Abstract
Background
α1-antitrypsin (α1-AT) is highly expressed in many tumors. However, to the best of our knowledge, its relationship to triple negative breast cancer (TNBC) has not yet been studied. Thus, in this research we first explored the influence of α1-AT silencing on the abilities of migration and invasion, and then further study its molecular mechanism in TNBC cells.
Material/Methods
The viability of MDA-MB-231 cells were detected using cell counting kit-8 (CCK-8). The abilities of migration and invasion were examined by Transwell assay. The metastasis-related factors were tested respectively by quantitative real-time PCR (qRT-PCR) and western blot assays.
Results
Our study results showed that α1-AT level in TNBC tissues was higher than non-triple negative breast cancer (n-TNBC) and adjacent normal breast tissues. The high expression of α1-AT was linked to type of cancer, tumor size, TNM stage and metastasis, but was not correlated with α1-AT expression and age. si-α1-AT suppressed the viability, migration, and invasion of cells. While si-α1-AT upregulated E-cadherin and the tissue inhibitor of metalloproteinases-2 (TIMP-2) levels, it downregulated metastasis associated 1 (MTA1), matrix metallopeptidase 2 (MMP2), phosphorylated-mammalian target of rapamycin (p-mTOR), phosphorylated-protein kinase B (p-Akt), and phosphorylated-phosphatidylinositol 3 kinase (p-PI3K) levels. We also found that the PI3K/Akt/mTOR pathway activator reversed the role of si-α1-AT in metastasis-related factors.
Conclusions
α1-AT was highly expressed in TNBC tissues, and its silencing suppressed the abilities of migration and invasion in TNBC cells and downregulated the PI3K/Akt/mTOR pathway. Thus, α1-AT may have a potential therapeutic effect on TNBC.
MeSH Keywords: Adrenergic alpha-1 Receptor Antagonists, Phosphatidylinositol 3-Kinase, Transcellular Cell Migration, Triple Negative Breast Neoplasms
Background
For decades, breast cancer has had the highest incidence rate for malignant tumors in women [1]. The estimated number of newly diagnosed cases and estimated deaths in China account for 12.2% and 9.6% of the world cases, respectively. Breast cancer seriously affects the physical and mental health of women [2,3]. Triple negative breast cancer (TNBC) is a special subtype of breast cancer and is called TNBC for its negative results on tests for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2). Noticeably, TNBC accounts for about 10% to 20% of the total number of breast cancer cases [4]. Increasing attention has been paid to TNBC for some of its characteristics such as early onset, high degree of malignancy, high recurrence rate, early metastasis, and poor prognosis [5]. In the early diagnosis of TNBC, the results of physical examination and imaging studies are often not satisfactory [6]. Therefore, a majority of patients with TNBC are diagnosed in the middle or late stages, which means that the best time for radical surgery is missed [5]. Currently, the main treatment for TNBC is chemotherapy and radiotherapy [7]. Compared with other types of breast cancer, TNBC has a higher sensitivity to chemotherapy and radiotherapy. Thus, the prognosis will remain poor if a patient merely receives conventional standard treatment [8]. For a better treatment of TNBC, we must seek new targets for anti-cancer treatment.
α1-antitrypsin (α1-AT), a member of the serine protease inhibitor superfamily, acts as a glycoprotein that is secreted by hepatocytes, monocytes, and various tumor cells [9]. α1-AT is located on chromosome 14, and the 70 allele regulates its synthesis [10]. Researchers found that α1-AT is involved in important physiological processes of blood coagulation and complement activation, and it has a vital role in regulating the body growth and cytotoxic effects of lymphocytes [11]. A number of studies have found that α1-AT is expressed in many tumors [12,13], for example, researchers have reported that the serum levels of α1-AT in pancreatic cancer patients are significantly higher than in normal control patients [14]. Studies also indicated that the risk of lung cancer increases in patients with anti-α1-AT allele deficiency [15]. However, to the best of our knowledge, no research has studied the relationship between α1-AT and TNBC.
Invasion and migration of tumor cells are the leading cause of poor prognosis and can cause death of patients who are diagnosed with breast cancer; meanwhile, invasion and migration of tumors are difficult to overcome in the clinic [16,17]. To explore the internal mechanism of breast cancer invasion and migration, finding, and even reversing, the biological target of invasion and migration have therefore become an urgent problem to be tackled in the clinic. Recent studies have shown that the PI3K/Akt/mTOR pathway plays a vital role in the process of tumor cell proliferation, angiogenesis, invasion, and metastasis, as well as radiotherapy and chemotherapy antagonism, and that it is frequently activated in breast cancers [18,19]. A previous study documented that PIK3CA was the most common mutated gene in TNBC [20]. The inhibition of the PI3K/Akt pathway potentiates the sensitivity of TNBC to EGFR kinase inhibitors [21]. Therefore, it can be concluded that the PI3K/Akt pathway may be a promising target in the treatment of TNBC. However, the effect of α1-AT on the PI3K/Akt pathway in TNBC is unclear.
In this study, we explored the relationship between α1-AT expression and TNBC, non-TNBC (n-TNBC), and adjacent normal breast tissues from breast cancer patients. Moreover, we assessed the influence of α1-AT silencing on the abilities of migration and invasion in TNBC cells, and we studied the PI3K/Akt/mTOR pathway.
