Abstract
The pannexin-1 (Panx1) channel has been reported to mediate the release of ATP that is involved in local tissue inflammation, obesity, and many chronic degenerative diseases. It remains unknown whether Panx1 is present in podocytes and whether this channel in podocytes mediates ATP release leading to glomerular inflammation or fibrosis. To answer these questions, we first characterized the expression of Panx channels in podocytes. Among the three known pannexins, Panx1 was the most enriched in podocytes, either cultured or native in mouse glomeruli. Using a Port-a-Patch planar patch-clamp system, we recorded a large voltage-gated outward current through podocyte membrane under the Cs+in/Na+out gradient. Substitution of gluconate or aspartate for chloride in the bath solution blocked voltage-gated outward currents and shifted the reversal potential of Panx1 currents to the right, indicating the anion permeability of this channel. Pharmacologically, the recorded voltage-gated outward currents were substantially attenuated by specific Panx1 channel inhibitors. Given the anti-inflammatory and intracellular ATP restorative effects of adiponectin, we tested whether this adipokine inhibits Panx1 channel activity to block ATP release. Adiponectin blocked Panx1 channel activity in podocytes. Mechanistically, inhibition of acid ceramidase (AC) remarkably enhanced Panx1 channel activity under control conditions and prevented the inhibition of Panx1 channel by adiponectin. Correspondingly, intracellular addition of AC products, sphingosine or sphingosine-1-phosphate (S1P), blocked Panx1 channel activity, while elevation of intracellular ceramide had no effect on Panx1 channel activity. These results suggest that adiponectin inhibits Panx1 channel activity in podocytes through activation of AC and associated elevation of intracellular S1P.
Keywords: Adiponectin, ATP release, pannexin-1 channel, acid ceramidase, podocytes, inflammasome
1. Introduction
Experimental and clinical studies have revealed that adipose tissue, especially visceral fat, generates bioactive substances that contribute to pathophysiologic renal hemodynamic and structural changes leading to obesity-associated glomerular injury [1]. These bioactive substances derived from adipose tissue include a number of adipokines such as leptin, adiponectin, and visfatin as well as various cytokines such as resistin, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) [1]. Recent studies have demonstrated that upregulation of visfatin, a pro-inflammatory adipokine, contributes to the development of chronic kidney disease (CKD) during obesity [2, 3] and diabetes [4, 5]. On the contrary, adiponectin as an anti-inflammatory adipokine has been reported to display protective actions on the development of various obesity-related diseases [6]. Low plasma adiponectin levels, known as hypoadiponectinemia, are closely associated with obesity, type II diabetes mellitus, and different cardiovascular diseases [7, 8], indicating that adiponectin may have beneficial or protective actions in obesity. These are proposed to include inhibition of pro-inflammatory cytokine release, enhancement of anti-inflammatory cytokine release, and restoration of intracellular ATP levels [6, 9, 10]. As a “find me” signal, ATP is released by apoptotic cells to recruit phagocytes to clear up the dying cells [11], which may trigger the inflammatory response to cell death or damage-associated molecular patterns (DAMPs). Indeed, activation of P2X7 receptors by extracellular ATP has been shown to activate inflammasomes in different cells [12, 13]. In the kidney, NLRP3 inflammasome activation was found to play a pivotal role in obesity-induced podocyte injury, an initiating event leading to glomerular damage [14, 15]. Given the fact that adiponectin attenuates obesity-induced podocyte injury [16–18], its actions to inhibit ATP efflux and NLRP3 inflammasome activation may represent an important mechanism in the protection of the kidney from obesity-associated injury. In contrast, reduced adiponectin may promote glomerular injury and lead to glomerular sclerosis during obesity.
In previous studies, pannexin-1 (Panx1) channel-dependent ATP release has been reported to play a critical role in NLRP3 inflammasome activation in a variety of mammalian cells under different physiological and pathological conditions [19–21]. The Panx family consists of Panx1, Panx2, and Panx3, which are homologous to the invertebrate gap junction innexins and have more distant similarities in their membrane topologies and pharmacological sensitivities to the connexins [22–24]. Among pannexins, Panx1 is ubiquitously expressed in animal and human tissues, which may work as a membrane channel to carry ions and signaling molecules between the extracellular space and cytoplasm, regulating cell and organ functions [25, 26]. Various pathogen-associated molecular patterns (PAMPS) and DAMPs such as lipopolysaccharide, uric acid, silica, alum crystals, ATP, hyaluronan, and heparin sulfate have been reported to activate Panx1 channels, which trigger the inflammatory response via activation of inflammasomes [27, 28]. To our knowledge, however, there are no reports about the expression and function of Panx1 in podocytes, and little is known regarding whether Panx1 possesses a regulatory role in these kidney cells. Since Panx1 expression increases in response to obesity and Panx1-dependent ATP release exerts paracrine effects initiating inflammatory cross talk between obese or dying adipocytes and tissue macrophages [29, 30], we wondered whether adiponectin as an anti-inflammatory adipokine exerts its action by targeting Panx1-mediated inflammasome activation and thereby protects podocytes from obesity-induced injury. The present study attempted to address this question.
