Abstract
Among their pleiotropic functions, scaffold proteins are required for the accurate coordination of signaling pathways. It has only been within the past 10 years that their roles in glucose homeostasis and metabolism have emerged. It is well appreciated that changes in the expression or function of signaling effectors, such as receptors or kinases, can influence the development of chronic diseases such as diabetes and obesity. However, little is known regarding whether scaffolds have similar roles in the pathogenesis of metabolic diseases. In general, scaffolds are often underappreciated in the context of metabolism or metabolic diseases. In the present review, we discuss various scaffold proteins and their involvement in signaling pathways related to metabolism and metabolic diseases. The aims of the present review were to highlight the importance of scaffold proteins and to raise awareness of their physiological contributions. A thorough understanding of how scaffolds influence metabolism could aid in the discovery of novel therapeutic approaches to treat chronic conditions, such as diabetes, obesity, and cardiovascular disease, for which the incidence of all continue to increase at alarming rates.
Transduction of receptor-mediated signaling pathways requires upstream receptors at the cell membrane and their downstream effector molecules, such as kinases or transcription factors. Precise coordination of signaling events is necessary to ensure that stimulus-dependent effects are accurately evoked in a particular cell type, and dysregulation of signaling events can lead to the development of diseases, including cancer (1–3). Molecular scaffolds or adaptor proteins (herein referred to as scaffolds) have been proposed to be the principal regulators that ensure such coordination (4, 5). Scaffolds can bind to one or more signaling effectors to facilitate their spatial or temporal localization within a cell and also regulate the cellular functions of various effectors, including those involved in metabolic signaling pathways (4–6). For example, scaffolds belonging to the 14-3-3 protein family interact with the phosphorylated form of the transcription factor, FOXO1, leading to it sequestration in the cytoplasm and inhibition of its transcriptional activity (7). Furthermore, they can regulate the enzymatic activity of kinases, such as hormone-sensitive lipase (HSL) (8).
Despite the knowledge that scaffolds are needed to accurately coordinate signaling pathways, it is only within the past 10 years that their roles in metabolism have emerged. For decades, receptors and kinases have been considered to be the key signaling effectors responsible for metabolic processes, such as glucose uptake, lipolysis, and insulin secretion (9, 10). However, little is known whether scaffolds have similar importance in metabolic processes or in the pathogenesis of metabolic diseases, such as diabetes and obesity. Thus, scaffolds have often been underappreciated in the context of metabolism and metabolic diseases.
In this mini-review, we have summarized the contributions of various scaffold proteins to metabolism and cardiometabolic diseases. We describe their participation in processes underlying whole-body metabolism and whether alterations in their function might contribute to disease development. Overall, we sought to highlight the importance of scaffolds in metabolism and metabolic diseases and to raise awareness of the need to consider their functions when developing new therapeutic targets for the treatment of obesity, diabetes, and cardiovascular disease.
Scaffold Proteins and Their Importance to Cellular Signaling/Processes
The role of scaffold proteins in coordinating cellular signaling events have been reviewed in several recent publications (11–15). We briefly discuss their importance in signal transduction pathways related to metabolic regulation.
Many hormones, such as incretins, catecholamines, and glucagon, exert their cellular effects through the activation of 7-transmembrane G protein–coupled receptors (GPCRs) (13, 16–20). Scaffolds of GPCRs might directly regulate receptor signaling by acting as allosteric modulators of receptor conformation (21). For example, after agonist binding, changes in receptor conformation can lead to enhanced interaction of scaffold proteins with G proteins and GPCRs (13, 21, 22). Most scaffolds that interact with GPCRs contain PDZ (PSD-95/Discs-large/ZO-1 homology) domains, which facilitate their interactions with the carboxyl-termini of other proteins (23). One of the best characterized scaffold families that interacts with GPCRs and G proteins are the β-arrestins, and their metabolic functions have been discussed in the subsequent sections.
Scaffolds proteins are also involved in promoting the association of ion channels with other signaling molecules in specific microdomains. For example, the activity and localization of transient receptor potential (TRP) channels, which are involved in intracellular Ca2+ homeostasis (24), are determined by their interactions with various scaffolds. Homer proteins, including Homer1, Homer2, and Homer3, are scaffolds that were first identified in the brain and have been shown to interact with TRP channels (25, 26). Enhanced spontaneous Ca2+ influx in cells from Homer1 knockout mice has been observed due to increased TRP channel activity (26). Impairment of Homer function might also attenuate thrombin-evoked Ca2+ entry and the maintenance of store-operated Ca2+ entry in human platelets, which demonstrates cell type-dependent functions (27). In addition to the Homer protein family, a number of additional scaffold proteins are associated with TRP proteins. TRPC4 contains a PDZ-interacting domain at the C-terminus, which is an interaction site with scaffolds such as EBP50/NHERF. A TRPC4 mutant lacking the ability to interact with EBP50/NHERF leads to intracellular accumulation. In contrast, wild-type channels that can bind to this scaffold will be uniformly distributed in the plasma membrane in HEK-293 cells (28).
The activity of the heat-activated ion channel TRPV1 is modulated by protein kinase A (PKA) and protein kinase C (PKC), in addition to the phosphatase calcineurin. The interactions between TRPV1 and PKA, PKC, or calcineurin are dependent on the ability of AKAP150 to organize and form a signaling complex (29). RACK-1 (receptor for activated C kinase 1) is another scaffold protein involved in the regulation of TRP channels and has been reported to inhibit TRPP3 channel activity (30). It also facilitates the association of TRPC3 with a number of proteins, including type I IP3R, STIM1, and Orai1 (31, 32). Finally, caveolins also display scaffold functions because they promote the localization TRPC1 channels within lipid raft domains and aid in the internalization of insulin receptors (33–35). Caveolin-1 has also been found to have a predominant role in insulin granule exocytosis and insulin signaling in metabolic target tissues (34, 36).
Receptor tyrosine kinases (RTKs) and their downstream signaling pathways have important roles in development and metabolic homeostasis. RTKs can include, but are not limited to, the epidermal growth factor receptor (EGFR), fibroblast growth factor receptor (FGFR), vascular endothelial growth factor receptor (VEGFR), and the insulin receptor families (37–40). In the case of RTKs, autophosphorylation at NXXY motifs recruit scaffolds that contain phosphotyrosine-binding domains. These scaffolds include members of the insulin-receptor substrate (IRS), Dok, FGF-receptor substrate 2, and Shc families (37–41). Once associated with an RTK, the scaffold protein is phosphorylated at tyrosine motifs, resulting in the recruitment of Src homology (SH) 2 domain proteins and the subsequent activation of intracellular pathways (42). This series of events has been well-described with respect to the scaffold Shc1 (43, 44). The Shc1 gene encodes three proteins of 46, 52, and 66 kDa that share an N-terminal phosphotyrosine-binding domain and a C-terminal SH2 domain, flanking a central region (CH1) containing key phosphorylation sites (Tyr239/240 and Tyr313) (44). Modification of these phosphorylation sites creates pYXN binding motifs for the SH2 domain of the Grb2 adaptor. Through its SH3 domains, Grb2 recruits proteins, such as Sos1 and Gab1, and cause downstream activation of ERK and phosphatidylinositol 3′-kinase (PI3K)/protein kinase B (Akt) pathways (45, 46). Collectively, the pleiotropic roles of scaffolds in various signaling pathways demonstrate their importance in cell biology; however, whether scaffolds are required for metabolic signaling pathways is unclear.
Scaffolds With Known Metabolic Roles
Although many scaffold proteins have been identified, it is not clear whether they are all involved in the regulation of metabolic homeostasis. Some scaffolds have multiple isoforms, suggesting that they might have redundant cellular functions in metabolic signaling pathways. However, those with well-established roles in metabolism have been discussed in subsequent paragraphs (Table 1).
Table 1.
Involvement of Scaffolds in Cardiometabolic Functions
| Scaffold Protein | Model | Modifications | Metabolic Outcome | Reference |
|---|---|---|---|---|
| β-Arrestin 1 | INS1 cells | siRNA knockdown | Impaired GSIS | Sonoda et al. (47) |
| Overexpression | Protection from glucose-apoptosis | Quoyer et al. (48) | ||
| Mouse | Systemic knockout | Increased adiposity | Ruderman et al. (49) | |
| Decreased insulin sensitivity | ||||
| Potentiated HFD-induced obesity | ||||
| Mouse cardiomyocytes | Overexpression | Resistance to apoptosis | Song et al. (50), Eguchi et al. (51), and Nackiewicz et al. (52) | |
| β-Arrestin-2 | Mouse | Liver-specific knockout | Increased gluconeogenesis | Zhu et al. (53) |
| Hepatocytes | Overexpression | Decreased gluconeogenesis | Zhu et al. (53) | |
| Mouse cardiomyocytes | Overexpression | Stress-induced cardiac damage | Eguchi et al. (51) | |
| Mouse | β-Cell specific knockout | Worsened glucose tolerance after HFD | Zhu et al. (54) | |
| GSIS- and KCl-induced insulin secretion reduced | ||||
| β-Cell overexpression | Enhanced GSIS | Zhu et al. (54) | ||
| Human EndoC-βH1 cells | siRNA knockdown | Impaired GSIS | Zhu et al. (54) | |
| AKAPs | β-Cell | AKAP150 systemic knockout | Impaired insulin secretion | Hinke et al. (55) |
| Impacts l-type Ca2+currents | ||||
| Attenuates cytoplasmic accumulation of Ca2+ and cAMP | ||||
| Muscle | AKAP150 systemic knockout | Increased insulin sensitivity | Hinke et al. (55) | |
| Mouse cardiomyocytes | AKAP18α overexpression | Regulate Ca2+ influx via PKA in response to β-adrenergic stimulation | Gray et al. (56) and Fraser et al. (57) | |
| AKAP5 systemic knockout | Impaired trafficking and signaling of β1AR | Klauck et al. (58) | ||
| KSR2 | C2C12 cells | Expression of KSR2 mutants with human mutations | Reduced glucose oxidation | Pearce et al. (59) |
| Increased fatty acid oxidation | ||||
| Mouse | Systemic knockout | Develops obesity | Liu et al. (60) | |
| Hyperinsulinemia | ||||
| Glucose intolerance | ||||
| Increased AMPK-dependent glucose uptake | ||||
| Hypophagic | ||||
| NCK1/2 | MIN6 cells | Nck1 knockdown by siRNA | Improves β-cell function | Yamani et al. (61) |
| Resistance to ER stress | ||||
| Mouse | Systemic Nck1 knockout | Improves glucose homeostasis | Latreille and Larose (62) | |
| Enhanced hepatic insulin signaling | ||||
| Protects against β-cell apoptosis | ||||
| Mouse | Systemic Nck2 knockout | Increased adiposity | Dusseault et al. (63) | |
| 14-3-3 Proteins | MIN6 cells | Pharmacological inhibition | Apoptosis | Lim et al. (4) |
| Mouse islets | Pharmacological inhibition | Apoptosis | Lim et al. (4) | |
| 14-3-3ζ | MIN6 cells | siRNA knockdown | Apoptosis | Lim et al. (4) |
| MIN6 cells | Overexpression | Protection from apoptosis | Lim et al. (4) | |
| 3T3-L1 preadipocyte | siRNA knockdown | Inhibition of adipogenesis | Lim et al. (64) | |
| Mouse | Systemic knockout | Impaired visceral adipogenesis | Lim et al. (64, 65) | |
| Insulin resistance | ||||
| Improved oral glucose tolerance via GLP-1 | ||||
| Mouse | Systemic overexpression | Enhanced age- and HFD-induced weight gain | Lim et al. (64) | |
| Homer1 | Human platelets | Knockdown | Increases TRP channel activity | Jardin et al. (27) |
| Enhanced spontaneous Ca2+ influx | ||||
| Caveolin-1 | Mouse | Systemic knockout | HFD-induced postprandial hyperinsulinemia | Cohen et al. (33) and Razani et al. (34) |
| Decreases insulin sensitivity | ||||
| Alterations in lipid homeostasis | ||||
| Resistant to HFD-induced obesity | ||||
| MIN6 cells | siRNA knockdown | Increases basal insulin secretion | Boothe et al. (35) | |
| MIN6 cells | siRNA knockdown | Impaired insulin receptor internalization and ERK activation | Nevins et al. (36) | |
| JIP1 | INS1 cells | siRNA knockdown | Increases susceptibility to apoptosis | Morel et al. (66) |
| Mouse | Systemic knockout | Insulin resistance | Jaeschke et al. (67) |
Abbreviations: AMPK, AMP-activated protein kinase; ER, endoplasmic reticulum; GSIS, glucose-induced insulin secretion; HFD, high-fat diet; KSR, kinase suppressor of RAS; Nck, noncatalytic region of tyrosine kinase; siRNA, small interfering RNA.