Material and Methods
Tissue source
The TNBC, n-TNBC,and adjacent normal breast tissues were obtained from 50 breast cancer patients who receive their treatments at the Second Affiliate Hospital of Kunming Medical University from 2010 to 2015. Normal breast tissue was adjacent to TNBC or n-TNBC tissues. The breast cancer tissue and normal breast tissue were sorted under the microscope. The patients signed informed protocols and agreed that their tissues would be used for research purposes. This project was reviewed by the Ethics Committee of the Second Affiliate Hospital of Kunming Medical University.
Cell culture
Normal human breast cell line Hs 578Bst, human breast cancer cell line SKBR3, ZR-75-30 and T47D, and human TNBC cell line MDA-MB-231 were purchased from Procell Biotechnology Co., Ltd. (Wuhan, China). Hs 578Bst cells were inoculated in a complete Dulbecco’s Modified Eagle Medium (DMEM; Huayueyang, Beijing, China) that contained 10% fetal bovine serum (FBS; MRC, Jiangsu, China) and a mixture of streptomycin and streptomycin (Solarbio, Beijing, China) in a 37°C humidified incubator (HWS-2000; Safu, Ningbo, China), with 5% CO2. SKBR3, MDA-MB-231, ZR-75-30 and T47D cells were inoculated in Roswell Park Memorial Institute-1640 (RPMI-1640, Huayueyang, Beijing, China) that was supplemented with 10% FBS, and a mixture of streptomycin and streptomycin in 37°C humidified incubator with 5% CO2.
Cell transfection and grouping
α1-AT-siRNA and unspecific scrambled siRNA plasmids were purchased from Genewiz Biotechnology Co., Ltd (Suzhou, China). MDA-MB-231 cells were transfected with plasmids at 50% to 60% confluence by lipofectamine™ 2000 transfection reagent (Thermo, Beijing, China). The unspecific scrambled siRNA was considered the negative control (NC). After transfection, cells were prepared for later research. The first half of the experiment was divided into 3 groups: the control (0.1% PBS) group, the NC (unspecific scrambled siRNA plasmid) group, and the si-α1-AT (α1-AT-siRNA plasmid) group. The second half was divided into 3 groups: the control (unspecific scrambled siRNA plasmid) group, the si-α1-AT (α1-AT-siRNA plasmid) group, and the si-α1-AT+PI3K (+) [α1-AT-siRNA plasmid and insulin-like growth factor-1 (IGF-1)] group.
Cell counting kit-8 (CCK-8) assay
The viability of cells was detected using CCK-8 (Beyotime, Shanghai, China) as described in a previous study [22]. To be more specific, cells were transferred to an incubator and cultured for 12, 24, 48, and 60 hours after transfection. Then, CCK-8 reagent was dripped into cells. Absorbance was tested at 450 nm by a microplate reader (SpectraMax iD3; Molecular devices, CA, USA) after 2.5 hours of incubation.
Transwell assay
The abilities of migration and invasion in MDA-MB-231 cells were studied using Transwell assay. The difference between the migration and invasion experiments lies in the use of Matrigel. Matrigel was required for the detection of invasion, however, for detecting migration, it was not needed. Matrigel (Qcbio, Shanghai, China, 356234) was used to cover the upper part of the Transwell plates at room temperature. RPMI-1640 medium was added for 90 minutes and then removed by suction. The medium with FBS was applied to the lower part of the Transwell plates. Cell suspension was added to the upper part of the Transwell plates for 24 hours. The Transwell plates were placed in paraformaldehyde for fixation, then taken out and dried at room temperature for 10 minutes. Cells were stained by hematoxylin for 20 minutes. Cells were observed and photographed with an inverted microscope (CX23; Olympus, Japan).
Quantitative real-time PCR (qRT-PCR) assay
The total RNA of cells and tissues were collected by RNA extraction kit (Takara, Beijing, China). We used 1 μg of RNA for synthesizing cDNA using an RT Master Mix kit (Takara, Beijing, China). SYBR Premix Taq™ II kit (Takara, Beijing, China) was used for amplifying cDNA. All primers sequence were as follows: α1-AT, forward: 5′-TCAAGGACACCGAGGAAGAG-3′, reverse: 5′-AGGTGCTGTAGTTTCCCCTC-3′, product: 190 bp. E-cadherin, forward: 5′-TTTGAAGATTGCACCGGTCG-3′, reverse: 5′-CAGCGTGACTTTGGTGGAAA-3′, product: 180 bp. Tissue inhibitor of metalloproteinases-2 (TIMP-2), forward: 5′-AGCACCACCCAGAAGAAGAG-3′, reverse: 5′-TGATGCAGGCGAAGAACTTG-3′, product: 175 bp. Metastasis associated 1 (MTA1), forward: 5′-CTACGACCCACAGCAGAAGA-3′, reverse: 5′-TGGTCGATCTGCTTGTCTGT-3′, product: 180 bp. Matrix metallopeptidase 2 (MMP2), forward: 5′-TGGCTACACACCTGATCTGG-3′, reverse: 5′-GAGTCCGTCCTTACCGTCAA-3′, product: 184 bp. β-actin, forward: 5′-CACCATGTACCCAGGCATTG-3′, reverse: 5′-TCGTACTCCTGCTTGCTGAT-3′, product: 180 bp. β-actin was treated as sample control. The formula 2−DDCT was used to calculate the gene expression.
