Abstract
Patient: Male, 53
Final Diagnosis: Clivus chordoma
Symptoms: Pain the eye
Medication: —
Clinical Procedure: —
Specialty: Radiology
Objective:
Rare disease
Background:
The clivus is a depression in the anterior occipital bone of the skull base, posterior to the dorsum sellae, at the junction with the sphenoid bone. Chordoma is a rare tumor arising from embryonic remnants of the notochord and can be locally aggressive with a tendency to recur. The optimal management of this rare tumor remains controversial. A report of a case of recurrent chordoma of the clivus is presented to illustrate the value of volumetric three-dimensional (3-D) reconstruction with computed tomography (CT) and magnetic resonance imaging (MRI) to determine optimal surgical management.
Case Report:
A 53-year-old man presented with pain in the right orbital cavity, right proptosis, swelling of the right cheek, and bilateral loss of vision. He also had adrenal insufficiency. CT and contrast-enhanced (gadolinium) T1-weighted MRI with multiplanar acquisition were performed with volumetric 3-D reconstruction of the tumor, to increase the chances of treatment success. Surgical resection was performed to remove the tumor and reduce the risk of recurrence. Histology of the tumor was consistent with chordoma, supported by positive immunohistochemical staining for S-100 and epithelial membrane antigen (EMA).
Conclusions:
This report highlighted the value of 3-D volume imaging in the diagnosis and treatment planning in a rare case of recurrent chordoma of the clivus. Analysis of tumor volume may be an indicator of the efficacy of surgery, complementing the Response Evaluation Criteria In Solid Tumors (RECIST) system and as a valuable tool to predict treatment outcome.
MeSH Keywords: Head and Neck Neoplasms; Imaging, Three-Dimensional; Magnetic Resonance Imaging
Background
The clivus is a depression in the anterior basilar occipital bone of the skull base, posterior to the dorsum sellae, at the junction with the sphenoid bone. Chordomas are rare malignant tumors, locally aggressive, arising from embryonic remnants of the primitive notochord, around which vertebral column and skull base develop [1]. Chordomas account for 0.2% of intracranial tumors [2] and usually become symptomatic following a slow growth pattern, and is difficult to treat surgically due to its location and propensity to recur [3].
The histopathological features of chordoma can vary from low-grade to high-grade, and the treatment can be challenging not only because of its proximity to critical neurovascular structures but also because of the biology of this tumor [4]. The differential diagnosis of a primary tumor arising from the clivus includes chordoma, chondrosarcoma, plasmacytoma, and clival metastases [5]. Chordoma can be a slow-growing and infiltrative tumor with a locally destructive growth pattern and a high-risk of local recurrence [3]. The treatment of chordoma arising in the clivus is complicated because of its deep location and invasive nature, including the difficulty in achieving complete surgical resection [3]. Imaging is performed for the diagnosis of the tumor and also for the selection of the optimal surgical approach. Computed tomography (CT) and magnetic resonance imaging (MRI) can be used to identify a chordoma of the clivus, based on its midline location and destruction of the clivus and adjacent bone and soft tissue, with CT and MRI routinely used for preoperative planning [6].
Currently, available imaging software allows 3-D anatomical reconstruction of the tumor and adjacent structures, based on 3-D rendering software and anatomical modeling and spatial information of the target structure [7,8]. Volumetric anatomical modeling of deeply located tumors can help to determine the surgical approach and parameters to be used to ensure a safer treatment and decrease the chances of tumor recurrence [9–11].
Previously published clinical studies have supported that the management of clival chordoma is problematic and the optimal treatment strategy involves removal of the tumor mass as extensively as possible, including removal of the connective tissue and bone surrounding the tumor [12]. Because chordoma can be highly infiltrative, it is essential to identify the dimensions and all margins of the tumor, so that the treatment can be as accurate and effective as possible, and with the least morbidity.
