Abstract
Background
Cantharidin (CTD) is one of the major active ingredients of blister beetles and has significant antitumor activity in many cancer cell lines. The aim of our study was to evaluate the effect of CTD on the apoptosis of human osteosarcoma cells MG-63 and MNNG/HOS, and to explore the possible molecular mechanism.
Material/Methods
Osteosarcoma cells MG-63 and MNNG/HOS were treated with varying concentrations of CTD. The proliferation inhibition of cells was detected by MTS. Flow cytometry and Hoechst 33258 staining were used to determine cell cycle arrest and apoptosis, and apoptosis-related protein levels were analyzed by Western blotting.
Results
Our current findings suggest that CTD could inhibit the proliferation of these 2 osteosarcoma cells. The cells treated with CTD showed an obvious apoptotic morphology, and CTD promoted cells apoptosis in a dose-dependent manner. In addition, cantharidin-induced apoptosis was accompanied by increased expression of Bax and PARP and decreased expression of Bcl-2, p-Akt, and p-Cdc2.
Conclusions
CTD accelerates the apoptosis of MG-63 and MNNG/HOS cells in a concentration-dependent manner through the mitochondria-dependent pathway, suggesting that use of CTD is a novel approach for the treatment of osteosarcoma.
MeSH Keywords: Apoptosis, Cantharidin, Mitochondria, Osteosarcoma
Background
Osteosarcoma is a malignant tumor originating from mesenchymal tissue and is the most common primary malignancy in children and adolescents. It accounts for about 20% of primary bone malignancies and 0.5% of malignant tumors [1–3]. With use of neoadjuvant chemotherapy, the survival rate of local limb osteosarcoma patients has been significantly improved; the 5-year survival rate has risen to more than 60%, but osteosarcoma is less sensitive to radiotherapy, chemotherapy and other treatment programs, and has poor prognosis [4–7]. Therefore, it is difficult to develop more effective means to prevent and treat osteosarcoma.
In the last few years, traditional Chinese medicine has been increasingly used in the treatment of osteosarcoma in order to overcome chemoresistance and promote apoptosis of osteosarcoma cells [8,9]. Apoptosis is active cell death regulated by a series of genes, and inducing apoptosis is a critical goal of developing new antitumor therapies at this stage. At present, the known apoptotic regulatory pathways include the death receptor pathway, the endoplasmic reticulum stress pathway, and the mitochondrial pathway [10]. Apoptosis through the death receptor-mediated pathway is mainly caused by Fas/FasL, caspase-8, and caspase-10 activation. The endoplasmic reticulum stress pathway appears to participate in Ca2+ and caspase-12 activation and other phenomena. Significant features of mitochondrial pathway activation include the decreased mitochondrial transmembrane potential, with large amounts of cytochrome c released from mitochondria into the cytoplasm [11], and the release of cytochrome C causes apoptosis of downstream apoptotic molecules such as caspase 9 and A series of cascade reactions [12]. Moreover, Bcl-2 family members and genes play a key role in regulating the mitochondrial pathway. Members of the Bcl-2 family are divided into anti-apoptotic and pro-apoptotic factors. Anti-apoptotic factors include Bcl-2 and Bcl-x and pro-apoptotic factors include Bax.Bad.Bak and Bid [13]. Cantharidin (CTD) is a monoterpene compound extracted from blister beetles; previous studies have shown it has a variety of biological activities [14,15]. Its molecular formula is C10H12O4 and it has a weight of 196.2. Cantharidin can inhibit protein synthesis in tumor cells, then affects the synthesis of RNA and DNA and the process of cell cycle, promotes apoptosis of tumor cells, and inhibits proliferation of tumor cells. It has been confirmed that it can exhibit potent anti-cancer activity in many types of human cancer cells, including bladder cancer [16], pancreas cancer [17], gastric cancer [18], and breast cancer [19].
In this study, we treated human osteosarcoma MG-63 and MNNG/HOS cells with cantharidin, then assessed the effect of CTD on their proliferation, apoptosis, and cell cycle, and also explored their possible molecular mechanisms. The results showed that cantharidin was effective in inhibiting proliferation, inducting of apoptosis, and blocking cell cycle in osteosarcoma cells.