Although two receptors for adiponectin, namely, adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2), have been cloned and several signaling pathways may mediate the actions of adiponectin through these receptors [31], the downstream signaling pathways that mediate the action of this adipokine via its receptors remain poorly understood. In particular, it is imperative to know how adiponectin acts on Panx1 channels or other effector molecules to produce its anti-inflammatory effects in podocytes during obesity. In this regard, there is evidence that adiponectin may bind to its receptors, either AdipoR1 or AdipoR2, to stimulate the activity of ceramidase, the enzyme responsible for catalyzing the breakdown of ceramide to sphingosine and fatty acids. Sphingosine can be further converted into sphingosine-1-phosphate (S1P), which may produce potent anti-inflammatory effects under different pathological conditions [32, 33]. These findings raised the possibility that ceramidase-dependent catabolism of ceramide and associated intracellular S1P production may contribute to the action of adiponectin on Panx1 activation, thereby decreasing ATP release and inhibiting NLRP3 inflammasome activation in podocytes.
In the present study, we first characterized the expression of Panx1 and its channel activity in cultured and native murine podocytes. Then, we went on to determine the inhibitory effects of adiponectin on Panx1 channel opening in these cells. We also explored the possible mechanism by which adiponectin suppresses Panx1 channel activity via a signaling pathway associated with ceramide metabolism by ceramidases. We demonstrate that adiponectin indeed exerts its inhibitory effects on Panx1 channel activity in podocytes via activation of acid ceramidase (AC) and increased production of intracellular S1P.
2. Materials and methods
2.1. Cell culture
Conditionally-immortalized mouse podocytes, kindly provided by Dr. Paul E. Klotman, were cultured and maintained as described previously [34]. Briefly, they were grown at the permissive temperature (33°C) on collagen I-coated flasks or plates in RPMI 1640 medium supplemented with 10% fetal bovine serum and 10 U/mL recombinant mouse interferon-γ. The podocytes were then passaged and allowed to differentiate at 37°C for 10–14 days without interferon-γ before use in the experiments described below.
2.2. Whole-cell patch clamp recording
Whole-cell planar patch-clamp recordings were performed using cultured murine podocytes. The planar patch-clamp technology combined with a pressure control system (Port-a-Patch, Nanion Technologies) was applied as previously described [35]. Ion currents were recorded, filtered, and analyzed using an Axopatch 200B amplifier, an Axon Digidata 1550B low-noise data acquisition system and the pClamp10 software (Axon instruments). Seal resistance was higher than 1 GΩ. Internal solution contained 50 mM CsCl, 10 mM NaCl, 60 mM CsF, 20 mM EGTA, and 10 mM HEPES/CsOH. External solution contained 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM D-glucose monohydrate, and 10 mM HEPES/NaOH. Seal enhancing solution contained 80 mM NaCl, 3 mM KCl, 10 mM MgCl2, 35 mM CaCl2, and 10 mM HEPES/HCl. Podocytes were stimulated by administration of compounds into the internal and external solutions. Carbenoxolone, probenecid, carmofur, ceranib-1, and ceranib-2 were purchased from Sigma-Aldrich. D-erythro-MAPP, C-16 ceramide (dissolved in dimethylformamide to 0.5 mg/mL and diluted to 40 μM with internal solution before addition), sphingosine, and sphingosine-1-phosphate (dissolved in methanol to 1 mg/mL and diluted to 40 μM with internal solution before addition) were purchased from Cayman. Recombinant mouse adiponectin protein was purchased from Abcam.
2.3. Western blot analysis
Western blot analysis was performed as described previously [36]. In brief, homogenates of mouse kidneys and cultured murine podocytes were prepared using sucrose buffer containing protease inhibitors. After boiling for 5 min at 95°C in a 5× loading buffer, total proteins (20 μg) were subjected to SDS-PAGE, transferred onto a PVDF membrane and blocked by solution with dry milk. Then, the membrane was probed with rabbit anti-pannexin-1 (1:1000; Abcam, Cambridge, MA, USA), rabbit anti-pannexin-2 (1:1000; Abcam, Cambridge, MA, USA), rabbit anti-pannexin-3 (1:1000; Abcam, Cambridge, MA, USA), or anti-β-actin (1:5000; Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) overnight at 4°C followed by incubation with horseradish peroxidase-labeled IgG (1:5000). The immunoreactive bands were detected by chemiluminescence methods and visualized on Kodak Omat X-ray films. Densitometric analysis of the images obtained from X-ray films was performed using the Image J software (NIH, Bethesda, MD, USA).