Chordoma arising in the clivus and involving the maxillary sinus is rare and challenging to treat, and the main complication following surgery is local tumor recurrence [13]. In the field of head and neck surgery, this case report and the approach used for pre-operative assessment of the tumor deserves special attention. The patient in this report presented with recurrent chordoma of the clivus, involving the right maxillary sinus with displacement of the right eye in a patient who had been previously treated unsuccessfully, multiple times by several methods, including surgery and radiotherapy. The volume analysis of the tumor, as well as the assessment of the shape and extent of the tumor, highlighted the degree of surrounding bone destruction.
The aim of this case report is to highlight the importance of the use of volume analysis of a recurrent chordoma of the clivus involving the right maxillary sinus using 3-D reconstruction with medical software. The recurrence of chordoma of the clivus involving the maxillary sinus is extremely rare, and its surgical management is complex with regard to the most appropriate surgical treatment approach to ensure a reduced rate of recurrence. Studies are ongoing in our center to evaluate the feasibility of 3-D volume imaging in patient follow-up, to study the progress of patients following surgery.
Case Report
A 53-year-old man, with no history of alcoholism or smoking, but with a previous history of hypothyroidism, and recurrent intracranial chordoma, presented with pain in the right orbital cavity, right proptosis, swelling of the right cheek, and bilateral loss of vision to the Department of Otolaryngology at the Clinics Hospital of the University of Campinas (UNICAMP) in February 2016. He also had adrenal insufficiency. His right eyeball was displaced from its original location, and he had become blind in both eyes. Computed tomography (CT) and magnetic resonance imaging (MRI) scans were performed.
Computed tomography (CT) data acquisition
A head CT scan was performed with a 64-detector row CT scanner (Aquillion 64) (Toshiba Medical Systems, Tochigi, Japan). The imaging slice thickness and reconstruction interval were both 3 mm, and the field-of-view (FOV) was 320 mm. The CT scan was acquired after injection of contrast agent (iodinated contrast media) to improve visualization of the tumor on imaging. The CT scanner operated at 120 kV and 400 mA.
Magnetic resonance imaging (MRI) data acquisition
MRI acquisition was performed with a 3-T Philips Achieva system according to the following parameters: multiplanar acquisition with T1-weighted and T2-weighted sequences, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI) before and after intravenous injection of gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA).
The field-of-view (FOV) was 240 mm, the voxel size was 0.68 mm, and the slice thickness was 5 mm. The recon-all voxel size was 0.29 mm, and the reconstruction matrix was 64×64 points.
CT and MRI analysis
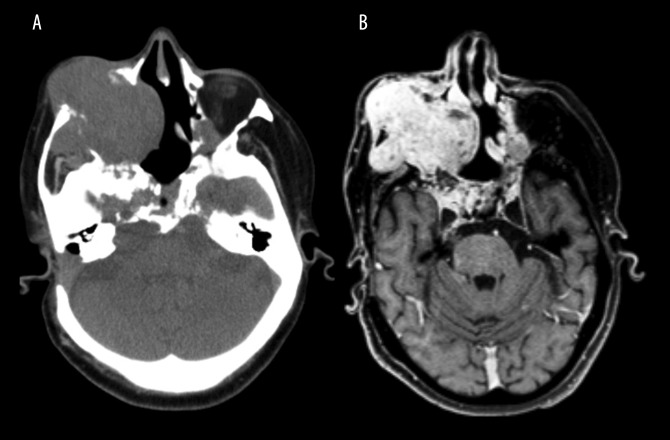
Images were reviewed by a radiologist with dentomaxillofacial, CT, and MRI experience. The CT and MRI scans confirmed the presence of an expansive lesion in the area corresponding to the right maxillary sinus. The tumor extended into the right maxilla, right maxillary sinus and soft tissues around the zygomatic area, with signs of infiltration into the right orbit, which explained the proptosis of the right eyeball. The tumor also infiltrated into the masticator space (Figure 1A). The imaging of the tumor was enhanced by the use of contrast material and was shown to be centered at the clivus, with a cystic and heterogeneous appearance on imaging, measuring 9.7×6.6×8.4 mm.
