Abstract
Background
The aim of this study was to investigate the expression of the BRAF V600E gene mutation and the RET/PTC gene rearrangement in the progression of papillary thyroid carcinoma (PTC) in 50 patients from Inner Mongolia.
Material/Methods
Clinical data, blood, and tissue samples were obtained from 50 patients with PTC and ten patients with benign thyroid adenoma. Expression of BRAF V600E, RET/PTC, nuclear factor-κB (NF-κB), interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, C-X-C motif chemokine ligand (CXCL)1, CXCL2, C-C motif chemokine ligand (CCL)2, and CCL3 were measured using polymerase chain reaction (PCR), immunohistochemistry, and an enzyme-linked immunosorbent assay (ELISA).
Results
Of the 50 patients with PTC, 37 patients expressed the BRAF V600E gene mutation, eight patients expressed RET/PTC, and five patients showed concomitant BRAF V600E and RET/PTC. Time to recurrence for patients with PTC with BRAF V600E was significantly increased compared with patients with concomitant BRAF V600E mutation and RET/PTC rearrangement (P<0.05). Expression of BRAF V600E, RET/PTC, and concomitant expression of BRAF V600E and RET/PTC were significantly associated with patient age and lymph node metastasis (P<0.05). Serum levels of NF-κB, IL-1β, IL-6, TNF-α, TGF-β and CCL3, and tumor tissue levels of IL-1β, IL-6, TNF-α, TGF-β, CXCL2 and CCL2 in patients with PTC were significantly increased compared with patients with benign thyroid adenoma, before and after surgery (P<0.05).
Conclusions
Expression of the BRAF V600E mutation and RET/PTC translocation promoted the activity of NF-κB, expression of inflammatory mediators, and lymph node metastases in patients with PTC.
MeSH Keywords: Inflammation, Lymphatic Metastasis, Proto-Oncogene Proteins B-raf, Receptor Activator of Nuclear Factor-kappa B, Thyroid Neoplasms
Background
Worldwide, thyroid cancer is the most common cancer of the endocrine system, accounting for approximately 1% of all newly diagnosed cancer cases, and the incidence has been increasing in the past few decades [1]. According to the International Cancer Research Institute, in 2010, the standardized incidence ratio of thyroid cancer in men was 1.9/10,000, which represented an increase of 26% from 2008 [2]. In 2010, the incidence of thyroid cancer in women was 6.1/10,000, which represented an increase of 29.8% from 2008 [2]. In China, thyroid cancer is the ninth most common cause of cancer, and the ratio of thyroid cancer between men and women is approximately 1: 3.3 [2]. There is still a need for more effective methods for the prevention, early diagnosis, and treatment of thyroid cancer.
There are four main histological types of thyroid cancer that include papillary, follicular, medullary, and anaplastic cancer, but papillary thyroid cancer (PTC) is the most common type, representing up to 80% of cases [3]. Depending on the geographic population being studied, epidemiology studies have shown 5-year and 10-year survival rates for PTC to be between 80–90% [4]. However, the survival rates for PTC vary, and patient outcome might be related to genetic factors.
BRAF is a gene that has a critical role in the mitogen-activated protein kinase (MAPK) signaling pathway [5]. The most common mutation in the BRAF gene is V600E, which occurs as a result of a base substitution in exon 15 of thymine for adenine that converts valine to glutamic acid at amino acid residue 600 (BRAF V600E) [6]. As the most common BRAF mutation, V600E promotes the continuous activation of BRAF kinase [6]. Previously published studies have shown that up to 45% of patients with PTC have a BRAF mutation, most of them being mutations in BRAF V600E [7].
In previously published studies on patients with PTC, BRAF mutations have been reported to be associated with increased patient age, tumor progression and metastasis, tumor phenotype, and tumor recurrence [8–11]. Kim et al. studied 72 cases of PTC and found that 49 cases (68.1%) were associated with BRAF mutation, and in three cases out of 49, BRAF V600E was found in tumor tissue and serum [12]. Kurt et al. found that 40 of 46 patients (87%) with PTC had a BRAF mutation and 4 of these 40 patients had distant metastases [13]. However, previously published studies have had limited patient sample size and limited molecular diagnostic technology, and the role of the BRAF V600E mutation in the progression of PTC remains unclear.
The human RET gene is located on chromosome 10q11.2 and encodes a transmembrane protein, RET, which is a receptor tyrosine kinase [14]. In studies from the past two decades, the RET gene has been shown to be activated by chromosome rearrangement in PTC [15,16]. RET gene rearrangement can result in the formation of a fusion protein, resulting in cell transformation in thyroid carcinoma [17]. In 1999, Miki et al. showed that expression of RET/PTC was significantly increased in patients with PTC who had local invasion [18]. Giannini et al. showed that H4-RET expression was significantly associated with lymph node metastasis in PTC [19]. The findings of these previously published studies support the relationship between RET/PTC and metastasis in thyroid cancer.
Nuclear factor-κB (NF-κB) has a role in cell apoptosis, in promoting inflammation, and in the immune response [20]. Ludwig et al. showed that high levels of expression of NF-κB were related to RET activation in a human medullary thyroid carcinoma cell line [21]. Palona et al. showed that anti-apoptosis and invasion of thyroid carcinoma cells regulated by BRAF were also regulated by NF-κB [22]. The findings of these previously published studies support a role for NF-κB in the progression and metastasis of PTC that express the BRAF or RET genes.
Previously published studies have also shown that the induction of RET gene rearrangement was associated with increased expression of inflammation-related genes in the tumor microenvironment of patients with primary PTC and lymph node metastasis [23–25]. Husain et al. found that in anaplastic thyroid carcinoma tissue, levels of vascular endothelial growth factor (VEGF)A, VEGFC, and interleukin (IL)-6 increased, and were associated with the expression of BRAF V600E [26]. The findings of these previously published studies support a role for the BRAF V600E mutation on inflammation in thyroid cancer.
In an in vitro study, we have previously published the findings protein expression, and cell proliferation and cell migration in four thyroid cancer cell lines, TPC-1 (BRAF WT/WT), BCPAP (BRAF V600E/V600E), PCCL3, and PTC3–5 (RET/PTC), which showed that BRAF V600E and RET/PTC both promoted thyroid cell proliferation and migration, and NF-κB was involved in these cellular processes [27].
Therefore, the aim of this study was to investigate the expression of the BRAF V600E gene mutation and the RET/PTC gene rearrangement in the progression of PTC in 50 patients from Inner Mongolia. The activity of NF-κB and serum inflammatory mediators were also investigated, with clinical characteristics, including the presence of lymph node metastases and time to tumor progression.