2.4. Immunofluorescence microscopy
Double-immunofluorescence staining was performed using cultured podocytes grown on collagen-coated glass cover slips and frozen mouse kidney sections as described previously [37, 38]. Briefly, after fixation the cells were incubated with goat anti-nephrin (1:200; R&D, Minneapolis, MN, USA) and rabbit anti-pannexin-1 (1:200) overnight at 4°C. Then, Alexa 488-labeled donkey anti-goat secondary antibody (1:200; Life Technologies, CA, USA) and Alexa 555-labeled donkey anti-rabbit secondary antibody (1:200; Life Technologies, CA, USA) were added to the cell slides and incubated for 1 hour at room temperature. Frozen mouse kidney slides were fixed in acetone, blocked, and then incubated with goat anti-nephrin and rabbit anti-pannexin-1 overnight at 4°C. Then, Alexa 488-labeled donkey anti-goat secondary antibody and Alexa 555-labeled donkey anti-rabbit secondary antibody were added to the cell slides and incubated for 1 hour at room temperature. Slides were then washed, mounted, and observed using a confocal laser scanning microscope (Fluoview FV1000, Olympus, Japan).
2.5. Immunohistochemistry
Kidneys were embedded with paraffin and 5 mm sections were cut from the embedded blocks. After heat-induced antigen retrieval, washing with 3% H2O2 and 30 min blocking with fetal bovine serum, slides were incubated with primary antibody diluted in phosphate-buffered saline (PBS) with 4% fetal bovine serum. Rabbit anti-pannexin-1 (1:200), rabbit anti-pannexin-2 (1:200), or rabbit anti-pannexin-3 (1:200) were used as primary antibody in this study. After incubation with the primary antibody overnight at 4°C, the sections were washed in PBS and incubated with biotinylated IgG (1:200) for 1 hour and then with streptavidin-HRP for 30 minutes at room temperature. 3,3’-Diaminobenzidine (DAB, 50 μL) was added to each kidney section and stained for 1 minute. After washing, the slides were counterstained with hematoxylin for 5 minutes. The slides were then mounted, observed under a microscope, and photomicrographed for later analysis [39].
2.6. Statistical analysis
All of the values are expressed as mean ± SEM. Significant differences among multiple groups were examined using ANOVA followed by a Student-Newman-Keuls test. P<0.05 was considered statistically significant.
3. Results
3.1. Expression of Panx1 in cultured podocytes
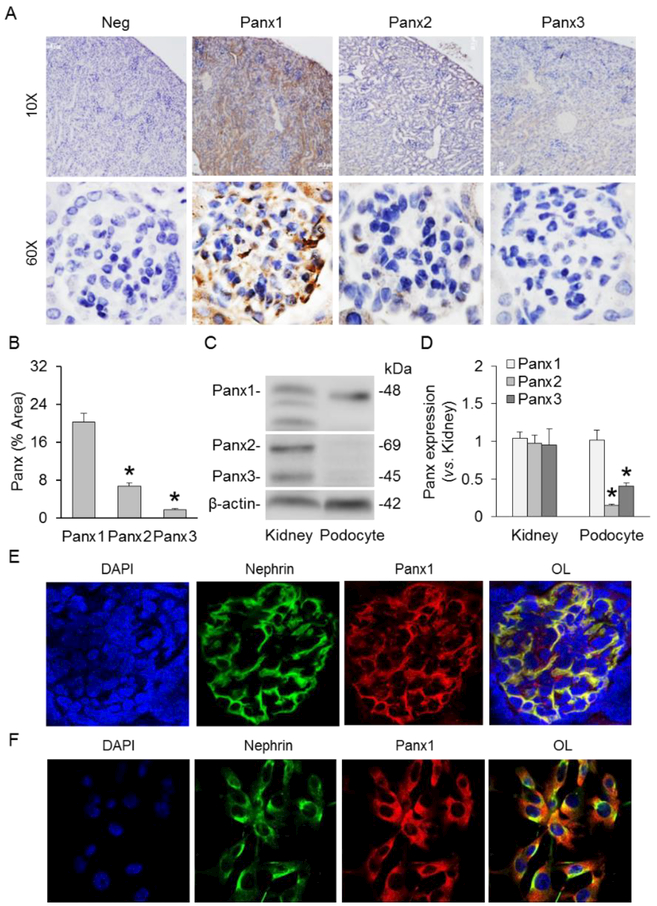
We first characterized the expression of Panx1 in murine podocytes. By immunohistochemistry, abundant expression of Panx1 was confirmed in renal glomeruli of C57BL/6J WT mice, which was remarkably higher than Panx2 and Panx3 (Fig. 1A and 1B). Western blot analysis demonstrated that Panx1 was highly expressed in both mouse kidneys and cultured murine podocytes. The expression levels of Panx2 and Panx3 were markedly higher in mouse kidneys compared with cultured murine podocytes (Figs. 1C and 1D). Using confocal microscopy, it was found that red fluorescence of Panx1 (Alexa 555) was highly colocalized with green fluorescence of nephrin (Alexa 488), a podocyte membrane marker, in renal glomeruli of C57BL/6J WT mice (Fig. 1E). Furthermore, high colocalization of Panx1 and nephrin was detected in cultured murine podocytes (Fig. 1F). These experiments provided the first evidence showing the expression of Panx1 in cultured and native podocytes.
