Abstract
Background
This study aimed to investigate the effect of deferoxamine (DFO) on leukemia in vitro, and to explore the underlying molecular mechanism.
Material/Methods
K562 leukemia cells were treated with various concentrations of DFO (10, 50, and 100 μmol/l) with or without 10 μmol/l ferric chloride for 12 h. Then, total cellular iron was detected. CCK-8 kit and flow cytometry were used for cell viability and apoptosis detection. In addition, expression of apoptosis-related genes was determined by Western blotting and qRT-PCR, respectively.
Results
The results suggested that DFO significantly inhibited K562 cell viability and induced cell apoptosis in a dose-dependent manner. We also found that the protein and mRNA levels of Bax, p53, and Fas dose-dependently increased in DFO-treated K562 cells, while the level of Bcl-2 markedly decreased in a dose-dependent manner. Moreover, the findings showed that ferric chloride eliminated these effects on K562 cells caused by DFO treatment.
Conclusions
Our results indicate that DFO plays a protective role in leukemia via inhibiting leukemia cell viability and inducing cell apoptosis by the regulation of apoptosis-related genes expression.
MeSH Keywords: Apoptosis; Cell Proliferation; Iron Chelating Agents; Leukemia, Myeloid, Acute
Background
Iron, an indispensable element of life, participates in a variety of cell cycle regulatory proteins and apoptosis-related gene expression, and plays an important role in tumor cell growth and differentiation. Iron chelators can affect the total cellular iron content and is mainly used in the clinical treatment of iron overload diseases. In recent years, it has gradually attracted researcher attention due to its anti-solid tumor and anti-liquid tumor effects, such as in neuroblastoma, liver cancer, breast cancer, ovarian cancer, and leukemia. Experiments [1–3] have confirmed that iron deprivation can inhibit the proliferation of a variety of tumor cells and induce apoptosis. Use of iron chelators is expected to become a new strategy for the treatment of cancer. Recent studies have shown that the iron chelator Triapine can inhibit ribonucleotide reductase activity and tumor growth in vivo and in vitro and has entered Phase I and Phase II clinical trials [4]. In addition, some other iron chelators also have potential anti-tumor activity, such as deferasirox [5], DpC [6], Dp44mT [6,7], aurintricarboxylic acid (ATA), and deferoxamine DFO [8]. DFO is the most widely used iron chelator for the treatment of iron overload diseases, and its anti-tumor effect has attracted more and more attention. A large number of in vitro and clinical studies have shown that DFO can inhibit the growth of tumor cells by chelating intracellular iron and has a clear anti-tumor cell proliferation effect [9–15].
Abnormal proliferation and apoptosis of cells are closely related to the occurrence of tumors. Apoptosis is one of the important mechanisms of anti-tumor therapy. Studies have shown that iron chelators can induce leukemia cell apoptosis and may be involved in a variety of apoptosis-related signal transduction pathways, but the mechanism of the induction of apoptosis is not entirely clear [16–19]. Iron chelators can inhibit the proliferation of tumor cells and induce cell apoptosis; thus, it has great clinical value in the treatment of leukemia. Therefore, iron chelators as a new class of anti-tumor agents are of great interest to scholars, but its anti-tumor mechanisms have not been fully elucidated.
The present study aimed to investigate the effect and underlying molecular mechanism of DFO on leukemia in vitro to provide a better theoretical basis for the treatment of leukemia.
Material and Methods
Cell culture and treatment
The K562 leukemia cell line was obtained from the American Type Culture Collection (ATCC). K562 cells were incubated in RPMI 1640 medium (Gibco) containing 10% fetal bovine serum, and 1% penicillin-streptomycin solution (Sigma) at 37°C with 5% CO2. Cells were passaged every 2–3 days.
K562 cells (3×l04 cells/well) were treated with various concentrations of DFO (10, 50, and 100 μmol/l) with or without 10 μmol/l ferric chloride for 12 h. Cells treated with saline instead of DFO served as the control group.
