Abstract
Background:
Linoleic acid (LA) is abundant in modern industrialized diets. Oxidized LA metabolites (OXLAMs) and reactive aldehydes, such as 4-hydroxy-2-nonenal (4-HNE), are present in heated vegetable oils and can be endogenously synthesized following consumption of dietary LA. OXLAMs have been implicated in cerebellar degeneration in chicks; 4-HNE is linked to neurodegenerative conditions in mammals. It unknown whether increasing dietary LA or OXLAMs alters the levels of oxidized fatty acids (oxylipins), precursor fatty acids, or 4-HNE in mammalian brain.
Objectives:
To determine the effects of increases in dietary OXLAMs and dietary LA, on levels of fatty acids, oxylipins, and 4-HNE in mouse brain tissues.
Methods:
Mice (n = 8 per group) were fed one of three controlled diets for 8 weeks: (1) a low LA diet, (2) a high LA diet, or (3) the low LA diet with added OXLAMs. Brain fatty acids, oxylipins, and 4-HNE were quantified in mouse cerebellum and cerebral cortex by gas chromatography-flame ionization detection, liquid chromatography-tandem mass spectrometry, and immunoblot, respectively.
Results:
Increasing dietary LA significantly increased omega-6 fatty acids, decreased omega-3 fatty acids, and increased OXLAMs in brain. Dietary OXLAMs had minimal effect on oxidized lipids but did decrease both omega-6 and omega-3 fatty acids. Neither dietary LA nor OXLAMs altered 4-HNE levels.
Conclusion:
Brain fatty acids are modulated by both dietary LA and OXLAMs, while brain OXLAMs are regulated by endogenous synthesis from LA, rather than incorporation of preformed OXLAMs.
Keywords: Oxylipins, Linoleic acid, OXLAMs, Cerebrum, Cerebellum, Peroxidation
1. Introduction
Linoleic acid (LA) is the most abundant polyunsaturated fatty acid in modern industrialized diets, accounting for approximately 3 to > 17% percent of energy (%E) intake in individuals worldwide [1–3]. Current LA intakes in industrialized populations are higher than historical and evolutionary norms of 2–3%E. The importance of LA to human health has, classically, been attributed to three primary functions: (i) its role as an “essential fatty acid” because small amounts of LA (about 0.5%E) in the diet are required for integrity of the epi-dermal water barrier [4]; (ii) its ability to reduce serum low density lipoprotein cholesterol when replacing dietary saturated fats [3,5]; and for being the precursor to arachidonic acid (AA); AA is en-zymatically converted to peroxidation products with well-established bioactivities, including prostanoids and leukotrienes (reviewed in [3] and [6]).
Like AA, docosahexaenoic acid (DHA) and other polyunsaturated fatty acids, LA contains a 1,4 cis-cis pentadiene system and thus can serve as the substrate for enzymatic peroxidation to synthesize biologically active oxygenated derivatives, collectively known as oxylipins. The subset of oxylipins derived from LA, which are known as oxidized linoleic acid metabolites (OXLAMs), include hydroperoxy-octadecadienoates (HpODEs), hydroxy-octadecadienoates (HODEs), oxo-octa-decadienoates (oxo-ODEs), epoxy-octadecenoates (EpOMEs), dihydroxy-octadecenoates acids (DiHOMEs), hydroxy-epoxides [6], keto-epoxides [6], and trihydroxy-octadecenoates (TriHOMEs) [2]. HpODEs and lipid peroxides derived from other omega-6 fatty acids are also precursors for α,β unsaturated reactive aldehyde degradation products including 4-hydroxy-2-nonenal (4-HNE) [7]. Since mammals cannot synthesize LA de novo, the LA content of diet is likely to be a critical determinant of accumulation of LA, OXLAMs, and 4-HNE in many tis-sues [2,8]. HpODEs and other OXLAMs can also be formed non-en-zymatically when vegetable oils rich in LA are cooked or otherwise heated [9–11]. A substantial portion of vegetable oils in industrialized populations, including those used in many processed and packaged foods, is heated prior to consumption; and these preformed OXLAMs could potentially be absorbed and incorporated into certain tissues in-cluding brain after consumption [12–14]. Therefore, the abundance of OXLAMs and 4-HNE in human and other mammalian tissues could potentially be affected by both consumption of non-oxidized LA, with subsequent conversion to OXLAMs in the body, and by consumption of preformed OXLAMs.
OXLAMs and 4-HNE have been mechanistically linked to several pathological conditions including cardiovascular disease [15], steatohepatitis [16,17], neurodegenerative diseases [18], and chronic pain [6,19,20], reviewed in [3,15,21]. Consumption of heated vegetable oils rich in LA [22,23], or intravenous administration of HpODEs [24], produces cerebellar necrosis and ataxia in chicks without damaging the cerebral cortex, indicating that OXLAMs could potentially have brainregion specific neurotoxic effects in some species. Plausible mechanisms exist whereby high exposure to OXLAMs could have neurotoxic effects in humans, including endothelial cell activation [25], generalized lipid and membrane peroxidation [26,27], mitochondrial dysfunction [28], and microglial activation [29–31]. 4-HNE, which forms chemical bonds with cysteine, lysine, and histidine residues [32] and has been implicated in protein misfolding and aggregation [33], is linked to the development or progression of neurofibrillary tangles and amyloid plaques that are characteristic of Alzheimer’s disease [33,34]. However, despite these plausible mechanisms, there is a lack of data to assess the effects of increasing dietary LA and dietary OXLAMs on the fatty acid, oxylipin, and aldehyde compositions in mammalian brain.
In the present paper, we examine if high intakes of LA (from unheated corn oil), or exogenously produced OXLAMs (from heated corn oil), both characteristic of modern industrialized diets, impact mammalian brain biochemistry. Findings support the hypothesis that diets enriched in LA and OXLAMs alter cerebral and cerebellar lipid accumulation and peroxidation in mammals.