Western blot assay
The western blot assays were performed as described in a previous article [23]. Total proteins of cells and tissues were collected and separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then bound to PVDF membrane (Reno, Hangzhou, China). Following this step, 5% non-fat milk was used to seal the membranes for 1.5 hours, and the membranes were hybridized to anti-α1-AT (Abcam, ab207303, 1: 1200), anti-E-cadherin (Abcam, ab15148, 1: 1000), anti-TIMP-2 (Abcam, ab180630, 1: 1000), anti-MTA1 (Abcam, ab71153, 1: 800), anti-MMP2 (Abcam, ab92536, 1: 1500), anti-phosphorylated-mammalian target of rapamycin (p-mTOR) (Invitrogen, 710216, 1;800), anti-mTOR (Invitrogen, 44-1125G, 1: 1000), anti-phosphorylated-protein kinase B (p-Akt) (Invitrogen, 44-623G, 1: 1200), anti-Akt (Invitrogen, 44-623G, 1: 1600), anti-phosphorylated-phosphatidylinositol 3 kinase (p-PI3K) (Invitrogen, PA5-12799, 1: 1600), anti-PI3K (Invitrogen, MA5-17149, 1: 1000), anti-β-actin (R&D, MAB8969, 1: 2000). After hybridization, the membrane was soaked in corresponding secondary antibodies (HRP mouse ant-goat IgG, Invitrogen, BA1074, 1: 7000; HRP mouse anti-rabbit, Invitrogen, BA1034, 1: 7000) at 37°C for 60 minutes. The protein was detected by ECL detection reagent (Taixin, Beijing, China).
Statistical analysis
Using Excel Software, results are shown as mean ± standard error of mean, unless otherwise specified. The differences among groups were analyzed by one-way analysis of variance (ANOVA), and the post hoc test was performed by Tukey test. Chi-square test was performed to study the relationship between α1-AT expression and clinicopathological characteristics of patients diagnosed with breast cancer. Each experiment was performed in triplicate and repeated 3 times. P value determination of <0.05 was considered statistically significant.
Results
si-α1-AT repressed the viability of MDA-MB-231 cells
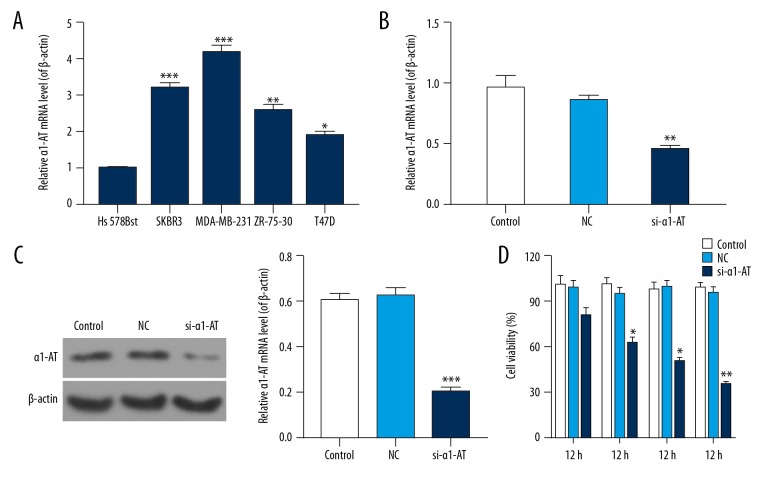
The mRNA and protein levels of α1-AT were detected using qRT-PCR and western blot assay. We found that the mRNA level of α1-AT was highest in MDA-MB-231 cells (Figure 1A, P<0.05). Evidence also show that transfection of si-α1-AT in MDA-MB-231, mRNA and protein levels of α1-AT were reduced, and levels of α1-AT in control were the same as the NC group (Figure 1B, 1C, P<0.05).
Figure 1.
si-α1-AT repressed the viability of MDA-MB-231 cells. (A) qRT-PCR was used to measure the mRNA level of α1-AT in normal human breast cell line Hs 578Bst, human breast cancer cell line SKBR3, ZR-75-30 and T47D, and human TNBC cell line MDA-MB-231. * P<0.05; ** P<0.01; *** P<0.001 vs. Hs 578Bst cells. (B) qRT-PCR was performed to assess mRNA level of α1-AT in MDA-MB-231 cells that were transfected with 0.1% PBS (control), α1-AT-siRNA (si-α1-AT), and unspecific scrambled siRNA (NC) plasmids. (C) Western blot was used to measure the protein level of α1-AT. β-actin was used as the control. The gray value was detected and counted by quality one. (D) CCK-8 was performed to measure the cell viability at 12, 24, 48, and 60 hours. * P<0.05; ** P<0.01; *** P<0.001, vs. NC.
The viability of cells was measured by CCK-8. Our results revealed that transfection of si-α1-AT in MDA-MB-231 cells decreased the viability of cells. However, no significant difference was observed at 12 hours. The viability of cell was 50% at 48 hours (Figure 1D, P<0.05).
si-α1-AT inhibited the abilities of migration and invasion in MDA-MB-231 cells
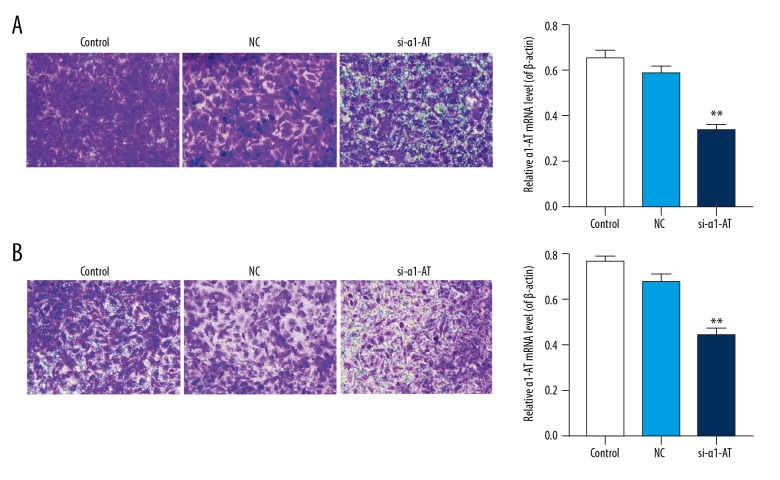
Transwell assay was performed to study the influence of si-α1-AT on the abilities of migration and invasion in MDA-MB-231 cells. The results revealed the silencing of α1-AT in MDA-MB-231 cells, and the numbers of cellular migration and invasion decreased significantly. Compare to the control and NC groups, the rate of migration in si-α1-AT was 50±1.2%, which decreased by 48±0.9%, and 38±0.6% respectively. Compare to the control and NC groups, the rate of invasion in si-α1-AT was 56±1.4%, which was decreased by 40±0.7% and 29±0.4% respectively (Figure 2, P<0.05).