Cellular total iron detection
After specific treatment, the cellular total iron level was assessed. K562 cells (5×106) were collected and then re-suspended in HBS (210 mmol/l HEPES and 150 mmol/l NaCl). Subsequently, the cells were added to an acid mixture (3 mol/l hydrogen chloride, 10% trichloroacetic acid, and 3% benzenesulfonic acid) and incubated at 37°C for 2 h. After centrifugation at 3000 rpm for 30 min, the supernatant was collected, then we added a color-substrate solution (0. 045% sodium acetate and 0.2% methylene). At the end of the experiment, absorbance was measured at 546 nm.
CCK-8 assay
K562 cells were seeded into a 96-well plate (2×105 cells/well) and cultured in RPMI 1640 medium at 37°C for 24 h. Then, the cells were treated with various concentrations of DFO (10, 50, and 100 μmol/l) with or without 10 μmol/l Fecl3 for 12 h. Cells treated with saline instead of DFO served as the control group. Finally, cell viability was detected using a CCK-8 kit according to the manufacturer’s instructions. The experiment was performed at least 3 times.
Cell apoptosis detection
After specific treatment, log-phase K562 cells were collected and washed with cold PBS at least 3 times. K562 cell apoptosis was measured by cell apoptosis assay. In brief, K562 cells (1×106) from groups were first re-suspended in binding buffer and then labeled with annexin V-FITC and propidium iodide (PI) (BD Pharmingen, San Diego, CA, USA) according to the manufacturer’s instructions. Flow cytometry (BD FACS Aria; BD Biosciences, Franklin Lakes, NJ, USA) was applied to analyze cell apoptosis. The experiment was performed at least 3 times.
Western blot analysis
After specific treatment, total cellular proteins from K562 cells were extracted by using RIPA Buffer (Auragene, Changsha, China). A BCA protein quantitative kit (Thermo, USA) was used to measure the concentration of protein samples. Equal amounts of protein samples were resolved by 12% SDS-PAGE and then transferred onto PVDF membranes. The membranes were blocked with 5% non-fat milk at room temperature for 1 h, followed by incubation with primary antibodies (anti-GAPDH; anti-Bax; anti-Bcl-2; anti-Fas; anti-p53; dilution for all, 1: 1000; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. Subsequently, membranes were incubated with an HRP-conjugated secondary antibody (Anti-rabbit IgG, HRP-linked antibody; 1: 5000) at room temperature for 2 h. To visualize the protein blots, an ECL kit (Applygen, Beijing, China) was used according to the manufacturer’s protocol.
QRT-PCR
TRIzol reagent (Takara, Japan) was used to extract total RNA from the K562 cells. GAPDH was used as an internal control. cDNAs were generated using the PrimeScript™ RT reagent kit (Takara, Japan) in line with the manufacturer’s instructions. SYBR Premix Ex Taq (Takara, Japan) was used to analyze the synthesized cDNAs according to the manufacturer’s instructions. Primer sequences used for real-time PCR were:
Bax: Forward: 5′CCTCAGGATGCGTCCACCAAGA3′;
Reverse: 3′TGTGTCCACGGCGGCAATCA5′.
Bcl-2: Forward: 5′GAGACCGAAGTCCGCAGAACCT3′;
Reverse: 3′GGAGACCACACTGCCCTGTTGA5′.
P53: Forward: 5′GGCTCTGACTGTACCACCATCC3′;
Reverse: 3′GGCACAAACACGCACCTCAAAG5′.
Fas: Forward: 5′GCTTCCTTCCCATCCTCCTGAC3′;
Reverse: 3′ACTCGTAAACCGCTTCCCTCAC5′.
GAPDH: Forward: 5′AGATCATCAGCAATGCCTCCT3′
Reverse: 3′TGAGTCCTTCCACGATACCAA5′.
Statistical analysis
All data are displayed as the mean ±SD. SPSS 17.0 statistical software (SPSS, Chicago, IL, USA) was used for statistical analyses. Comparisons between groups were performed using the t test or ANOVA. p<0.05 was considered as statistically significant.