2. Methods
Wild type male C57BL/6 mice (n = 8 per group) were fed ad-libitum one of three controlled diets designed to contain 40% fat by weight in g/kg, for 8 weeks: (i) a low LA diet designed to contain 4%E as LA, (ii) a high LA diet designed to contain 17%E as LA or (iii) the low 4% E LA diet enriched with dietary OXLAMs from thermally-stressed corn oil. The three study diets were prepared by Dyets Inc. (Bethlehem, PA) using unheated and thermally stressed oils of known fatty acid composition. Despite being designed to contain the same amount of LA as the Low LA diet, the Low LA + OXLAMs diet was observed to contain substantially less LA on gas chromatography analysis. Fatty acid and OXLAM concentrations of the three diets are shown in Table 1. Fatty acid percent compositions are shown in Table S1. This animal protocol followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23) and was approved by the University of California, San Diego Institutional Animal Care and Use Committee (protocol number S11200).
Table 1.
Concentrations of fatty acids and oxidized linoleic acid metabolites (OXLAMs) in study diets.
| Low LA diet | High LA dieta | Low LA + OXLAM dieta | |
|---|---|---|---|
| Fatty acids (mg/g) | |||
| LA | 28.0 ± 3.9 | 92.0 ± 5.5 | 16.5 ± 0.3 |
| ALA | 10.6 ± 1.5 | 11.3 ± 0.6 | 9.1 ± 0.3 |
| SFA | 122.6 ± 13.1 | 42.7 ± 2.1 | 91.0 ± 3.7 |
| MUFA | 16.0 ± 2.2 | 49.5 ± 12.5 | 18.6 ± 0.7 |
| OXLAMs (nmol/g) | |||
| Total OXLAMs | 20.2 ± 4.7 | 33.4 ± 2.0 | 259.6 ± 21.6 |
| 9-HODE | 6.8 ± 1.6 | 7.9 ± 0.5 | 20.0 ± 4.1 |
| 9-oxo-ODE | 0.4 ± 0.1 | 0.5 ± 0.1 | 3.6 ± 0.8 |
| 9(10)-EpOME | 0.9 ± 0.2 | 1.0 ± 0.1 | 51.8 ± 6.0 |
| 9,10-DiHOME | 1.9 ± 0.3 | 8.0 ± 0.4 | 2.2 ± 0.2 |
| 13-HODE | 7.1 ± 2.5 | 8.8 ± 1.0 | 67.5 ± 12.8 |
| 13-oxo-ODE | 0.0 ± 0.0 | 0.3 ± 0.1 | 1.9 ± 0.7 |
| 12(13)-EpOME | 1.5 ± 0.4 | 2.0 ± 0.4 | 109.3 ± 7.5 |
| 12,13-DiHOME | 1.5 ± 0.2 | 4.9 ± 0.1 | 3.4 ± 0.2 |
Data are mean ± standard deviation. LA, linoleic acid; ALA, alpha-linolenic acid; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; HODE, hydroxy-octadecadienoic acid; oxo-ODE, oxo-octadecadienoic acid; EpOME, epoxy-octadecenoic acid; DiHOME, dihydroxy-octadecenoic acid.
Indicates that the although the Low LA diet and the Low LA plus OXLAM diet were designed contain the same amount of non-oxidized LA, the Low LA plus OXLAM diet had substantially less non-oxidized LA on GC analysis. Diets did not contain arachidonic acid, eicosapentaenoic acid, or docosahexaenoic acid.
2.1. Preparation of heated corn oil
Thermally stressed corn oil was prepared by heating 2 kg of Crisco™ brand corn oil purchased from a local grocery store in a shallow cast iron pan in an oven at 115 °C for approximately 4 weeks, with daily stirring of the oil. Progress was monitored by 1H NMR (400 MHz) in deuterated chloroform by comparing the decrease in integration of the bis-allylic protons (centered around 2.76 ppm) of the heated oil with the glyceryl methylene protons (multiplets at 4.13 ppm and 4.28 ppm) which remained unchanged (referenced to a sample taken from the corn oil immediately upon opening). Lipid peroxide composition was assessed by estimating the integration of newly formed peaks in regions that were sufficiently separated from potential interference of peaks arising from the vitamin E in the corn oil. These corresponded to the conjugated cis, trans, and trans, trans dienes of HpODEs (5.40–5.55 ppm, 5.86–6.11 ppm, smaller multiplets at 6.12–6.32 ppm) as described by Guillen et al. [35]. Based on LA composition of corn oil as 55%, in the 2KG sample there were 192 g of HpODEs present in the total oil mixture at reaction end as calculated from the NMR integration results. Further oxidation products were also observed as minor aldehyde peaks (9.40–9.56 ppm).
2.2. Fatty acid and oxylipin analysis of study diets
Dietary fatty acids in the food pellets were analyzed by gas chromatography (GC) with a flame ionization detector (FID) as previously reported [36]. Briefly, food pellets were crushed with pestle and mortar. 0.4 mL toluene, 3 mL methanol and 0.6 mL 8% HCl in methanol were added to 30 mg of powder after adding 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine as an internal standard. Samples were heated for 1 h at 90 °C. One mL of hexane and 1 mL water were added, and the samples were allowed to sit at room temperature for a few minutes. The upper hexane layer containing the fatty acid methyl esters was transferred to a new tube containing 0.45 mL water, vortexed and centrifuged for 2 min at 13,000 rpm. The hexane layer was separated, dried under nitrogen, reconstituted in 0.2 mL hexane and subjected to gas-chromatography analysis. Fatty acid methyl esters were analyzed with a Clarus 500 GC system equipped with FID (Perkin Elmer, CA, USA) and a fused silica capillary column (DB-FFAP, 30 m, 0.25 mm i.d, 0.25 μm film thickness, Agilent, Santa Clara, CA, USA). The injector and detector temperatures were set to 240 °C and 300 °C, respectively. The oven temperature program was set at 80 °C for 2 min, increased to 185 °C at the rate of 10 °C/min, and to 240 °C at the rate of 5 °C/min, and held at 240 °C for 13 min. Helium was the carrier gas and was maintained at a flow rate of 1.3 mL/min.