Figure 1.
Computed tomography (CT) and contrast-enhanced T1-weighted magnetic resonance imaging (MRI) shows an infiltrative and expansive lesion arising in the clivus. (A) Axial slice of a computed tomography (CT) scan showing an expansive, infiltrative, lobulated, and heterogeneous lesion. The lesion is centered on the clivus and extends to the right maxilla, right maxillary sinus, and the soft tissues of the zygomatic region. The clivus is a depression in the anterior basilar occipital bone of the skull base, posterior to the dorsum sellae, at the junction with the sphenoid bone. (B) Axial slice of a contrast-enhanced (gadolinium), T1-weighted magnetic resonance imaging (MRI) with multiplanar acquisition shows an infiltrative and expansive lesion with cystic areas and adjacent calcified material. Signs of previous surgical treatment are present.
Axial contrast-enhanced T1-weighted MRI showed a high signal intensity lesion with marked contrast enhancement (Figure 1B). There was evidence of previous surgery, with imaging showing turbinectomy with previous surgery involving the nasal septum, right medial nasal conchae, mastoidectomy, and thickening of the intracranial dura mater (pachymeningitis). Gliosis and hemosiderin deposition were found in the inferior frontal gyrus, which was more pronounced on the right.
Past medical and surgical history
The patient had a previous history of chordoma of the clivus treated with radiotherapy in 2005 and with surgery in 2013. The previous radiotherapy treatment consisted of 61.2 Gy of radiation fractionated in 34 sessions (1.8 Gy per session). All anatomical structures where the tumor was present were irradiated with a safety margin. The previous surgical procedures were performed with a combination of approaches, which included an open approach and an endoscopic technique.
Surgical procedure on the current hospital admission
Following clinical examination and imaging, a biopsy was performed under local anesthesia. The morphological findings and immunohistochemical profile were compatible with recurrent clivus chordoma in the right maxillary sinus. The patient was prepared and submitted for surgery involving orbital exenteration, maxillectomy, and temporal rotation flap. The surgical procedure was performed in February 2016, and the patient progressed well in the postoperative period. The patient was also referred to the Dentistry Division of the UNICAMP Clinic Hospital for the manufacture of a palatal prosthesis. During the follow-up appointment, this case was selected for 3-D imaging analysis using InVesalius software.
Histopathology and immunohistochemistry of the chordoma
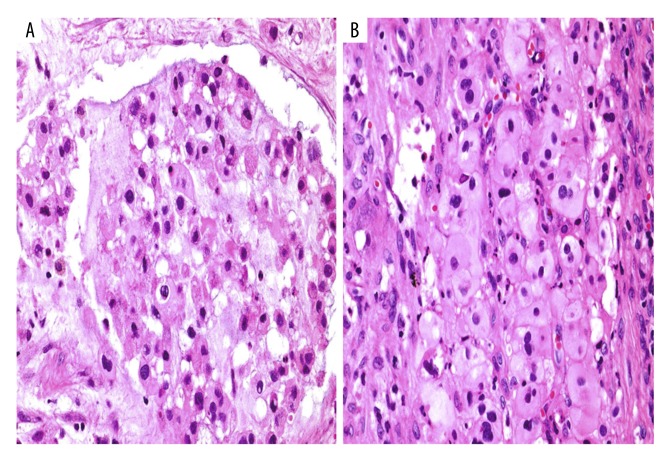
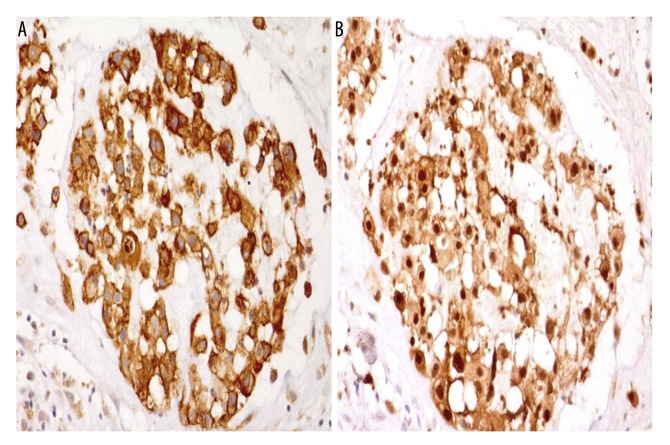
Histology showed the presence of tumor cells with cytoplasmic vacuoles in a basophilic stroma (Figure 2A) and strands of epithelioid cells with eosinophilic cytoplasm (Figure 2B). Tumor cells expressed epithelial membrane antigen (EMA) (membranous and cytoplasmic positivity) and S-100 protein (nuclear and cytoplasmic positivity) (Figures 3A, 3B).