β-Arrestins
Receptors belonging to the β-adrenergic receptor (βAR; βAR1, βAR2, and βAR3) are GPCRs that have been studied extensively in terms of their structure, regulation, and downstream signaling pathways (13, 68, 69). Scaffold proteins have been found to be necessary for efficient internalization of GPCRs and the initiation of downstream signaling events (68, 70–72). For example, β-arrestin 1 and 2 are prototypical GPCR scaffolds that translocate to the plasma membrane after agonist stimulation, where they are recruited to βARs and phosphorylated by GPCR kinases. This results in agonist-induced desensitization of βARs, as a result of their internalization or degradation and subsequent recycling to the plasma membrane (Fig. 1) (73–75). Although this has demonstrated that β-arrestins can have negative effects on GPCR signaling (76, 77), recent studies have also revealed that β-arrestins are required for GPCR-dependent metabolic signaling pathways (78–82). Activation of the glucagon-like peptide-1 (GLP-1) receptor (GLP-1R) involves Gs-proteins for cAMP production, and β-arrestin 1 was shown to be required for GLP-1–induced cAMP synthesis and insulin secretion from INS-1 β-cells (47). GLP-1 potentiates insulin secretion from β-cells in a glucose-dependent manner, and β-arrestin 1 knockdown in INS1 cells impaired GLP-1–induced insulin release due to reduced cAMP production and decreased activation of PKA and PKA-independent pathways (47, 83–85). Also, β-arrestin 1 was found to mediate the antiapoptotic effect of GLP-1 in β-cells by influencing GLP-1–induced ERK activation and promoting the phosphorylation of Bad on Ser112, an established inhibitory phosphorylation site (48). In pancreatic β-cells, GLP-1R signaling events associated with insulin secretion or β-cell survival have been well-characterized. However, when considering the role of scaffolds such as β-arrestin 1, this has demonstrated the increased complexity of GLP-1 actions. Despite a high degree of homology, β-arrestin 2 and its functions in β-cells differ. Systemic deletion in mice has been associated with impaired β-cell mass expansion in response to a high-fat diet (86). Furthermore, systemic β-arrestin 2 knockout mice do not display defects in insulin secretion, and Zhu et al. (54) reported that β-cell–specific deletion or overexpression of β-arrestin 2 impaired or potentiated insulin secretion, respectively. Moreover, depletion of β-arrestin 2 in the human β-cell line, EndoC-βH1, was also associated with decreased insulin secretory function (54).
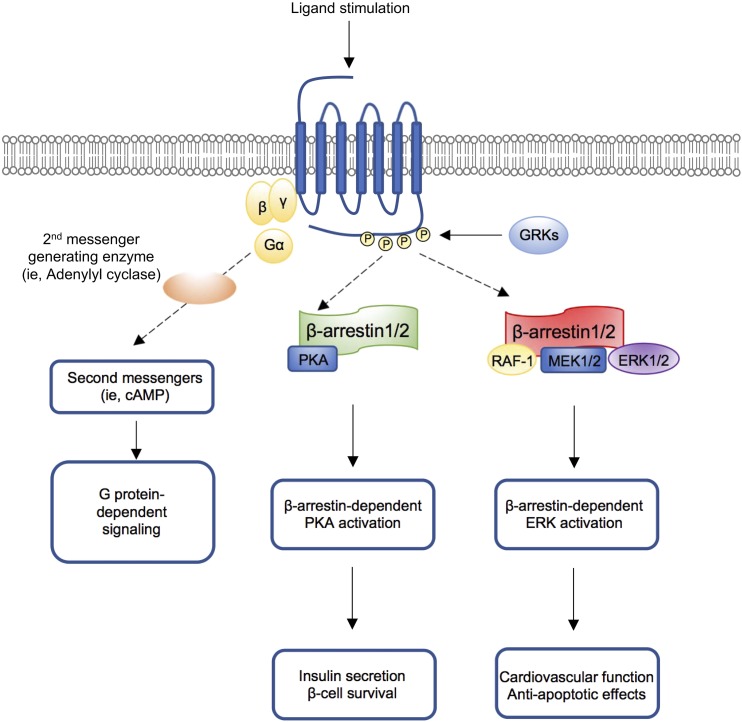
Figure 1.
A model for β-arrestin–mediated regulation of metabolic processes. Ligand binding stimulates the activation of a GPCR, leading to the dissociation of the G protein heterotrimeric complex and subsequent initiation of G protein-dependent signaling. The ligand-occupied GPCR is phosphorylated by G protein–related kinases (GRKs), followed by the recruitment of β-arrestin 1/2 to the phosphorylated GPCR. β-Arrestin 1/2 subsequently interacts with proteins belonging to cAMP–PKA or ERK1/2 pathways to promote metabolic responses.
β-Arrestins were also found to be involved in liver metabolism. Selective inactivation of β-arrestin 2 in hepatocytes of adult mice results in glucose intolerance and enhanced hepatic glucagon receptor signaling and glucagon-induced glucose mobilization (53). Hepatic insulin sensitivity and β-adrenergic signaling were not affected. Hepatocyte-specific overexpression of β-arrestin 2 greatly reduced hepatic glucagon receptor signaling and protected mice against the metabolic deficits caused by the consumption of a high-fat diet. Control mice displayed glucose intolerance, increased gluconeogenesis, and enhanced glucagon sensitivity compared with mutant mice (68).
cAMP-dependent kinase–anchoring proteins
cAMP-dependent kinase–anchoring proteins (AKAPs) are scaffolds involved in GPCR signaling and play central roles in facilitating GPCR association with protein kinases and phosphatases (Fig. 2) (6, 87, 88). AKAPs can also associate with phosphodiesterases that degrade the second messenger, cAMP (89). Multiple AKAP proteins have been identified, and one, AKAP150 (encoded by AKAP5), has been shown to coordinate key aspects of adrenergic signaling, such as Ca2+ cycling and excitation–contraction in cardiomyocytes (90). In addition to binding to PKA, AKAP150 can also bind to the phosphatase calcineurin (also known as PP2B), which has been identified as a central regulator of cardiac hypertrophy (91). Specific to glucose homeostasis, AKAP150-null mice secrete less insulin from β-cells but display improved glucose handling owing to increased insulin sensitivity in skeletal muscle (55). This metabolic phenotype is retained in AKAP150ΔPIX mice that lack a seven amino acid sequence required for interactions with calcineurin (55). Hence, AKAP150 has pleiotropic functions in the regulation of cardiomyocyte function and metabolic homeostasis.
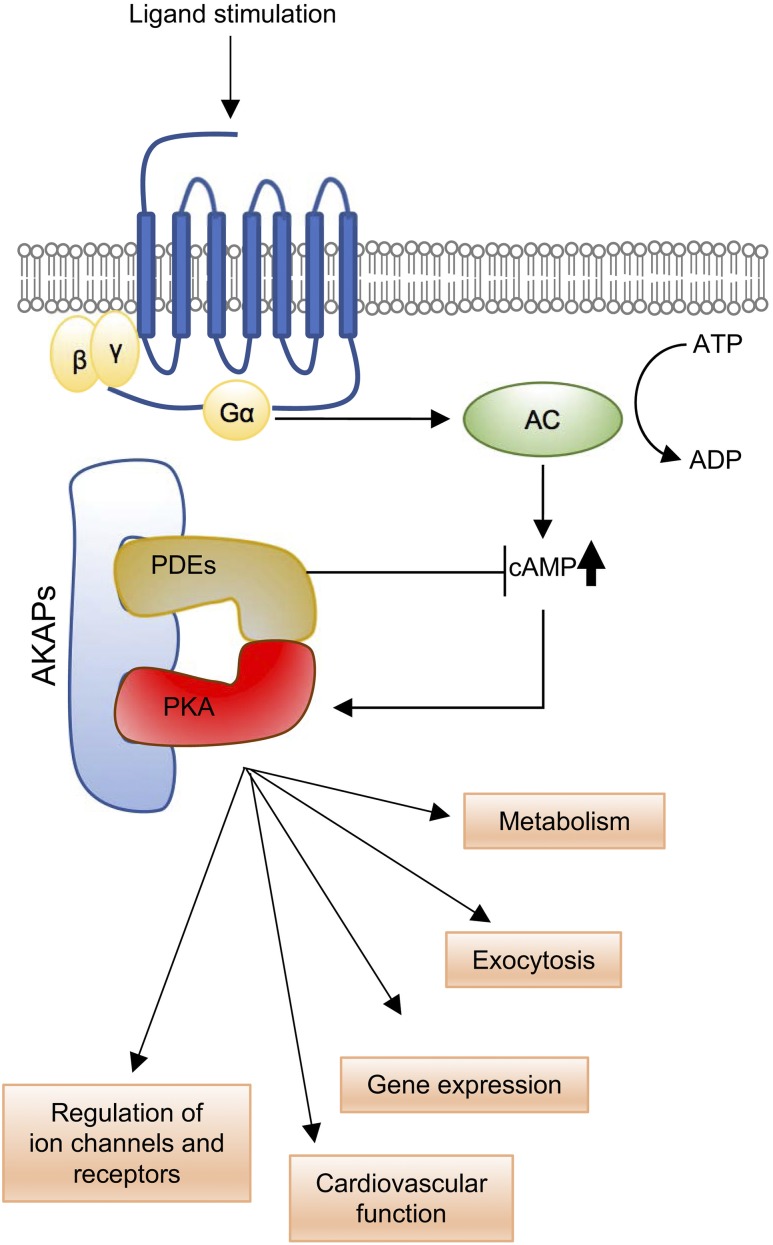
Figure 2.