Diet OXLAM concentrations were measured in 30 mg of a crushed food pellet dissolved in 200 μL ice-cold methanol containing 0.1% acetic acid and 0.1% BHT and spiked with 10 μL antioxidant mix and 10 μL surrogate standard containing 5 pmol of d11–11(12)-EpETrE, d11–14,15-DiHETrE, d4–6-keto-PGF1a, d4–9-HODE, d4-LTB4, d4-PGE2, d4-TXB2, d6–20-HETE, and d8–5-HETE dissolved in methanol. The antioxidant solution contained three antioxidants mixed at a 1:1:1 ratio (v/v/v) consisting of 0.6 mg/mL ethylenediaminetetraacetic acid in water, 0.6 mg/ml BHT in methanol, and 0.6 mg/ml triphenylphosphine in water:methanol (1:1; v/v), that were filtered through a Millipore filter to remove solid particles. Samples were hydrolyzed and oxylipins extracted with solid phase extraction (SPE) and analyzed by LC-MS/MS as described below for the mouse brain tissue analysis.
2.3. Mouse tissue collection and fatty acid, oxylipin, and 4-HNE analyses
Mice were killed by CO2 overdose. Tissue samples were immediately collected, frozen on dry-ice chilled isobutane, and stored at −80 °C. Tissue oxylipins were analyzed as previously described [37]. Briefly, two hundred microliters of ice-cold methanol containing 0.1% acetic acid and 0.1% BHT was added to approximately 30–50 mg of frozen tissue, following the addition of 10 μL of the antioxidant mix and 10 μL surrogate standards as described above. The brain samples containing the extraction solvent, antioxidant mix, and surrogate standards were cooled in −80 °C freezer for 30 min and then homogenized for 2 min using a bead homogenizer. The homogenized samples were stored for 30 min at −80 °C freezer, followed by centrifugation at 13,000 rpm (15,870 g) in a 5424R microcentrifuge (Eppendorf) for 10 min at 0 °C. The supernatant was transferred to a new tube for the oxylipin analysis, while the pellet was kept at −80 °C for fatty acid analysis. The supernatant was hydrolyzed in equal volumes of 0.5 M sodium carbonate solution (26.5 mg per ml of 1:1 v/v methanol/water) at 60 °C for 30 min. The samples were acidified to pH 4 to 6 with 25 μL acetic acid and 1575 μL water was added to dissolve the resulting salts. The solution containing hydrolyzed oxidized lipids (oxylipins) was subjected to SPE. Samples were poured onto 60 mg Waters Oasis HLB 3 cc cartridges (Waters, Milford, MA, USA) pre-rinsed with one volume ethyl acetate and two volumes methanol and conditioned with two volumes of SPE buffer (5% methanol and 0.1% acetic acid in ultrapure water). The columns were rinsed twice with SPE buffer and subjected to vacuum (≈15–20 psi) for 20 min. Oxylipins were eluted with 0.5 mL of methanol and 1.5 mL ethyl acetate, dried under nitrogen, reconstituted in 100 μL methanol and filtered by centrifugation in a UltrafreeMC-VV centrifugal filter (0.1 μm; Millipore Sigma, MA, USA). Oxylipins were quantified on an Agilent 1290 Infinity UHPLC system coupled to a 6460 triple-quadrupole tandem mass spectrometer with electrospray ionization (Agilent Corporation, Palo Alto, CA, USA), as previously described [38]. The pellet kept for fatty acid analysis was reconstituted in 0.4 mL toluene and subjected to direct transesterification with methanolic HCl, using the same protocol described above for the diet analysis [36]. Samples were analyzed by GC using the column and temperature program described above. The DHA peak coeluted with nervonic acid, based on the retention times of the authentication standards. However, nervonic acid is negligible in brain tissue [39].
4-HNE-modified protein adducts in one hemisphere of cerebral cortex were analyzed by immunoblot using Anti-4-HNE (1:1000, Abcam, Cambridge, UK) and the intensity of bands were quantified using ImageJ. Briefly, cortex tissues were homogenized in RIPA buffer (Cell Signaling, Danvers, MA, USA) containing protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). For im-munoblot analysis 40 μg of protein lysate was resolved on Any kD™ Mini-PROTEAN® TGX™ Precast polyacrylamide gels (Biorad, Hercules, CA, USA), transferred to nitrocellulose membrane, blocked in 5% Blottinggrade Blocker (Biorad, Hercules, CA, USA), and incubated with anti-4-HNE antibody (1:1000, Abcam, Cambridge, UK) and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:10000, Genetex, Irvine, CA, USA) for normalization, followed by incubation with peroxidase-conjugated secondary antibody (Cell Signaling, Danvers, MA, USA). Protein bands were visualized with enhanced chemiluminescence reagent and digitized using a CCD camera (ChemiDoc®, Biorad, Hercules, CA, USA). Densitometric analysis was performed with ImageJ after background subtraction and normalization to GAPDH (1:10,000, Genetex, Irvine, CA, USA).
2.4. Data analysis and graphical representation
Statistical analyses were performed using Stata version 13.1. Nonparametric analyses were employed due to the presence of non-normal distributions. A Kruskal–Wallis test was used for between-group comparisons, and the Dunn’s test of multiple comparisons was used to compare the high LA group and Low LA + OXLAM group to the Low LA reference group. P-values for the multiple comparisons were Sidak-adjusted. Diet-induced changes in selected oxylipins and their precursor n-3 and n-6 fatty acids were graphed using boxplots with medians and interquartile ranges.
