 and PD-1 pathway blockade: Combinations in the clinic. Front Oncol 4:385, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen PL, Roh W, Reuben A, et al. : Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 6:827-837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]