Abstract
Background
The primary aim of this study was to evaluate the level of autophagy and apoptosis enzymes in patients with coronary artery disease (CAD). Furthermore, we investigated the role of autophagy and apoptosis in the progression of coronary collateral and coronary total occlusion (TO).
Material/Methods
We enrolled 115 patients in this prospective, observational, controlled study, who were categorized into 3 groups as follows: group 1, patients with chronic TO (n=49); group 2, patients with acute TO such as myocardial infarction (n=36); and group 3, healthy control patients (n=30). We used the enzyme-linked immunosorbent assay (ELISA) kit for autophagy-related protein 5 (ATG5) and apoptosis (M30) in the plasma for these 3 groups.
Results
Autophagy levels significantly varied among the groups (13.7±5.3 ng/mL, 11.7±3.4 ng/mL, and 7.5±3, respectively; P<0.001). In addition, apoptosis levels significantly varied among the groups (78.6±33.4 ng/mL, 64.9±30.6 ng/mL, and 47.6±18.2, respectively; P<0.001). The subgroup analysis revealed significant positive correlations between the autophagy level and the Rentrop score in contrast to apoptosis in group 1 (r=0.463; P<0.001).
Conclusions
This study determined that autophagy and apoptosis levels were higher in patients with CAD than in healthy controls. In contrast to the serum apoptosis level, serum autophagy levels demonstrated a significant positive correlation with the Rentrop score. Hence, an elevated autophagy level might be a potential activator and marker of the process by which the body protects itself in CAD.
MeSH Keywords: Apoptosis Regulatory Proteins, Autophagy, Coronary Artery Disease
Background
Coronary artery disease (CAD) is a leading cause of death worldwide and is characterized by different manifestations such as acute myocardial infarction (AMI) and chronic coronary total occlusion (TO). TO is defined as ≥99% epicardial coronary artery stenosis [1], which is both acute and chronic. Reportedly, several mechanisms play a role in the body during the progression of TO.
Apparently, apoptosis plays a crucial role in several diseases and physiological processes such as embryogenesis, normal tissue homeostasis, aging, cancer, and some infectious, autoimmune, and neurological diseases [2–6]. In addition, it plays a role in AMI, left ventricular remodeling, and development of heart failure [7].
Autophagy is a distinct form of programmed cell death that is a naturally regulated self-protective degradation mechanism of living cells or organisms under various stress conditions. It appears to play an essential role in the progression of CAD. Some studies of acute ischemic events conducted in animals have revealed that autophagy is protective in the ischemic phase because of an adaptive response of the heart [8–11].
However, little research has been conducted on assessing the relationship between autophagy and apoptosis levels in patients with acute and chronic heart disease.
This study investigated the role of and relationship between autophagy and apoptosis in acute and chronic ischemic heart disease and assessed the role of autophagy and apoptosis in the progression of coronary collateral and TO.
Material and Methods
Study design
This was a prospective, observational, controlled study.
Study population
Between 2016 and 2017 we examined 115 consecutive patients at a university hospital who were diagnosed with at least 1 major, chronic, or acute TO of the coronary artery. The number of study participants was determined by power analysis. We evaluated all participants during coronary angiography (CAG) and categorized them into 3 groups as follows: group 1, patients with chronic TO (n=49); group 2, patients with acute TO such as myocardial infarction (n=36); and group 3, healthy controls (n=30). Participants in groups 1 and 3 presented a positive scintigraphy scan or exercise test result. However, patients in group 2 demonstrated ST-segment elevation myocardial infarction and underwent primary percutaneous intervention (PCI). PCI was performed in these patients within 3 h after the appearance of symptoms, with no signs of infection. Furthermore, we excluded patients with uncontrolled hypertension, renal dysfunction, connective tissue diseases, thyroid function disorders, or prior CAD. This study was conducted according to the principles of the Declaration of Helsinki, and its protocol was approved by İstanbul Bilim University Hospital Ethics Committee. We obtained informed consent from all participants.
Biochemical measurements
After CAG, we collected approximately 2 mL of blood from the median cubital veins of each participant into EDTA-containing tubes. In addition, we collected blood samples from all participants during the CAG process. All blood samples were centrifuged and the plasma samples were stored at −80°C. In addition, we tested the plasma with the enzyme-linked immunosorbent assay (ELISA) kit for autophagy-related protein 5 (Cloud-Clone Corp., USA) (ATG5) and apoptosis (PEVIVA, USA, Canada, Japan) (M30). Accordingly, biochemical and hematological parameters were assessed on the same day.