Figure 2.
Photomicrographs of the histopathology of the tumor, consistent with a diagnosis of chordoma. (A) Photomicrograph of the light microscopy of the routinely prepared tissue section. A moderately cellular tumor with a lobulated architecture and basophilic myxoid stroma consists of regularly shaped cells with some cytoplasmic vacuoles, consistent with chordoma. Hematoxylin and eosin (H&E). Magnification, ×40. (B) Photomicrograph of the light microscopy of the routinely prepared tissue section. A moderately cellular tumor includes strands of epithelioid cells with eosinophilic cytoplasm, consistent with chordoma. H&E. Magnification, ×40.
Figure 3.
Photomicrographs of the immunohistochemistry (IHC) of the tumor showing positive immunostaining for epithelial membrane antigen (EMA) and S-100, consistent with a diagnosis of chordoma. (A) Photomicrograph of the immunohistochemical localization (brown) of epithelial membrane antigen (EMA) shows cell membrane and cytoplasmic positivity. Magnification, ×40. (B) Photomicrograph of the immunohistochemical localization (brown) of S-100 protein shows nuclear and cytoplasmic positivity. Magnification, ×40.
Data analysis: 3-D volumetric reconstruction
Two oral dentomaxillofacial radiologists determined the threshold level and opacity curve for tumor segmentation and surrounding structures (Figure 4), using the open-source software InVesalius version 3.0 (Technology Information Center, Campinas, Brazil) (www.cti.gov.br/invesalius). Once the relevant structures were isolated, 3-D volume reconstruction analysis was performed.
Figure 4.
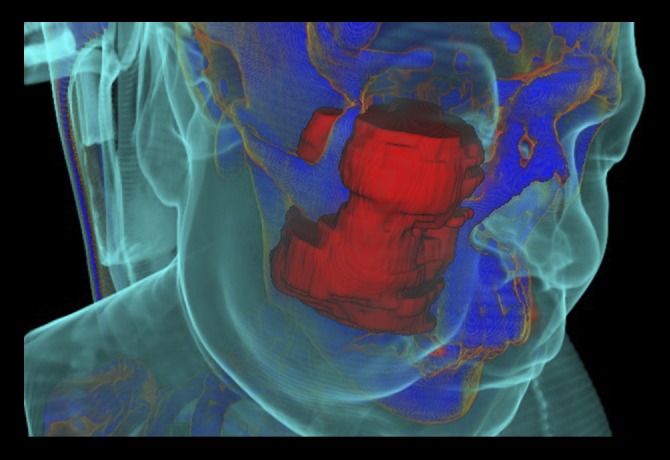
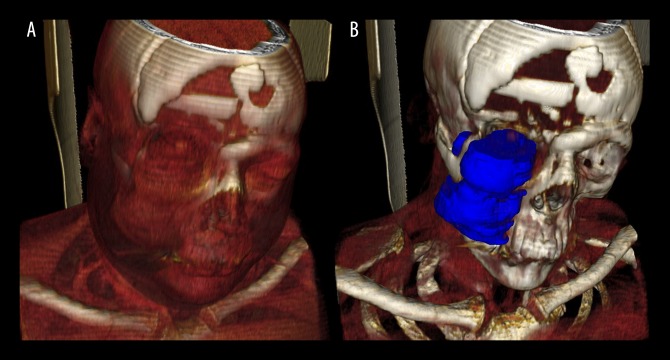
InVesalius three-dimensional (3-D) software filters applied to the computed tomography (CT) scan shows the extent of local invasion of the chordoma arising from the clivus. Soft tissues (light blue), in contrast to the tumor (red) and bone structure (dark blue), allows visualization of the swollen area of the cheek caused by the invasion of the tumor into the right zygomatic arch, maxilla, maxillary sinus, and masticator space.