Material and Methods
Reagents and antibodies
CTD was purchased from Sigma-Aldrich (St. Louis, MO, USA) and it has a purity of more than 99% tested by high-performance liquid chromatography (HPLC). Its molecular formula is C10H12O4 and the relative molecular mass is 196.2 (Figure 1) [20]. CTD was used as a stock solution in dimethyl sulfoxide (DMSO) with a concentration of 100 mg/ml and was stored at −20°C. The CTD stock solution was freshly diluted to a desired concentration before each experiment. The main antibodies for GAPDH, Akt, poly ADP ribose polymerase (PARP), Bax, and Bcl-2 were obtained from Abcam, Inc. (Cambridge, UK). The antibodies specific for phosphor-Akt (Thr308), p-Cdc2 were purchased from Cell Signaling Technology (Beverly, MA, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti- mouse secondary antibodies were purchased from Pierce. Hoechst 33258 was from Sigma-Aldrich and MTS was purchased from Promega (Madison, WI, USA).
Figure 1.
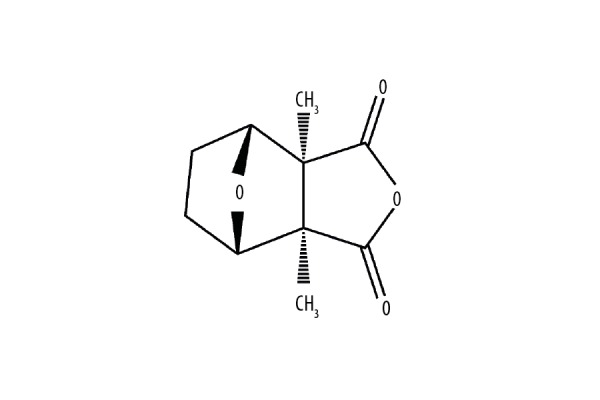
The chemical structure of cantharidin. The molecular formula of cantharidin is C10H12O4 and its molecular weight is 196.2.
Cell lines and cultures
The human osteosarcoma cell lines MNNG/HOS (CRL-1547TM, ATCC) and MG-63 (CRL-1427TM, ATCC) were from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China), where they were analyzed and identified. These procedures included cross-species checks, DNA identification, and quarantine. Cell lines used in this study were cultured for less than 6 months. MG-63 and MNNG/HOS were incubated in Eagle’s Minimum Essential Medium, supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin mixture and both were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Cell proliferation analysis by MTS assay
MTS assay was used to determine the proliferation of human osteosarcoma cells treated with CTD in 96-well plates; 4–8×103 cells/well were seeded in 100 ul medium and allowed to adhere overnight [21]. Cells were treated with different concentrations of CTD (0–5 mg/ml) for different periods of time (0–72 h). Two hours before the end of the incubation, MTS (20 µl in 100 µl Eagle’s Minimum Essential Medium) was added to each well and the cells were incubated at 37°C for 2 h. Cell viability was calculated by measuring the absorbance at 490 nm using a MR7000 microplate reader (Dynatech) and IC50 values were calculated using the probit model.
Hoechst staining
Hoechst staining [22] was used to detect apoptosis of human osteosarcoma cells treated with CTD. The cells were treated with CTD and then cultured for 24 h. Hoechst 33258 (5–10 μg/ml) was added to stain in the dark for 10 min, washed with PBS, and then resuspended, and the morphological changes of apoptosis were observed by inverted fluorescence microscopy. Hoechst 33258 penetrated the cell membrane and stained the nuclear DNA blue. During the apoptosis stage, Hoechst 33258 enhanced the combination with apoptotic cells, and apoptotic cells were identified by staining with strong blue fluorescence.
Annexin V-phycoerythrin (PE)/7-amino-actinomycin D (7-AAD) double-staining
The apoptosis of human osteosarcoma cells MG-63 and MNNG/HOS treated with CTD was quantified by Annexin V-PE/7-AAD double-staining. Cells treated with CTD (0–4 mg/ml) and (0–6 mg/ml), respectively, were seeded at a concentration of 2×105 cells/well in 6-well plates and cultured for 24 h. Afterwards, the cells were stained using the annexin V-PE/7-AAD double-fluorescence apoptosis detection kit (BD Biosciences, San Diego, CA, USA) as described in the manufacturer’s instruction. Samples were tested using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) to obtain data within 30 min after staining.