3. Results
Body weight in the Low LA + OXLAM diet group was significantly lower than the other two groups (median weights in grams were 39.2 g (IQR 34.1–42.2 g), 40.3 g (IQR 33.5–42.5 g), and 30.0 g (IQR 27.5–34.2 g) for the Low LA, High LA, and Low LA + OXLAMs diets, respectively; p < 0.01).
3.1. Effects of dietary LA and OXLAMs on brain n-6 fatty acids
Increasing dietary LA significantly increased the abundance of LA (18:2n-6), the LA elongation product eicosadienoic acid (EDA, 20:2n-6), and the LA elongation and desaturation product arachidonic acid (AA, 20:4n-6), in both cerebellum and cerebrum (Fig. 1) (Tables S2–S3). The Low LA + OXLAM diet group had lower abundance of LA and EDA in cerebellum and decreased LA in cerebral cortex but had no effect on arachidonic acid in either tissue.
Fig. 1. Dietary LA and OXLAM-induced changes in n-6 fatty acid content of cerebellum and cerebrum.
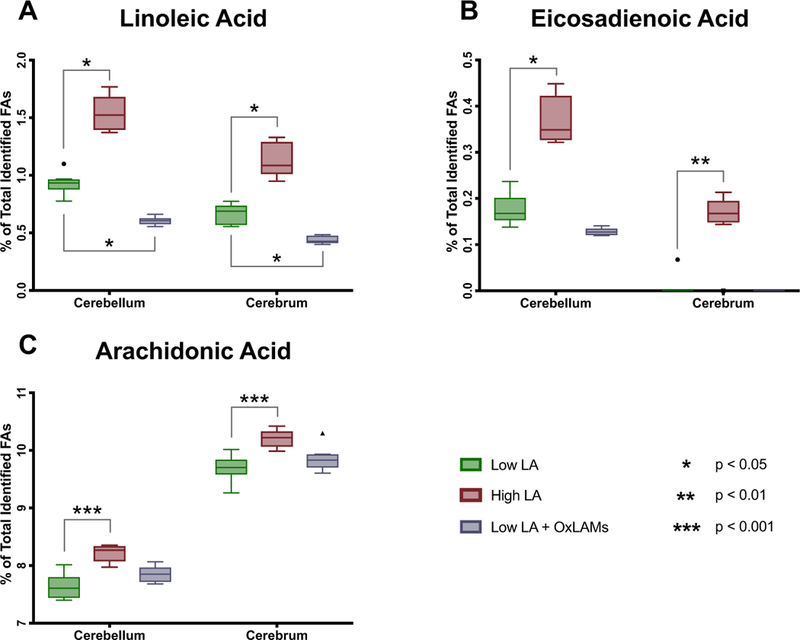
N = 8 tissue samples per group. Y-axis ranges differ in each graph. Box plots are based on the Tukey method. P-values are derived from Dunn’s test of multiple compar-isons using rank sums, comparing the Low LA reference group vs each of the other two groups. P-values are Sidak-adjusted. FAs, Fatty Acids; LA, Linoleic Acid; OXLAM, Oxidized Linoleic Acid Metabolite.
3.2. Effects of dietary LA and OXLAMs on brain n-3 fatty acids
Increasing dietary LA significantly decreased the abundance of both eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6–3) in cerebellum, and decreased the abundance of EPA but had no effect on DHA in cerebrum (Fig. 2) (Tables S2–S3). The Low LA + OXLAM diet group had lower abundance of EPA in both cerebellum and cerebrum, and decreased DHA in cerebral cortex, but had no effect on DHA in cerebellum.
Fig. 2. Dietary LA and OXLAM-induced changes in n-3 fatty acid content of cerebellum and cerebrum.
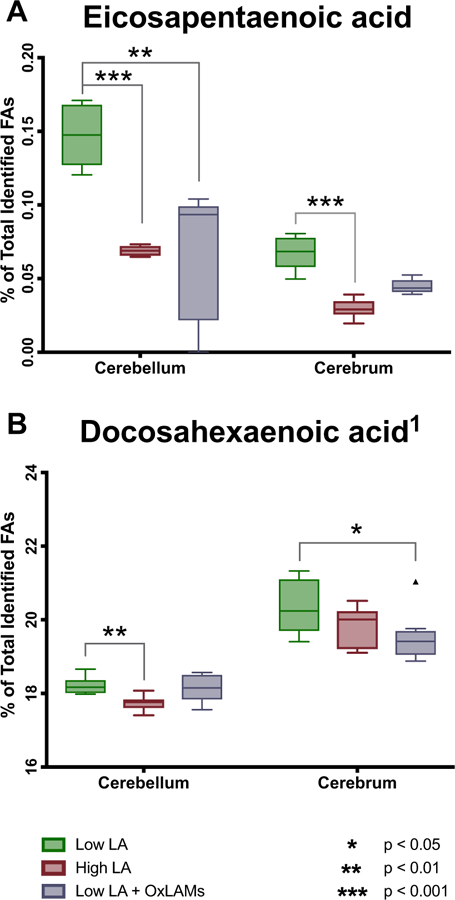
N = 8 tissue samples per group. Y-axis ranges differ in each graph. Box plots are based on the Tukey method. P-values are derived from Dunn’s test of multiple comparisons using rank sums, reporting the Low LA reference group vs each of the other two groups. P-values are Sidak adjusted. LA, linoleic acid; OXLAM, Oxidized Linoleic Acid Metabolite.
3.3. Effects of dietary LA and OXLAMs on brain oxylipins
Increasing dietary LA significantly increased the abundance of OXLAMs, including epoxy- and hydroxy-LA derivatives, in both cerebellum (Fig. 3) and cerebral cortex (Fig. 4), but had comparatively minor effects on oxylipins derived from AA, EPA or DHA. Increasing dietary OXLAMs decreased the abundance of 12-HETE in cerebellum and cerebral cortex, and decreased TxA2 in cerebral cortex, without altering any other oxylipins (Fig. 4) (Tables S4–S5).