Material and Methods
Patients studied and ethical approval
Clinical data and tumor samples were collected from 50 patients with papillary thyroid carcinoma (PTC) with tumor recurrence, and from ten patients with benign thyroid adenoma as the control group. Both groups of patients had histologically confirmed diagnoses and were investigated and treated at the Endocrinology Department of Inner Mongolia Peoples’ Hospital, between January 2016 to January 2017. All patients studied were matched by age and gender and lived in Inner Mongolia for more than 20 years. Patients with a diagnosis of PTC underwent tumor resection and therapeutic neck dissection. The tumor tissues were sent to the Department of Pathology for examination, preparation the tissue sections and slides for light microscopy. Informed consent was obtained for each patient who participated in the study. The study was approved by the Medical Ethics Committees of Inner Mongolia Peoples’ Hospital.
Detection of the βRAF V600E mutation using polymerase chain reaction (PCR)
DNA was extracted from the thyroid tumor tissue using a DNA extraction kit (Omega Bio-Tek, Inc.) according to the manufacturer’s instructions. The following primers were prepared for polymerase chain reaction (PCR):
BRAF V600E (forward: 5′-TCATAATGCTTGCTCTGATGGGA-3′ and reverse: 5′-GGCCAAAAATTTAATCAGTGGA-3′) and
GAPDH (forward: 5′-TGCACCACCAACTGCTTAGC-3′ and reverse: 5′-AGCTCAGGGATGACCTTGCC-3′).
The primers were synthesized by Invitrogen (Thermo Fisher Scientific Inc. Shanghai, China). Polymerase chain reaction (PCR) was performed using 25 μl samples, with the following reaction schedule, that included 30 cycles: pre-denatured at 95°C for 5 min; denaturated at 95°C for 30 sec; annealed at 55°C for 30 sec; extension at 72°C for 30 sec; and a final extension at 72°C for 8 min. The PCT product was analyzed using a 1.5% agarose gel (Sigma-Aldrich Co., Shanghai, China). Specific bands were recovered and sent to BGI Genomics Co., Ltd (Shenzhen, China) for DNA sequencing.
Detection of RET/PTC and nuclear factor-κB (NF-κB) (p65) using immunohistochemistry and immunofluorescence
Immunohistochemistry was used to detect the expression of RET/PTC and nuclear factor (NF)- κB (p65) in tissues containing PTC (n=50) and benign thyroid adenoma (n=10). Tissue sections on glass slides were dried at 68°C for 20 min, and de-waxed in graded ethanol solutions, incubated in 3% H2O2 at 37°C for 10 min, and washed in phosphate buffered saline (PBS) (Boster Biological Technology Co., Ltd. Wuhan, China) and incubated with citrate buffer solution (0.01M) at 95°C for 20 min, cooled to 25°C, and blocked with normal goat serum (Beijing China Ocean Co., Ltd.) at 37°C for 10 min.
Tissue sections on glass slides were incubated with primary rabbit anti-RET antibody (1: 500) (Abcam, Shanghai, China) and primary antibody to NF-κB (p65) (1: 500) (Abcam, Shanghai, China) at 4°C, overnight, and then incubated with goat anti-rabbit antibody (1: 200) (Abcam, Shanghai, China) or donkey anti-rabbit IgG conjugated with DyLight 488 Amine-Reactive Dye (1: 500) (Abcam, Shanghai, China) at 37°C for 30 min, for immunofluorescence analysis. For immunohistochemistry analysis, following washing, 3,3’-diaminobenzidine (DAB) (Beyotime Biotechnology, Shanghai, China) was incubated on the slides at 25°C for between 3–30 min until the color label developed. After washing, counterstaining of the tissue sections was performed with hematoxylin and eosin (H&E) (Beyotime Biotechnology, Shanghai, China) at 25°C for 2 min. After washing, the stained tissue sections on the glass slides were sealed with neutral resin (Bioway Biotechnology Co., Ltd. Beijing, China), coverslipped, and observed under a light microscope (Olympus Corporation, Beijing, China).
Detection of inflammatory mediators using enzyme-linked immunosorbent assay (ELISA)
Fasting venous blood samples (10 ml) were collected from each patient, before and after surgery, in glass tubes containing the anticoagulant, ethylenediaminetetraacetic acid (EDTA) (Corning, Shanghai, China). Blood samples were centrifuged at 4°C and 3,000×g for 15 min. The supernatant was collected and placed in a cryogenic tube (Corning, Shanghai, China) and stored at −20°C.
Tumor tissues were cut into pieces and homogenized in a 0.86% saline solution and fully homogenized (Shanghai Jingxin Industrial Development Co., Ltd., China) at 4°C. The homogenized samples were centrifuged at 4°C and 4,000×g for 15 min. The supernatant was collected in epoxy epoxide (EP) (Corning, Shanghai, China) and stored at −20°C.
Serum and tissue interleukin (IL)-1β (Bioway Biotechnology Co., Ltd. Beijing, China), IL-6 (Bioway Biotechnology Co., Ltd. Beijing, China), tumor necrosis factor (TNF)-α (Bioway Biotechnology Co., Ltd. Beijing, China), transforming growth factor (TGF)-β (Bioway Biotechnology Co., Ltd. Beijing, China), C-X-C motif chemokine ligand (CXCL)1 (Jianglai Industrial Company, Shanghai, China), CXCL2 (Jianglai Industrial Company, Shanghai, China), C-C motif chemokine ligand (CCL)2 (Jianglai Industrial Company, Shanghai, China) and CCL3 (Jianglai Industrial Company, Shanghai, China) were measured using an enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer’s instructions.
Statistical analysis
Data were presented as the mean ± standard deviation (SD) and analyzed using SPSS version 20.0. One-way analysis of variance (ANOVA) and a Pearson’s chi-squared (χ2) test were used for comparative analysis. A test of least significant difference (LSD) was performed with the homogeneity of variance, and the Dunnett’s T3 test was performed for analysis of heterogeneity. Pearson’s correlation was performed on the association between BRAF V600E or RET/PTC and age, gender, lymph node metastasis, and NF-κB expression in patients with PTC. A P-value of <0.05 was considered to be statistically significant.