Cell cycle analysis by flow cytometry
Human osteosarcoma cells MNNG/HOS and MG-63 were seeded in 6-well plates at a concentration of 10×105 cells/well and various concentration gradients of CTD (0–4mg/ml) and (0–6 mg/ml) were then added to grow for 24 h. After that, cells of each well were collected, washed with PBS, and centrifuged. Cells were then fixed with 70% pre-cooled ethanol overnight and stained with 40 µg/ml propidium iodide (PI) for 30 min in the dark. Cell DNA content was detected by a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot analysis
MNNG/HOS and MG-63 cells were each treated with different concentrations of CTD (0–4 mg/ml) and (0–6 mg/ml) at the exponential growth phase for 24 h. Then, the total protein was extracted using a total protein extraction kit (Boster Biological Technology, USA). The protein level was quantified with a bicinchoninic acid protein assay kit from Boster according to the manufacturer’s protocol. The protein extract was separated using a 10–15% SDS–polyacrylamide gel electrophoresis and then transferred to the polyvinylidene fluoride (PVDF) membranes, then membranes were blocked in 5% skimmed milk dissolved in TBST for 1 h. The membranes were incubated at 4°C overnight with a primary antibody containing GAPDH, Akt, Bcl-2, Bax, PARP, and phosphor-Akt (Thr308), p-Cdc2, and then incubated with horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse secondary antibody at room temperature for 1 h. The protein expression was determined using enhanced chemiluminescence reagents (Millipore, Waltham, MA, USA). GAPDH was used as an internal control.
Statistical analysis
All experiments were done in biological triplicate. Results are shown as the mean ± standard deviation. The t test was used to assess the statistically significant differences between the experimental and control groups. IC50 values and 95% confidence intervals were calculated from the MTS assay data by probability regression. All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) software. P<0.05 was considered statistically significant.
Results
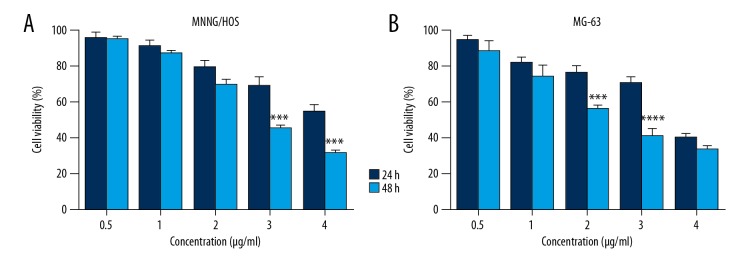
CTD inhibits the proliferation of human osteosarcoma cells in a time- and concentration-dependent manner
In order to study the proliferation of osteosarcoma cells, we studied the growth inhibition of human osteosarcoma cell lines MG-63 and MNNG/HOS by MTS assays. Osteosarcoma cells were treated with different concentrations of CTD for 48 h. The results showed that the inhibitory effect of cantharidin on MG-63 and MNNG/HOS cells occurred in a time- and concentration-dependent manner (Figure 2). The IC50 values of CTD on MNCG/HOS cells were 2.783 μg/ml, and 3.4 μg/ml on MG-63 cells.
Figure 2.
Cantharidin (CTD) inhibits proliferation of human osteosarcoma cells in a concentration-and time-dependent manner. (A) MNNG/HOS and (B) MG-63 cells were treated with different concentrations of CTD for different time periods. The cells were examined using the MTS assay and the absorbance was obtained at 490 nm using a microplate reader. The inhibition rate is expressed as the percentage of cell inhibition compared with the control group. The data are expressed as the mean ±SD from samples in triplicate.