Fig. 3. Effects of increases in dietary LA and dietary OXLAMs on cerebellar oxylipins.
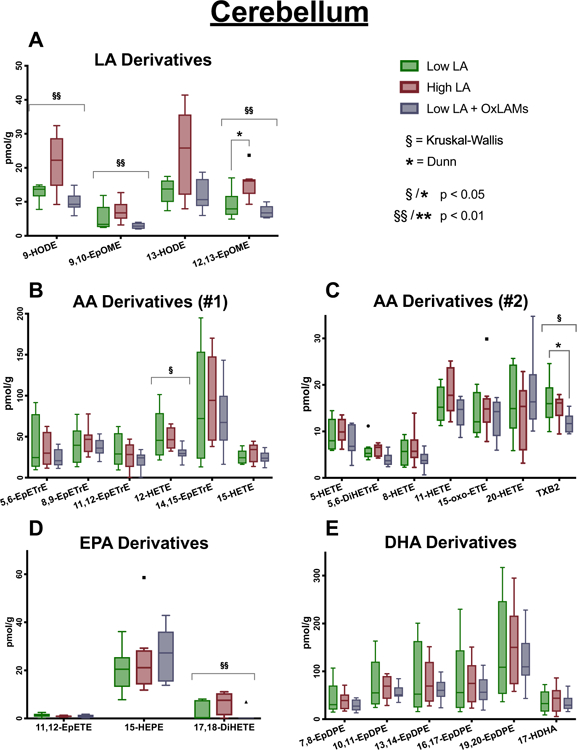
N = 8 tissue samples per group. Y-axis ranges differ in each graph. Box plots are based on the Tukey method. P-values with asterisks are derived from Dunn’s test of multiple comparisons using rank sums, comparing the Low LA reference group vs each of the other two groups. These P-values are also Sidak-adjusted. P-values with section signs are derived from the Kruskal-Wallis nonparametric rank-sum test across all three groups. LA, linoleic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosaheaxaenoic acid.
Fig. 4. Effects of increases in dietary LA and dietary OXLAMs on cerebral cortex oxylipins.
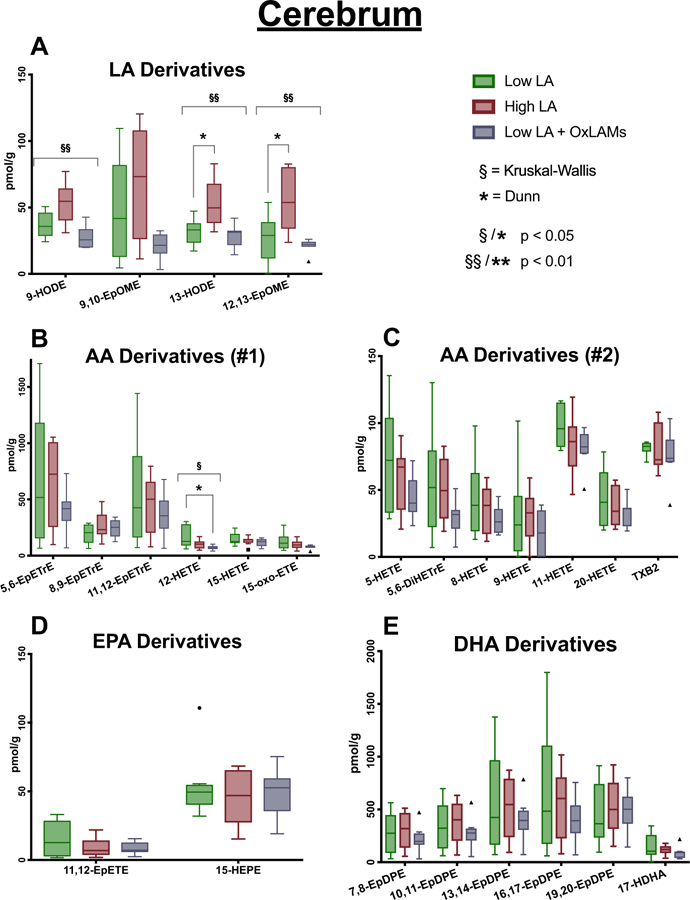
N = 8 tissue samples per group. Y-axis scales differ in each graph. Box plots are based on the Tukey method. P-values are derived from Dunn’s test of multiple comparisons using rank sums, reporting the Low LA reference group vs each of the other two groups. P-values are Sidak adjusted. LA, linoleic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosaheaxaenoic acid.
3.4. Effects of dietary LA and OXLAMs on brain 4-HNE
4-HNE, a secondary product of fatty acid peroxidation, was not significantly altered in cerebral cortex by increases in dietary LA or dietary OXLAMs (Fig. 5).
Fig. 5. Effects of increases in dietary LA and dietary OXLAMs on 4-HNE in mouse cerebral cortex.
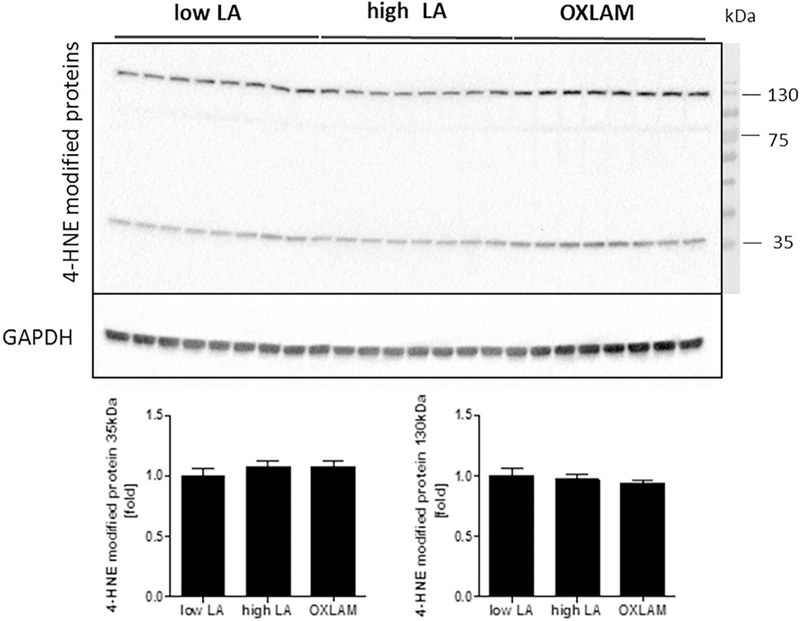
N = 8 tissue samples per group. Immunoblot analysis of cerebral cortex protein lysates for the detection of 4-HNE-modified proteins. Glyceraldehyde 3-phos-phate dehydrogenase (GAPDH) was used as a loading control. Densitometric analysis was performed on background-subtracted blots and normalized to GAPDH. The Low LA group was used as a reference control and set to 1.