Basic scheme of AKAP–PKA signaling. Ligand-activated GPCRs that transduce via the Gα subunit of the heterotrimeric G protein complex cause the activation of adenylyl cyclase (AC), which catalyzes the formation of cAMP. Binding of cAMP to the PKA complex leads to dissociation of the catalytic subunits from the holoenzyme to activate PKA. Attenuation of cAMP-dependent signaling is facilitated by phosphodiesterases (PDEs), which hydrolyze cAMP to modulate the duration and extent of cAMP-dependent signaling. AKAP family members function as scaffolds to bring together PKA and PDEs in close proximity to ensure precise cAMP-dependent PKA signaling. Reductions in AKAP expression or function can lead to deleterious effects on metabolic and cardiovascular physiology.
Insulin receptor substrates
Although not often thought of as scaffolds, IRS proteins represent a critical group of proteins required for downstream transduction of insulin and IGF-1 receptor signaling events, including activation of PI3K. Among its pleiotropic metabolic effects, insulin is essential for stimulating glucose uptake in peripheral tissues, and defects at various steps of the insulin-signaling pathway promote insulin resistance, which is one of the earliest metabolic abnormalities detected in individuals with the metabolic syndrome (92, 93). Tyrosine phosphorylation of IRS molecules is normally required for transducing the signals from activated receptors; however, phosphorylation of serine/threonine residues on IRS proteins has negative effects on their function (94, 95). For example, c-Jun NH2-terminal kinase (JNK) phosphorylates IRS1 at serine and threonine residues, which prevents the recruitment of IRS1 to the insulin receptor and inhibits insulin signaling (96).
JNK-interacting proteins (JIPs) are scaffold proteins involved in coordinating JNK signaling (97, 98), and they have also been found to interact with the mixed-lineage protein kinase group of MAPKs, MKK7, and AKT (97). IRS and JIP scaffold proteins might function independently to regulate insulin- and JNK-dependent signal transduction, respectively; however, these scaffolds can exhibit cross-talk. This was exemplified by a study using Jip1 knockout mice, which exhibit decreased high-fat diet-induced JNK activation in white adipose tissue, phosphorylation of IRS1 on Ser-307, and insulin resistance (67).
Kinase suppressor of RAS
Several scaffold proteins have been identified in different MAPK pathways; however, it is unclear whether they all have roles in metabolic signaling. One of best characterized MAPK scaffolds is the kinase suppressor of RAS (KSR) 2, which is required for ERK1/2 signaling (Fig. 3) (99). KSR was originally identified in RAS-dependent genetic screens in Drosophila melanogaster and Caenorhabditis elegans (100–102). Variants in KSR2 disrupt Raf-MEK-ERK signaling and can impair cellular fatty acid oxidation and glucose oxidation (59). In C2C12 cells overexpressing KSR2 variants with identified human mutations markedly reduced glucose oxidation was observed. In contrast, overexpression of wild-type KSR2 increased palmitate-stimulated oxygen consumption, and this effect could be attenuated by cotransfection of KSR2 mutants (59). Preincubation of cells with metformin increased palmitate-stimulated oxygen consumption in nontransfected cells, and the increase in basal fatty acid oxidation due to wild-type KSR2 overexpression could be further increased with metformin. The reduced basal level of fatty acid oxidation seen with the KSR2 mutations was completely rescued in all cases by the addition of metformin (59).
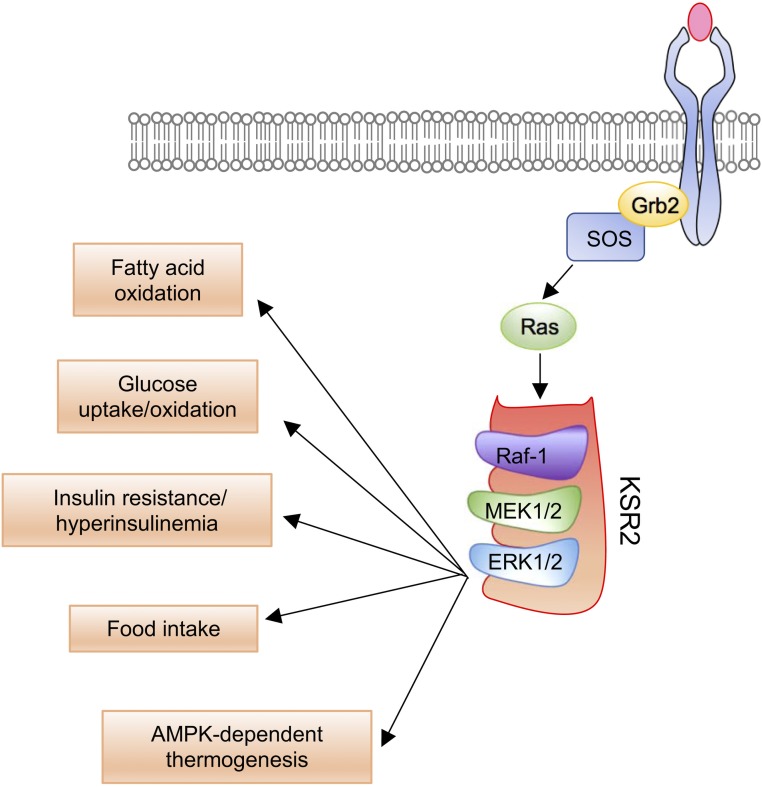
Figure 3.
The role of KSR in ERK-dependent metabolic signaling. Following receptor stimulation, Grb2 recruits SOS to activate Ras. This is necessary for the recruitment of Raf to the membrane. Translocation of kinase suppressor of ras 2 (KSR2) to the cell membrane leads to the formation of a protein complex with activated Raf-1, MAPK kinase (MEK1/2), and ERK1/2. This complex amplifies ERK1/2-dependent signaling to facilitate various metabolic outcomes.
Noncatalytic region of tyrosine kinase proteins
Noncatalytic region of tyrosine kinase (Nck) scaffold proteins include Nck1 and Nck2 (103, 104). Nck1 and Nck2 are involved in critical biological processes, including embryonic development, actin cytoskeletal reorganization, axonal guidance, proliferation, and the unfolded protein response (105–112). In MIN6 insulinoma cells, Nck1 enhances protein kinase R–like endoplasmic reticulum kinase (PERK) basal activity, leading to PERK-dependent insulin biosynthesis (61). In addition, Yamani et al. (61) reported that silencing Nck1 in MIN6 cells is protective against cell death induced by chemical inducers of endoplasmic reticulum stress. Depletion of Nck1 in MIN6 cells upregulates the unfolded protein response, as demonstrated by enhanced basal PERK activity, phosphorylation of eIF2α, and increased ATF4 mRNA and nuclear protein levels (61). Thus, silencing Nck1 improves β-cell function and resistance to endoplasmic reticulum stress. Whole body Nck1 knockout mice display improved glucose homeostasis and enhanced hepatic insulin signaling compared with obese littermate controls (62). Nck1 also has regulatory roles in the activation of the PI3K/Akt pathway via a protein tyrosine phosphatase 1B–dependent mechanism. Primary hepatocytes isolated from Nck1 knockout mice display enhanced insulin-mediated Akt activation due to Nck1-dependent regulation of protein tyrosine phosphatase 1B expression (113).
In contrast, Nck2 has been found to regulate adipogenesis and glucose homeostasis, because Nck2 deletion in mice increases adiposity through adipocyte hypertrophy, promotes glucose intolerance, and decreases insulin sensitivity. In addition, Nck2 knockdown has been found to enhance adipocyte differentiation in vitro (63). Recent work has provided pharmacological evidence of abrogation of the interaction between Nck1 and PERK to improve β-cell function and survival (114). Through the use of the TAT-pY561 phosphopeptide, increases in basal PERK activation was observed, resulting in protection against glucolipotoxicity-induced apoptosis and enhanced insulin production and secretion from pancreatic β-cells.
14-3-3 Proteins
14-3-3 Proteins are a ubiquitously expressed family of scaffolds consisting of seven isoforms (β, ε, γ, η, θ, σ, and ζ). They bind to phosphorylated proteins containing specific phosphoserine or phosphothreonine motifs (RSxpS/TxP or R'pS/TxP, respectively) (115–117). Different roles of 14-3-3 proteins in cell cycle progression, development, and neurite growth have been described (115, 118, 119); however, their roles in energy metabolism are still poorly understood. In vitro studies have documented the abilities of 14-3-3 proteins to regulate enzyme activity, protein phosphorylation status, protein stability, and granule transport and exocytosis (115, 120–124). Regarding metabolism and glucose homeostasis, 14-3-3 isoforms have demonstrated to interact with effectors in the insulin signaling pathway, such as IRS-1, Raf-1, AS160/TBD1C4, and FOXO1 (Fig. 4) (4, 64, 120, 121, 125–129). Collectively, these data suggest that 14-3-3 proteins could have important metabolic roles in vivo. In the context of lipid and glucose metabolism, 14-3-3 proteins interact with enzymes involved in fatty acid synthesis and glycolysis, such as fatty acid synthase and fructose-2,6-bisphosphate kinase/phosphatase, respectively (130–132). 14-3-3 Proteins might also regulate PKA-dependent HSL activity. It has been suggested that the phosphorylation of HSL by PKA during lipolysis leads to the creation of phosphorylation-binding sites for 14-3-3 proteins (133). Site-directed mutagenesis of these phosphorylation-binding sites is associated with decreased HSL activity (8, 134). Further studies using in vivo models are required to assess when 14-3-3 proteins can have such pleiotropic effects in glucose homeostasis and metabolism.