Fig. 1.
Expression of Panxs in cultured and native podocytes. A. Immunohistochemical staining of Panx1, Panx2, and Panx3 in mouse glomeruli. B. Summarized data showing the expressions of Panx1, Panx2, and Panx3 in mouse glomeruli (n=4–5). C. Representative Western blot images showing the expressions of Panx1, Panx2, and Panx3 in mouse kidneys and cultured murine podocytes. D. Summarized data showing the expressions of Panx1, Panx2, and Panx3 in mouse kidneys and cultured murine podocytes (n=5). E. Representative image of confocal microscopy showing the colocalization of nephrin and Panx1 in mouse glomerulus (n=6). F. Representative image of confocal microscopy showing the colocalization of nephrin and Panx1 in cultured murine podocytes (n=4). * p<0.05 vs. Panx1 (panel B) or mouse kidneys (panel D). Panx, pannexins; Neg, negative; OL, overlay.
3.2. Characterization of Panx1 channel activity in cultured podocytes
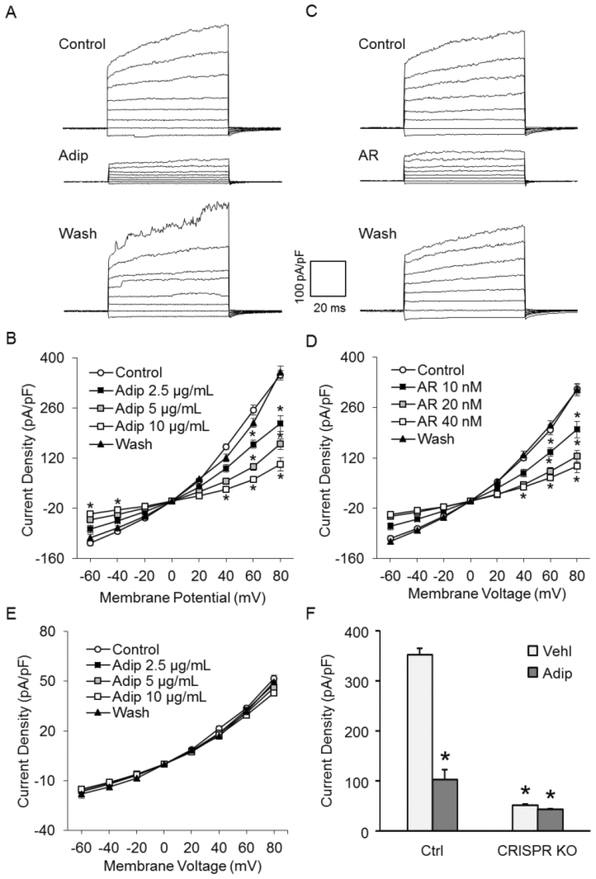
Using a Port-a-Patch automatic patch clamp system, we next confirmed the activity of Panx1 as a large transmembrane channel in podocytes. Fig. 2A shows representative recordings of large voltage-gated outward current at holding potentials from −70 to 80 mV under the Cs+in/Na+out gradient. Fig. 2B summarizes the relationship of holding potential and channel current amplitude. It is clear that the channel current increased with the elevation of holding potential, and the whole-cell current was 283.9±10.9 pA/pF at +80 mV. Pharmacologically, the currents were reversibly blocked by carbenoxolone (CBX) and probenecid (PBNC) to 19.0±2.6% and 26.2±2.1% of control, respectively. The sensitivity of whole-cell currents to CBX and PBNC was detected at negative voltage, which signifies the voltage-gated property of these currents and confirms the contribution of Panx1 to these currents. To further confirm the recorded whole-cell currents were mediated by Panx1 channel, Panx1 CRISPR/cas9 knockout (KO) plasmid was transfected into podocytes before whole-cell patch clamp recording. Western blot analysis demonstrated that Panx1 CRISPR/cas9 KO plasmid markedly reduced Panx1 channel expression in podocytes (supplementary figure 1). As shown in Fig. 4E and 4F, the whole-cell currents of podocytes transfected with Panx1 CRISPR/cas9 KO plasmid were remarkably lower than normal podocytes under control condition. The inhibition of Panx1 channel activity by CBX was insignificant. These results further confirmed the contribution of Panx1 channel to these recorded whole-cell currents.
Fig. 2.