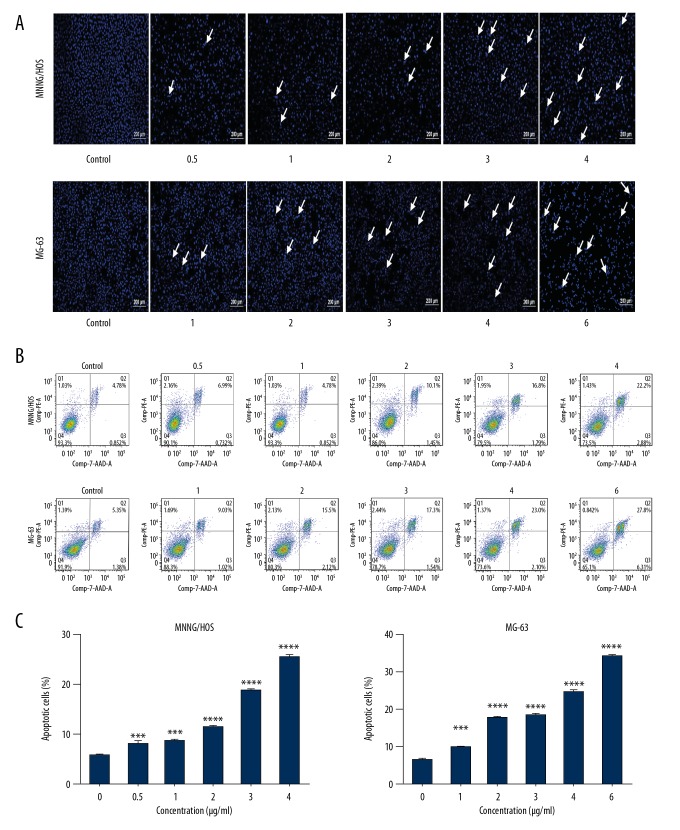
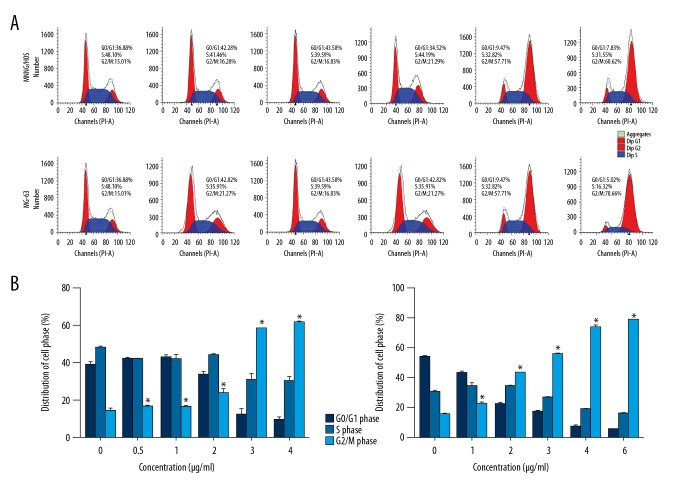
CTD induces the apoptosis and G2/M phase arrest of human osteosarcoma cells
To study the mechanism of CTD-induced apoptosis of human osteosarcoma cells, MG-63 and MNNG/HOS cells were treated with CTD and apoptotic cells were examined by Hoechst 33258 staining and flow cytometry, as detailed in previous studies [23]. The results in Figure 3A demonstrate that the nuclei of MG-63 and MNNG/HOS cells in the control group showed weak fluorescence, which was the expression of normal cells. In the experimental group, the nuclei of the cells were bright blue, nuclei showed condensation or fragmentation, chromatin showed condensation and marginalization, and apoptotic bodies were visible; these are characteristic morphological changes of apoptosis. Moreover, we found that in the osteosarcoma cell lines, the number of late apoptotic cells increased at higher CTD levels. Maximum cell death occurred within 48 h of drug exposure (data not shown). Thus, the CTD was chosen to be exposed for 24 h as the appropriate time point for the following experiments. In addition, quantitative results of cell apoptosis and cell cycle distribution by using flow cytometry assay indicated that cells treated with high- concentrations of CTD showed higher apoptosis rates compared to cells treated with low concentrations (Figure 3B, 3C), and these effects were concentration-dependent. As shown in Figure 4, MG-63 and MNNG/HOS cells were exposed to different doses of CTD and incubated for 24 h; in G2/M phase, CTD increased the percentage of cells in a dose-dependent manner, mainly due to G2/M phase arrest. All results revealed that the cell proliferation of cantharidin-treated cells was inhibited by the induction of apoptotic cell death mechanisms.
Figure 3.
Cantharidin induces apoptosis in human osteosarcoma cells. (A) MNNG/HOS and MG-63 cells were separately treated with 0, 0.5, 1, 2, 3, and 4 μg/ml and 0, 1, 2, 3, 4, and 6 μg/ml of cantharidin for 24 h, then cells were collected to detect apoptosis by Hoechst staining under fluorescence microscope (original magnification, 100×, bar=200 µm). (B) Annexin V-Phycoerythrin (PE)/7-Amino-Actinomycin D (7-AAD) double-staining of osteosarcoma cells treated with graded concentrations of Cantharidin. Data were calculated from separate experiments. (C) The results are expressed as a percentage of apoptosis. Significantly different from the control group at *** p<0.01; **** p<0.001.
Figure 4.
Cantharidin induces cell cycle arrest in human osteosarcoma cells. (A) MNNG/HOS and MG-63 cells were separately treated with 0, 0.5, 1, 2, 3, and 4 μg/ml and 0, 1, 2, 3, 4, and 6 μg/ml of cantharidin for 24 h, and flow cytometry was used to analyze cell cycle distribution. (B) The results are expressed as a percentage of cell cycle distribution. Significantly different from the control group at * p<0.05.