CAG analysis
We subjected all patients to CAG by the Judkins technique using the femoral approach. During the procedure, we recorded images at a speed of 15 square/s on a digital angiographic system (ACOM.PC; Siemens AG, Germany). We used iopromide (Ultravist 370; Schering AG, Berlin, Germany) as the contrast agent. The recordings were examined by 2 independent cardiologists. Angiographic CAD was defined as ≥50% luminal diameter stenosis of at least 1 major epicardial coronary artery. The CAD severity was determined on the basis of the Gensini score [12]. Furthermore, the coronary collateral degree was determined using the Rentrop score [13]. TO was defined as ≥99% epicardial coronary artery stenosis [1].
Statistical analysis
We used SPSS for Windows version 15.0 software (SPSS, Inc., Chicago, IL) for statistical analysis. All continuous variables are expressed as mean ±SD and categorical variables are defined as numbers and percentages. Furthermore, categorical data were compared using the χ2 test and continuous variables were compared among the groups using the t test or the Mann-Whitney U test, depending on their distribution, which was assessed with the Shapiro-Wilk test. In addition, we used the analysis of variance and least significant difference from post hoc tests for intragroup comparisons of continuous variables. Finally, we used the Pearson and Spearman correlation analyses to estimate the relationship between the test parameters, and we considered P<0.05 as statistically significant.
Results
Overall, we enrolled 115 participants in this study, of whom 85 had TO and 30 had a healthy coronary artery. Table 1 lists the baseline clinical, demographic, and echocardiographic parameters of participants. Although age, sex, body mass index (BMI), smoking status, and dyslipidemia were analogous among the 3 groups, hypertension was different among the groups (P=0.036).
Table 1.
Baseline characteristics and laboratory and echocardiographic parameters of the study population.
| Variable | Chronic TO (n=49) | Acute TO (n=36) | Controls (n=30) | p Value |
|---|---|---|---|---|
| Age, years | 62.5±7.7 | 58.6.5±8.4 | 60.1±10 | 0.105 |
| Sex, Female/Male | 12/37 | 10/26 | 10/20 | 0.696 |
| BMI, kg/m2 | 25.9±1.8 | 26.2±2.9 | 27±1.6 | 0.107 |
| Dyslipidemia, n (%) | 22 (44) | 17 (46) | 19 (63) | 0.253 |
| Hypertension, n (%) | 45 (91) | 28 (77) | 29 (96) | 0.036 |
| Smokers, n (%) | 37 (75) | 26 (72) | 17 (56) | 0.192 |
| WBC | 8690±2061 | 11943±2828 | 7145±1623 | 0.001 |
| Hemoglobin | 14±1.5 | 14.2±1.7 | 12.9±1.1 | 0.001 |
| Platelet | 246979±52268 | 261441±72731 | 260200±51652 | 0.462 |
| LVEDD, mm | 49.5±4.3 | 50.5±5.1 | 47.6±3.5 | 0.028 |
| LVESD, mm | 34.9±4 | 36.6±5.7 | 31.7±3.6 | 0.001 |
| LA, mm | 40.1±3 | 40.9±3.6 | 39.1±2.4 | 0.052 |
| IVS, mm | 11.6±1.2 | 11.1±1.1 | 11.2±0.8 | 0.164 |
| PW, mm | 10.8±0.9 | 10.4±0.9 | 10.4±0.8 | 0.115 |
| LVEF, % | 52.2±5.2 | 46.6±6.7 | 58±1.7 | 0.001 |
| Gensini Score | 67.8±25.1 | 68.5±30.5 | 0 | 0.053 |
| Autophagy | 13.7±5.3 | 11.7±3.4 | 7.5±3 | 0.001 |
| Apoptosis | 78.6±33.4 | 64.9±30.6 | 47.6±18.2 | 0.001 |
BMI – body mass index; IVS – interventricular septum; LA – left atrium; LVEDD – left ventricular end-diastolic diameter; LVEF – left ventricular ejection fraction; LVESD – left ventricular end-systolic diameter; PW – posterior wall; WBC – white blood cell count.
The results of the left ventricular (LV) echocardiographic and hematological parameters significantly varied among the 3 groups (Table 1), especially the LV end-diastolic diameter (49.5±4.3, 50.5±5.1, and 47.6±3.5 mm, respectively; P=0.028), LV end-systolic diameter (34.9±4, 36.6±5.7, and 31.7±3.6 mm, respectively; P<0.001), ejection fraction (52.2±5.2, 46.6±6.7, and 58±1.7, respectively; P<0.001), white blood cells (8690±2061, 11,943±2828, and 7145±1623, respectively; P<0.001), and hemoglobin (14±1.5, 14.2±1.7, and 12.9±1.1, respectively; P<0.001). Furthermore, autophagy levels were significantly different among the 3 groups (13.7±5.3, 11.7±3.4, and 7.5±3 ng/mL, respectively; P<0.001; Figure 1, Table 1). Notably, apoptosis levels were also significantly different among the groups (78.6±33.4 ng/mL, 64.9±30.6 ng/mL, and 47.6±18.2, respectively; P<0.001; Figure 2, Table 1).