Recording and pharmacological inhibition of Panx1 channel activity in podocytes. Podocyte patches were held at −50 mV and polarized by 100 ms voltage pulses between −70 and 80 mV. A. Representative whole-cell currents of podocytes with or without CBX. B. I-V curves of steady-state currents recorded in podocytes with or without CBX (25 μM) (n=10 batches of experiments). C. Representative whole-cell currents of podocytes in the presence or the absence of PBNC. D. I-V curves of steady-state currents in podocytes in the absence or presence of PBNC (1 mM) (n=12 batches of experiments). E. I-V curves of steady-state currents in podocytes transfected with Panx1 CRISPR/cas9 KO plasmid in the absence or presence of CBX (25 μM) (n=5 batches of experiments). F. Summarized data showing the steady-state currents at 80 mV in podocytes under different conditions. * p<0.05 vs. control (panel B and D) or Ctrl-Vehl (panel F). CBX, carbenoxolone; PBNC, probenecid; CRISPR KO, Panx1 CRISPR/cas9 KO plasmid.
Fig. 4.
Concentration-dependent inhibition of Panx1 channel activity by adiponectin. A. Representative whole-cell currents of podocytes with or without adiponectin. B. I-V curves of steady-state currents of podocytes with or without adiponectin (n=9 batches of experiments). C. Representative whole-cell currents of podocytes with or without AdipoRon. D. I-V curves of steady-state currents of podocytes with or without AdipoRon (n=9 batches of experiments). E. IV curves of steady-state currents in podocytes transfected with Panx1 CRISPR/cas9 KO plasmid in the absence or presence of adiponectin (n=5 batches of experiments). F. Summarized data showing the steady-state currents at 80 mV in podocytes under different conditions. * p<0.05 vs. control (panel B and D) or Ctrl-Vehl (panel F). Adip, adiponectin; AR, AdipoRon; CRISPR KO, Panx1 CRISPR/cas9 KO plasmid.
As another known characteristic of Panx1, anion permeability was also determined by the substitution of extracellular chloride for a larger anion [40]. During whole-cell current recording, 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, and 2 mM CaCl2 were substituted with 140 mM Naaspartate, 4 mM K-aspartate, 1 mM Mg-aspartate2, 2 mM Ca-aspartate2. As shown in Fig. 3A, the replacement of chloride with aspartate (131 Da) remarkably decreased the outward currents and shifted the reversal potential of Panx1 channel to the right. Similar data was obtained when we replaced chloride with gluconate (195 Da) in the external solution (Fig. 3C). Restoration of extracellular chloride totally recovered the whole-cell currents and the reversal potential of Panx1 channel. Summarized data demonstrated that the down-regulation of whole-cell currents by chloride replacement was significant (Figs. 3B and 3D). Altogether, these observations indicate that Panx1 is a voltage-gated transmembrane channel with anion permeability in podocytes.
Fig. 3.
Anion permeability of Panx1 channels. Panx1 currents in podocytes were recorded with different anions in the external solution. A. Representative whole-cell currents of podocytes with chloride (control) or aspartate in the external solution. B. I-V curves illustrating shifts of Panx1 current reversal potentials upon replacement of extracellular chloride with aspartate (n=5 batches of experiments). C. Representative whole-cell currents of podocytes with chloride or gluconate in the external solution. D. I-V curves illustrating shifts of Panx1 current reversal potentials upon replacement of extracellular chloride with gluconate (n=8 batches of experiments). * p<0.05 vs. chloride.
3.3. Concentration-dependent inhibition of Panx1 channel activity by adiponectin
Further experiments were designed to determine whether these channels can be inhibited by adiponectin because the major goal of the present study was to explore the mechanism by which this protective adipokine increases intracellular ATP content. As shown in Fig. 4A, addition of adiponectin (Adip) into the extracellular solution remarkably decreased Panx1 channel activity. Fig. 4B summarizes the results showing that adiponectin at 2.5 μg/mL, 5 μg/mL, and 10 μg/mL significantly inhibited the whole-cell currents in a dose-dependent manner. When the dose of adiponectin reached 10 μg/mL, the activity of Panx1 channel decreased to 29.4±5.3% of control, a level similar to that achieved by addition of CBX and PBNC. As a well-known selective agonist of adiponectin receptor 1, the effect of AdipoRon on Panx1 channel activity was also tested. As shown in Fig. 4C and 4D, AdipoRon (AR) produced remarkable inhibitory effects on Panx1 channel activity in a dose-dependent manner, which was similar to adiponectin. To further confirm the Panx1 specificity of whole-cell current reduction by adiponectin, the effect of adiponectin on podocytes transfected with Panx1 CRISPR/cas9 KO plasmid was detected. As shown in Fig. 4E and 4F, whole-cell currents of Panx1 CRISPR/cas9 KO plasmid-transfected cells was remarkably lower than normal cells under control condition. The inhibition of Panx1 channel activity by adiponectin was insignificant. These results further confirmed that adiponectin reversibly inhibited Panx1 channel activity in a dose-dependent manner.