Results
Effect of iron chelators on proliferation of K562 cells
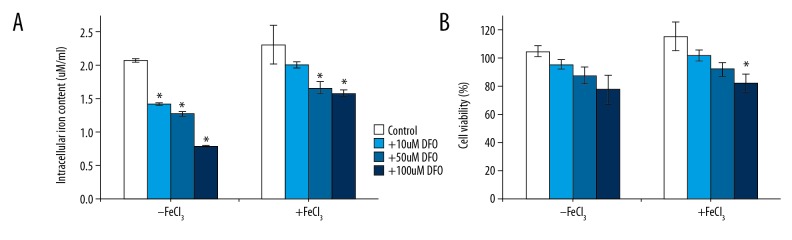
In this study we used DFO as the iron chelator. K562 cells (3×l04 cells/well) were treated with various concentrations of DFO (10, 50, and 100 μmol/l) with or without 10 μmol/l ferric chloride for 12 h. Cells treated with saline instead of DFO served as the control group. Results showed that cells of the control group had higher iron content, and DFO treatment significantly decreased the iron content in K562 cells in a dose-dependent manner (Figure 1A).
Figure 1.
Effect of iron chelators on proliferation of myeloma cell lines. The cells treated with DFO (0,10, 50, 100 μmol/l) and then with or without FeCl3 for 12 h. Ion content analysis (A). MTT assay was used to detect cell proliferation (B). * P<0.05 vs. the respective control group, t test.
CCK-8 assay was used to detect cell proliferation ability. We found that when treated with DFO, the proliferation ability of K562 cells was inhibited in a dose-dependent manner (Figure 1B). Quenching of the iron chelator prior to cell treatment markedly diminished its antiproliferative effect, thereby demonstrating that iron chelation was in fact responsible for suppression of proliferation of the K562 cell lines.
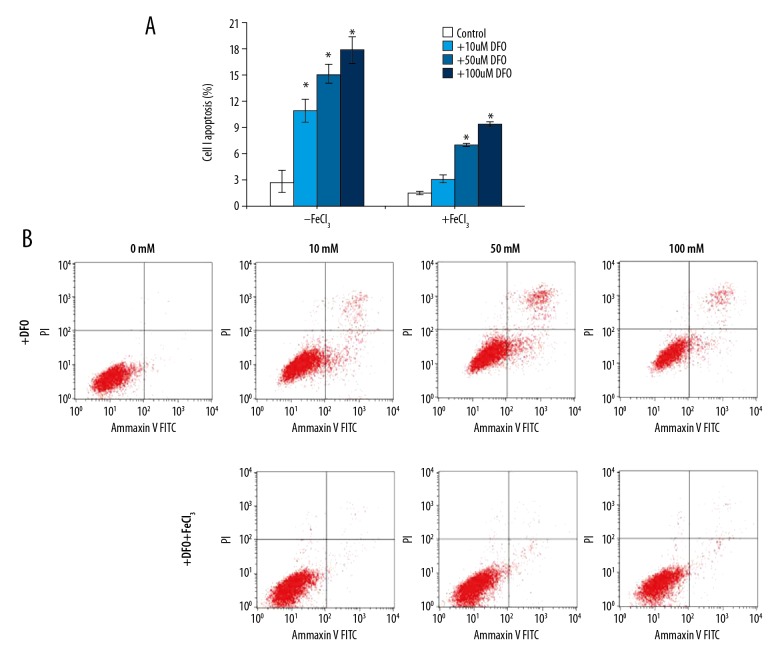
Induction of K562 cell apoptosis by DFO
K562 cells apoptosis was measured by flow cytometry assay (Figure 2B) and cell apoptosis rate was calculated (Figure 2A). The results from flow cytometry assay showed that compared with the control group, DFO dose-dependently increased the apoptosis of K562 cells. These results demonstrated that DFO can increase cell apoptosis.
Figure 2.