Figure 1.
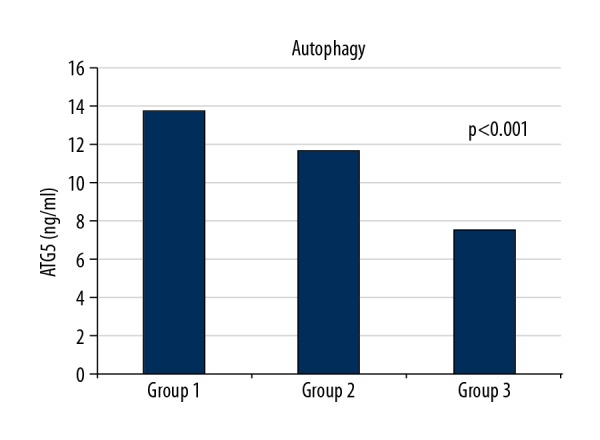
Autophagy levels of groups.
Figure 2.
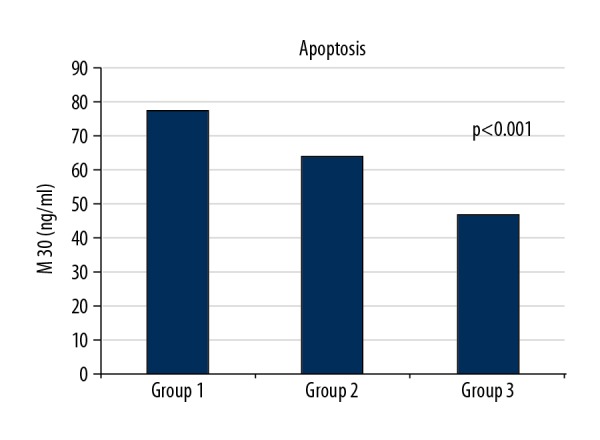
Apoptosis levels of groups.
The subgroup analysis and comparison revealed a significant difference between autophagy and apoptosis levels among the groups. Both autophagy and apoptosis level were higher when comparing groups 1 and 2 (13.7±5.3 ng/mL and 11.7±3.4 ng/mL, respectively, P=0.041; 78.6±33.4 ng/mL and 64.9±30.6 ng/mL, respectively, P=0.041). In addition, autophagy and apoptosis levels in groups 1 and 2 were higher than in controls, and both levels of participants with TO did not significantly differ from each other. In contrast, autophagy and apoptosis levels of participants with and without TO were significantly different (P<0.001; Table 2).
Table 2.
Comparison of autophagy and apoptosis levels of coronary total occlusion.
| TO | N | Median | p | |
|---|---|---|---|---|
| Autophagy | Control | 30 | 7.6000* | <0.001 |
| LAD | 34 | 12.6893** | ||
| Cx | 7 | 13.6100** | ||
| RCA | 44 | 12.4700** | ||
| Apoptosis | Control | 30 | 41.2550* | 0.001 |
| LAD | 34 | 67.1750** | ||
| Cx | 7 | 66.6900* | ||
| RCA | 44 | 66.9350** |
Normal coronary artery;
coronary artery with total occlusion.
LAD – left anterior descending coronary artery; CX – circumflex coronary artery; RCA – right coronary artery.
Despite observing similar increases in autophagy and apoptosis levels in all groups, no correlation was found between apoptosis and autophagy levels. However, the subgroup analysis revealed a significant positive correlation between the autophagy level and the Rentrop score in group 1 in contrast to apoptosis (r=0.463; P<0.001).
Discussion
This study demonstrated that both autophagy and apoptosis levels were higher in patients with CAD, and patients with chronic TO exhibited the highest autophagy and apoptosis levels. In addition, serum autophagy levels demonstrated a significant positive correlation with the collateral degree in contrast to apoptosis levels.
The results of this study suggest that autophagy and apoptosis are similar processes in the body, which despite working together, segregate during the coronary collateral formation. In addition, it is assumed that high autophagy levels might lead to coronary collateral formation. Although autophagy and apoptosis levels exhibited similar increases in our study groups, a significant positive correlation between the autophagy level and the Rentrop score was only determined in the chronic TO group. This feature might lend protection to the body. During the body protection, apoptosis and autophagy might stop working together.