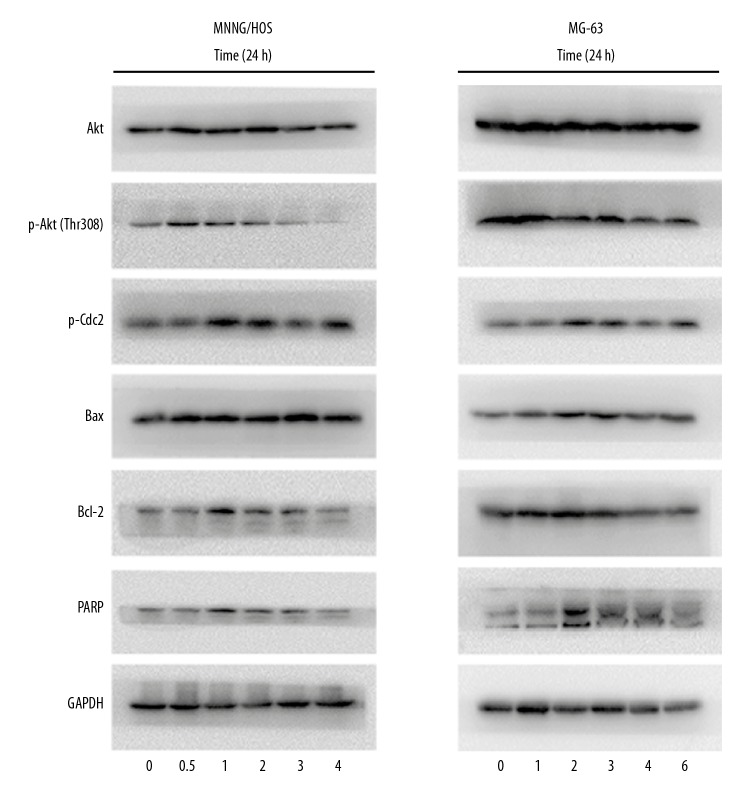
CTD induced apoptosis of human osteosarcoma cells by activating the mitochondrial pathway
In order to investigate how the expression of mitochondrial apoptosis-related proteins affected the mechanism of CTD-induced apoptosis, we performed Western blot analysis. Figure 5 shows the results in cells treated with different concentrations of CTD for 24 h. With increased concentration, the expression of the pro-apoptotic protein Bax was significantly increased, and the expression level of the anti-apoptotic protein Bcl-2 was decreased. CTD increased the protein level of PARP (Figure 5), but the cell cycle protein p-Cdc2 caused G2/M arrest and phosphor-Akt (Thr308) were decreased, leading to apoptosis. Thus, these results suggest that CTD-induced apoptosis of human osteosarcoma cells is mediated by mitochondrial pathways.
Figure 5.
Cantharidin affects the protein expression of G2/M arrest and apoptosis in MNNG/HOS and MG-63 cells. The cells were separately treated with 0, 0.5, 1, 2, 3, and 4 μg/ml and 0, 1, 2, 3, 4, and 6 μg/ml of cantharidin for 24 h, and Western blot analysis was used to determine protein levels of p-Cdc2, Akt, Bcl-2, Bax, PARP, and p-Akt (Thr308), respectively.
Discussion
Extraction of active ingredients from natural plants and from animals for the treatment of malignant tumors has made considerable progress, such as the discovery that gedunin has the potential to exert antiproliferative effect on human embryonal carcinoma (NTERA-2) [24] and that Mangifera zeylanica bark has potential anti-cancer activity in breast and ovarian cancer [25]. In addition, paclitaxel has been used for clinical treatment of breast cancer, ovarian cancer, and other tumors, oridonin has been used for the treatment of lung cancer, esophageal cancer, and liver cancer, and retinoic acid has shown efficacy in treatment of leukemia [26–28]. There have been numerous studies showing that CTD induces cell cycle arrest and apoptosis in a variety of human cancers, including pancreatic cancer [29], bladder cancer [16], colorectal cancer [30], and hepatocellular carcinoma [31].
In this study, we demonstrated the antitumor activity of CTD in MG-63 and MNNG/HOS cells. These 2 cell lines were treated with various concentrations of CTD, and the results showed that CTD promoted the apoptosis of MNNG/HOS and MG-63 cells in a concentration- and time-dependent manner. The apoptotic process was achieved by activating the mitochondrial pathway.