Results
BRAF V600E and RET/PTC were present in papillary thyroid carcinoma (PTC) tissue but not in benign thyroid adenoma tissue
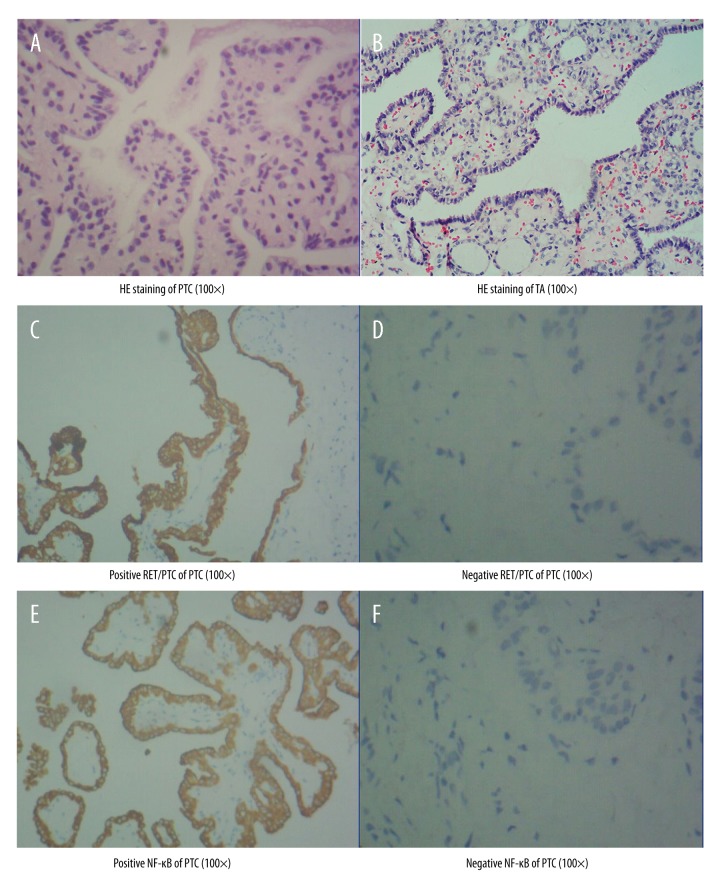
The clinical data of 50 patients with papillary thyroid carcinoma (PTC) patients and ten patients with benign thyroid adenoma are shown in Table 1. The histology of the tumors was evaluated using light microscopy with the tissue sections routinely stained with hematoxylin and eosin (H&E). The histological appearances of PTC and benign thyroid adenoma tissues are shown in Figure 1A and Figure 1B, respectively. Figure 1C shows positive immunostaining for RET/PTC in tissue from a case of papillary thyroid carcinoma (PTC).
Table 1.
Clinical data of PTC and TA patients.
| PTC | TA | F/χ2 | P | |||||
|---|---|---|---|---|---|---|---|---|
| BRAFV600E | RET/PTC | BRAFV600E & RET/PTC | BRAFV600E | RET/PTC | BRAFV600E & RET/PTC | |||
| N | 50 | 10 | ||||||
| n/% | 37/74.00 | 8/16.00 | 5/10.00 | – | – | – | 41.542 | <0.001* |
| Gender | ||||||||
| Male | 18/36.00 | 6/60.00 | 3.015 | 0.037* | ||||
| 10/27.03 | 6/75.00 | 2/40.00 | – | – | – | |||
| Female | 32/64.00 | 4/40.00 | ||||||
| 27/72.97# | 2/25.00# | 3/60.00 | – | – | – | |||
| F/χ2 | 3.579 | – | ||||||
| P | 0.036* | – | ||||||
| Age | 53.06±11.42 | 48.60±11.61 | 6.614 | 0.001* | ||||
| 51.65±10.30 | 49.13±11.62 | 71.80±3.11@& | – | – | – | |||
| F/χ2 | 9.542 | – | ||||||
| P | <0.001 | – | ||||||
| r | 0.399 | – | ||||||
| 95%CI | 0.023~0.629 | – | ||||||
| P | 0.004* | – | ||||||
| Recurrent time | 0.73~15.12 | 0.46~8.37 | 0.36~5.08 | – | – | – | ||
| Average recurrent time | 7.27±4.65 | 5.48±3.06 | 2.43±2.00@ | – | – | – | ||
| F/χ2 | 6.499 | – | ||||||
| P | 0.027* | – | ||||||
| Lymphatic metastasis | 38/76.00 | – | ||||||
| 15/40.54 | 8/100.00@ | 5/100.00@ | – | – | – | |||
| F/χ2 | 8.961 | – | ||||||
| P | 0.001 | – | ||||||
| r | −0.486 | – | ||||||
| 95%CI | −0.634~−0.350 | – | ||||||
| P | <0.001* | – | ||||||
| Positive NF-κB | 43/86.00 | 2/20.00 | 9.619 | <0.001* | ||||
| 32/86.49 | 6/75.00 | 5/100.00 | – | – | – | |||
| F/χ2 | 0.790 | – | ||||||
| P | 0.460 | – | ||||||
PTC – papillary thyroid carcinoma; TA – thyroid adenoma thyroid adenoma; NF-κB – nuclear factor-κB; CI – confidence interval;
P<0.05;
comparing between male and female, P<0.05;
comparing to BRAFV600E, P<0.05;
comparing to RET/PTC, P<0.05.
Figure 1.
Photomicrographs of the light microscopy of papillary thyroid carcinoma (PTC) and benign thyroid adenoma, routinely stained with hematoxylin and eosin (H&E) and using immunohistochemistry (IHC) for RET/PTC and nuclear factor-κB (NF-κB) (p65). (A) Tissue section showing the histological findings in papillary thyroid carcinoma (PTC). Hematoxylin and eosin (H&E). (B) Tissue section showing the histological findings in benign thyroid adenoma (TA). (H&E). (C) Immunohistochemistry shows positive immunostaining for RET/PTC in tissue from a case of papillary thyroid carcinoma (PTC). (D) Negative immunostaining for RET/PTC. (E) Immunohistochemistry shows positive immunostaining for nuclear factor-κB (NF-κB) (p65) in tissue from a case of papillary thyroid carcinoma (PTC). (F) Negative immunostaining for NF-κB.
Of the 50 patients with PTC, 37 patients expressed the BRAF V600E gene mutation, eight patients expressed RET/PTC, and five patients showed concomitant BRAF V600E and RET/PTC. There was no positive BRAF V600E or RET/PTC in ten patients with benign thyroid adenoma (F=41.542, P<0.05).
In the tumor tissues from the 50 patients with PTC, the positive rate of expression of BRAF V600E (74%) was significantly greater compared with the expression of RET/PTC (16%), and the positive rate of concomitant BRAF V600E and RET/PTC (10%) was significantly lower (P<0.05). These expression patterns were not found in the tissue of benign thyroid adenoma.