3.4. Enhancement of Panx1 channel activity by AC inhibitors
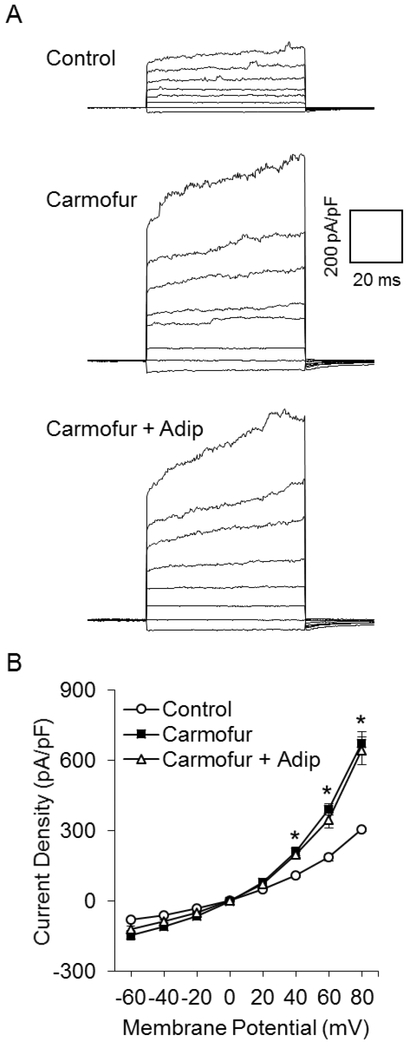
To explore the mechanisms by which adiponectin exerts its inhibitory effect on Panx1, we tested whether activation of either acid or neutral ceramidase contributes to inhibition of Panx1 by adiponectin, given that previous studies showed the regulation of acid and neutral ceramidase activity by adiponectin [41–43]. We first determined the effects of acid ceramidase (AC) inhibitors on Panx1 currents. In Fig. 5A, representative patch clamp recordings showed that blockade of AC activity by carmofur, a specific AC inhibitor, markedly enhanced Panx1 channel activity in podocytes. Summarized data demonstrated that carmofur significantly enhanced Panx1 channel activity in a dose-dependent manner (Fig. 5B). We also obtained similar when D-e-MAPP, another selective AC inhibitor, was added into the external solution during whole-cell current recording. Panx1 channel activity was significantly elevated by D-e-MAPP in a dose-dependent manner (Fig. 5C and 5D). At the highest concentration used, AC inhibitors increased Panx1 channel activity by more than 3-fold.
Fig. 5.
Enhancement of Panx1 channel activity by AC inhibitors. A. Representative whole-cell currents of podocytes with or without carmofur. B. I-V curves of steady-state currents of podocytes with or without carmofur (n=8 batches of experiments). C. Representative whole-cell currents of podocytes in the presence or the absence of D-e-MAPP. D. I-V curves of steady-state currents of podocytes in the presence or the absence of D-e-MAPP (n=6 batches of experiments). * p<0.05 vs. control.
We also tested whether inhibition of neutral ceramidase (NC) alters Panx1 channel activity. was found that inhibition of NC activity by its selective NC inhibitor, ceranib-1, had significant effect on Panx1 activity in podocytes. Similarly, another NC selective inhibitor, ceranib-2, also failed to affect Panx1 channel activity in podocytes (supplementary figure 2). Together, these data suggest that NC is not involved in the regulation of Panx1 channel activity in podocytes.
3.5. Dependence of adiponectin-induced inhibition of Panx1 channel activity upon AC
To determine the role of AC in adiponectin-induced inhibition of Panx1 activity, we examined whether pretreatment of podocytes with acid or neutral ceramidase inhibitor prevents reduction of Panx1 channel activity induced by adiponectin. Fig. 6A shows representative recordings, which depict a remarkable increase in Panx1 activity after addition of carmofur. In the presence of carmofur, adiponectin failed to produce any inhibitory effect on Panx1 channel activity. Fig. 6B presents summarized data showing that carmofur significantly increased current intensity and blocked adiponectin-induced inhibition of this channel activity. However, the inhibition of NC by ceranib-1 had no effect on the Panx1 channel activity in podocytes regardless of the presence or absence of adiponectin (supplementary figure 3).
Fig. 6.
Dependence of adiponectin-induced inhibition of Panx1 channel activity upon AC activity. A. Representative whole-cell currents of podocytes before and after addition of adiponectin to the external solution in the presence of carmofur. B. I-V curves of steady-state currents of podocytes before and after addition of adiponectin (10 μg/mL) to the external solution in the presence of carmofur (50 nM) (n=6 batches of experiments). * p<0.05 vs. control. Adip, adiponectin.