Figure 2.
si-α1-AT inhibited the abilities of migration and invasion in MDA-MB-231 cells. (A) The rate of migration was analyzed by Transwell assay in MDA-MB-231 cells. (B) The rate of invasion was tested by Transwell assay. * P<0.05; ** P<0.01; *** P<0.001, vs. NC.
si-α1-AT regulated the migration-related factors in MDA-MB-231 cells
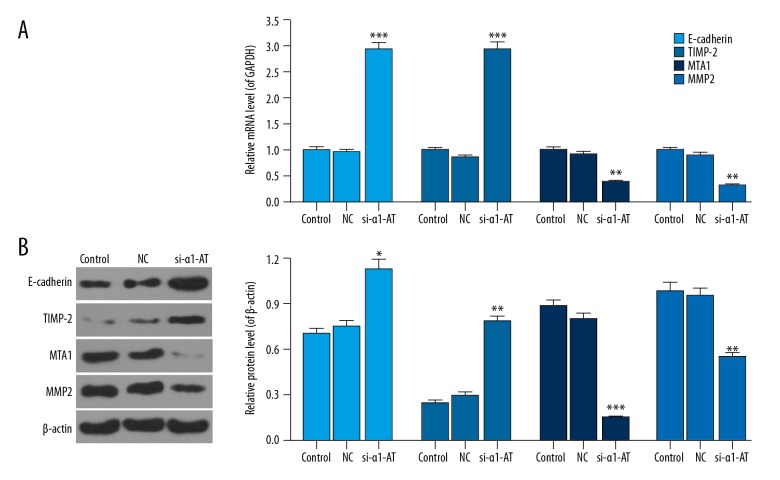
qRT-PCR and western blot assays were performed to measure the mRNA and protein levels of migration-related factors. The data showed that mRNA levels of E-cadherin and TIMP-2 increased, while the mRNA levels of MTA1 and MMP2 decreased in α1-AT silencing MDA-MB-231 cells. Meanwhile, the protein levels of E-cadherin, TIMP-2, MTA1, and MMP2 were the same as that of mRNA (Figure 3, P<0.05).
Figure 3.
si-α1-AT regulated the migration-related factors in MDA-MB-231 cells. (A) The mRNA levels of E-cadherin, TIMP-2, MTA1, and MMP2 were investigated by qRT-PCR. (B) The protein levels of E-cadherin, TIMP-2, MTA1, and MMP2 were assessed by western bolt. * P<0.05; ** P<0.01; *** P<0.001, vs. NC.
si-α1-AT blocked the PI3K/Akt/mTOR pathway
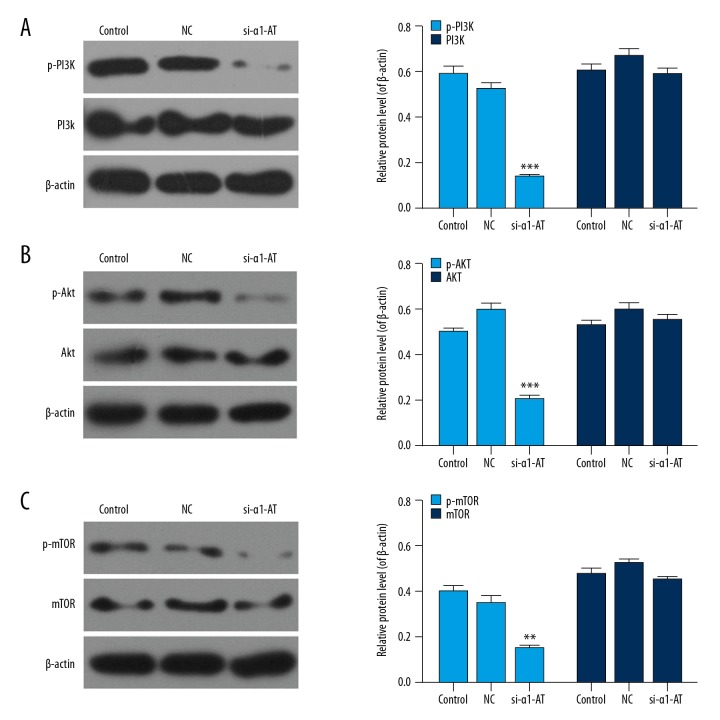
Western blot assay was performed to analyze the protein levels of PI3K/Akt/mTOR pathway-related factors. We found that phosphorylation levels of PI3K, Akt, and mTOR were downregulated in α1-AT silencing MDA-MB-231 cells. Moreover, the protein levels of PI3K, Akt, and mTOR remained stable in different groups. (Figure 4, P<0.05).