BRAF V600E and RET/PTC expression were associated with the gender of patients with PTC
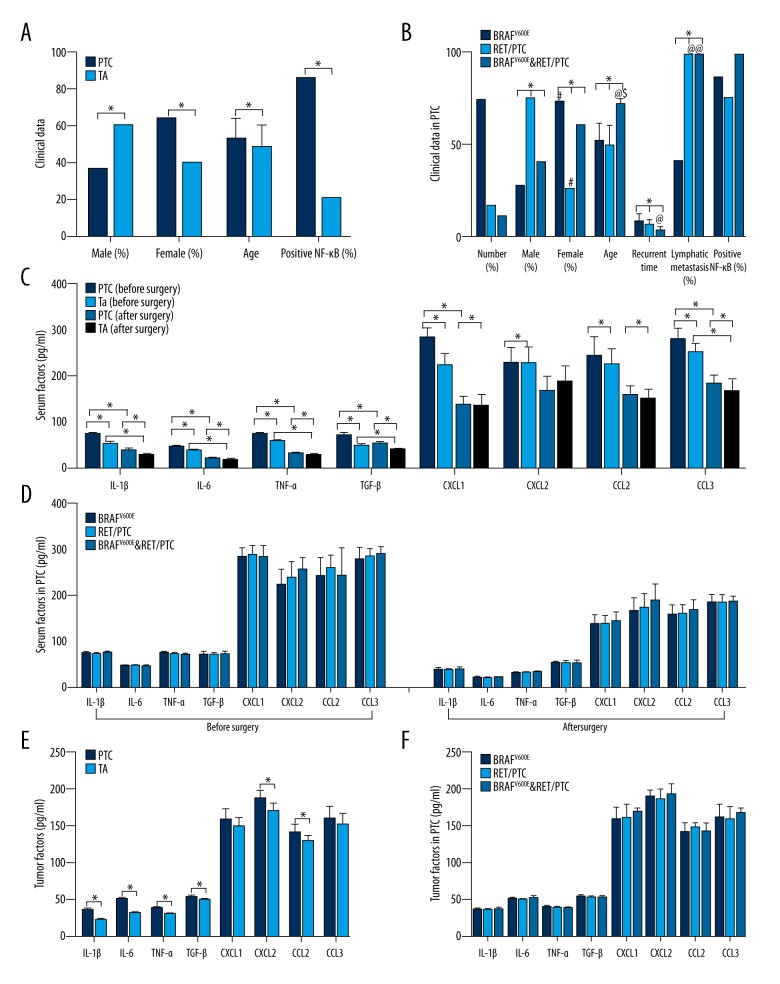
There were significant differences in the gender distribution of positive BRAF V600E, RET/PTC and concomitant BRAF V600E and RET/PTC in patients with PTC compared with benign thyroid adenoma (F=3.015, P=0.037, P<0.05) (Figure 2A), as no positive BRAF V600E or RET/PTC expression was found in patients with benign thyroid adenoma.
Figure 2.
Distribution of gender, age, time to recurrence, lymph node metastases, and positive expression of nuclear factor-κB (NF-κB) in patients with papillary thyroid carcinoma (PTC) and patients with benign thyroid adenoma (TA). (A) Comparison of clinical data between patients with papillary thyroid carcinoma (PTC) and patients with benign thyroid adenoma. (B) Comparison of clinical data among patients with different types of PTC. (C) Comparison of serum factors between patients with PTC and patients with benign thyroid adenoma, before and after surgery. (D) Comparison of serum factors among patients with different kinds of PTC, before and after surgery. (E) Comparison of tumor tissue factors between patients with PTC and patients with benign thyroid adenoma. (F) Comparison of tumor tissue factors among patients with different kinds of PTC. * Comparing between different groups, P<0.05; # Comparison between male and female patients, P<0.05; @ Comparisons of expression of BRAF V600E, P<0.05; $ Comparison of expression of RET/PTC, P<0.05.
In patients with PTC, the gender distributions of positive BRAF V600E, RET/PTC and concomitant BRAF V600E and RET/PTC showed significant differences (F=3.579, P=0.036, P<0.05). Positive BRAF V600E was present in 72.97% of female patients with PTC, which was significantly greater compared with male patients with PTC (27.03%). Positive RET/PTC was present in 75.00% of male patients with PTC, which was significantly greater compared with female patients with PTC (25%) (P<0.05) (Figure 2B).
BRAF V600E and RET/PTC expression correlated with the age of patients with PTC
There were significant differences in age distributions of BRAF V600E, RET/PTC and concomitant BRAF V600E and RET/PTC expression between patients with PTC and benign thyroid adenoma (F=6.614, P=0.001, P<0.05) (Figure 2A).
In patients with PTC, the age distributions of positive BRAF V600E, RET/PTC and concomitant BRAF V600E and RET/PTC expression in patients with PTC showed significant differences (F=9.542, P<0.001). Significant differences were found between patients with PTC with BRAF V600E and those with concomitant BRAF V600E and RET/PTC (P<0.001), as well as between RET/PTC and concomitant BRAF V600E and RET/PTC (P=0.002, P<0.05) (Figure 2B). Correlation analysis showed that the age of patients with PTC was positively correlated with BRAF V600E, RET/PTC and concomitant BRAF V600E and RET/PTC, as the older the patients with PTC, the higher the positive rate of RET/PTC or concomitant BRAF V600E and RET/PTC was, and the lower the positive rate of BRAF V600E was (r=0.399; 95% CI, 0.023–0.629; P=0.004, P<0.05).
βRAF V600E and RET/PTC expression were associated with time to recurrence in patients with PTC
In patients with PTC, the distribution of time to tumor recurrence in patients with BRAF V600E, RET/PTC and concomitant BRAF V600E and RET/PTC showed significant differences (F=6.224, P=0.045, P<0.05) (Figure 2B). Time to recurrence in patients with PTC carrying BRAF V600E was significantly longer compared with patients with concomitant expression of BRAF V600E and RET/PTC (P=0.005, P<0.05) (Table 1).
BRAF V600E and RET/PTC expression correlated with lymph node metastasis in patients with PTC
The presence of lymph node metastases and the expression of BRAF V600E, RET/PTC and concomitant BRAF V600E and RET/PTC in patients with PTC showed significant differences (F=8.961, P=0.001, P<0.05). There was a significant difference between patients with BRAF V600E and RET/PTC (P=0.001, P<0.05), as well as between BRAF V600E and concomitant BRAF V600E and RET/PTC expression (P=0.006, P<0.05). The presence of lymph node metastasis in patients with PTC was positively correlated with BRAF V600E, RET/PTC and concomitant expression of BRAF V600E and RET/PTC. Patients with PTC with positive RET/PTC or concomitant BRAF V600E and RET/PTC having a significantly increased rate of lymph node metastases (r=−0.486; 95% CI, −0.634–0.350; P<0.05).
BRAF V600E and RET/PTC expression were associated with NF-κB in patients with PTC
There was a significant difference in the distribution of NF-κB in patients with PTC who were positive for BRAF V600E, RET/PTC and concomitant BRAF V600E and RET/PTC compared with patients with benign thyroid adenoma (F=9.619, P<0.05) (Figure 2A). However, no significant difference was found between patients with PTC and BRAF V600E, RET/PTC or concomitant BRAF V600E and RET/PTC expression (Figure 2B).
Correlation between age, lymph node metastases, time to recurrence, and NF-κB in patients with PTC
The age of the patients with PTC was significantly correlated with the presence of lymph node metastases, as the older the patients were, the higher the rate of lymph node metastasis was (r=−0.373; 95% CI, −0.619–0.105; P=0.008, P<0.05). Time to recurrence of PTC was correlated with the presence of lymph node metastases or with positive expression of NF-κB, shown by the significant association between time to recurrence and the higher the rate of lymph node metastasis (r=−0.515; 95% CI, −0.711–0.300; P<0.001) and between time to recurrence and the positive rate of expression of NF-κB (r=−0.485; 95% CI, −0.625–0.302; P<0.001).