Induction of K562 cells apoptosis by DFO. K562 cells viability was assessed using a CCK-8 kit (A). Apoptosis in 562 cells was measured by flow cytometry assay (B). * P<0.05 vs. the respective control group, t test.
Apoptosis-related protein and mRNA expression
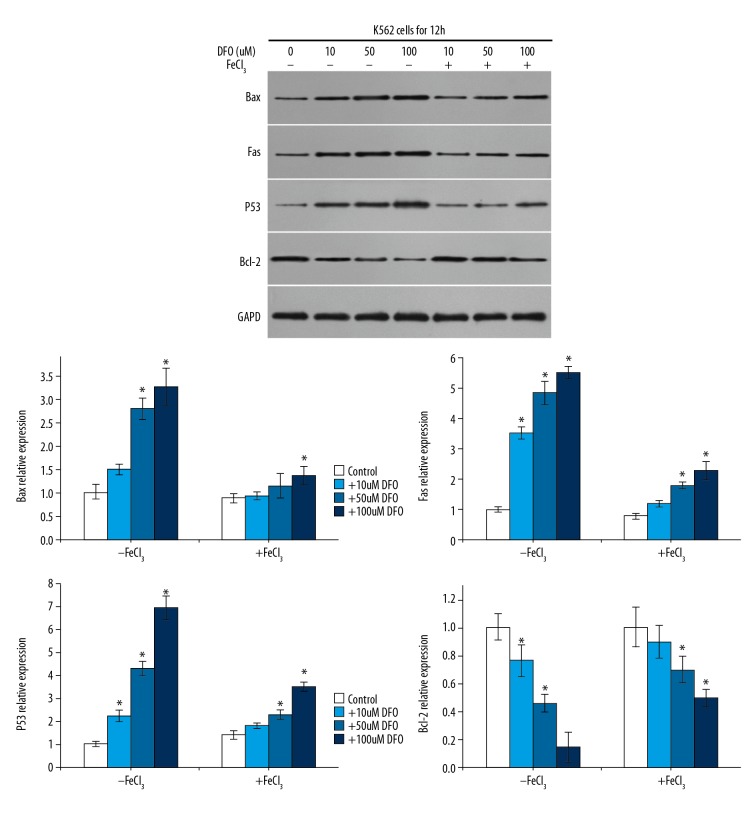
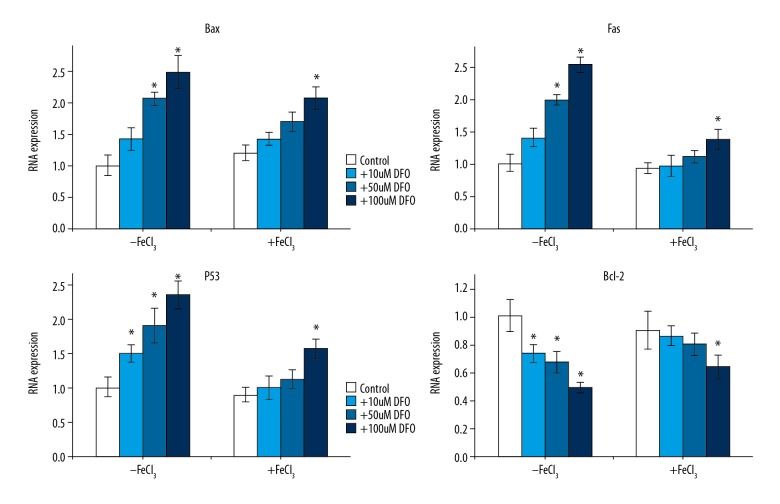
To explore the underlying mechanism of the effect of DFO on K562 cells, we assessed the expression of apoptosis-related genes Bax, p53, and Fas by Western blotting and qRT-PCR, respectively. As expected, we found that the protein (Figure 3) and mRNA (Figure 4) levels of Bax, p53, and Fas were dose-dependently increased in DFO-treated K562 cells, while the level of Bcl-2 markedly decreased in a dose-dependent manner. These results suggest that DFO plays a protective role in leukemia by inhibiting leukemia cell viability and inducing cell apoptosis via regulating apoptosis-related genes expression.