Figure 4.
si-α1-AT blocked the PI3K/Akt/mTOR pathway. (A) The protein levels of PI3K and p-PI3K were determined using western bolt. (B) The protein levels of p-Akt and Akt were assessed using western bolt. (C) The protein levels of p-mTOR, and mTOR were measured using western bolt. * P<0.05; ** P<0.01; *** P<0.001, vs. NC.
si-α1-AT regulated of migration-related factors in MDA-MB-231 cells by downregulating PI3K/Akt/mTOR pathway
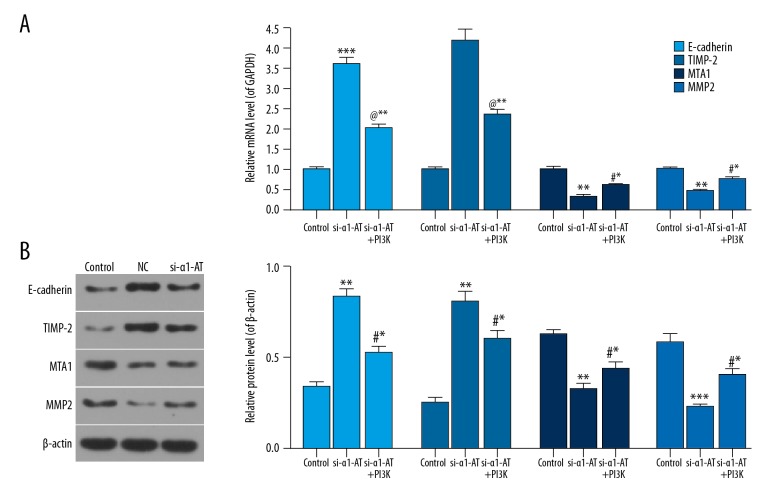
To further explore the role of si-α1-AT in migration-related factors by regulating the PI3K/Akt/mTOR pathway, the levels of migration-related factors were evaluated in MDA-MB-231 cells treated with PI3K/Akt/mTOR signal agonist (IGF-1) under α1-AT silencing. In comparison with the si-α1-AT group, the data showed that the levels of E-cadherin and TIMP-2 in si-α1-AT+PI3K(+) were downregulated, whereas MTA1 and MMP2 levels were upregulated (Figure 5, P<0.05).
Figure 5.
si-α1-AT regulated the migration-related factors in MDA-MB-231 cells by downregulating the PI3K/Akt/mTOR pathway. (A, B) The mRNA and protein expression of E-cadherin, TIMP-2, MTA1, and MMP2 in cells exposed to unspecific scrambled siRNA plasmid (control), α1-AT siRNA (si-α1-AT), si-α1-AT, and IGF-1 [si-α1-AT+PI3K (+)] using qRT-PCR (A) and western blot (B). * P<0.05; ** P<0.01; *** P<0.001, vs. control. # P<0.05; @ P<0.01; $ P<0.001, vs. si-α1-AT.
α1-AT level was enhanced in breast cancer patients, and was associated with the patient’s clinicopathological characteristics and survival time
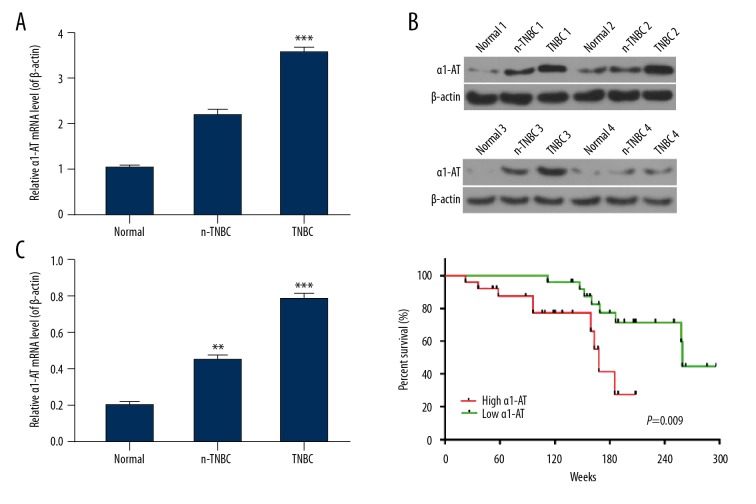
qRT-PCR was used to analyze the expression levels of α1-AT in TNBC, n-TNBC, and adjacent normal breast tissues from breast cancer patients. The results revealed that the mRNA level of α1-AT in TNBC tissue was higher than that in n-TNBC tissue and normal tissue; noticeably, the mRNA level of α1-AT in normal tissue was the lowest. In addition, we detected α1-AT protein level with western blot in a sample of 12 randomly chosen cases of TNBC (TNBC1/2/3/4), n-TNBC (n-TNBC1/2/3/4), and adjacent normal breast tissues (normal 1/2/3/4) from breast cancer patients. Gray value showed that the trend of α1-AT protein in TNBC1/2/3/4, n-TNBC1/2/3/4, and normal 1/2/3/4 tissues was similar to mRNA level tendency (Figure 6A, 6B, P<0.05). Moreover, we studied the relationship between α1-AT expression and survival time of breast cancer patients. Our data showed that patients with α1-AT high expression had 166±10.6 weeks for survival time, whereas the survival time of breast cancer patients with α1-AT low expression was 260±12.3 weeks. (Figure 6C, P<0.05).
Figure 6.