Inflammatory mediators
As shown in Table 2 and Figure 2C, serum levels of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, C-X-C motif chemokine ligand (CXCL)1, CXCL2, C-C motif chemokine ligand (CCL)2, and CCL3 levels in patients with PTC significantly decreased after surgery when compared with levels before surgery (P<0.05). In patients with benign thyroid adenoma, serum levels of IL-1β, IL-6, TNF-α, TGF-β, CXCL1, CCL2 and CCL3 also significantly decreased after surgery when compared with levels before surgery (P<0.05).
Table 2.
Serum inflammatory factors in PTC and TA patients before and after surgery (χ±SDs, pg/ml).
| PTC | TA | F/χ2 | P | |||
|---|---|---|---|---|---|---|
| BRAFV600E | RET/PTC | BRAFV600E & RET/PTC | ||||
| IL-1β | ||||||
| Before | 72.28±4.35 | 51.07±4.80 | 191.53 | <0.001* | ||
| After | 36.75±4.16 | 27.01±4.24 | 45.437 | <0.001* | ||
| t | 41.664 | 12.487 | ||||
| P | <0.001* | <0.001* | ||||
| Before | 72.56±4.24 | 70.09±4.58 | 73.66±4.58 | |||
| F/χ2 | 1.356 | |||||
| P | 0.268 | |||||
| After | 36.95±4.04 | 35.73±3.72 | 36.97±6.18 | |||
| F/χ2 | 0.281 | |||||
| P | 0.757 | |||||
| IL-6 | ||||||
| Before | 45.49±1.50 | 35.79±1.89 | 320.105 | <0.001* | ||
| After | 19.43±2.46 | 16.31±2.49 | 13.333 | 0.001* | ||
| t | 61.240 | 16.794 | ||||
| P | <0.001* | <0.001* | ||||
| Before | 45.56±1.40 | 45.33±1.86 | 45.23±1.86 | |||
| F/χ2 | 0.160 | |||||
| P | 0.852 | |||||
| After | 19.66±2.54 | 18.18±2.61 | 19.74±0.46 | |||
| F/χ2 | 1.254 | |||||
| P | 0.295 | |||||
| TNF-α | ||||||
| Before | 72.52±3.75 | 57.15±4.24 | 134.009 | <0.001* | ||
| After | 30.45±2.42 | 27.70±3.63 | 8.975 | 0.004* | ||
| t | 66.548 | 12.348 | ||||
| P | <0.001* | <0.001* | ||||
| Before | 73.08±3.76 | 71.47±3.80 | 70.09±2.81 | |||
| F/χ2 | 1.830 | |||||
| P | 0.172 | |||||
| After | 30.30±2.31 | 30.08±2.77 | 32.07±2.51 | |||
| F/χ2 | 0.284 | |||||
| P | 0.969 | |||||
| TGF-β | ||||||
| Before | 69.64±6.16 | 47.41±4.10 | 118.816 | <0.001* | ||
| After | 51.03±5.22 | 38.62±3.57 | 51.304 | <0.001* | ||
| t | 15.443 | 5.755 | ||||
| P | <0.001* | <0.001* | ||||
| Before | 69.62±6.22 | 69.37±5.48 | 70.24±7.94 | |||
| F/χ2 | 0.031 | |||||
| P | 0.969 | |||||
| After | 51.03±4.98 | 51.42±5.88 | 50.38±7.00 | |||
| F/χ2 | 0.058 | |||||
| P | 0.943 | |||||
| CXCL1 | ||||||
| Before | 283.15±20.70 | 222.39±25.67 | 66.271 | <0.001* | ||
| After | 135.99±20.58 | 133.10±26.16 | 0.149 | 0.701 | ||
| t | 32.615 | 7.569 | ||||
| P | <0.001* | <0.001* | ||||
| Before | 282.58±20.39 | 286.63±21.92 | 281.77±25.19 | |||
| F/χ2 | 0.133 | |||||
| P | 0.876 | |||||
| After | 135.26±21.22 | 136.15±18.62 | 141.12±22.12 | |||
| F/χ2 | 0.173 | |||||
| P | 0.842 | |||||
| CXCL2 | ||||||
| Before | 227.40±35.22 | 227.12±35.50 | 0.001 | 0.982 | ||
| After | 166.53±32.58 | 186.24±36.07 | 2.945 | 0.091 | ||
| t | 8.471 | 2.111 | ||||
| P | <0.001* | 0.064 | ||||
| Before | 221.63±34.61 | 237.21±35.57 | 254.38±28.04 | |||
| F/X2 | 2.405 | |||||
| P | 0.101 | |||||
| After | 162.76±32.05 | 171.48±30.68 | 186.52±37.87 | |||
| F/χ2 | 1.296 | |||||
| P | 0.283 | |||||
| CCL2 | ||||||
| Before | 242.95±42.99 | 223.04±37.04 | 1.862 | 0.178 | ||
| After | 156.75±21.96 | 148.60±21.86 | 1.150 | 0.288 | ||
| t | 13.362 | 5.041 | ||||
| P | <0.001* | 0.001* | ||||
| Before | 240.01±43.25 | 257.75±29.09 | 241.08±61.62 | |||
| F/χ2 | 0.555 | |||||
| P | 0.578 | |||||
| After | 155.16±22.31 | 158.18±20.54 | 166.28±23.36 | |||
| F/χ2 | 0.574 | |||||
| P | 0.567 | |||||
| CCL3 | ||||||
| Before | 279.09±26.26 | 250.60±20.74 | 10.420 | 0.002* | ||
| After | 183.02±18.52 | 164.45±28.71 | 6.884 | 0.011* | ||
| t | 19.860 | 7.252 | ||||
| P | <0.001* | <0.001* | ||||
| Before | 276.78±28.62 | 283.75±18.36 | 288.74±16.73 | |||
| F/χ2 | 0.596 | |||||
| P | 0.555 | |||||
| After | 183.06±19.38 | 181.83±19.56 | 184.70±12.20 | |||
| F/χ2 | 0.036 | |||||
| P | 0.965 | |||||
PTC – papillary thyroid carcinoma; TA – thyroid adenoma thyroid adenoma;
P<0.05.
Before surgery, except for CXCL2 and CCL2, serum IL-1β (F=191.53, P<0.001), IL-6 (F=320.105, P<0.001), TNF-α (F=134.009, P<0.001), TGF-β (F=118.816, P<0.001), CXCL1 (F=66.271, P<0.001) and CCL3 (F=10.420, P=0.002) levels were significantly higher in patients with PTC compared with patients with benign thyroid adenoma (P<0.05). After surgery, except for CXCL1, CXCL2 and CCL2, serum IL-1β (F=45.437, P<0.001), IL-6 (F=13.333, P=0.001), TNF-α (F=8.975, P=0.004), TGF-β (F=51.304, P<0.001) and CCL3 (F=6.884, P=0.011) levels were significantly higher in patients with PTC compared with patients with benign thyroid adenoma (P<0.05). No significance in the levels of serum inflammatory mediators was found before and after surgery among different patients with PTC (Table 2, Figure 2D).