To determine the effect of long-term adiponectin treatment on Panx1 expression in podocytes, we treated podocytes with adiponectin and/or carmofur for 24 hours. The western blot analysis showed that adiponectin remarkably decreased Panx1 expression in podocytes. On the other hand, co-treatment with carmofur, the AC inhibitor, significantly attenuated the inhibiting effect of adiponectin on Panx1 expression (supplementary figure 4). These results demonstrated that adiponectin negatively regulated Panx1 through both acute and chronic mechanisms.
3.6. Effects of AC products on Panx1 activity
To assess which sphingolipids associated with AC may inhibit the activity of the Panx1 channel, the AC substrate, ceramide, and its metabolite, sphingosine, as well as a further downstream bioactive product formed by sphingosine kinase, S1P, were used to treat podocytes during patch clamp recordings. As depicted in Fig. 7A, intracellular addition (internal solution) of sphingosine remarkably decreased the Panx1 activity. Summarized results showed that inhibition of Panx1 channel activity by intracellular elevation of sphingosine was significant (Fig. 7B). Fig. 7C shows that Panx1 activity was also markedly suppressed by intracellular increases in S1P. In Fig. 7D, summarized data show that intracellular addition of S1P significantly reduced the current intensity of Panx1 channel in podocytes. However, intracellular addition of ceramide had no effect on Panx1 channel activity (supplementary figure 5). These results suggest that AC products within podocytes have inhibitory effects on the Panx1 channel activity.
Fig. 7.
Effects of AC products on Panx1 activity. A. Representative Panx1 channel currents in podocytes with or without sphingosine. B. I-V curves of steady-state Panx1 channel current density in podocytes with or without sphingosine (4 μM) in the internal solution (n=8 batches of experiments). C. Representative Panx1 channel currents in podocytes with or without S1P in the internal solution. D. I-V curves of steady-state Panx1 channel current density in podocytes with or without S1P (4 μM) in the internal solution (n=6 batches of experiments). * p<0.05 vs. control. Sph, sphingosine; S1P, sphingosine-1-phosphate.
3.7. Effects of adiponectin on ATP release through Panx1 channel
To confirm the effects of adiponectin on Panx1 channel activity, we measured the amount of ATP released from podocytes treated with adiponectin, carmofur, and ceranib-1. The Panx1 inhibitors, CBX (25 μM) and PBNC (1 mM), were used as positive controls of ATP release inhibition. As shown in Fig. 8, it was found that adiponectin (10 μg/mL) reduced the amount of ATP released from podocytes to the similar level of CBX and PBNC treatments. On the contrary, inhibition of AC activity by carmofur (50 nM) remarkably enhanced ATP release from podocytes and totally reversed the inhibition of ATP release by adiponectin. Although, inhibitions of ATP release by CBX and PBNC were not influenced by carmofur. Consistent with whole-cell patch clamp results, inhibition of NC by ceranib-1 (50 μM) had no significant effects on ATP release from podocytes. These results further confirmed that adiponectin inhibited ATP release through Panx1 channel in an AC-dependent manner.
Fig. 8.
Effects of adiponectin on ATP release through Panx1 channel. Summarized data of ATP released from podocyte under different treatments. * p<0.05 vs. Ctrl-Vehl. # p<0.05 vs. Adip-Vehl. Ctrl, control; Vehl, vehicle; Adip, adiponectin; CBX, carbenoxolone; PBNC, probenecid.
4. Discussion
The major goal of the present study was to determine whether adiponectin inhibits Panx1 channel activity via acid ceramidase (AC)-mediated signaling pathway in podocytes. After characterization of Panx1 in cultured and native podocytes, we demonstrated that recombinant adiponectin significantly blocked Panx1 channel opening in these kidney cells to similar levels induced by known Panx1 channel inhibitors. It was also confirmed that the inhibitory effect of adiponectin on Panx1 channel activity was attributed to the activation of AC and consequent production of sphingosine and S1P. These results together provide the first evidence that inhibition of Panx1 channel activity is an important mechanism that mediates the action of adiponectin to release ATP in podocytes.
We first characterized the expression and activity of the Panx1 channel in murine podocytes, which is considered as a large transmembrane channel responsible for ATP release [44]. By various approaches such as Western blot analysis, immunohistochemistry, and confocal microscopy, it was found that, among Panxs, only Panx1 was abundantly expressed in murine podocytes. Using a Port-a-Patch planar patch-clamp system, we recorded a large voltage-gated outward current through podocyte membranes, which was almost completely blocked by carbenoxolone (CBX) and probenecid (PBNC), two specific Panx1 channel inhibitors. It was also confirmed that this Panx1 channel in podocytes was anionic, because replacement of anions in the bath solution shifted their reversal potential to right, a much higher holding potential in the patch. These biophysical and pharmacological characteristics indeed define the recorded channels as Panx1, because this anion permeability of Panx1 channels was confirmed in previous studies [45–48]. To our knowledge, these results for the first time demonstrated the presence of Panx1 in podocytes and its functional activity as a channel in plasma membrane of these kidney cells. In previous studies, these features of Panx1 channels have also been shown in many other cell types such as endothelial cells, smooth muscle cells, erythrocytes, leukocytes and platelets [11, 46, 49–51].