The development of malignant tumors is closely related to the imbalance between cell proliferation and apoptosis regulation. Apoptosis plays a negative role in tumor development and can inhibit tumor growth [32]. MTS assay showed that the survival rate of MG-63 and MNNG/HOS cells decreased with the increase of drug concentration after 48 h, suggesting that CTD can promote the apoptosis of osteosarcoma cells, and the concentration was positively correlated. We demonstrated that CTD could induce apoptosis of osteosarcoma MG-63 and MNNG/HOS cells by flow cytometry and Hoechst33258 staining and had a significant dose-effect relationship. It also suggests that CTD may be useful in the treatment of osteosarcoma.
In addition, the expression of apoptosis-related proteins in the mitochondrial pathway after treatment with CTD in human osteosarcoma cells was detected by Western blotting. The anti-apoptotic molecule Bcl-2 and the pro-apoptotic molecule Bax determine the state of cell survival or apoptosis, which is an important regulator of apoptosis in the endogenous mitochondrial pathway. The results showed that the protein level of PARP decreased, the expression of Bcl-2 decreased, and the expression of Bax increased, which reduced the Bcl-2/Bax ratio, leading to the decrease of mitochondrial membrane potential and the release of Cyto-C from the mitochondria into the cytoplasm. Furthermore, in mitochondrial release of Cyto-C to the cytoplasm, caspase enzyme family members are cut and activated, as are PARP, DNA2PK, and other dead substrates, making the apoptosis signal cascade amplify and thus promoting the occurrence of apoptosis [33,34].
Western blotting also showed that the protein level of p-Cdc2 decreased, and MG-63 and MNNG/HOS cells treated with CTD showed G2/M arrest in the cell cycle distribution. In human cells, the cell cycle-dependent protein kinase CDK1 is a key protein that regulates G2/M phase. CDK1 activation is a necessary condition for G2 phase to enter M phase [35]. The 14th threonine and the 15th tyrosine residues on the phosphorylated CDK1 cause CDK1 hyperphosphorylation [36]. The results of our study show that the effect of the G2/M phase arrest was also accompanied by upregulation of phosphorylated CDK1 (Tyr-15) expression, indicating that the effect of CTD in arresting the G2/M phase in human osteosarcoma MNNG/HOS and MG-63 cells is related to the decreased activity due to hyperphosphorylated CDK1.
Moreover, our study has shown that CTD can still induce apoptosis through other molecular pathways as follows. 1) By activation of NF-κB signaling pathway. IKK (IκB kinase) is an upstream kinase of the NF-κB signaling pathway. PP2A can dephosphorylate IKK and cantharidin as a PP2A inhibitor can promote IKK phosphorylation and activate NF-κB pathway. Activation of the NF-κB pathway in some cases can induce apoptosis [37]. 2) By promoting the expression of ROS, and then downregulating Bcl-2 expression, increasing pro-apoptotic Bax expression, and activating the mitochondrial apoptosis pathway [38,39]. 3) By activation of the MAPK and ERK signal transduction pathway [40,41].
Conclusions
In summary, although the role of CTD in tumor cells is not clearly understood, these data indicate that CTD effectively inhibits the growth of human osteosarcoma cells by activating the mitochondrial pathway. Further research on these mechanisms using in vivo and in vitro osteosarcoma models is needed.
Footnotes
Source of support: This research was supported by grants from the National Natural Science Foundation of China (NO. 81472504, NO. 81401822, and 81572177), the Science and Technology Foundation of Zhejiang Province (2016C33151), the Medical Science and Technology Project of Zhejiang Province of China (2016146428), and the Natural Science Funds of Zhejiang Province (LY17H160033, LQ14H060002, and LY15H060003)
Conflict of interest
None.