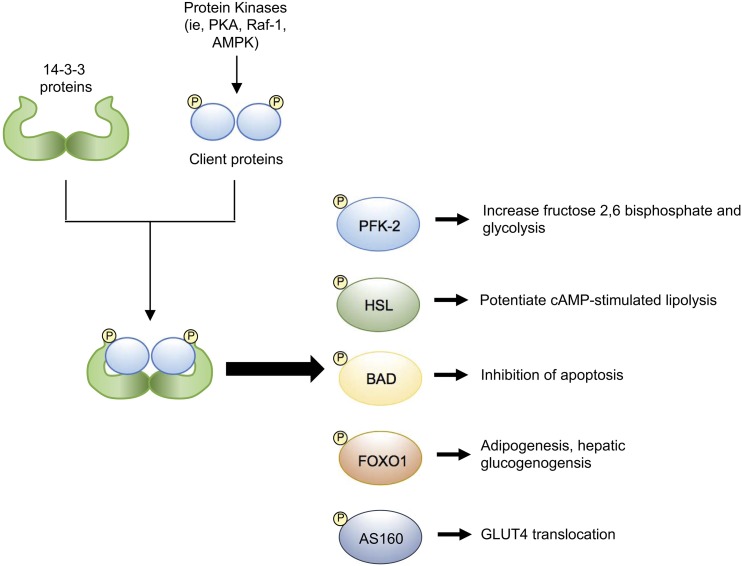
Figure 4.
Metabolic roles of 14-3-3 proteins via their interactions with client proteins. Serine or threonine phosphorylation of client proteins by various kinases generates recognition sites for dimeric 14-3-3 proteins. The interactomes of 14-3-3 proteins are large and diverse, and the interactions of 14-3-3 proteins with various metabolic effectors have been identified. This suggests regulatory roles for 14-3-3 proteins in processes such as glucose uptake, pancreatic β-cell survival, and adipogenesis.
Of the seven isoforms, we determined that 14-3-3ζ was essential for adipocyte differentiation in vitro and in vivo (64). 14-3-3ζ knockout mice were markedly lean from birth with specific reductions in visceral fat depots. In contrast, transgenic overexpression of 14-3-3ζ in mice potentiated obesity without metabolic complications (64). Through the use of isoform-specific small interfering RNAs (siRNAs), we found that only the 14-3-3ζ isoform was essential for 3T3-L1 adipogenesis, and 14-3-3ζ depletion promoted autophagy-dependent degradation of C/EBP-δ, preventing induction of the master adipogenic factors, Pparγ and C/EBP-α (64). Given their ability to bind to proteins phosphorylated by multiple kinases, including PKA, Akt, and Raf-1, the interactomes of 14-3-3 proteins are large and diverse (122, 133, 135–138) and suggest that novel regulators of adipocyte differentiation could be identified within the 14-3-3ζ interactome. Thus, we used proteomics to elucidate the 14-3-3ζ interactome during adipogenesis and observed >100 proteins that were unique to adipocyte differentiation, 56 of which were novel interacting partners (138). siRNA-mediated depletion of RNA splicing factors, found to be highly abundant within the 14-3-3ζ interactome during adipocyte differentiation, revealed that some factors such as Hnrpf have essential roles in adipogenesis and in the alternative splicing of Pparg (138).
With respect to glucose homeostasis, we demonstrated that systemic 14-3-3ζ deletion improves glucose tolerance through a GLP-1–dependent mechanism (65). 14-3-3ζ Knockout mice displayed improved glucose tolerance after an oral glucose gavage compared with an intraperitoneal glucose bolus. This improvement was associated with significantly elevated fasting GLP-1 levels, and this improvement in oral glucose tolerance could be abrogated through the use of a GLP-1 receptor antagonist (65). Collectively, these findings suggest that 14-3-3ζ and perhaps its related isoforms have critical roles in the regulation of metabolism, glucose homeostasis, and adipogenesis.
Contributions of Scaffold Proteins to Cardiometabolic Diseases
Alterations in the expression of scaffolds, such as 14-3-3 proteins, have been suggested to lead to detrimental outcomes, notably carcinogenesis and neurologic disorders (115, 118, 139, 140). Also, it is not well-appreciated whether changes in the function or expression of scaffolds can have detrimental effects on metabolic health. We have provided an overview of notable examples of scaffold proteins linked to metabolic diseases.
Obesity
Obesity is a major risk for insulin resistance, type 2 diabetes, and cardiovascular disease and is associated with significantly increased adipose tissue mass (141). Multiple, complex pathways facilitate adipogenesis, which demonstrates the requirement of scaffold proteins to coordinate signaling events for accuracy. This raises the question of whether alterations in their expression or function could potentiate the development of obesity and other metabolic diseases.
Recently, we identified critical roles for the 14-3-3 protein family and, in particular, 14-3-3ζ in the regulation of visceral adipogenesis and adipocyte differentiation (64). Mice lacking 14-3-3ζ were smaller and had substantially reduced fat mass compared with their wild-type littermates. However, transgenic overexpression of 14-3-3ζ exacerbated age-onset weight gain and potentiated high-fat diet–induced obesity (64). To the best of our knowledge, this was the first study to report how changes in 14-3-3ζ levels could affect metabolic health. It was previously reported that adipose tissue from obese individuals had increased levels of 14-3-3ζ and other isoforms (142, 143), and a single nucleotide protein close to YWHAZ (gene encoding 14-3-3ζ) was significantly associated with weight gain (144). Taken together, these lines of evidence suggest an intimate role of 14-3-3ζ and perhaps it related isoforms in the pathogenesis of obesity.
Rare variants in KSR2 have been associated with the development of early-onset obesity and severe insulin resistance in humans (59). These KSR2 variants promote changes in food intake, basal metabolic rate, fatty acids, and glucose oxidation in humans. When expressed in C2C12 cells, KSR2 mutations cause impaired fatty acid and glucose oxidation (59). Through the use of proteomics, KSR2 has been found to interact with multiple proteins, including AMP-activated protein kinase (AMPK) (60, 145). This interaction is thought to be essential for metabolism, because Ksr2 knockout mice develop obesity, hyperinsulinemia, and impaired glucose tolerance (145–147). Moreover, Ksr2 knockout mice are hypophagic and expend less energy than wild-type mice (145). However, deletion of Ksr2 also impaired the thermogenic actions of AMPK activation, which suggests that Ksr2 might bridge the activity of both ERK1/2 and AMPK signaling pathways for metabolic homeostasis (145).
SH2 B adaptor protein 1 (SH2B1) is a scaffold protein involved in different RTK and cytokine receptor signaling pathways that require Janus kinases. Mice lacking the Sh2b1 gene display leptin resistance, increased food intake, severe obesity, and insulin resistance (148, 149). Four SH2B1 gene variants in humans (P90H, T175N, P322S, and F344Lfs*20) identified via the Genetics of Obesity Study cohort are associated with early-onset obesity and insulin resistance (150).
Although no gene variants or mutations for the β-arrestins in humans have been reported, β-arrestin 1 knockout mice are susceptible to diet-induced obesity, and they exhibit increased fat mass accumulation and decreased whole body insulin sensitivity when fed a high-fat diet (151). Collectively, these observations indicate that scaffold proteins themselves could influence the development of obesity and demonstrate the need for further detailed study into their contributions to obesity.
Type 2 diabetes and insulin resistance
In addition to the progressive decline in pancreatic β-cell function and mass, insulin resistance is known to contribute to the development of type 2 diabetes (49, 152). Despite the different causal factors and pathogenic mechanisms, alterations in the activity of scaffold proteins themselves could be a causal factor that promotes the development of type 2 diabetes.
It has been reported that the gene encoding the scaffold protein JIP1, MAPK8IP1, is a candidate type 2 diabetes gene in humans (153). Knockdown by siRNA or expression of a protein variant harboring a missense mutation (559N) in an insulinoma cell line were also associated with decreased INS transcriptional activity and increased susceptibility to apoptosis (153). Mice that express a point mutation in Jip1 (Thr103Ala) displayed decreased Jnk activation, which results in a protection against high-fat diet–associated insulin resistance (66).
Luan et al. (154) have proposed a relationship between β-arrestin 2 and insulin resistance. They reported that β-arrestin 2 expression in muscle and liver is severely downregulated in insulin-resistant animal models (154), and a similar relationship was observed in liver samples from a small cohort of patients with type 2 diabetes. In wild-type mice, deletion of β-arrestin 2 caused postprandial hyperglycemia and glucose intolerance, both hallmarks of insulin resistance. In contrast, administration of β-arrestin 2 in db/db mice via adenoviral delivery improved insulin sensitivity. These studies also revealed that insulin stimulates the formation of a β-arrestin 2 signaling complex, in which β-arrestin 2 scaffolds Akt and Src to the insulin receptor (154).
It has been proposed that 14-3-3 proteins might bind to various components of the insulin signaling network (4, 64, 120, 121, 125–129), suggesting that these proteins could have important roles in various insulin-induced metabolic effects in different tissues. For example, decreased levels of 14-3-3ζ are associated with decreased insulin sensitivity in humans (155). We found that systemic 14-3-3ζ knockout mice displayed decreased insulin sensitivity after peripheral administration of exogenous insulin (64). Collectively, these lines of evidence suggest that changes in 14-3-3ζ expression might cause defects in glucose transporter type 4–mediated glucose uptake in skeletal muscle or fat. The Rab GTPase-activating protein AS160/TBC1D4 (TBC1 domain family member 4) controls a distal signaling event that regulates translocation of glucose transporter type 4–containing vesicles to the plasma membrane, and its inhibitory activity is attenuated through interactions with 14-3-3 proteins (127, 156). Transgenic mice overexpressing an AS160/TBC1D4 mutant that is unable to bind to 14-3-3 proteins leads to decreased insulin sensitivity, presumably owing to the inability of 14-3-3 proteins to inhibit AS160 activity (127).