4. Discussion
Per capita mean dietary LA has increased markedly in industrialized populations over the past century [1]. Current LA intakes are much higher than historical and evolutionary norms of 2–3%E and depend on industrial oil processing to create concentrated oils, which scarcely resemble their unprocessed food sources. For example, LA accounts for 55% of calories in corn oil versus 0.5% of calories in whole corn [40]. The LA in concentrated oil is susceptible to exogenous oxidation when oils are thermally stressed from frying, cooking, during deodorization and refinement of liquid vegetable oils, or when processed foods con-taining high LA oils are heated [9,41]. Because a substantial portion of the dietary LA in industrialized populations is heated prior to consumption, modern populations have substantially increased intakes of both LA and preformed LA peroxidation products. Consumption of heated oils rich in LA without adequate vitamin E [22,23], or intravenous administration of HPODEs [24], can produce cerebellar necrosis and ataxia in chicks, suggesting that dietary OXLAMs might potentially have adverse effects in CNS tissues. However, it is not yet known whether eating nonoxidized or heated vegetable oils has biochemical consequences in mammalian brain tissues.
Here we showed that increasing dietary LA specifically increased LA peroxidation products in cerebellum and cerebrum, without sub-stantially affecting oxylipins derived from AA, EPA or DHA. By contrast, the consumption of preformed LA peroxidation products (OXLAMs) had minimal effect on the concentrations of brain oxylipins. Neither dietary LA nor dietary OXLAMs altered cerebral 4-HNE levels. These collective findings suggest that the altered mammalian brain lipid oxidation observed after LA feeding is driven by either endogenous enzymatic LA peroxidation, or endogenous free-radical mediated LA peroxidation, rather than by absorption of preformed LA oxidation products present in the diet. Specific LA peroxidation products that were increased by high LA diet in the brain—including HODEs, EpOMEs, and DiHOMEs—have shown diverse bioactivities in preclinical models, and have been implicated in the pathogenesis of conditions outside of the CNS that are characterized by inflammation and oxidative stress, in-cluding cardiovascular disease [3,15], non-alcoholic steatohepatitis [16,17], acute respiratory distress syndrome [42–44] asthma [45,46], and chronic pain [6,19,20], reviewed in [3,15,21]. The present finding that the body weight of the Low LA + OXLAM diet group was significantly lower than the other two groups, suggests that dietary OX-LAMs could potentially have unfavorable effects on growth in mammals. However, future studies are needed to determine whether weight reducing effects of heated oils are due to decreased food intake, partial displacement of the energy provided by fatty acids with non-caloric oxidized lipids, or to other effects on metabolism.
Consistent with previous reports [8,19,47], results of the present study indicate that increasing dietary non-oxidized LA shifts the bal-ance of brain precursor polyunsaturated fatty acids away from the n-3 family (EPA and DHA) towards the n-6 family (increases in LA, eicosadienoic acid, and AA). However, unlike LA, the observed diet-induced changes in EPA, DHA, and AA did not translate to changes in their oxylipin derivatives. Interestingly, the Low LA + OXLAMs diet appeared to significantly decrease the abundance of n-3 DHA and EPA in CNS tissues, despite containing less dietary LA than the Low LA diet alone. DHA and EPA are proposed to play critical structural and functional roles in CNS tissues [48,49]. Thus, future studies are warranted to determine the effects of high intakes of thermally stressed high LA oils on developmental, behavioral, or cognitive endpoints.
Since heated oils are major source of dietary OXLAMs in modern diets, the use of heated corn oil is a strength of the present study. However, to gain a better understanding of the effects of specific components in heated oils, future studies should consider adding specific oxidation products (e.g. HpODEs) and degradation products (e.g. aldehydes) that are present in heated high LA oils to study diets. Since inclusion of oxidized oils may also alter the taste and texture of food pellets to influence food consumption, future studies should consider gavaging study oils to optimize the controlled nature of study diets. Future dietary OXLAM studies should consider using more tightly controlled diets that isolate OXLAMs as a controlled variable (keeping non-oxidized LA constant), to isolate the specific effects of individual OXLAMs.
In summary, the present study fills an important gap by establishing proof of principle in a mammalian model that diets enriched in LA and OXLAMs alter cerebral and cerebellar lipid accumulation and peroxidation. Mammalian brain OXLAM concentrations appear to be regulated by endogenous synthesis from dietary LA, rather than direct incorporation of preformed OXLAMs that are in the diet. Future studies are warranted to investigate whether and how diets enriched in LA and OXLAMs impact behavior, neurodevelopment, or neurodegeneation.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Programs of the National Institute on Aging and the National Institute on Alcohol Abuse and Alcoholism (C.E.R), U01 AA022489–01A1 from the National Institute on Alcohol Abuse and Alcoholism (A.E.F.), the National Health and Medical Research Council of Australia Centre of Research Excellence Grant 1035530 (R.A.G), U.S.DA National Institute of Food and Agriculture Project 1008787 (A.Y.T.), and the German Research Foundation (SCHU3146/1–2 to S.S.). The authors acknowledge the intellectual contributions of Duong Nguyen.