As shown in Table 3 and Figure 2E, except for CXCL1 and CCL3 levels, IL-1β (F=453.823, P<0.001), IL-6 (F=622.606, P<0.001), TNF-α (F=149.515, P<0.001), TGF-β (F=16.927, P<0.001), CXCL2 (F=20.494, P<0.001) and CCL2 (F=9.445, P=0.003) levels in tumor tissues were significantly higher in patients with PTC compared with patients with benign thyroid adenoma (P<0.05). No significant differences in the expression of tumor tissue factors were found among between different types of PTC tumor (Figure 2F).
Table 3.
Tumor inflammatory factors in PTC and TA patients (χ±SDs, pg/ml).
| PTC | TA | F/χ2 | P | |||
|---|---|---|---|---|---|---|
|
| ||||||
| BRAFV600E | RET/PTC | BRAFV600E & RET/PTC | ||||
| IL-1β | 35.89±1.83 | 22.63±1.61 | 453.823 | <0.001* | ||
| 35.91±1.81 | 35.30±2.07 | 36.64±1.65 | ||||
| F/χ2 | 0.845 | |||||
| P | 0.436 | |||||
|
| ||||||
| IL-6 | 50.59±2.26 | 32.23±1.12 | 622.606 | <0.001* | ||
| 50.70±2.32 | 49.50±1.54 | 51.54±2.57 | ||||
| F/χ2 | 1.443 | |||||
| P | 0.246 | |||||
|
| ||||||
| TNF-α | 53.30±2.80 | 31.04±1.11 | 149.515 | <0.001* | ||
| 38.85±2.01 | 38.59±1.96 | 38.13±1.24 | ||||
| F/χ2 | 0.326 | |||||
| P | 0.724 | |||||
|
| ||||||
| TGF-β | 53.30±2.80 | 49.27±2.94 | 16.927 | <0.001* | ||
| 53.71±2.78 | 52.11±2.81 | 52.17±2.61 | ||||
| F/χ2 | 1.550 | |||||
| P | 0.223 | |||||
|
| ||||||
| CXCL1 | 158.58±15.36 | 149.47±12.35 | 3.098 | 0.084 | ||
| 157.14±15.73 | 159.65±17.25 | 167.50±4.60 | ||||
| F/χ2 | 1.027 | |||||
| P | 0.366 | |||||
|
| ||||||
| CXCL2 | 187.62±10.87 | 170.62±10.65 | 20.494 | <0.001* | ||
| 187.91±10.01 | 184.55±13.07 | 190.44±14.79 | ||||
| F/χ2 | 0.489 | |||||
| P | 0.616 | |||||
|
| ||||||
| CCL2 | 141.07±11.59 | 129.17±8.64 | 9.445 | 0.003* | ||
| 140.02±12.31 | 146.32±6.90 | 140.40±11.49 | ||||
| F/χ2 | 0.982 | |||||
| P | 0.382 | |||||
|
| ||||||
| CCL3 | 160.21±16.96 | 151.95±16.86 | 1.984 | 0.164 | ||
| 159.96±18.10 | 157.62±16.63 | 166.24±5.69 | ||||
| F/χ2 | 0.404 | |||||
| P | 0.670 | |||||
PTC – papillary thyroid carcinoma; TA – thyroid adenoma thyroid adenoma;
P<0.05.
Discussion
Recently, BRAF gene mutations and the RET/PTC gene re-arrangement have been found in patients with papillary thyroid carcinoma (PTC) by fine-needle aspiration biopsy (FNAB), a finding that has attracted clinical attention to these factors in PTC [28,29]. Although not all tissues from patients with PTC contain BRAF mutations or the RET/PTC gene re-arrangement, the association between BRAF mutation or RET/PTC re-arrangement and the pathogenesis and progression of PTC remains unclear.
The present study included tumor tissue samples from 50 patients with histologically confirmed diagnoses of PTC and tissue samples from ten patients with benign thyroid adenoma, which allowed comparison of the findings from detection of expression of BRAF V600E or RET/PTC or concomitant expression of both. The findings of this study showed that 37 patients with PTC carried the BRAF V600E mutation, eight patients had the RET/PTC translocation, and five patients had concomitant BRAF V600E and RET/PTC, but no positive BRAF V600E or RET/PTC were present in patients with benign thyroid adenoma. These results indicated that BRAF V600E and RET/PTC only occurred in malignant thyroid tumors, specifically in PTC in this study, but not in benign thyroid tumors. The findings of the present study are supported by the findings from previously published studies [30–35].
There have been previous studies that have reported the concomitant expression of BRAF V600E and RET/PTC in PTC tumor tissue. Wang et al. studied 125 Chinese patients with PTC and found that 19% of patients had tumors that expressed both BRAF V600E and RET/PTC [36]. Ying et al. studied patients with recurrent PTC and found that 9.3% of patients with PTC expressed both BRAF V600E and RET/PTC [37]. In the present study, five out of 50 patients (10%) with recurrent PTC expressed concomitant BRAF V600E and RET/PTC. These findings indicate that concomitant expression of BRAF V600E and RET/PTC in patients with PTC is associated with an increased risk of tumor recurrence.
In the present study, gender differences were investigated, which showed that positive BRAF V600E in female patients with PTC was significantly more prevalent in female patients compared with male patients (27: 10), while RET/PTC was significantly more prevalent in male patients with PTC when compared with female patients (6: 2), but no significant difference between male and female patients was found for the concomitant expression of BRAF V600E and RET/PTC in this study. According to the findings of a previous study by Ito et al., there was no significant difference between men and women for the expression of BRAF V600E based on a study that included 631 Japanese patients with PTC [38]. Li et al., investigated 3,437 patients with PTC, and their results also showed no differences between expression of the BRAF V600E mutation and gender [39]. However, in a study by Sadtzki et al., the findings showed an increased prevalence of positive RET/PTC in male patients with PTC compared with female patients with PTC [40]. Ying et al. analyzed the data of 68 patients with PTC and found positive BRAF V600E expression in female patients (70.3%), which was significantly greater when compared with male patients, while positive RET/PTC in male patients (75%) was significantly greater when compared with female patients [37]. The findings from these studies show gender variations that may be due to regional differences in the populations studied.