The regulatory roles of the immune system on metabolism has become an emerging area of research of importance (157), and it is possible that the scaffolds required for coordinating immune signaling could also have metabolic roles. Toll-like receptor 4 (TLR4) represents one of several immune receptors that link immune system function to metabolic health, and it has been found to be involved in the development of obesity, insulin resistance, and pancreatic islet inflammation and dysfunction (50–52, 158, 159). SAM and SH3 domain-containing protein 1 (SASH1) is a scaffold that coordinates TLR4 signaling (160), and interactions of SASH1 with different downstream effectors of TLR4, including nuclear factor κ-light chain enhancer of activated B, JNK, and p38 MAPK, is required for production of proinflammatory cytokines (160). These observations suggest a possible role for SASH1 in the development of these metabolic disorders through the production of proinflammatory cytokines and warrants further investigation. With numerous proinflammatory factors having effects on metabolic regulation (157, 161), identifying the scaffolds that coordinate the downstream pathways of their respective receptors represents an unexplored and necessary area of study.
Cardiovascular diseases
Cardiovascular diseases are the most common cause of mortality in developed countries. Chronic Gs protein activity due to prolonged β-adrenergic signaling can lead to increased myocyte size, apoptosis, and contractile failure (162–164). To date, one of the most effective treatments of heart failure is to target the βARs (β1AR, β2AR). Antagonists for these receptors function by attenuating G-protein–mediated signaling and G-protein–independent mechanisms, such as those involving receptor phosphorylation by G-protein receptor kinases and the recruitment of β-arrestins (Fig. 1). Transactivation of the EGFR has been shown to have cardioprotective effects in response to chronic catecholamine stress, and this is thought to be through a β-arrestin 1–mediated mechanism (165). Comparisons of transgenic mice overexpressing wild-type β1AR or a mutant β1AR lacking G-protein receptor kinase phosphorylation sites under conditions of chronic catecholamine stress revealed that mutant mice failed to exhibit β-arrestin–mediated EGFR transactivation and had pronounced myocyte apoptosis and left ventricular dilatation (165). Taken together, these findings demonstrate the importance of β-arrestin 1 in cardiovascular function.
In contrast, β-arrestin 2 has been identified as a potential pathogenic factor in cardiac ischemia-reperfusion (I/R) injury (166). Three major findings were observed. First, β-arrestin 2 in the heart is upregulated in response to cardiac oxidative stress and I/R injury. Second, upregulation of β-arrestin 2 triggers cardiomyocyte death and exaggerates oxidative stress-induced cardiac damage and cardiomyopathy. Finally, β-arrestin 2 binds to the p85 subunit of PI3K and subsequently prevents its interaction with CaV3, resulting in impaired activation of the PI3K-Akt-GSK3β prosurvival pathway. Moreover, downregulation of β-arrestin 2 was found to have cardioprotective effects against I/R-induced myocardial injury.
Angiotensin II type IA receptors are another family of GPCRs that require β-arrestins and are implicated in heart failure. Mechanical stretch of heart tissue can trigger a conformational change in angiotensin II type IA receptors, leading to β-arrestin recruitment in the absence of ligand (167). Hearts from mice lacking β-arrestin or angiotensin II type IA receptors failed to induce a cardioprotective response to mechanical stretch. Also, the same heart displayed altered ERK and Akt activation, impaired transactivation of the EGFR, and enhanced myocyte apoptosis. Collectively, these findings support differential roles for β-arrestins in the context of cardiovascular disease.
Another scaffold protein family implicated in βARs signaling in the heart is the AKAP family. AKAP members have differential roles in cardiac physiology. For example, AKAP18α (AKAP7α) targets l-type calcium channels to regulate Ca2+ influx in response to β-adrenergic stimulation (56, 57). Murine AKAP150 and human AKAP79 have been shown to interact with different enzymes such as PKA, PKC, and calcineurin and their potential substrates, including l-type calcium channels, β-ARs, caveolin-3, and adenylyl cyclase (58, 73, 168–174). Moreover, loss of AKAP150 disrupts intracellular trafficking of β-ARs in cardiomyocytes (175). Defects in agonist-internalized β1-AR recycling have been observed in AKAP5 knockout myocytes, and this could be rescued when full-length AKAP5 was reintroduced (175). This suggested that AKAP5 exerted specific and profound effects on β1-AR recycling in mammalian cells.
Some observations have proposed a link between MAPK/ERK signaling and cardiac hypertrophy. For example, the four and a half LIM domain protein-1 (FHL1) is a novel MAPK scaffold protein that mediates MAPK signaling responses at the sarcomere during stress-induced cardiac hypertrophy (176). FHL1 is ubiquitously expressed in mice and humans and increase in the heart during hypertrophic conditions (177–181). In vivo studies using transgenic mice overexpressing a constitutively active form of Gq selectively in the heart demonstrated that FHL1 influences Gαq signaling, as Fhl1 deficiency prevented Gq-dependent cardiomyopathies (176).
Taken together, the differential roles of scaffold proteins with respect to cardiomyopathies have been identified, demonstrating their importance to cardiovascular health and disease.
Concluding Remarks
Scaffold proteins have emerged as multifunctional molecules with essential roles in cell signaling. Thus, a greater understanding of the contributions of scaffolds to metabolism and cardiometabolic diseases should provide a framework for the identification of new, potential targets for the treatment of these diseases. For example, increasing our current understanding of how scaffolds regulate and interact with their target proteins could provide additional information on the pathogenic mechanisms of potential therapeutic targets. One method to understand in greater detail how a scaffold regulates multiple pathways within metabolic tissues will be to examine the interactomes of various scaffolds under physiological and pathophysiological conditions. Such studies will expand our understanding of their metabolic functions and potentially open new avenues of research to better metabolism and metabolic disorders.
Acknowledgments
The authors would like to apologize to those authors whose work was not cited in this review due to space limitations.
Financial Support: This work was supported by Canadian Institutes of Health Research (CIHR) Project Grant PJT-153144 (to G.E.L.) and National Sciences and Engineering Research Council (NSERC) Discovery Grant RGPIN-2017-05209 (to G.E.L.), and a Discovery Award from the Banting Research Foundation. Salary support for G.E.L. was provided through a Fonds de recherche Québec Santé (FRQS) Research Scholar (Junior 1) award.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AKAP
cAMP-dependent kinase–anchoring protein
- Akt
protein kinase B
- AMPK
AMP-activated protein kinase
- EGFR
epidermal growth factor receptor
- FGFR
fibroblast growth factor receptor
- FHL1
four and a half LIM domain protein-1
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- GPCR
G protein–coupled receptor
- HSL
hormone-sensitive lipase
- I/R
ischemia/reperfusion
- IRS
insulin-receptor substrate
- JNK
c-Jun NH2-terminal kinase
- KSR
kinase suppressor of RAS
- Nck
noncatalytic region of tyrosine kinase
- PERK
protein kinase R–like endoplasmic reticulum kinase
- PI3K
phosphatidylinositol 3′-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- RTK
receptor tyrosine kinase
- SASH1
SAM and SH3 domain-containing protein 1
- SH
Src homology
- SH2B1
SH2 B adaptor protein 1
- siRNA
small interfering RNA
- TLR4
toll-like receptor 4
- TRP
transient receptor potential
- VEGFR
vascular endothelial growth factor receptor
- βAR
β-adrenergic receptor
References
- 1. Gotoh N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 2008;99(7):1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5(4):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giancotti FG. Deregulation of cell signaling in cancer. FEBS Lett. 2014;588(16):2558–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim GE, Piske M, Johnson JD. 14-3-3 Proteins are essential signalling hubs for beta cell survival. Diabetologia. 2013;56(4):825–837. [DOI] [PubMed] [Google Scholar]
- 5. Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278(5346):2075–2080. [DOI] [PubMed] [Google Scholar]
- 6. Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326(5957):1220–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su YW, Hao Z, Hirao A, Yamamoto K, Lin WJ, Young A, Duncan GS, Yoshida H, Wakeham A, Lang PA, Murakami K, Hermeking H, Vogelstein B, Ohashi P, Mak TW. 14-3-3sigma regulates B-cell homeostasis through stabilization of FOXO1. Proc Natl Acad Sci USA. 2011;108(4):1555–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marvyn PM, Bradley RM, Button EB, Mardian EB, Duncan RE. Fasting upregulates adipose triglyceride lipase and hormone-sensitive lipase levels and phosphorylation in mouse kidney. Biochem Cell Biol. 2015;93(3):262–267. [DOI] [PubMed] [Google Scholar]
- 9. Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122(Pt 7):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. [DOI] [PubMed] [Google Scholar]
- 11. Pan CQ, Sudol M, Sheetz M, Low BC. Modularity and functional plasticity of scaffold proteins as p(l)acemakers in cell signaling. Cell Signal. 2012;24(11):2143–2165. [DOI] [PubMed] [Google Scholar]
- 12. Buday L, Tompa P. Functional classification of scaffold proteins and related molecules. FEBS J. 2010;277(21):4348–4355. [DOI] [PubMed] [Google Scholar]
- 13. Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res. 2002;91(8):672–680. [DOI] [PubMed] [Google Scholar]
- 14. Kleppe R, Martinez A, Døskeland SO, Haavik J. The 14-3-3 proteins in regulation of cellular metabolism. Semin Cell Dev Biol. 2011;22(7):713–719. [DOI] [PubMed] [Google Scholar]