Footnotes
The authors have no competing interests to declare.
References
- [1].Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR, Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century, Am. J. Clin. Nutr 93 (5) (2011) 950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, et al. , Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid me-tabolites in humans, Prostaglandins Leukot. Essent. Fat. Acids 87 (4–5) (2012) 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, et al. , Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73), BMJ 353 (2016) i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hansen AE, Haggard ME, Boelsche AN, Adam DJ, Wiese HF, Essential fatty acids in infant nutrition. III. Clinical manifestations of linoleic acid deficiency, J. Nutr 66 (4) (1958) 565–576. [DOI] [PubMed] [Google Scholar]
- [5].Mensink RP, Zock PL, Kester AD, Katan MB, Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials, Am. J. Clin. Nutr 77 (5) (2003) 1146–1155. [DOI] [PubMed] [Google Scholar]
- [6].Ramsden CE, Domenichiello AF, Yuan ZX, Sapio MR, Keyes GS, Mishra SK, et al. , A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch, Sci. Signal 10 (493) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schneider C, Porter NA, Brash AR, Autoxidative transformation of chiral omega6 hydroxy linoleic and arachidonic acids to chiral 4-hydroxy-2E-nonenal, Chem. Res. Toxicol 17 (7) (2004) 937–941. [DOI] [PubMed] [Google Scholar]
- [8].Taha AY, Hennebelle M, Yang J, Zamora D, Rapoport SI, Hammock BD, et al. , Regulation of Rat Plasma and Cerebral Cortex Oxylipin Concentrations With Increasing Levels of Dietary Linoleic Acid. Prostaglandins, Leukotrienes, and Essential Fatty Acids, (2016). [DOI] [PMC free article] [PubMed]
- [9].Morales A, Marmesat S, Dobarganes MC, Marquez-Ruiz G, Velasco J, Quantitative analysis of hydroperoxy-, keto- and hydroxy-dienes in refined vege-table oils, J. Chromatogr. A 1229 (2012) 190–197. [DOI] [PubMed] [Google Scholar]
- [10].Marmesat S, Velasco J, Dobarganes MC, Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures, J. Chromatogr. A 1211 (1–2) (2008) 129–134. [DOI] [PubMed] [Google Scholar]
- [11].Marmesat S, Morales A, Velasco J, Dobarganes M Carmen, Influence of fatty acid composition on chemical changes in blends of sunflower oils during thermoxidation and frying, Food Chem 135 (4) (2012) 2333–2339. [DOI] [PubMed] [Google Scholar]
- [12].Wilson R, Lyall K, Smyth L, Fernie CE, Riemersma RA, Dietary hydroxy fatty acids are absorbed in humans: implications for the measurement of ‘oxidative stress’in vivo, Free Radic. Biol. Med 32 (2) (2002) 162–168. [DOI] [PubMed] [Google Scholar]
- [13].Ferreiro-Vera C, Priego-Capote F, Mata-Granados JM, Luque de Castro MD, Short-term comparative study of the influence of fried edible oils intake on the metabolism of essential fatty acids in obese individuals, Food Chem 136 (2) (2013) 576–584. [DOI] [PubMed] [Google Scholar]
- [14].Wilson R, Fernie CE, Scrimgeour CM, Lyall K, Smyth L, Riemersma RA, Dietary epoxy fatty acids are absorbed in healthy women, Eur. J. Clin. Investig 32 (2) (2002) 79–83. [DOI] [PubMed] [Google Scholar]
- [15].Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, et al. , Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis, BMJ 346 (2013) e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu H, Beier JI, Arteel GE, Ramsden CE, Feldstein AE, McClain CJ, et al. , Transient receptor potential vanilloid 1 gene deficiency ameliorates hepatic injury in a mouse model of chronic binge alcohol-induced alcoholic liver disease, Am. J. Pathol 185 (1) (2015) 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, et al. , Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, J. Lipid Res 51 (10) (2010) 3046–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Di Domenico F, Tramutola A, Butterfield DA, Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurode-generative disorders, Free Radic. Biol. Med 111 (2017) 253–261. [DOI] [PubMed] [Google Scholar]
- [19].Ramsden CE, Ringel A, Majchrzak-Hong SF, Yang J, Blanchard H, Zamora D, et al. , Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids: implications for idiopathic pain syndromes? Mol. Pain 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, Gaylord S, et al. , Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial, Pain 154 (11) (2013) 2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Budowski P, Bartov I, Dror Y, Frankel EN, Lipid oxidation products and chick nutritional encephalopathy, Lipids 14 (9) (1979) 768–772. [DOI] [PubMed] [Google Scholar]
- [22].Fischer VW, Nelson JS, Cerebrovascular changes in tocopherol-depleted chicks, fed linoleic acid, J. Neuropathol. Exp. Neurol 32 (3) (1973) 474–483. [DOI] [PubMed] [Google Scholar]
- [23].Dror Y, Budowski P, Lysosomal acid phosphatase decrease in nutritional encephalopathy in chicks, Nutr. Metab 24 (3) (1980) 154–160. [DOI] [PubMed] [Google Scholar]
- [24].Nishida T, Tsuchiyama H, Inoue M, Kummerow FA, Effect of intravenous injection of oxidized methyl esters of unsaturated fatty acids on chick en-cephalomalacia, Proc. Soc. Exp. Biol. Med 105 (1960) 308–312. [DOI] [PubMed] [Google Scholar]
- [25].Pacifici EH, McLeod LL, Peterson H, Sevanian A, Linoleic acid hydroperoxide-induced peroxidation of endothelial cell phospholipids and cytotoxicity, Free Radic. Biol. Med 17 (4) (1994) 285–295. [DOI] [PubMed] [Google Scholar]