Figure 3.
Apoptosis-related protein expression. Western blot was used to analyze apoptosis-related protein expression. Bax, p53, and Fas dose-dependently increased in CFO-treated K562 cells, while Bcl-2 markedly decreased in a dose-dependent manner.
Figure 4.
Apoptosis-related mRNA expression. qRT-PCR was used to analyze apoptosis-related mRNA expression. Bax, p53, and Fas dose-dependently increased in CFO-treated K562 cells, while Bcl-2 markedly decreased in a dose-dependent manner.
Discussion
The present study investigated the effect of DFO on leukemia in vitro and explored the underlying molecular mechanism. We found that DFO inhibited leukemia cell proliferation ability and induced cell apoptosis through regulating the apoptosis-related genes expression.
Iron is essential for the metabolic processes of many cells, including DNA synthesis, and it is also essential for cancer cell proliferation before DNA synthesis begins [20]. Iron chelators are often used to treat iron overload disease. Recent studies have shown that iron chelators play an antiproliferative role in cancer [21–25]. In recent years, more and more studies have been conducted on the anti-leukemia effect of iron chelators. Chang et al. reported the strong anti-leukemia activity of deferasirox [26]. DFO has been reported to be dramatically affected by T cell acute lymphoblastic leukemia/lymphoma cell survival to induce apoptosis, and it displayed synergistic activity with 3 ALL-specific drugs, including dexamethasone, doxorubicin, and L-asparaginase [27]. DFO is the most widely used iron chelator for the treatment of iron load disease, and it also has some anti-tumor activity. A large number of in vitro and clinical studies have shown that DFO can inhibit the growth of tumor cells by chelating the iron in cells and has a clear anti-tumor cell proliferation effect [9–15]. However, the specific effect and underlying mechanism of DFO in leukemia remain largely unclear. Therefore, we conducted the present study.
Abnormal proliferation and apoptosis of cells are closely related to the occurrence of cancer. Iron chelators can inhibit the proliferation of tumor cells and induce apoptosis of tumor cells, which is of great clinical value in the treatment of leukemia [28,29]. Therefore, iron chelating agents as a class of new anti-tumor drugs are of great concern to scholars, but its anti-tumor mechanism of action has not been fully elucidated. Consistent with previous studies, we found that DFO significantly inhibited leukemia cell proliferation. Apoptosis is an important mechanism of anti-cancer therapy. Our studies have shown that DFO can induce leukemia cell apoptosis and may involve a variety of apoptosis-related signal transduction pathways.
We explored the underlying molecular mechanisms of the effects of DFO on leukemia cells and some cell apoptosis- and proliferation-related genes, including tumor suppressor p53, proto-oncogene Bcl-2, pro-apoptotic protein Bax, and Fas. Bcl-2 is the main gene that regulates apoptosis, and studies have shown that Bcl-2 over-expression can prolong survival, inhibit apoptosis, and reduce the sensitivity of leukemia cells to chemotherapeutic drugs. Mutations p53, an important tumor-suppressor gene, occur in some leukemia patients. A previous study reported that in iron deficiency, p53 gene expression was up-regulated and p21C1P1/WAF1 expression was down-regulated, which in turn induced apoptosis of leukemia cells. The present study found that DFO treatment dose-dependently increased the level of Bax, p53, and Fas in leukemia cells, while the level of Bcl-2 was decreased.