The 3-D model could be rotated and its different components removed or made transparent so that any structure at any angle could be examined, with its anatomical associations, to determine distances from vital structures and analyze topography. Also, the interaction between the tumor and bone structure could also be visualized. The 3-D volume reconstruction of the tumor allowed a series of characteristics to be highlighted.
It was also possible to confirm that the tumor invaded ante-riorly into the right maxilla, right maxillary sinus, and superficial soft tissues of the zygomatic area. Laterally, the tumor invaded the masticator space, including the masseter muscle and temporal muscle, as well as the zygomatic arch. Another benefit of the 3-D model and volumetric analysis, as well as the proper application of the software filters, was the differentiation and separation of tissues in different colors on the images. For example, the swollen area of the patient’s cheek could be highlighted, which was caused by the expansion of the tumor (Figure 4). Anteriorly, invasion of the inferior portion of the right orbital cavity was also demonstrated, which explained the clinical sign of proptosis of the right eyeball. The alteration in the contrast levels of the images allowed for investigation of the effects of the tumor on soft tissues, such as the muscles (Figure 5A), as well as demonstrating the anatomical topography of the tumor (Figure 5B). Posteriorly, there were signs of involvement and erosion of the clivus, orbital apices, pterygopalatine fossa and bilateral cavernous sinus. Medially, the lesion was found to involve the right nasal fossa and nasal bone, and close contact of the tumor with the nasal septum was also seen, as were the signs of previous surgical treatment (frontal craniotomy). Imaging confirmation of the tumor’s extent and analysis of the tumor volume can be used to increase the accuracy and safety of the surgical management.
Figure 5.
Three-dimensional (3-D) skeletal reconstruction showing local invasion of the chordoma into the right maxillary sinus and the right orbit. (A) Three-dimensional (3-D) skeletal reconstruction highlights the soft tissues. There is a tumor in the region of the right maxillary sinus and close contact of the tumor with the nasal bone. (B) 3-D skeletal reconstruction highlights the tumor topography (dark blue), and shows infiltration of the tumor into the lower portion of the right orbit, which resulted clinically in proptosis of the right eyeball.
Discussion
There remains controversy regarding the most accurate and effective management of chordoma arising from the region of the clivus. Some clinicians have advised that surgical resection is the definitive treatment modality for clival chordoma, whereas others have advocated the use of radiotherapy, either as the primary therapy or as an adjuvant therapy [14]. Gamma knife radiosurgery and proton therapy have also been reported for the management of cases of recurrence [15,16].
However, it has also been shown that the role of surgery and adjuvant radiation therapy for chordomas of the base of the cranium is not well established [17]. The debate on the optimal management has continued for several decades, with the acknowledgment that attempts at radical removal of the tumor might lead to significant surgical morbidity. Skull base chordomas require a broad surgical approach strategy based on their location and surgeon´s preference. Endoscopic techniques, along with trans-sphenoidal, transmaxillary, transnasal, high anterior cervical, retropharyngeal, and trans-oral approaches, have been well documented in the literature [18].