- 15. Patwari P, Lee RT. An expanded family of arrestins regulate metabolism. Trends Endocrinol Metab. 2012;23(5):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem. 2012;287(9):6421–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang X, Dai FF, Gaisano G, Giglou K, Han J, Zhang M, Kittanakom S, Wong V, Wei L, Showalter AD, Sloop KW, Stagljar I, Wheeler MB. The identification of novel proteins that interact with the GLP-1 receptor and restrain its activity. Mol Endocrinol. 2013;27(9):1550–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emery AC. Catecholamine receptors: prototypes for GPCR-based drug discovery. Adv Pharmacol. 2013;68:335–356. [DOI] [PubMed] [Google Scholar]
- 19. Yang DH, Zhou CH, Liu Q, Wang MW. Landmark studies on the glucagon subfamily of GPCRs: from small molecule modulators to a crystal structure. Acta Pharmacol Sin. 2015;36(9):1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim GE, Brubaker PL. Glucagon-like peptide 1 secretion by the L-cell. Diabetes. 2006;55(Suppl 2):S70–S77. [Google Scholar]
- 21. Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60(1):653–688. [DOI] [PubMed] [Google Scholar]
- 22. Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38(1):289–319. [DOI] [PubMed] [Google Scholar]
- 23. Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24(1):1–29. [DOI] [PubMed] [Google Scholar]
- 24. Smani T, Dionisio N, Lopez JJ, Berna-Erro A, Rosado JA. Cytoskeletal and scaffolding proteins as structural and functional determinants of TRP channels. Biochim Biophys Acta. 2014;1838(2):658–664. [DOI] [PubMed] [Google Scholar]
- 25. Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Mouse brain and muscle tissues constitutively express high levels of Homer proteins. Eur J Biochem. 2000;267(3):634–639. [DOI] [PubMed] [Google Scholar]
- 26. Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114(6):777–789. [DOI] [PubMed] [Google Scholar]
- 27. Jardín I, López JJ, Salido GM, Rosado JA. Functional relevance of the de novo coupling between hTRPC1 and type II IP3 receptor in store-operated Ca2+ entry in human platelets. Cell Signal. 2008;20(4):737–747. [DOI] [PubMed] [Google Scholar]
- 28. Mery L, Strauss B, Dufour JF, Krause KH, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci. 2002;115(Pt 17):3497–3508. [DOI] [PubMed] [Google Scholar]
- 29. Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59(3):450–461. [DOI] [PubMed] [Google Scholar]
- 30. Yang J, Wang Q, Zheng W, Tuli J, Li Q, Wu Y, Hussein S, Dai XQ, Shafiei S, Li XG, Shen PY, Tu JC, Chen XZ. Receptor for activated C kinase 1 (RACK1) inhibits function of transient receptor potential (TRP)-type channel Pkd2L1 through physical interaction. J Biol Chem. 2012;287(9):6551–6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bandyopadhyay BC, Ong HL, Lockwich TP, Liu X, Paria BC, Singh BB, Ambudkar IS. TRPC3 controls agonist-stimulated intracellular Ca2+ release by mediating the interaction between inositol 1,4,5-trisphosphate receptor and RACK1. J Biol Chem. 2008;283(47):32821–32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woodard GE, López JJ, Jardín I, Salido GM, Rosado JA. TRPC3 regulates agonist-stimulated Ca2+ mobilization by mediating the interaction between type I inositol 1,4,5-trisphosphate receptor, RACK1, and Orai1. J Biol Chem. 2010;285(11):8045–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003;285(1):C222–C235. [DOI] [PubMed] [Google Scholar]
- 34. Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277(10):8635–8647. [DOI] [PubMed] [Google Scholar]
- 35. Boothe T, Lim GE, Cen H, Skovsø S, Piske M, Li SN, Nabi IR, Gilon P, Johnson JD. Inter-domain tagging implicates caveolin-1 in insulin receptor trafficking and Erk signaling bias in pancreatic beta-cells. Mol Metab. 2016;5(5):366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nevins AK, Thurmond DC. Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J Biol Chem. 2006;281(28):18961–18972. [DOI] [PubMed] [Google Scholar]
- 37. Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol. 2007;19(2):124–134. [DOI] [PubMed] [Google Scholar]
- 38. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114(Pt 5):853–865. [DOI] [PubMed] [Google Scholar]
- 39. Neilson KM, Friesel R. Ligand-independent activation of fibroblast growth factor receptors by point mutations in the extracellular, transmembrane, and kinase domains. J Biol Chem. 1996;271(40):25049–25057. [DOI] [PubMed] [Google Scholar]
- 40. Keegan K, Johnson DE, Williams LT, Hayman MJ. Isolation of an additional member of the fibroblast growth factor receptor family, FGFR-3. Proc Natl Acad Sci USA. 1991;88(4):1095–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol. 2005;345(1):1–20. [DOI] [PubMed] [Google Scholar]
- 43. Luzi L, Confalonieri S, Di Fiore PP, Pelicci PG. Evolution of Shc functions from nematode to human. Curr Opin Genet Dev. 2000;10(6):668–674. [DOI] [PubMed] [Google Scholar]
- 44. van der Geer P, Wiley S, Gish GD, Pawson T. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr Biol. 1996;6(11):1435–1444. [DOI] [PubMed] [Google Scholar]
- 45. Pawson T. Dynamic control of signaling by modular adaptor proteins. Curr Opin Cell Biol. 2007;19(2):112–116. [DOI] [PubMed] [Google Scholar]
- 46. Bisson N, James DA, Ivosev G, Tate SA, Bonner R, Taylor L, Pawson T. Selected reaction monitoring mass spectrometry reveals the dynamics of signaling through the GRB2 adaptor. Nat Biotechnol. 2011;29(7):653–658. [DOI] [PubMed] [Google Scholar]
- 47. Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. Beta-arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc Natl Acad Sci USA. 2008;105(18):6614–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quoyer J, Longuet C, Broca C, Linck N, Costes S, Varin E, Bockaert J, Bertrand G, Dalle S. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem. 2010;285(3):1989–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123(7):2764–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Song MJ, Kim KH, Yoon JM, Kim JB. Activation of toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346(3):739–745. [DOI] [PubMed] [Google Scholar]
- 51. Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15(4):518–533. [DOI] [PubMed] [Google Scholar]
- 52. Nackiewicz D, Dan M, He W, Kim R, Salmi A, Rütti S, Westwell-Roper C, Cunningham A, Speck M, Schuster-Klein C, Guardiola B, Maedler K, Ehses JA. TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair beta cell insulin gene expression via IL-1 and IL-6. Diabetologia. 2014;57(8):1645–1654. [DOI] [PubMed] [Google Scholar]
- 53. Zhu L, Rossi M, Cui Y, Lee RJ, Sakamoto W, Perry NA, Urs NM, Caron MG, Gurevich VV, Godlewski G, Kunos G, Chen M, Chen W, Wess J. Hepatic β-arrestin 2 is essential for maintaining euglycemia. J Clin Invest. 2017;127(8):2941–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu L, Almaça J, Dadi PK, Hong H, Sakamoto W, Rossi M, Lee RJ, Vierra NC, Lu H, Cui Y, McMillin SM, Perry NA, Gurevich VV, Lee A, Kuo B, Leapman RD, Matschinsky FM, Doliba NM, Urs NM, Caron MG, Jacobson DA, Caicedo A, Wess J. β-Arrestin-2 is an essential regulator of pancreatic β-cell function under physiological and pathophysiological conditions. Nat Commun. 2017;8:14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hinke SA, Navedo MF, Ulman A, Whiting JL, Nygren PJ, Tian G, Jimenez-Caliani AJ, Langeberg LK, Cirulli V, Tengholm A, Dell’Acqua ML, Santana LF, Scott JD. Anchored phosphatases modulate glucose homeostasis. EMBO J. 2012;31(20):3991–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272(10):6297–6302. [DOI] [PubMed] [Google Scholar]
- 57. Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17(8):2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271(5255):1589–1592. [DOI] [PubMed] [Google Scholar]
- 59. Pearce LR, Atanassova N, Banton MC, Bottomley B, van der Klaauw AA, Revelli JP, Hendricks A, Keogh JM, Henning E, Doree D, Jeter-Jones S, Garg S, Bochukova EG, Bounds R, Ashford S, Gayton E, Hindmarsh PC, Shield JP, Crowne E, Barford D, Wareham NJ, O’Rahilly S, Murphy MP, Powell DR, Barroso I, Farooqi IS; UK10K Consortium . KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell. 2013;155(4):765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu L, Channavajhala PL, Rao VR, Moutsatsos I, Wu L, Zhang Y, Lin LL, Qiu Y. Proteomic characterization of the dynamic KSR-2 interactome, a signaling scaffold complex in MAPK pathway. Biochim Biophys Acta. 2009;1794(10):1485–1495. [DOI] [PubMed] [Google Scholar]
- 61. Yamani L, Li B, Larose L. Nck1 deficiency improves pancreatic β cell survival to diabetes-relevant stresses by modulating PERK activation and signaling. Cell Signal. 2015;27(12):2555–2567. [DOI] [PubMed] [Google Scholar]
- 62. Latreille M, Laberge MK, Bourret G, Yamani L, Larose L. Deletion of Nck1 attenuates hepatic ER stress signaling and improves glucose tolerance and insulin signaling in liver of obese mice. Am J Physiol Endocrinol Metab. 2011;300(3):E423–E434. [DOI] [PubMed] [Google Scholar]
- 63. Dusseault J, Li B, Haider N, Goyette MA, Côté JF, Larose L. Nck2 deficiency in mice results in increased adiposity associated with adipocyte hypertrophy and enhanced adipogenesis. Diabetes. 2016;65(9):2652–2666. [DOI] [PubMed] [Google Scholar]
- 64. Lim GE, Albrecht T, Piske M, Sarai K, Lee JT, Ramshaw HS, Sinha S, Guthridge MA, Acker-Palmer A, Lopez AF, Clee SM, Nislow C, Johnson JD. 14-3-3ζ coordinates adipogenesis of visceral fat. Nat Commun. 2015;6(1):7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lim GE, Piske M, Lulo JE, Ramshaw HS, Lopez AF, Johnson JD. Ywhaz/14-3-3ζ deletion improves glucose tolerance through a GLP-1-dependent mechanism. Endocrinology. 2016;157(7):2649–2659. [DOI] [PubMed] [Google Scholar]
- 66. Morel C, Standen CL, Jung DY, Gray S, Ong H, Flavell RA, Kim JK, Davis RJ. Requirement of JIP1-mediated c-Jun N-terminal kinase activation for obesity-induced insulin resistance. Mol Cell Biol. 2010;30(19):4616–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jaeschke A, Czech MP, Davis RJ. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 2004;18(16):1976–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sibley DR, Strasser RH, Benovic JL, Daniel K, Lefkowitz RJ. Phosphorylation/dephosphorylation of the beta-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci USA. 1986;83(24):9408–9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1: a role in receptor sequestration. J Biol Chem. 2001;276(22):18953–18959. [DOI] [PubMed] [Google Scholar]
- 70. Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273(2):685–688. [DOI] [PubMed] [Google Scholar]
- 71. Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–661. [DOI] [PubMed] [Google Scholar]
- 72. Goodman OB Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–450. [DOI] [PubMed] [Google Scholar]