In summary, our results indicate that DFO can inhibit leukemia cell viability and induce cell apoptosis through regulating the expression of apoptosis-related genes, which provides an important basis for the study of leukemia. However, research on the effect of deferoxamine in leukemia is still in the preliminary stage, and it needs much in-depth research and exploration. In addition, there are still many problems to be explored and solved regarding the application of iron chelation therapy in treatment of leukemia. Questions to be answered include: At which stage of the course of the disease (at diagnosis, or at complete remission to prevent relapse) is this strategy planned to be applied? Which types of AMLs is this strategy effective against? What are the adverse effects of chelation treatment in AML patients? When AML patients have low ferritin levels (low iron storages), how can chelation treatment be applied? Why are thalassemic patients who receive DFO regularly as chelation treatment still diagnosed with AML or other malignancies? In the future, we will try to answer these questions and perform an in-depth study of the role of DFO in leukemia progression.
Conclusions
Our results indicate that DFO protects against leukemia by inhibiting leukemia cell viability, inducing cell apoptosis, and regulating expression of apoptosis-related genes, providing an important basis for the treatment of leukemia.
Footnotes
Source of support: This study was supported by the Nanjing Municipal Health Bureau (Grant no: YKK15087)
Conflicts of interests
None.
References
- 1.Buss JL, Torti FM, Torti SV. The role of iron chelation in cancer therapy. Curr Med Chem. 2003;10:1021–34. doi: 10.2174/0929867033457638. [DOI] [PubMed] [Google Scholar]
- 2.Pullarkat V, Meng Z, Donohue C, et al. Iron chelators induce autophagic cell death in multiple myeloma cells. Leuk Res. 2014;38:988–96. doi: 10.1016/j.leukres.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Marques O, da Silva MB, Porto G, Lopes C. Iron homeostasis in breast cancer. Cancer Lett. 2014;347:1–14. doi: 10.1016/j.canlet.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Chitambar CR, Antholine WE. Iron-targeting antitumor activity of gallium compounds and novel insights into Triapine(®)-metal complexes. Antioxid Redox Signal. 2013;18:956–72. doi: 10.1089/ars.2012.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeki I, Yamamoto N, Yamasaki T, et al. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:8967–77. doi: 10.3748/wjg.v22.i40.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu YX, Zeng ML, Yu D, et al. In vitro assessment of the role of DpC in the treatment of head and neck squamous cell carcinoma. Oncol Lett. 2018;15:7999–8004. doi: 10.3892/ol.2018.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JL, Kang HN, Kang MH, et al. The oral iron chelator deferasirox induces apoptosis in myeloid leukemia cells by targeting caspase. Acta Haematol. 2011;126:241–45. doi: 10.1159/000330608. [DOI] [PubMed] [Google Scholar]
- 8.Kuban-Jankowska A, Sahu KK, Gorska-Ponikowska M, et al. Inhibitory activity of iron chelators ATA and DFO on MCF-7 breast cancer cells and phosphatases PTP1B and SHP2. Anticancer Res. 2017;37:4799–806. doi: 10.21873/anticanres.11886. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Cui Y, Shi M. Deferoxamine promotes MDA-MB-231 cell migration and invasion through increased ROS-Dependent HIF-1α accumulation. Cell Physiol Biochem. 2014;33:1036–46. doi: 10.1159/000358674. [DOI] [PubMed] [Google Scholar]
- 10.Bajbouj K, Shafarin J, Hamad M. High-dose deferoxamine treatment disrupts intracellular iron homeostasis, reduces growth, and induces apoptosis in metastatic and nonmetastatic breast cancer cell lines. Technol Cancer Res Treat. 2018;17 doi: 10.1177/1533033818764470. 1533033818764470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olcay L, Hazırolan T, Yıldırmak Y, et al. Biochemical, radiologic, ultrastructural, and genetic evaluation of iron overload in acute leukemia and iron-chelation therapy. J Pediatr Hematol Oncol. 2014;36:281–92. doi: 10.1097/MPH.0b013e3182a11698. [DOI] [PubMed] [Google Scholar]