α1-AT level was enhanced in breast cancer patients and associated with the patient’s clinicopathological characteristics and survival time. (A) qRT-PCR was used to test mRNA levels of α1-AT in triple negative breast cancer (TNBC), non-triple negative breast cancer (n-TNBC) as well as adjacent normal breast tissues from breast cancer patients. Normal breast tissue is adjacent to TNBC or n-TNBC tissues. (B) Western blot was used to analyze protein levels of α1-AT in randomized 12 cases of TNBC, n-TNBC, and normal breast tissues from breast cancer patients. The randomized 4 cases of TNBC were called TNBC1/2/3/4, the randomized 4 cases of TNBC were called n-TNBC1/2/3/4, the adjacent normal breast tissues were called normal 1/2/3/4. β-actin was considered as control. The gray value was detected and counted by quality one. (C) Quantification of the relationship between α1-AT expression and survival time of patients with breast cancer was analyzed by GraphPad prism 6.0 Software. * P<0.05; ** P<0.01; *** P<0.001, vs. normal tissues.
Chi-square test was performed to assess the correlation between α1-AT expression and clinicopathological characteristics of patients diagnosed with breast cancer. The results showed that the expression level of α1-AT was linked to the type of cancer, tumor size, TNM stage, and metastasis, however, it was not linked to α1-AT expression or age (Table 1).
Table 1.
The correlation between α1-AT expression and clinicopathological characteristics of patients with breast cancer.
| Groups | Number of patients | Low PYGB expression | High PYGB expression | P value |
|---|---|---|---|---|
| Cancer type | ||||
| Triple negative breast cancer | 25 | 8 | 17 | 0.001** |
| Non-triple negative breast cancer | 25 | 20 | 5 | |
| Age (years) | 0.054 | |||
| <50 | 18 | 4 | 14 | |
| ≥50 | 32 | 16 | 16 | |
| Tumor size (cm) | 0.001** | |||
| <5 | 28 | 8 | 18 | |
| ≥5 | 22 | 18 | 4 | |
| TNM stage | 0.011* | |||
| I–II | 23 | 7 | 16 | |
| III–IV | 27 | 18 | 9 | |
| Metastasis | 0.021* | |||
| No | 30 | 19 | 11 | |
| Yes | 20 | 6 | 14 | |
P<0.05,
P<0.01, Chi-square test.
Discussion
The current studies find that α1-AT is highly expressed in the breast cancer cell line MCF-7 [24]. Research has shown that breast cancer patients express α1-AT antibodies with a specificity of 96%, which means that α1-AT might be a serum marker for early screening and diagnosis of breast cancer [25]. We therefore made the hypothesis that since α1-AT had been shown to be highly expressed in breast cancer, it might play a role in TNBC as well. Migration and invasion are the most important biological characteristics of malignant tumors and are the 2 main causes of death in tumor patients [16]. Migration and invasion of TNBC have been previously studied [26,27]. One study showed that the abilities of growth and invasion are enhanced in breast cancer cells with high expression of α1-AT [25]. Thus, we suspected that the silencing of α1-AT could have an inhibitory effect on viability, migration, and invasion of the human TNBC cell line MDA-MB-231. Our study showed that, by RNA interference, si-α1-AT repressed viability, migration, and invasion of MDA-MB-231 cells.
E-cadherin is an intercellular adhesion molecule, and it has been shown that downregulation of E-cadherin inhibits intercellular adhesion and polarity [28]. In breast cancer patients, activity of MMP-2 in invasive carcinoma is obviously higher than that in non-invasive carcinoma [29]. As we know, TIMP-2 is an important endogenous regulatory factor, which inhibits the activity of MMP-2 [30,31]. In addition, MTA1 has been recently discovered and isolated, and it is a class of important tumor metastasis-associated genes, and over-expression of MTA1 greatly increases the probability of metastasis [32]. Thus, we tested the expression levels of TIMP-2, MTA1, E-cadherin, and MMP2 in MDA-MB-231 cells, and found that the levels of E-cadherin and TIMP-2 were upregulated, while MTA1 and MMP2 levels were downregulated in α1-AT silencing MDA-MB-231 cells. These results demonstrated that si-α1-AT inhibited the abilities of migration and invasion in MDA-MB-231 cells by regulating E-cadherin, TIMP-2, MTA1, and MMP2 factors.
Not only is the PI3K/Akt/mTOR pathway vital to the occurrence, development, treatment, and prognosis of malignant tumors [33–36], but it is also the most frequent mutation found in breast cancer [37,38]. The mutation of PIK3CA was reported to the most common mutation in TNBC [20]. Evidence has also shown that suppression of the PI3K/Akt/mTOR pathway decreases cisplatin resistance in TNBC cells [35]. An article reported that the PI3K/AKT/mTOR pathway participates in regulation of TNBC progression, and that it is a potential therapeutic target in TNBC [39]. In addition, the activation of PI3K/Akt was considered a potential marker for TNBC [40]. Based on the aforementioned research, we investigated the effect of si-α1-AT on MDA-MB-231 cells on regulating this pathway. Similar to the aforementioned study, our results found that the MDA-MB-231 cells treated with si-α1-AT reduced phosphorylation levels of PI3K, Akt, and mTOR. In addition, we found that when the PI3K/Akt/mTOR pathway was activated under si-α1-AT in MDA-MB-231 cells, the expression of E-cadherin, TIMP-2, MTA1 and MMP2 was reversed. We therefore confirmed that si-α1-AT regulated the migration-related factors in MDA-MB-231 cells by downregulating the PI3K/Akt/mTOR pathway.