The present study included an analysis of the age of the patients with PTC, and the results showed that the average age of patients with PTC carrying positive BRAF V600E or RET/PTC was significantly lower compared with those expressing concomitant BRAF V600E and RET/PTC. Also, the expression of both BRAF V600E and RET/PTC was positively correlated with age but negatively correlated with time to recurrence. These results indicated an association between concomitant expression of BRAF V600E and RET/PTC and increased age in the patients with PTC with a shorter was the time to tumor recurrence in PTC, indicating a worse prognosis. Previously published studies have shown conflicting results for the association between clinical outcome in PTC, patient age, and BRAF V600E or RET/PTC expression. In the present study, in primary PTC, BRAF mutations were correlated with age, and most of the patients with PTC carrying BRAF mutations were less than 45 years-of-age, but in previous studies that included patients more than 45 years-of-age, higher positive rates for RET/PTC occurred in the primary tumor [34,41–43].
The results of the present study showed that a there was a positive rate of lymph node involvement in patients with PTC carrying RET/PTC, or concomitant BRAF V600E and RET/PTC, which was 100%. For patients with PTC carrying BRAF V600E, the presence of lymph node metastases was 40.54%. Although Lu et al. analyzed 150 patients with primary PTC and found an association with positive lymph node involvement in patients with PTC carrying BRAF V600E (66.9%), which did not reach statistical significance [44]. Several previous studies have shown that the presence of RET/PTC, RET/PTC subtypes, and geographical area, to be associated with lymph node metastases in patients with PTC [18,19,45].
Nuclear factor-κB (NF-κB) has a role in promoting tumor metastasis and has been previously shown to be involved the progression of PTC [46]. Bommarito et al., found that NF-κB was regulated by BRAF V600E in promoting the development and progression of PTC [47]. Neely et al., showed a significant correlation between RET/PTC expression and NF-κB activity [48]. However, there has been no previously published evidence to show an association between the expression of NF-κB and the development of benign thyroid adenoma. In the present study, 86% of cases of PTC were positive for the expression of NF-κB. However, 20% of cases of benign thyroid adenoma were also positive for NF-κB. The positive expression NF-κB in patients with PTC and was most significantly associated with concomitant expression of BRAF V600E and RET/PTC. Therefore, from the findings of this study, it is possible to propose that both BRAF V600E and RET/PTC could promote the activity of NF-κB and the progression of PTC. Also, the findings of this study showed that time to recurrence of PTC was positively and significantly correlated with NF-κB activity in PTC, but not in benign thyroid adenoma. These findings are of interest, and further studies are recommended on the associations between the expression of BRAF V600E and RET/PTC with NF-κB and with patients prognosis in PTC.
Both malignant thyroid carcinoma and benign thyroid adenoma can be associated with inflammation, and inflammation can be associated with an increase in gene mutations, as has been shown for BRAF mutations, RAS mutations, and RET/PTC gene re-arrangement [49]. In the present study, the results showed significant differences in the levels of serum inflammatory mediators between patients with PTC and patients with benign thyroid adenoma. In patients with PTC, before surgery, serum levels of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, C-X-C motif chemokine ligand (CXCL)1, and CCL3 were significantly increased when compared with patients with benign thyroid adenoma. After surgery, for patients with PTC, all the serum levels of inflammatory mediators decreased significantly, but serum levels of IL-1β, IL-6, TNF-α, TGF-β, and CCL3 in patients with PTC were still significantly greater post-operatively when compared with serum levels in patients with benign thyroid adenoma. IL-1β, IL-6, TNF-α, TGF-β, CXCL2, and CCL2 in tumor tissue of patients with PTC were significantly increased compared with patients with benign thyroid adenoma, which supported an increased degree of inflammation in PTC compared with benign thyroid adenoma.
This study had several limitations. The study included a small sample size of 50 patients with PTC, which is a factor that may have affected the results and their interpretation. Also, clinical data from the patients included in the study was only collected during a two-year period, and longer patient follow-up should be undertaken in future studies. This study was based on an Asian population from the Inner Mongolia region of China. Future studies are recommended that include a larger patient sample population and comparison with regional variations in patients with PTC.
Conclusions
In a patient population in Inner Mongolia with papillary thyroid carcinoma (PTC), the expression of the BRAF V600E mutation and the RET/PTC translocation promoted the activity of NF-κB, expression of inflammatory mediators, and lymph node metastases and showed a significant association with age, gender, and time to recurrence.
Footnotes
Source of support: The costs of the study were supported by the corresponding author and Zhu Jiang Hospital of Southern Medical University
Conflict of interest
None.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Database Report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83(12):2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Yu GP, Li JC, Branovan D, et al. Thyroid cancer incidence and survival in the National Cancer Institute Surveillance, Epidemiology, and End Results race/ethnicity groups. Thyroid. 2010;20(5):465–73. doi: 10.1089/thy.2008.0281. [DOI] [PubMed] [Google Scholar]
- 3.Sherman SI, Angelos P, Ball DW, et al. Thyroid carcinoma. Lancet. 2003;361(9356):501–11. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 4.Gimm O. Thyroid cancer. Cancer Lett. 2001;163(2):143–56. doi: 10.1016/s0304-3835(00)00697-2. [DOI] [PubMed] [Google Scholar]
- 5.Marais R, Light Y, Paterson HF, et al. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272(7):4378–83. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 6.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 7.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Nat Cancer Inst. 2003;95(8):625–27. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 8.Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hotspot BRAF mutation,V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88(9):4393–97. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88(11):5399–404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 10.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90(12):6373–79. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 11.Riescoeizaguirre G, Gutiérrezmartínez P, Garcíacabezas MA, et al. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Rel Cancer. 2006;13(1):257–69. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 12.Kim BH, Kim IJ, Lee BJ, et al. Detection of plasma BRAF(V600E) mutation is associated with lung metastasis in papillary thyroid carcinomas. Yonsei Med J. 2015;56(3):634–40. doi: 10.3349/ymj.2015.56.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurt B, Yalçın S, Alagöz E, et al. The relationship of the BRAF V600E mutation and the established prognostic factors in papillary thyroid carcinomas. Endocrine Pathol. 2012;23(3):135–40. doi: 10.1007/s12022-012-9218-7. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi M. Structure and expression of the RET transforming gene. IARC Sci Publ. 1988;92(92):189–97. [PubMed] [Google Scholar]