Until recently, imaging-based diagnosis of clival chordoma has relied on computed tomography (CT) and magnetic resonance imaging (MRI) as the ‘gold standard’ to evaluate the degree of bone involvement [1], to identify patterns of calcification within the lesion, and to differentiate chordomas from other focal lesions pre-operatively [5]. Although the Response Evaluation Criteria in Solid Tumors (RECIST) remains the current standard for assessing therapy response in solid malignant tumors, volumetric assessment has been reported to provide more representative data of actual tumor size, and as clival chordomas can mimic other lesions, presenting with similar symptoms and imaging features, 3-D volumetric assessment is gaining support in clinical diagnosis and pre-operative patient assessment [19,20]. A previously published study has demonstrated that a non-mid-line growth pattern can help to differentiate between chordoma and chondrosarcoma on MRI in a majority of patients [19].
Patients with complete resection of chordomas arising from the base of the cranium have a prolonged 5-year progression-free survival (PFS) and overall survival (OS) [17], and so it is essential that imaging findings be as clear as possible regarding the tumor location and morphology, since these images will serve to guide surgery. Patient survival and local control are associated with the ability to achieve wide surgical margins during excision. However, surgical morbidity may be significant due to the propensity for this tumor to surround neural, vascular, and visceral structures [21].
Early diagnosis of chordoma and the involvement of a multi-disciplinary team are essential for optimal management [21], and the use of the 3-D imaging model followed by volumetric analysis may be an important approach to improve the pre-operative evaluation of the tumor and improve postoperative patient survival [22]. Also, past cases where the treatment consisted exclusively of radiotherapy, we consider that the calculation of the tumor volume can serve as an auxiliary tool to determine treatment efficacy. The use of 3-D reconstruction and volumetric assessment are particularly important in the evaluation of intracranial chordoma as the margins of this tumor are recognized to be difficult to assess [20]. Because of these challenges in the imaging of chordoma, the segmentation process of this case was performed in duplicate to ensure that the 3-D volume data was accurate.
A follow-up CT scan performed in February 2018 showed that in this patient, the chordoma had progressed even after all the previous treatments, including the surgical resections. At present, at 26 months following the most recent hospital admission and the latest surgery, the patient is alive, but with a poor prognosis.
Conclusions
Considering that survival rate and progression-free survival of patients affected by clivus chordoma depend on a surgical approach that aims to keep the surgical resection margins free of tumor cells, it is essential to use both computed tomography (CT) and magnetic resonance imaging (MRI). This case report has highlighted the value of 3-D volume imaging in the diagnosis and treatment planning in a rare case of chordoma of the clivus. Analysis of tumor volume may be an indicator of the efficacy of surgery, complementing the Response Evaluation Criteria In Solid Tumors (RECIST) system and serving as a valuable tool to predict treatment outcomes.
Footnotes
Conflict of interests
None.
References:
- 1.Ahmed SK, Murata H, Draf W. Clivus Chordoma: Is it enough to image the primary site? Skull Base. 2010;20:111–13. doi: 10.1055/s-0029-1225533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowery RD. A case report: Maxillotomy for removal of a clival chordoma. J Neurosci Nurs. 1999;31:303–8. doi: 10.1097/01376517-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Harbour JW, Lawton MT, Criscuolo GR, et al. Clivus chordoma: A report of 12 recent cases and review of the literature. Skull Base Surg. 1991;1:200–6. doi: 10.1055/s-2008-1057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell RG, Prevedello DM, Ditzel FL, et al. Contemporary management of clival chordomas. Curr Opin Otolaryngol Head Neck Surg. 2015;23:153–61. doi: 10.1097/MOO.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 5.DeConde AS, Sanaiha Y, Suh JD, et al. Metastatic disease to the clivus mimicking clival chordomas. J Neuro Surg B Skull Base. 2013;74:292–99. doi: 10.1055/s-0033-1348027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashim H, Rosman AK, Abdul Aziz A, et al. Atypical clival chordoma in an adolescent without imaging evidence of bone involvement. Malays J Med Sci. 2014;21:78–82. [PMC free article] [PubMed] [Google Scholar]