To further confirm the role of α1-AT in TNBC, we detected the expression of α1-AT in the patients with TNBC. We found that the expression level of α1-AT in TNBC tissue was higher than that in n-TNBC tissue and normal tissue. Moreover, our results indicated that the high expression α1-AT in breast cancer patients predicted poor clinical prognosis. Nevertheless, our sample data was small and, thus, cannot represent larger population-based data. Therefore, the role of α1-AT in TNBC should be investigated from the large-scale studies.
Conclusions
Our studies have proven that α1-AT is highly expressed in TNBC tissues of breast cancer patients, and that its high expression in breast cancer patients predicted poor clinical prognosis. Moreover, the silencing of α1-AT suppressed the viability, migration, and invasion of human TNBC cell line MDA-MB-231, accompanied by downregulated of the PI3K/Akt/mTOR pathway. Therefore, α1-AT might be a promising target for TNBC treatment in the future; studies using in vivo molecular and cell signaling dynamics are needed to validate these findings.
Footnotes
Source of support: Departmental sources
Conflict of Interest
None.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. Cancer J Clin. 2011;61(6):409–18. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–89. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 3.Warner E. Clinical practice. Breast-cancer screening. N Engl J Med. 2011;365(11):1025–32. doi: 10.1056/NEJMcp1101540. [DOI] [PubMed] [Google Scholar]
- 4.Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer (Auckl) 2016;10:25–36. doi: 10.4137/BCBCR.S32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer (Dove Med Press) 2016;8:93–107. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fosu-Mensah N, Peris MS, Weeks HP, et al. Advances in small-molecule drug discovery for triple-negative breast cancer. Future Med Chem. 2015;7(15):2019–39. doi: 10.4155/fmc.15.129. [DOI] [PubMed] [Google Scholar]
- 7.Lumachi F, Chiara GB, Foltran L, Basso SM. Proteomics as a guide for personalized adjuvant chemotherapy in patients with early breast cancer. Cancer Genomics Proteomics. 2015;12(6):385–90. [PubMed] [Google Scholar]
- 8.Wang M, Li A, Sun G, et al. Association between mesothelin expression and survival outcomes in patients with triple-negative breast cancer: a protocol for a systematic review. Syst Rev. 2016;5(1):133. doi: 10.1186/s13643-016-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelvyte I, Sjogren HO, Janciauskiene S. Effects of native and cleaved forms of alpha1-antitrypsin on ME 1477 tumor cell functional activity. Cancer Detect Prev. 2002;26(4):256–65. doi: 10.1016/s0361-090x(02)00090-9. [DOI] [PubMed] [Google Scholar]
- 10.Janciauskiene S, Tumpara S, Wiese M, et al. Alpha1-antitrypsin binds hemin and prevents oxidative activation of human neutrophils: Putative pathophysiological significance. J Leukoc Biol. 2017;102(4):1127–41. doi: 10.1189/jlb.3A0317-124R. [DOI] [PubMed] [Google Scholar]
- 11.Ikari Y, Mulvihill E, Schwartz SM. alpha 1-Proteinase inhibitor, alpha 1-antichymotrypsin, and alpha 2-macroglobulin are the antiapoptotic factors of vascular smooth muscle cells. J Biol Chem. 2001;276(15):11798–803. doi: 10.1074/jbc.M008503200. [DOI] [PubMed] [Google Scholar]
- 12.El-Akawi ZJ, Abu-Awad AM, Sharara AM, Khader Y. The importance of alpha-1 antitrypsin (alpha1-AT) and neopterin serum levels in the evaluation of non-small cell lung and prostate cancer patients. Neuro Endocrinol Lett. 2010;31(1):113–16. [PubMed] [Google Scholar]
- 13.Li Y, Miao L, Yu M, et al. alpha1-antitrypsin promotes lung adenocarcinoma metastasis through upregulating fibronectin expression. Int J Oncol. 2017;50(6):1955–64. doi: 10.3892/ijo.2017.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tountas Y, Sparos L, Theodoropoulos C, Trichopoulos D. Alpha 1-antitrypsin and cancer of the pancreas. Digestion. 1985;31(1):37–40. doi: 10.1159/000199175. [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Wentzlaff KA, Katzmann JA, et al. Alpha1-antitrypsin deficiency allele carriers among lung cancer patients. Cancer Epidemiol Biomarkers Prev. 1999;8(5):461–65. [PubMed] [Google Scholar]
- 16.Jacquemet G, Hamidi H, Ivaska J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr Opin Cell Biol. 2015;36:23–31. doi: 10.1016/j.ceb.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Noel A, Gilles C, Foidart JM. [Invasion and metastatic dissemination in breast cancer: mechanisms]. Rev Med Liege. 2011;66(5–6):274–78. [in French] [PubMed] [Google Scholar]
- 18.Li H, Zhang B, Liu Y, Yin C. EBP50 inhibits the migration and invasion of human breast cancer cells via LIMK/cofilin and the PI3K/Akt/mTOR/MMP signaling pathway. Med Oncol. 2014;31(9):162. doi: 10.1007/s12032-014-0162-x. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Xu Y, Xu J, et al. Role of TM4SF1 in regulating breast cancer cell migration and apoptosis through PI3K/AKT/mTOR pathway. Int J Clin Exp Pathol. 2015;8(8):9081–88. [PMC free article] [PubMed] [Google Scholar]