- 73. Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ, Hall RA. Beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem. 2000;275(49):38659–38666. [DOI] [PubMed] [Google Scholar]
- 74. Xu J, Paquet M, Lau AG, Wood JD, Ross CA, Hall RA. Beta 1-adrenergic receptor association with the synaptic scaffolding protein membrane-associated guanylate kinase inverted-2 (MAGI-2): differential regulation of receptor internalization by MAGI-2 and PSD-95. J Biol Chem. 2001;276(44):41310–41317. [DOI] [PubMed] [Google Scholar]
- 75. Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401(6750):286–290. [DOI] [PubMed] [Google Scholar]
- 76. Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–517. [DOI] [PubMed] [Google Scholar]
- 77. Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. Beta-arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248(4962):1547–1550. [DOI] [PubMed] [Google Scholar]
- 78. Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24(5):643–652. [DOI] [PubMed] [Google Scholar]
- 79. Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69(1):451–482. [DOI] [PubMed] [Google Scholar]
- 80. Wu N, Hanson SM, Francis DJ, Vishnivetskiy SA, Thibonnier M, Klug CS, Shoham M, Gurevich VV. Arrestin binding to calmodulin: a direct interaction between two ubiquitous signaling proteins. J Mol Biol. 2006;364(5):955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mangmool S, Shukla AK, Rockman HA. Beta-arrestin-dependent activation of Ca(2+)/calmodulin kinase II after beta(1)-adrenergic receptor stimulation. J Cell Biol. 2010;189(3):573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda). 2008;23:151–159. [DOI] [PubMed] [Google Scholar]
- 83. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. [DOI] [PubMed] [Google Scholar]
- 84. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113(3):546–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 2004;53(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ravier MA, Leduc M, Richard J, Linck N, Varrault A, Pirot N, Roussel MM, Bockaert J, Dalle S, Bertrand G. β-Arrestin2 plays a key role in the modulation of the pancreatic beta cell mass in mice. Diabetologia. 2014;57(3):532–541. [DOI] [PubMed] [Google Scholar]
- 87. Lin F, Wang H, Malbon CC. Gravin-mediated formation of signaling complexes in beta 2-adrenergic receptor desensitization and resensitization. J Biol Chem. 2000;275(25):19025–19034. [DOI] [PubMed] [Google Scholar]
- 88. Fan G, Shumay E, Wang H, Malbon CC. The scaffold protein gravin (cAMP-dependent protein kinase-anchoring protein 250) binds the beta 2-adrenergic receptor via the receptor cytoplasmic Arg-329 to Leu-413 domain and provides a mobile scaffold during desensitization. J Biol Chem. 2001;276(26):24005–24014. [DOI] [PubMed] [Google Scholar]
- 89. Kapiloff MS, Chandrasekhar KD. A-kinase anchoring proteins: temporal and spatial regulation of intracellular signal transduction in the cardiovascular system. J Cardiovasc Pharmacol. 2011;58(4):337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li L, Li J, Drum BM, Chen Y, Yin H, Guo X, Luckey SW, Gilbert ML, McKnight GS, Scott JD, Santana LF, Liu Q. Loss of AKAP150 promotes pathological remodelling and heart failure propensity by disrupting calcium cycling and contractile reserve. Cardiovasc Res. 2017;113(2):147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55(2):261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3(1):1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB Sr, Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes. 2005;54(11):3252–3257. [DOI] [PubMed] [Google Scholar]
- 94. Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes. 2006;55(8):2392–2397. [DOI] [PubMed] [Google Scholar]
- 95. Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275(12):9047–9054. [DOI] [PubMed] [Google Scholar]
- 97. Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19(1):91–118. [DOI] [PubMed] [Google Scholar]
- 98. Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–149. [DOI] [PubMed] [Google Scholar]
- 99. Morrison DK. KSR: a MAPK scaffold of the Ras pathway? J Cell Sci. 2001;114(Pt 9):1609–1612. [DOI] [PubMed] [Google Scholar]
- 100. Kornfeld K, Hom DB, Horvitz HR. The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell. 1995;83(6):903–913. [DOI] [PubMed] [Google Scholar]
- 101. Sundaram M, Han M. The C. elegans ksr-1 gene encodes a novel Raf-related kinase involved in Ras-mediated signal transduction. Cell. 1995;83(6):889–901. [DOI] [PubMed] [Google Scholar]
- 102. Therrien M, Chang HC, Solomon NM, Karim FD, Wassarman DA, Rubin GM. KSR, a novel protein kinase required for RAS signal transduction. Cell. 1995;83(6):879–888. [DOI] [PubMed] [Google Scholar]
- 103. Braverman LE, Quilliam LA. Identification of Grb4/Nckbeta, a src homology 2 and 3 domain-containing adapter protein having similar binding and biological properties to Nck. J Biol Chem. 1999;274(9):5542–5549. [DOI] [PubMed] [Google Scholar]
- 104. Chen M, She H, Davis EM, Spicer CM, Kim L, Ren R, Le Beau MM, Li W. Identification of Nck family genes, chromosomal localization, expression, and signaling specificity. J Biol Chem. 1998;273(39):25171–25178. [DOI] [PubMed] [Google Scholar]
- 105. Bladt F, Aippersbach E, Gelkop S, Strasser GA, Nash P, Tafuri A, Gertler FB, Pawson T. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol Cell Biol. 2003;23(13):4586–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1). J Biol Chem. 1996;271(42):25746–25749. [DOI] [PubMed] [Google Scholar]
- 107. Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271(35):20997–21000. [DOI] [PubMed] [Google Scholar]
- 108. Garrity PA, Rao Y, Salecker I, McGlade J, Pawson T, Zipursky SL. Drosophila photoreceptor axon guidance and targeting requires the dreadlocks SH2/SH3 adapter protein. Cell. 1996;85(5):639–650. [DOI] [PubMed] [Google Scholar]
- 109. Li W, Hu P, Skolnik EY, Ullrich A, Schlessinger J. The SH2 and SH3 domain-containing Nck protein is oncogenic and a common target for phosphorylation by different surface receptors. Mol Cell Biol. 1992;12(12):5824–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kebache S, Cardin E, Nguyên DT, Chevet E, Larose L. Nck-1 antagonizes the endoplasmic reticulum stress-induced inhibition of translation. J Biol Chem. 2004;279(10):9662–9671. [DOI] [PubMed] [Google Scholar]
- 111. Nguyên DT, Kebache S, Fazel A, Wong HN, Jenna S, Emadali A, Lee EH, Bergeron JJ, Kaufman RJ, Larose L, Chevet E. Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol Biol Cell. 2004;15(9):4248–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Latreille M, Larose L. Nck in a complex containing the catalytic subunit of protein phosphatase 1 regulates eukaryotic initiation factor 2alpha signaling and cell survival to endoplasmic reticulum stress. J Biol Chem. 2006;281(36):26633–26644. [DOI] [PubMed] [Google Scholar]
- 113. Li H, Dusseault J, Larose L. Nck1 depletion induces activation of the PI3K/Akt pathway by attenuating PTP1B protein expression. Cell Commun Signal. 2014;12(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kefalas G, Jouvet N, Baldwin C, Estall JL, Larose L. Peptide-based sequestration of the adaptor protein Nck1 in pancreatic β cells enhances insulin biogenesis and protects against diabetogenic stresses. J Biol Chem. 2018;293(32):12516–12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009;19(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell. 1997;91(7):961–971. [DOI] [PubMed] [Google Scholar]
- 117. Petosa C, Masters SC, Bankston LA, Pohl J, Wang B, Fu H, Liddington RC. 14-3-3zeta binds a phosphorylated Raf peptide and an unphosphorylated peptide via its conserved amphipathic groove. J Biol Chem. 1998;273(26):16305–16310. [DOI] [PubMed] [Google Scholar]
- 118. Hermeking H, Benzinger A. 14-3-3 Proteins in cell cycle regulation. Semin Cancer Biol. 2006;16(3):183–192. [DOI] [PubMed] [Google Scholar]
- 119. Meek SE, Lane WS, Piwnica-Worms H. Comprehensive proteomic analysis of interphase and mitotic 14-3-3-binding proteins. J Biol Chem. 2004;279(31):32046–32054. [DOI] [PubMed] [Google Scholar]
- 120. Barry EF, Felquer FA, Powell JA, Biggs L, Stomski FC, Urbani A, Ramshaw H, Hoffmann P, Wilce MC, Grimbaldeston MA, Lopez AF, Guthridge MA. 14-3-3:Shc scaffolds integrate phosphoserine and phosphotyrosine signaling to regulate phosphatidylinositol 3-kinase activation and cell survival. J Biol Chem. 2009;284(18):12080–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fantl WJ, Muslin AJ, Kikuchi A, Martin JA, MacNicol AM, Gross RW, Williams LT. Activation of Raf-1 by 14-3-3 proteins. Nature. 1994;371(6498):612–614. [DOI] [PubMed] [Google Scholar]
- 122. Mackintosh C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J. 2004;381(Pt 2):329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fu H, Subramanian RR, Masters SC. 14-3-3 Proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40(1):617–647. [DOI] [PubMed] [Google Scholar]
- 124. Roth D, Burgoyne RD. Stimulation of catecholamine secretion from adrenal chromaffin cells by 14-3-3 proteins is due to reorganisation of the cortical actin network. FEBS Lett. 1995;374(1):77–81. [DOI] [PubMed] [Google Scholar]
- 125. Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, Dalal SN, DeCaprio JA, Greenberg ME, Yaffe MB. 14-3-3 Transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol. 2002;156(5):817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen S, Synowsky S, Tinti M, MacKintosh C. The capture of phosphoproteins by 14-3-3 proteins mediates actions of insulin. Trends Endocrinol Metab. 2011;22(11):429–436. [DOI] [PubMed] [Google Scholar]
- 127. Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 2011;13(1):68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kosaki A, Yamada K, Suga J, Otaka A, Kuzuya H. 14-3-3beta protein associates with insulin receptor substrate 1 and decreases insulin-stimulated phosphatidylinositol 3′-kinase activity in 3T3L1 adipocytes. J Biol Chem. 1998;273(2):940–944. [DOI] [PubMed] [Google Scholar]