- 15.Fusco A, Grieco M, Santoro M, et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature. 1987;328(6126):170–72. doi: 10.1038/328170a0. [DOI] [PubMed] [Google Scholar]
- 16.Grieco M, Santoro M, Berlingieri MT, et al. PTC is a novel rearranged form of the RET proto-oncogene and frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60(4):557–63. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- 17.Powell DJ, Russell J, Nibu K, et al. The RET/PTC3 oncogene: Metastatic solid-type papillary carcinomas in murine thyroids. Cancer Res. 1998;58(23):5523–28. [PubMed] [Google Scholar]
- 18.Miki H, Kitaichi M, Masuda E, et al. RET/PTC expression may be associated with local invasion of thyroid papillary carcinoma. J Surg Oncol. 1999;71(2):81–82. doi: 10.1002/(sici)1096-9098(199906)71:2<76::aid-jso4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Giannini R, Salvatore G, Monaco C, et al. Identification of a novel subtype of H4-RET rearrangement in a thyroid papillary carcinoma and lymph node metastasis. Int J Oncol. 2000;16(3):485–89. doi: 10.3892/ijo.16.3.485. [DOI] [PubMed] [Google Scholar]
- 20.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig L, Kessler H, Wagner M, et al. Nuclear factor-kappaB is constitutively active in C-cell carcinoma and required for RET-induced transformation. Cancer Res. 2001;61(11):4526–35. [PubMed] [Google Scholar]
- 22.Palona I, Namba H, Mitsutake N, et al. BRAF V600E promotes invasiveness of thyroid cancer cells through nuclear factor kB activation. Endocrinology. 2006;147(12):5699–707. doi: 10.1210/en.2006-0400. [DOI] [PubMed] [Google Scholar]
- 23.Castellone MD, Guarino V, De FV, et al. Functional expression of the CXCR4 chemokine receptor is induced by RET/PTC oncogenes and is a common event in human papillary thyroid carcinomas. Oncogene. 2004;23(35):5958–67. doi: 10.1038/sj.onc.1207790. [DOI] [PubMed] [Google Scholar]
- 24.Borrello MG, Alberti L, Fischer A, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Nat Acad Sci USA. 2005;102(102):14825–30. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melillo RM, Castellone MD, Guarino V, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115(4):1068–81. doi: 10.1172/JCI22758. [RETRACTED ARTICLE] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Husain A, Hu N, Sadow PM, Nucera C. Expression of angiogenic switch, cachexia and inflammation factors at the crossroad in undifferentiated thyroid carcinoma with BRAF V600E. Cancer Lett. 2015;380(2):577–85. doi: 10.1016/j.canlet.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou D, Li Z, Bai X. BRAF V600E and RET/PTC promote proliferation and migration of papillary thyroid carcinoma cells in vitro by regulating nuclear factor-κB. Med Sci Monit. 2017;23:5321–29. doi: 10.12659/MSM.904928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung KW, Yang SK, Lee GK, et al. Detection of BRAF V600E mutation on fine needle aspiration specimens of thyroid nodule refines cytopathology diagnosis, especially in BRAF 600E mutation-prevalent area. Clin Endocrinol. 2006;65(5):660–66. doi: 10.1111/j.1365-2265.2006.02646.x. [DOI] [PubMed] [Google Scholar]
- 29.Sapio MR, Posca D, Raggioli A, et al. Detection of RET/PTC, TRK and BRAF mutations in preoperative diagnosis of thyroid nodules with indeterminate cytological findings. Clin Endocrinol. 2007;66(5):678–83. doi: 10.1111/j.1365-2265.2007.02800.x. [DOI] [PubMed] [Google Scholar]
- 30.Elisei R, Ugolini C, Viola D, et al. BRAF V600E mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–49. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 31.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14):1493–501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing M. BRAF mutation in thyroid cancer. Endocrine Rel Cancer. 2005;12(2):245–62. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 33.Viglietto G, Chiappetta G, Martineztello FJ, et al. RET/PTC oncogene activation is an early event in thyroid carcinogenesis. Oncogene. 1995;11(6):1207–10. [PubMed] [Google Scholar]
- 34.Elisei R, Romei C, Vorontsova T, et al. RET/PTC rearrangements in thyroid nodules: Studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab. 2001;86(7):3211–16. doi: 10.1210/jcem.86.7.7678. [DOI] [PubMed] [Google Scholar]
- 35.Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocrine Pathol. 2002;13(1):3–16. doi: 10.1385/ep:13:1:03. [DOI] [PubMed] [Google Scholar]
- 36.Wang YL, Wang JC, Wu Y, et al. Incidentally simultaneous occurrence of RET/PTC, H4-PTEN and BRAF mutation in papillary thyroid carcinoma. Cancer Lett. 2008;263(1):44–52. doi: 10.1016/j.canlet.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15(2):485–91. doi: 10.1158/1078-0432.CCR-08-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito Y, Yoshida H, Maruo R, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: Its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocrine J. 2009;56(1):89–97. doi: 10.1507/endocrj.k08e-208. [DOI] [PubMed] [Google Scholar]
- 39.Li F, Chen G, Sheng C, et al. BRAF V600E mutation in papillary thyroid microcarcinoma: A meta-analysis. Endocr Relat Cancer. 2015;22(2):159–68. doi: 10.1530/ERC-14-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadetzki S, Calderonmargalit R, Modan B, et al. RET/PTC activation in benign and malignant thyroid tumors arising in a population exposed to low-dose external-beam irradiation in childhood. J Clin Endocrinol Metab. 2004;89(5):2281–89. doi: 10.1210/jc.2003-030481. [DOI] [PubMed] [Google Scholar]
- 41.Nikiforov YE, Rowland JM, Bove KE, et al. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57(9):1690–94. [PubMed] [Google Scholar]
- 42.Fenton CL, Lukes Y, Nicholson D, et al. The RET/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000;85(3):1170–75. doi: 10.1210/jcem.85.3.6472. [DOI] [PubMed] [Google Scholar]
- 43.Nakazawa T, Kondo T, Kobayashi Y, et al. RET gene rearrangements (RET/PTC1 and RET/PTC3) in papillary thyroid carcinomas from an iodine-rich country (Japan) Cancer. 2005;104(5):943–51. doi: 10.1002/cncr.21270. [DOI] [PubMed] [Google Scholar]
- 44.Lu J, Gao J, Zhang J, et al. Association between BRAF V600E mutation and regional lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2015;8(1):793–99. [PMC free article] [PubMed] [Google Scholar]
- 45.Wang YL, Zhang RM, Luo ZW, et al. High frequency of level II–V lymph node involvement in RET/PTC positive papillary thyroid carcinoma. Eur J Surg Oncol. 2008;34(1):77–81. doi: 10.1016/j.ejso.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Pyo JS, Kang G, Kim DH, et al. Activation of nuclear factor-κB contributes to growth and aggressiveness of papillary thyroid carcinoma. Pathol Res Practice. 2013;209(4):228–32. doi: 10.1016/j.prp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Bommarito A, Richiusa P, Carissimi E, et al. BRAFV600E mutation, TIMP-1 upregulation, and NF-κB activation: Closing the loop on the papillary thyroid cancer trilogy. Endocr Rel Cancer. 2011;18(6):669–85. doi: 10.1530/ERC-11-0076. [DOI] [PubMed] [Google Scholar]
- 48.Neely RJ, Brose MS, Gray CM, et al. The RET/PTC-3 oncogene activates classical NF-κB by stabilizing NIK. Oncogene. 2011;30(1):87–96. doi: 10.1038/onc.2010.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guarino V, Castellone MD, Avilla E, Melillo RM. Thyroid cancer and inflammation. Mol Cell Endocrinol. 2010;321(1):94–102. doi: 10.1016/j.mce.2009.10.003. [DOI] [PubMed] [Google Scholar]