Although autophagy and apoptosis occur constitutively in the normal myocardium, they have more roles to play in acute and chronic events in the body. Previous studies and the present study show that autophagy and apoptosis are activated in acute ischemic heart disease [14–18], and apoptosis is also activated in chronic heart disease [19]. The study by Kanamori is especially similar to our study, but they examined mouse hearts at 0, 4, 5, and 24 h after coronary ligation. We examined the blood within 3 h after the symptoms started. AMI in mice is mechanical but in humans it is physiologic. Atherosclerosis and thrombosis both have roles. Only myocardial damage appears to be similar between the 2 species. Thus, their data partly support our study results. In addition, our study determined that autophagy and apoptosis levels were both higher in patients with chronic TO than in those with acute TO. Despite the obvious role of apoptosis in acute and chronic heart disease, the relationship between apoptosis and autophagy, the role of autophagy in patients with chronic TO, and their roles in patients all remain unclear.
Several studies have revealed that autophagy and apoptosis are essential processes of the human body, but these highly sophisticated series of physiological events with complicated activation are incompletely understood [20–24]. Furthermore, this study also highlights an issue related to these 2 mechanisms that is not entirely understood: we determined a significant difference between LV echocardiographic and hematological parameters among our study groups. Perhaps, differences in hematological parameters and structural parameters of the heart could account for different autophagy and apoptosis levels.
Finally, finding a novel and sensitive apoptotic and autophagy marker would facilitate the prognostic stratification of patients with acute and chronic TO and offer new therapeutic strategies. The inhibition of apoptosis or activation of autophagy with a novel therapy could enhance the prognosis of these patients. Understanding these 2 mechanisms might help in delaying or preventing cell death. However, among several impending concerns, the most challenging ones are assessing which autophagy level determines the coronary collateral degree and protects the body and how much apoptosis can be inhibited without harming the body.
Study limitations
This study has some limitations. First, it was a single-center study that comprised a small study population. Furthermore, we could not completely evaluate the prognostic value of autophagy and apoptosis levels in patients with TO. Hence, extensive studies with large patient cohorts are warranted to overcome these limitations.
Conclusions
This study reveals that autophagy and apoptosis levels were both higher in patients with CAD than in healthy controls and were also higher in patients with chronic TO than in those with acute TO. In contrast to the serum apoptosis level, serum autophagy levels demonstrated a significant positive correlation with the Reentrop score. Hence, an elevated autophagy level could be a potential activator and marker of the process by which the body protects itself in CAD.
Footnotes
Source of support: Departmental sources
References
- 1.Shah PB. Management of coronary chronic total occlusion. Circulation. 2011;123:1780–84. doi: 10.1161/CIRCULATIONAHA.110.972802. [DOI] [PubMed] [Google Scholar]
- 2.Brill A, Torchinsky A, Carp H, Toder V. The role of apoptosis in normal and abnormal embryonic development. J Assist Reprod Genet. 1999;16(10):512–19. doi: 10.1023/A:1020541019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tower J. Programmed cell death in aging. Ageing Res Rev. 2015;23(Pt A):90–100. doi: 10.1016/j.arr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter TG. Apoptosis and cancer: The genesis of a research field. Nat Rev Cancer. 2009;9(7):501–7. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 5.Lev N, Melamed E, Offen D. Apoptosis and Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):245–50. doi: 10.1016/S0278-5846(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 6.Mines MA, Beurel E, Jope RS. Regulation of cell survival mechanisms in Alzheimer’s disease by glycogen synthase kinase-3. Int J Alzheimers Dis. 2011;2011 doi: 10.4061/2011/861072. 861072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbate A, Biondi-Zoccai GG, Bussani R, et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41(5):753–60. doi: 10.1016/s0735-1097(02)02959-5. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Liu H, Foyil SR, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125:3170–81. doi: 10.1161/CIRCULATIONAHA.111.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 10.Kanamori H, Takemura G, Goto K, et al. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc Res. 2011;91:330–39. doi: 10.1093/cvr/cvr073. [DOI] [PubMed] [Google Scholar]
- 11.Kanamori H, Takemura G, Goto K, et al. Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol. 2011;300:H2261–71. doi: 10.1152/ajpheart.01056.2010. [DOI] [PubMed] [Google Scholar]
- 12.Gensini GG. A more meaningful scoring system for determining the severity of coronary artery disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 13.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–92. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Vatner DE, Kim SJ, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA. 2005;102:13807–12. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–58. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivetti G, Quaini F, Sala R, et al. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–16. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 18.Abbate A, Melfi R, Patti G, et al. Apoptosis in recent myocardial infarction. Clin Ter. 2000;151:247–51. [PubMed] [Google Scholar]
- 19.Baldi A, Abbate A, Bussani R, et al. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol. 2002;34(2):165–74. doi: 10.1006/jmcc.2001.1498. [DOI] [PubMed] [Google Scholar]
- 20.Ganley IG, Lam du H, Wang J, et al. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–61. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephan JS, Yeh YY, Ramachandran V, et al. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Natl Acad Sci USA. 2009;106:17049–54. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galluzzi L, Bravo-San Pedro JM, Vitale I, et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]