- 12.Choi JG, Kim JL, Park J, et al. Effects of oral iron chelator deferasirox on human malignant lymphoma cells. Korean J Hematol. 2012;47:194–201. doi: 10.5045/kjh.2012.47.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saletta F, Rahmanto YS, Noulsri E, et al. Iron chelator-mediated alterations in gene expression: identification of novel iron-regulated molecules that are molecular targets of hypoxia-inducible factor-1alpha and p53. Mol Pharmacol. 2010;77:443–58. doi: 10.1124/mol.109.061028. [DOI] [PubMed] [Google Scholar]
- 14.Phiwchai I, Thongtem T, Thongtem S, Pilapong C. Deferoxamine-conjugated AgInS2 nanoparticles as new nanodrug for synergistic therapy for hepatocellular carcinoma. Int J Pharm. 2017;524:30–40. doi: 10.1016/j.ijpharm.2017.03.058. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Shen G, Yin T. In vitro assessment of deferoxamine on mesenchymal stromal cells from tumor and bone marrow. Environ Toxicol Pharmacol. 2017;49:58–64. doi: 10.1016/j.etap.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Jang PS, Chung NG, et al. Deferasirox shows in vitro and in vivo antileukemic effects on murine leukemic cell lines regardless of iron status. Exp Hematol. 2013;41:539–46. doi: 10.1016/j.exphem.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Ohyashiki JH, Kobayashi C, Hamamura R, et al. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 2009;100:970–77. doi: 10.1111/j.1349-7006.2009.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benadiba J, Rosilio C, Nebout M, et al. Iron chelation: An adjuvant therapy to target metabolism, growth and survival of murine PTEN-deficient T lymphoma and human T lymphoblastic leukemia/lymphoma. Leuk Lymphoma. 2017;58:1433–45. doi: 10.1080/10428194.2016.1239257. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Rahmanto YS, Richardson DR. Bp44mT: An orally active iron chelator of the thiosemicarbazone class with potent anti-tumour efficacy. Br J Pharmacol. 2012;165:148–66. doi: 10.1111/j.1476-5381.2011.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–95. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Gutierrez E, Kovacevic Z, et al. Iron chelators for the treatment of cancer. Curr Med Chem. 2012;19:2689–702. doi: 10.2174/092986712800609706. [DOI] [PubMed] [Google Scholar]
- 22.Torti SV, Torti FM. Iron and cancer: More ore to be mined. Nat Rev Cancer. 2013;13:342–55. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamihara Y, Takada K, Sato T, et al. The iron chelator deferasirox induces apoptosis by targeting oncogenic Pyk2/β-catenin signaling in human multiple myeloma. Oncotarget. 2016;7:64330–41. doi: 10.18632/oncotarget.11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harima H, Kaino S, Takami T, et al. Deferasirox, a novel oral iron chelator, shows antiproliferative activity against pancreatic cancer in vitro and in vivo. BMC Cancer. 2016;16:702. doi: 10.1186/s12885-016-2744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeki I, Yamamoto N, Yamasaki T. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:8967–77. doi: 10.3748/wjg.v22.i40.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang YC, Lo WJ, Huang YT, et al. Deferasirox has strong anti-leukemia activity but may antagonize the anti-leukemia effect of doxorubicin. Leuk Lymphoma. 2017;58:1–12. doi: 10.1080/10428194.2017.1280604. [DOI] [PubMed] [Google Scholar]
- 27.Benadiba J, Rosilio C, Nebout M, et al. Iron chelation: an adjuvant therapy to target metabolism, growth and survival of murine PTEN-deficient T lymphoma and human T lymphoblastic leukemia/lymphoma. Leuk Lymphoma. 2017;58:1433–45. doi: 10.1080/10428194.2016.1239257. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Zheng X, Shou K, et al. The iron chelator Dp44mT suppresses osteosarcoma’s proliferation, invasion and migration: In vitro and in vivo. Am J Transl Res. 2016;8:5370–85. [PMC free article] [PubMed] [Google Scholar]
- 29.Moussa RS, Park KC, Kovacevic Z, Richardson DR. Ironing out the role of the cyclin-dependent kinase inhibitor, p21 in cancer: Novel iron chelating agents to target p21 expression and activity. Free Radic Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.03.027. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]