- 129. Neukamm SS, Ott J, Dammeier S, Lehmann R, Häring HU, Schleicher E, Weigert C. Phosphorylation of serine 1137/1138 of mouse insulin receptor substrate (IRS) 2 regulates cAMP-dependent binding to 14-3-3 proteins and IRS2 protein degradation. J Biol Chem. 2013;288(23):16403–16415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pozuelo Rubio M, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N, Mackintosh C. 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem J. 2004;379(Pt 2):395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pozuelo Rubio M, Peggie M, Wong BH, Morrice N, MacKintosh C. 14-3-3s regulate fructose-2,6-bisphosphate levels by binding to PKB-phosphorylated cardiac fructose-2,6-bisphosphate kinase/phosphatase. EMBO J. 2003;22(14):3514–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, Metalnikov P, O’Donnell P, Taylor P, Taylor L, Zougman A, Woodgett JR, Langeberg LK, Scott JD, Pawson T. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol. 2004;14(16):1436–1450. [DOI] [PubMed] [Google Scholar]
- 133. Lim GE, Johnson JD. 14-3-3ζ: a numbers game in adipocyte function? Adipocyte. 2015;5(2):232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Holm C, Davis RC, Osterlund T, Schotz MC, Fredrikson G. Identification of the active site serine of hormone-sensitive lipase by site-directed mutagenesis. FEBS Lett. 1994;344(2-3):234–238. [DOI] [PubMed] [Google Scholar]
- 135. Siersbæk R, Nielsen R, John S, Sung MH, Baek S, Loft A, Hager GL, Mandrup S. Extensive chromatin remodelling and establishment of transcription factor “hotspots” during early adipogenesis. EMBO J. 2011;30(8):1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Siersbæk R, Rabiee A, Nielsen R, Sidoli S, Traynor S, Loft A, Poulsen LC, Rogowska-Wrzesinska A, Jensen ON, Mandrup S. Transcription factor cooperativity in early adipogenic hotspots and super-enhancers. Cell Reports. 2014;7(5):1443–1455. [DOI] [PubMed] [Google Scholar]
- 137. Johnson C, Tinti M, Wood NT, Campbell DG, Toth R, Dubois F, Geraghty KM, Wong BH, Brown LJ, Tyler J, Gernez A, Chen S, Synowsky S, MacKintosh C. Visualization and biochemical analyses of the emerging mammalian 14-3-3-phosphoproteome. Mol Cell Proteomics. 2011;10(10):M110005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mugabo Y, Sadeghi M, Fang NN, Mayor T, Lim GE. Elucidation of the 14-3-3ζ interactome reveals critical roles of RNA-splicing factors during adipogenesis. J Biol Chem. 2018;293(18):6736–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Martin HL, Bedford R, Heseltine SJ, Tang AA, Haza KZ, Rao A, McPherson MJ, Tomlinson DC. Non-immunoglobulin scaffold proteins: precision tools for studying protein-protein interactions in cancer. N Biotechnol. 2018;45:28–35. [DOI] [PubMed] [Google Scholar]
- 140. Luo P, Li X, Fei Z, Poon W. Scaffold protein Homer 1: implications for neurological diseases. Neurochem Int. 2012;61(5):731–738. [DOI] [PubMed] [Google Scholar]
- 141. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Insenser M, Montes-Nieto R, Vilarrasa N, Lecube A, Simó R, Vendrell J, Escobar-Morreale HF. A nontargeted proteomic approach to the study of visceral and subcutaneous adipose tissue in human obesity. Mol Cell Endocrinol. 2012;363(1-2):10–19. [DOI] [PubMed] [Google Scholar]
- 143. Capobianco V, Nardelli C, Ferrigno M, Iaffaldano L, Pilone V, Forestieri P, Zambrano N, Sacchetti L. miRNA and protein expression profiles of visceral adipose tissue reveal miR-141/YWHAG and miR-520e/RAB11A as two potential miRNA/protein target pairs associated with severe obesity. J Proteome Res. 2012;11(6):3358–3369. [DOI] [PubMed] [Google Scholar]
- 144. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman ÅK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stančáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson Å, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van’t Hooft FM, Vinkhuyzen AAE, Westra HJ, Zheng W, Zondervan KT, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin MR, Jöckel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Völker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGCICBPMAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium . Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Costanzo-Garvey DL, Pfluger PT, Dougherty MK, Stock JL, Boehm M, Chaika O, Fernandez MR, Fisher K, Kortum RL, Hong EG, Jun JY, Ko HJ, Schreiner A, Volle DJ, Treece T, Swift AL, Winer M, Chen D, Wu M, Leon LR, Shaw AS, McNeish J, Kim JK, Morrison DK, Tschöp MH, Lewis RE. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10(5):366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Brommage R, Desai U, Revelli JP, Donoviel DB, Fontenot GK, Dacosta CM, Smith DD, Kirkpatrick LL, Coker KJ, Donoviel MS, Eberhart DE, Holt KH, Kelly MR, Paradee WJ, Philips AV, Platt KA, Suwanichkul A, Hansen GM, Sands AT, Zambrowicz BP, Powell DR. High-throughput screening of mouse knockout lines identifies true lean and obese phenotypes. Obesity (Silver Spring). 2008;16(10):2362–2367. [DOI] [PubMed] [Google Scholar]
- 147. Revelli JP, Smith D, Allen J, Jeter-Jones S, Shadoan MK, Desai U, Schneider M, van Sligtenhorst I, Kirkpatrick L, Platt KA, Suwanichkul A, Savelieva K, Gerhardt B, Mitchell J, Syrewicz J, Zambrowicz B, Hamman BD, Vogel P, Powell DR. Profound obesity secondary to hyperphagia in mice lacking kinase suppressor of ras 2. Obesity (Silver Spring). 2011;19(5):1010–1018. [DOI] [PubMed] [Google Scholar]
- 148. Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005;2(2):95–104. [DOI] [PubMed] [Google Scholar]
- 149. Duan C, Yang H, White MF, Rui L. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol. 2004;24(17):7435–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pearce LR, Joe R, Doche ME, Su HW, Keogh JM, Henning E, Argetsinger LS, Bochukova EG, Cline JM, Garg S, Saeed S, Shoelson S, O’Rahilly S, Barroso I, Rui L, Farooqi IS, Carter-Su C. Functional characterization of obesity-associated variants involving the α and β isoforms of human SH2B1. Endocrinology. 2014;155(9):3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhuang LN, Hu WX, Zhang ML, Xin SM, Jia WP, Zhao J, Pei G. Beta-arrestin-1 protein represses diet-induced obesity. J Biol Chem. 2011;286(32):28396–28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. [DOI] [PubMed] [Google Scholar]
- 153. Waeber G, Delplanque J, Bonny C, Mooser V, Steinmann M, Widmann C, Maillard A, Miklossy J, Dina C, Hani EH, Vionnet N, Nicod P, Boutin P, Froguel P. The gene MAPK8IP1, encoding islet-brain-1, is a candidate for type 2 diabetes. Nat Genet. 2000;24(3):291–295. [DOI] [PubMed] [Google Scholar]
- 154. Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457(7233):1146–1149. [DOI] [PubMed] [Google Scholar]
- 155. Elbein SC, Kern PA, Rasouli N, Yao-Borengasser A, Sharma NK, Das SK. Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes. 2011;60(3):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem. 2006;281(39):29174–29180. [DOI] [PubMed] [Google Scholar]
- 157. Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47(3):406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–1285. [DOI] [PubMed] [Google Scholar]
- 159. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Dauphinee SM, Clayton A, Hussainkhel A, Yang C, Park YJ, Fuller ME, Blonder J, Veenstra TD, Karsan A. SASH1 is a scaffold molecule in endothelial TLR4 signaling. J Immunol. 2013;191(2):892–901. [DOI] [PubMed] [Google Scholar]
- 161. Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev. 2015;36(3):245–271. [DOI] [PubMed] [Google Scholar]
- 162. Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280(5363):574–577. [DOI] [PubMed] [Google Scholar]
- 163. Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98(13):1329–1334. [DOI] [PubMed] [Google Scholar]
- 164. Molkentin JD, Dorn GW II. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63(1):391–426. [DOI] [PubMed] [Google Scholar]
- 165. Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117(9):2445–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang Y, Jin L, Song Y, Zhang M, Shan D, Liu Y, Fang M, Lv F, Xiao RP, Zhang Y. β-Arrestin 2 mediates cardiac ischemia-reperfusion injury via inhibiting GPCR-independent cell survival signalling. Cardiovasc Res. 2017;113(13):1615–1626. [DOI] [PubMed] [Google Scholar]
- 167. Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3(125):ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19(1):185–196. [DOI] [PubMed] [Google Scholar]
- 169. Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, Scott JD. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23(6):925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem. 2006;281(44):33537–33553. [DOI] [PubMed] [Google Scholar]
- 171. Shcherbakova OG, Hurt CM, Xiang Y, Dell’Acqua ML, Zhang Q, Tsien RW, Kobilka BK. Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol. 2007;176(4):521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J Jr. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113(5):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107(6):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Navedo MF, Nieves-Cintrón M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102(2):e1–e11. [DOI] [PubMed] [Google Scholar]
- 175. Li X, Nooh MM, Bahouth SW. Role of AKAP79/150 protein in β1-adrenergic receptor trafficking and signaling in mammalian cells. J Biol Chem. 2013;288(47):33797–33812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW II, Brown JH, Peterson KL, Omens JH, McCulloch AD, Chen J. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118(12):3870–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Chu PH, Ruiz-Lozano P, Zhou Q, Cai C, Chen J. Expression patterns of FHL/SLIM family members suggest important functional roles in skeletal muscle and cardiovascular system. Mech Dev. 2000;95(1-2):259–265. [DOI] [PubMed] [Google Scholar]
- 178. Gaussin V, Tomlinson JE, Depre C, Engelhardt S, Antos CL, Takagi G, Hein L, Topper JN, Liggett SB, Olson EN, Lohse MJ, Vatner SF, Vatner DE. Common genomic response in different mouse models of beta-adrenergic-induced cardiomyopathy. Circulation. 2003;108(23):2926–2933. [DOI] [PubMed] [Google Scholar]
- 179. Hwang DM, Dempsey AA, Wang RX, Rezvani M, Barrans JD, Dai KS, Wang HY, Ma H, Cukerman E, Liu YQ, Gu JR, Zhang JH, Tsui SK, Waye MM, Fung KP, Lee CY, Liew CC. A genome-based resource for molecular cardiovascular medicine: toward a compendium of cardiovascular genes. Circulation. 1997;96(12):4146–4203. [DOI] [PubMed] [Google Scholar]
- 180. Hwang DM, Dempsey AA, Lee CY, Liew CC. Identification of differentially expressed genes in cardiac hypertrophy by analysis of expressed sequence tags. Genomics. 2000;66(1):1–14. [DOI] [PubMed] [Google Scholar]
- 181. Lim DS, Roberts R, Marian AJ. Expression profiling of cardiac genes in human hypertrophic cardiomyopathy: insight into the pathogenesis of phenotypes. J Am Coll Cardiol. 2001;38(4):1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]