- [26].Polivoda BI, Damage of cell membranes by linoleic acid hydroperoxide, Biofizika 31 (3) (1986) 453–455. [PubMed] [Google Scholar]
- [27].Nakagawa K, Kato S, Miyazawa T, Determination of phosphatidylcholine hydro-peroxide (PCOOH) as a marker of membrane lipid peroxidation, J. Nutr. Sci. Vitaminol. (Tokyo) 61 (Suppl) (2015) S78–S80. [DOI] [PubMed] [Google Scholar]
- [28].Yin H, Zhu M, Free radical oxidation of cardiolipin: chemical mechanisms, detection and implication in apoptosis, mitochondrial dysfunction and human diseases, Free Radic. Res 46 (8) (2012) 959–974. [DOI] [PubMed] [Google Scholar]
- [29].Obinata H, Hattori T, Nakane S, Tatei K, Izumi T, Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A, J. Biol. Chem 280 (49) (2005) 40676–40683. [DOI] [PubMed] [Google Scholar]
- [30].Obinata H, Izumi T, G2A as a receptor for oxidized free fatty acids, Prostaglandins Other Lipid Mediat 89 (3–4) (2009) 66–72. [DOI] [PubMed] [Google Scholar]
- [31].Sheikh AM, Nagai A, Ryu JK, McLarnon JG, Kim SU, Masuda J, Lysophosphatidylcholine induces glial cell activation: role of rho kinase, Glia 57 (8) (2009) 898–907. [DOI] [PubMed] [Google Scholar]
- [32].Zarkovic K, Jakovcevic A, Zarkovic N, Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases, Free Radic. Biol. Med 111 (2017) 110–126. [DOI] [PubMed] [Google Scholar]
- [33].Nieva J, Shafton A, Altobell LJ 3rd, Tripuraneni S, Rogel JK, Wentworth AD, et al. , Lipid-derived aldehydes accelerate light chain amyloid and amorphous aggregation, Biochemistry 47 (29) (2008) 7695–7705. [DOI] [PubMed] [Google Scholar]
- [34].Montine KS, Olson SJ, Amarnath V, Whetsell WO Jr., Graham DG, Montine TJ, Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer’s disease is associated with inheritance of APOE4, Am. J. Pathol 150 (2) (1997) 437–443. [PMC free article] [PubMed] [Google Scholar]
- [35].Guillen MD, Goicoechea E, Oxidation of corn oil at room temperature: primary and secondary oxidation products and determination of their concentration in the oil liquid matrix from 1H nuclear magnetic resonance data, Food Chem 116 (1) (2009) 183–192. [Google Scholar]
- [36].Zhang Z, Richardson CE, Hennebelle M, Taha AY, Validation of a one step method for extracting fatty acids from salmon, chicken and beef samples, J. Food Sci 82 (10) (2017) 2291–2297. [DOI] [PubMed] [Google Scholar]
- [37].Hennebelle M, Zhang Z, Metherel AH, Kitson AP, Otoki Y, Richardson CE, et al. , Linoleic acid participates in the response to ischemic brain injury through oxidized metabolites that regulate neurotransmission, Sci. Rep 7 (1) (2017) 4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang J, Schmelzer K, Georgi K, Hammock BD, Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry, Anal. Chem 81 (19) (2009) 8085–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Taha AY, Basselin M, Ramadan E, Modi HR, Rapoport SI, Cheon Y, Altered lipid concentrations of liver, heart and plasma but not brain in HIV-1 transgenic rats, Prostaglandins Leukot. Essent. Fat. Acids 87 (4–5) (2012) 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory, USDA National Nutrient Database for Standard Reference, Release 28 (2018) Internet: /nea/bhnrc/ndl. [Google Scholar]
- [41].Dobarganes C, Marquez-Ruiz G, Possible adverse effects of frying with vegetable oils, Br. J. Nutr 113 (Suppl. 2) (2015) S49–S57. [DOI] [PubMed] [Google Scholar]
- [42].Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD, Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome, Am. J. Respir. Cell Mol. Biol 25 (4) (2001) 434–438. [DOI] [PubMed] [Google Scholar]
- [43].Ozawa T, Sugiyama S, Hayakawa M, Satake T, Taki F, Iwata M, et al. , Existence of leukotoxin 9,10-epoxy-12-octadecenoate in lung lavages from rats breathing pure oxygen and from patients with the adult respiratory distress syndrome, Am. Rev. Respir. Dis 137 (3) (1988) 535–540. [DOI] [PubMed] [Google Scholar]
- [44].Hu JN, Taki F, Sugiyama S, Asai J, Izawa Y, Satake T, et al. , Neutrophil-derived epoxide, 9,10-epoxy-12-octadecenoate, induces pulmonary edema, Lung 166 (6) (1988) 327–337. [DOI] [PubMed] [Google Scholar]
- [45].Mabalirajan U, Rehman R, Ahmad T, Kumar S, Singh S, Leishangthem GD, et al. , Linoleic acid metabolite drives severe asthma by causing airway epithelial injury, Sci. Rep 3 (2013) 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Panda L, Gheware A, Rehman R, Yadav MK, Jayaraj BS, Madhunapantula SV, et al. , Linoleic acid metabolite leads to steroid resistant asthma features partially through NF-kappaB, Sci. Rep 7 (1) (2017) 9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sakayori N, Kikkawa T, Tokuda H, Kiryu E, Yoshizaki K, Kawashima H, et al. , Maternal dietary imbalance between omega-6 and omega-3 polyunsaturated fatty acids impairs neocortical development via epoxy metabolites, Stem Cells 34 (2) (2016) 470–482. [DOI] [PubMed] [Google Scholar]
- [48].Lee JW, Huang BX, Kwon H, Rashid MA, Kharebava G, Desai A, et al. , Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function, Nat. Commun 7 (2016) 13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Matsuoka YJ, Sawada N, Mimura M, Shikimoto R, Nozaki S, Hamazaki K, et al. , Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: a population-based prospective cohort study, Transl. Psychiatry 7 (9) (2017) e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