- 20.Munzone E, Gray KP, Fumagalli C, et al. Mutational analysis of triple-negative breast cancers within the International Breast Cancer Study Group (IBCSG) Trial 22-00. Breast Cancer Res Treat. 2018 doi: 10.1007/s10549-018-4767-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Yi YW, Hong W, Kang HJ, et al. Inhibition of the PI3K/AKT pathway potentiates cytotoxicity of EGFR kinase inhibitors in triple-negative breast cancer cells. J Cell Mol Med. 2013;17(5):648–56. doi: 10.1111/jcmm.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen K, Sparks J, Omoruyi FO. Investigation of the cytotoxicity, antioxidative and immune-modulatory effects of Ligusticum porteri (Osha) root extract on human peripheral blood lymphocytes. J Integr Med. 2016;14(6):465–72. doi: 10.1016/S2095-4964(16)60280-7. [DOI] [PubMed] [Google Scholar]
- 23.Han BH, Lee YJ, Yoon JJ, et al. Hwangryunhaedoktang exerts anti-inflammation on LPS-induced NO production by suppressing MAPK and NF-kappaB activation in RAW264.7 macrophages. J Integr Med. 2017;15(4):326–36. doi: 10.1016/S2095-4964(17)60350-9. [DOI] [PubMed] [Google Scholar]
- 24.Mbeunkui F, Metge BJ, Shevde LA, Pannell LK. Identification of differentially secreted biomarkers using LC-MS/MS in isogenic cell lines representing a progression of breast cancer. J Proteome Res. 2007;6(8):2993–3002. doi: 10.1021/pr060629m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Arias E, Aguilar-Lemarroy A, Felipe Jave-Suarez L, et al. Alpha 1-antitrypsin: A novel tumor-associated antigen identified in patients with early-stage breast cancer. Electrophoresis. 2012;33(14):2130–37. doi: 10.1002/elps.201100491. [DOI] [PubMed] [Google Scholar]
- 26.Liang S, Chen Z, Jiang G, et al. Corrigendum to “Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-kappaB/IL-6 signals” [Cancer Lett. 386 (2017) 12-23] Cancer Lett. 2018;414:310. doi: 10.1016/j.canlet.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Liew SK, Azmi MN, In L, et al. Anti-proliferative, apoptotic induction, and anti-migration effects of hemi-synthetic 1′S-1′-acetoxychavicol acetate analogs on MDA-MB-231 breast cancer cells. Drug Des Devel Ther. 2017;11:2763–76. doi: 10.2147/DDDT.S130349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieters T, van Roy F. Role of cell-cell adhesion complexes in embryonic stem cell biology. J Cell Sci. 2014;127(Pt 12):2603–13. doi: 10.1242/jcs.146720. [DOI] [PubMed] [Google Scholar]
- 29.Katunina AI, Gershtein ES, Ermilova VD, et al. Matrix metalloproteinases 2, 7, and 9 in tumors and sera of patients with breast cancer. Bull Exp Biol Med. 2011;151(3):359–62. doi: 10.1007/s10517-011-1330-z. [DOI] [PubMed] [Google Scholar]
- 30.Chia CY, Kumari U, Casey PJ. Breast cancer cell invasion mediated by Galpha12 signaling involves expression of interleukins-6 and -8, and matrix metalloproteinase-2. J Mol Signal. 2014;9:6. doi: 10.1186/1750-2187-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Wang F, Xu P, et al. Perfluorooctanoic acid stimulates breast cancer cells invasion and upregulates matrix metalloproteinase-2/-9 expression mediated by activating NF-kappaB. Toxicol Lett. 2014;229(1):118–25. doi: 10.1016/j.toxlet.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Zhong H, Tang Y, Wang Y, Gu W. [Relationship between MTA1 expression and prognosis of chinese lung cancer patients: A meta-analysis]. Zhongguo Fei Ai Za Zhi. 2017;20(10):683–89. doi: 10.3779/j.issn.1009-3419.2017.10.04. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araki K, Miyoshi Y. Mechanism of resistance to endocrine therapy in breast cancer: The important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer. 2017 doi: 10.1007/s12282-017-0812-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Gasparri ML, Bardhi E, Ruscito I, et al. PI3K/AKT/mTOR pathway in ovarian cancer treatment: Are we on the right track? Geburtshilfe Frauenheilkd. 2017;77(10):1095–103. doi: 10.1055/s-0043-118907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gohr K, Hamacher A, Engelke LH, Kassack MU. Inhibition of PI3K/Akt/mTOR overcomes cisplatin resistance in the triple negative breast cancer cell line HCC38. BMC Cancer. 2017;17(1):711. doi: 10.1186/s12885-017-3695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osaki M, Kase S, Adachi K, et al. Inhibition of the PI3K-Akt signaling pathway enhances the sensitivity of Fas-mediated apoptosis in human gastric carcinoma cell line, MKN-45. J Cancer Res Clin Oncol. 2004;130(1):8–14. doi: 10.1007/s00432-003-0505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capodanno A, Camerini A, Orlandini C, et al. Dysregulated PI3K/Akt/PTEN pathway is a marker of a short disease-free survival in node-negative breast carcinoma. Hum Pathol. 2009;40(10):1408–17. doi: 10.1016/j.humpath.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Knowles E, O’Toole SA, McNeil CM, et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer. 2010;126(5):1121–31. doi: 10.1002/ijc.24831. [DOI] [PubMed] [Google Scholar]
- 39.Shao F, Sun H, Deng CX. Potential therapeutic targets of triple-negative breast cancer based on its intrinsic subtype. Oncotarget. 2017;8(42):73329–44. doi: 10.18632/oncotarget.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto K, Tsuda H, Koizumi F, et al. Activated PI3K/AKT and MAPK pathways are potential good prognostic markers in node-positive, triple-negative breast cancer. Ann Oncol. 2014;25(10):1973–79. doi: 10.1093/annonc/mdu247. [DOI] [PubMed] [Google Scholar]