- 7.Nascimento Falcão I, Cal Alonso MBC, da Silva LH, et al. 3-D morphology analysis of TMJ articular eminence in magnetic resonance imaging. Int J Dent. 2017;2017:5130241. doi: 10.1155/2017/5130241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira JM, Alonso MB, de Souza E, Tucunduva MJ, et al. Volumetric study of sphenoid sinuses: Anatomical analysis in helical computed tomography. Surg Radiol Anat. 2017;39:367–74. doi: 10.1007/s00276-016-1743-5. [DOI] [PubMed] [Google Scholar]
- 9.Gomes JPP, Costa ALF, Chone CT, et al. Three-dimensional volumetric analysis of ghost cell odontogenic carcinoma using 3-D reconstruction software: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:170–75. doi: 10.1016/j.oooo.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Eley KA, Watt-Smith SR, Golding SJ. Magnetic resonance imaging-based tumor volume measurements predict outcome in patients with squamous cell carcinoma of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:255–62. doi: 10.1016/j.oooo.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Eley KA, Watt-Smith SR, Boland P, et al. MRI pre-treatment tumor volume in maxillary complex squamous cell carcinoma treated with surgical resection. J Maxillofac Surg. 2014;42:119–24. doi: 10.1016/j.jcms.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Tamaki M, Aoyagi M, Kuroiwa T, et al. Clinical course and autopsy findings of a patient with clival chordoma who underwent multiple surgeries and radiation during a 10-year period. Skull Base. 2007;17:331–40. doi: 10.1055/s-2007-986438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen RP, Salzman KL, Stambuk HE, et al. Extraosseous chordoma of the nasopharynx. Am J Neuroradiol. 2009;30:803–7. doi: 10.3174/ajnr.A1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maira G, Pallini N, Anile C, et al. Surgical treatment of clival chordomas: The transsphenoidal approach revisited. J Neurosurg. 1996;85:784–92. doi: 10.3171/jns.1996.85.5.0784. [DOI] [PubMed] [Google Scholar]
- 15.Ito E, Saito K, Okada T, et al. Long-term control of clival chordoma with initial aggressive surgical resection and gamma knife radiosurgery for recurrence. Acta Neurochir. 2010;152:57–67. doi: 10.1007/s00701-009-0535-7. [DOI] [PubMed] [Google Scholar]
- 16.Deraniyagala RL, Yeung D, Mendenhall WM, et al. Proton therapy for skull base chordomas: An outcome study from the University of Florida Proton Therapy Institute. J Neurol Surg B Skull Base. 2014;75:53–57. doi: 10.1055/s-0033-1354579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Maio S, Temkin N, Ramanathan D, Sekhar LN. Current comprehensive management of cranial base chordomas: 10-years meta-analysis of observational studies. J Neurosurg. 2011;115:1094–105. doi: 10.3171/2011.7.JNS11355. [DOI] [PubMed] [Google Scholar]
- 18.Walcott BP, Nahed BV, Mohyelding A, et al. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012;13:69–76. doi: 10.1016/S1470-2045(11)70337-0. [DOI] [PubMed] [Google Scholar]
- 19.Müller U, Kubik-Huch RA, Ares C, et al. Is there a role for conventional MRI and MR diffusion-weighted imaging for distinction of skull base chordoma and chondrosarcoma? Acta Radiol Online. 2015;57:225–32. doi: 10.1177/0284185115574156. [DOI] [PubMed] [Google Scholar]
- 20.Fenerty KE, Patronas NJ, Heery CR, et al. Resources required for semi-automatic volumetric measurements in metastatic chordoma: Is potentially improved tumor burden assessment worth the time burden? J Digit Imaging. 2016;29:357–64. doi: 10.1007/s10278-015-9846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sciubba DM, Cheng JJ, Petteys RJ, et al. Chordoma of the sacrum and vertebral bodies. J Am Acad Orthop Surg. 2009;17:708–17. doi: 10.5435/00124635-200911000-00005. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MW, Gutin PH. Image-guided surgery for skull base neoplasms using the ISG viewing wand. Anatomic and technical considerations. Neurosurg Clin N Am. 1996;7:285–95. [PubMed] [Google Scholar]