References
- 1.Kobayashi E, Hornicek FJ, Duan Z. MicroRNA Involvement in Osteosarcoma. Sarcoma. 2012;2012 doi: 10.1155/2012/359739. 359739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampson VB, Yoo S, Kumar A, et al. MicroRNAs and potential targets in osteosarcoma: Review. Front Pediatr. 2015;3:69. doi: 10.3389/fped.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Yang Z, Li Y, et al. Cell apoptosis, autophagy and necroptosis in osteosarcoma treatment. Oncotarget. 2016;7(28):44763–78. doi: 10.18632/oncotarget.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Yao Y, Wang Z, et al. Therapeutic effect of pirarubicin-based chemotherapy for osteosarcoma patients with lung metastasis. J Chemother. 2010;22(2):119–24. doi: 10.1179/joc.2010.22.2.119. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013;25(4):398–406. doi: 10.1097/CCO.0b013e3283622c1b. [DOI] [PubMed] [Google Scholar]
- 7.He A, Yang X, Huang Y, et al. CD133(+) CD44(+) cells mediate in the lung metastasis of osteosarcoma. J Cell Biochem. 2015;116(8):1719–29. doi: 10.1002/jcb.25131. [DOI] [PubMed] [Google Scholar]
- 8.Wu HY, Lin TK, Kuo HM, et al. Phyllanthus urinaria induces apoptosis in human osteosarcoma 143B Cells via activation of Fas/FasL- and mitochondria-mediated pathways. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/925824. 925824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao LJ, Zhou XD, Shen CC, et al. Tetrandrine induces apoptosis and triggers a caspase cascade in U2-OS and MG-63 cells through the intrinsic and extrinsic pathways. Mol Med Rep. 2014;9(1):345–49. doi: 10.3892/mmr.2013.1761. [DOI] [PubMed] [Google Scholar]
- 10.Rossi D, Gaidano G. Messengers of cell death: Apoptotic signaling in health and disease. Haematologica. 2003;88(2):212–18. [PubMed] [Google Scholar]
- 11.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–19. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otera H, Mihara K. Mitochondrial dynamics: functional link with apoptosis. Int J Cell Biol. 2012;2012 doi: 10.1155/2012/821676. 821676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Rafiuddin-Shah M, Tu HC, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8(12):1348–58. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Xie L, Chen Z, et al. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci. 2010;101(5):1226–33. doi: 10.1111/j.1349-7006.2010.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honkanen RE. Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Lett. 1993;330(3):283–86. doi: 10.1016/0014-5793(93)80889-3. [DOI] [PubMed] [Google Scholar]
- 16.Su CC, Liu SH, Lee KI, et al. Cantharidin induces apoptosis through the calcium/PKC-regulated endoplasmic reticulum stress pathway in human bladder cancer cells. Am J Chin Med. 2015;43(3):581–600. doi: 10.1142/S0192415X15500366. [DOI] [PubMed] [Google Scholar]
- 17.Shen M, Wu MY, Chen LP, et al. Cantharidin represses invasion of pancreatic cancer cells through accelerated degradation of MMP2 mRNA. Sci Rep. 2015;5:11836. doi: 10.1038/srep11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, Chen Z, Zhou X, et al. Cantharidin induces G2/M phase arrest and apoptosis in human gastric cancer SGC-7901 and BGC-823 cells. Oncol Lett. 2014;8(6):2721–26. doi: 10.3892/ol.2014.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo JH, Shih TY, Lin JP, et al. Cantharidin induces DNA damage and inhibits DNA repair-associated protein expressions in TSGH8301 human bladder cancer cell. Anticancer Res. 2015;35(2):795–804. [PubMed] [Google Scholar]
- 20.Dorn DC, Kou CA, Png KJ, Moore MA. The effect of cantharidins on leukemic stem cells. Int J Cancer. 2009;124(9):2186–99. doi: 10.1002/ijc.24157. [DOI] [PubMed] [Google Scholar]
- 21.Jin LB, Zhu J, Liang CZ, et al. Paeoniflorin induces G2/M cell cycle arrest and caspase-dependent apoptosis through the upregulation of Bcl-2 X-associated protein and downregulation of B-cell lymphoma 2 in human osteosarcoma cells. Mol Med Rep. 2018;17(4):5095–101. doi: 10.3892/mmr.2018.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ediriweera MK, Tennekoon KH, Samarakoon SR, et al. Induction of apoptosis in MCF-7 breast cancer cells by Sri Lankan endemic mango (Mangifera zeylanica) fruit peel through oxidative stress and analysis of its phytochemical constituents. Journal of Food Biochemistry. 2017;41(1) [Google Scholar]
- 23.Jiang L, Zhao YD, Chen WX. The function of the novel mechanical activated ion channel piezo1 in the human osteosarcoma cells. Med Sci Monit. 2017;23:5070–82. doi: 10.12659/MSM.906959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tharmarajah L, Samarakoon SR, Ediriweera MK, et al. In vitro anticancer effect of gedunin on human teratocarcinomal (NTERA-2) cancer stem-like cells. Biomed Res Int. 2017;2017 doi: 10.1155/2017/2413197. 2413197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ediriweera MK, Tennekoon KH, Samarakoon SR, et al. A study of the potential anticancer activity of Mangifera zeylanica bark: Evaluation of cytotoxic and apoptotic effects of the hexane extract and bioassay-guided fractionation to identify phytochemical constituents. Oncol Lett. 2016;11(2):1335–44. doi: 10.3892/ol.2016.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khayat D, Antoine EC, Coeffic D. Taxol in the management of cancers of the breast and the ovary. Cancer Invest. 2000;18(3):242–60. doi: 10.3109/07357900009031828. [DOI] [PubMed] [Google Scholar]
- 27.Asou N. 2. All-trans retinoic acid in the treatment of acute promyelocytic leukemia. Intern Med. 2007;46(2):91–93. doi: 10.2169/internalmedicine.46.1780. [DOI] [PubMed] [Google Scholar]
- 28.Li CY, Wang EQ, Cheng Y, Bao JK. Oridonin: An active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43(5):701–4. doi: 10.1016/j.biocel.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Wu MY, Xie X, Xu ZK, et al. PP2A inhibitors suppress migration and growth of PANC-1 pancreatic cancer cells through inhibition on the Wnt/beta-catenin pathway by phosphorylation and degradation of beta-catenin. Oncol Rep. 2014;32(2):513–22. doi: 10.3892/or.2014.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B, Gao HC, Xu JW, et al. Apoptosis of colorectal cancer UTC116 cells induced by Cantharidinate. Asian Pac J Cancer Prev. 2012;13(8):3705–8. doi: 10.7314/apjcp.2012.13.8.3705. [DOI] [PubMed] [Google Scholar]
- 31.Wang CC, Wu CH, Hsieh KJ, et al. Cytotoxic effects of cantharidin on the growth of normal and carcinoma cells. Toxicology. 2000;147(2):77–87. doi: 10.1016/s0300-483x(00)00185-2. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Luna JL. Apoptosis regulators as targets for cancer therapy. Clin Transl Oncol. 2007;9(9):555–62. doi: 10.1007/s12094-007-0103-7. [DOI] [PubMed] [Google Scholar]
- 33.Kolenko VM, Uzzo RG, Bukowski R, Finke JH. Caspase-dependent and -independent death pathways in cancer therapy. Apoptosis. 2000;5(1):17–20. doi: 10.1023/a:1009677307458. [DOI] [PubMed] [Google Scholar]
- 34.Han B, Wang TD, Shen SM, et al. Annonaceous acetogenin mimic AA005 induces cancer cell death via apoptosis inducing factor through a caspase-3-independent mechanism. BMC Cancer. 2015;15:139. doi: 10.1186/s12885-015-1133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obaya AJ, Sedivy JM. Regulation of cyclin-Cdk activity in mammalian cells. Cell Mol Life Sci. 2002;59(1):126–42. doi: 10.1007/s00018-002-8410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79(4):563–71. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 37.Ji BC, Hsiao YP, Tsai CH, et al. Cantharidin impairs cell migration and invasion of A375.S2 human melanoma cells by suppressing MMP-2 and -9 through PI3K/NF-kappaB signaling pathways. Anticancer Res. 2015;35(2):729–38. [PubMed] [Google Scholar]
- 38.Kuo JH, Chu YL, Yang JS, et al. Cantharidin induces apoptosis in human bladder cancer TSGH 8301 cells through mitochondria-dependent signal pathways. Int J Oncol. 2010;37(5):1243–50. doi: 10.3892/ijo_00000775. [DOI] [PubMed] [Google Scholar]
- 39.Hsia TC, Yu CC, Hsu SC, et al. Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. Int J Oncol. 2014;45(1):245–54. doi: 10.3892/ijo.2014.2428. [DOI] [PubMed] [Google Scholar]
- 40.Hsia TC, Yu CC, Hsiao YT, et al. Cantharidin impairs cell migration and invasion of human lung cancer NCI-H460 cells via UPA and MAPK signaling pathways. Anticancer Res. 2016;36(11):5989–97. doi: 10.21873/anticanres.11187. [DOI] [PubMed] [Google Scholar]
- 41.Gu XD, Xu LL, Zhao H, et al. Cantharidin suppressed breast cancer MDA-MB-231 cell growth and migration by inhibiting MAPK signaling pathway. Braz J Med Biol Res. 2017;50(7):e5920. doi: 10.1590/1414-431X20175920. [DOI] [PMC free article] [PubMed] [Google Scholar]