Next, we tested whether adiponectin activates Panx1 channel activity in podocytes, which may contribute to ATP release shown in previous studies [52]. Our results showed that both adiponectin and AdipoRon, an adiponectin receptor agonist, reversibly inhibited Panx1 channel activity in podocytes in a dose-dependent manner, suggesting that a normal level of adiponectin in the extracellular space or outside of podocytes may importantly inhibit Panx1 channel activity, controlling ATP release from these cells. As an adipocyte-derived hormone, adiponectin is essential for maintenance of podocyte function and prevention of albuminuria under normal condition [16, 17]. During obesity, reduced adiponectin may lead to enhancement of Panx1 channel activity and thereby participate in the development of obesity-associated glomerular inflammatory response. In this regard, previous studies have indeed demonstrated that the activation of Panx1 channels can trigger the inflammatory response via activation of inflammasomes [27, 28] and that Panx1-dependent ATP release initiates inflammatory cross talk between obese or dying adipocytes and tissue macrophages [29, 30]. It has also been reported that knockout of the adiponectin gene induced podocyte dysfunction and albuminuria in mice and treatment with adiponectin could recover podocyte function and attenuate albuminuria [16], while overexpression of adiponectin prevented caspase-8-mediated apoptosis in podocytes and consequent albuminuria and interstitial fibrosis [53]. These pathological changes in podocytes and glomeruli associated with altered adiponectin levels may be related to its effects on Panx1 activity and consequent ATP retention within or release from podocytes, because the latter is critical in triggering NRLP3 inflammasome activation [19–21]. In this regard, a previous study by Qu et al has used Panx1 knockout mice to demonstrate that Panx1 channel-mediated ATP release is not required for inflammasome activation [54]. However, the expression level of Panx1 channel in bone marrow-derived macrophages (BMDMs) on which their study focused was remarkably lower than other Panx1-detected tissues, such as kidney, lung, spleen, and brain, which indicates that the activity of Panx1 may not be the most important mechanism mediating inflammasome activation in BMDMs. Also, their study focused on whether cleavage of procaspase-1 and release of pro-inflammatory cytokines are dependent on Panx1 channel, which are downstream activities of extracellular ATP-induced inflammasome activation. Therefore, their findings made no conclusions on whether Panx1 channel is essential for ATP release from BMDMs [54].
Since adiponectin has been reported to activate ceramidase to produce a regulatory role on cell functions [41] [42, 43], we tested whether any specific ceramidases activated by adiponectin are regulators of Panx1 channel activity in podocytes. It was found that AC inhibition by its selective inhibitors remarkably enhanced Panx1 channel activity in murine podocytes. In the presence of AC inhibitors, adiponectin failed to decrease Panx1 channel activity. However, NC inhibition by its selective inhibitors neither changed basal Panx1 activity nor affected adiponectin-induced suppression of Panx1 openings. Based on these results, it appears that the AC activation may mediate the inhibitory action of adiponectin on Panx1 channel activity. It has been reported that adiponectin-induced activation of ceramidase may enhance ceramide catabolism to form sphingosine and S1P within cells. To determine which sphingolipids associated with AC are attributed to the action of adiponectin-induced activation of Panx1 channels, we tested the effects of ceramide (an AC substrate), sphingosine (an AC product), and S1P (phosphorylated sphingosine via sphingosine kinase 1) by administrating them into the internal solution of the patches. Both sphingosine and S1P, but not ceramide, were found to inhibit Panx1 channel activity. These findings suggest that AC activation by adiponectin may produce sphingosine and S1P and thereby inhibit Panx1 channel activity. Since sphingosine is relatively short-lived in the cytoplasm and easily converted into S1P [55] even under normal physiological conditions, it is believed that S1P is the major messenger mediating the action of AC activation. Based on these observations and our data in the podocyte model, it is proposed that the inhibitory effect of adiponectin on Panx1 channel activity is mediated by activation of AC and subsequent increases in S1P. The present study did not attempt to define the mechanism by which S1P inhibits Panx1 channel activity. However, previous studies have shown that S1P is a multifunctional signaling molecule [56], which may act either at an intracellular site or be transported outside of cells to exert its action via S1P receptors on the plasma membrane [56–58]. Since we administrated S1P in the intracellular site, it is possible that in our preparation S1P acts on the intracellular regulatory domains of Panx1 to produce inhibitory effects. This idea will be further tested in more detail in our ongoing projects. Furthermore, it has been found that long-term treatment with adiponectin significantly decreased Panx1 channel expression in podocytes and this inhibitory effect was totally blocked by Carmofur. In this regard, a previous study has demonstrated that the expression of AC may be regulated by activation of adiponectin receptors (AdipoRs) [59]. In diabetic mice, expressions of both AdipoRs and AC have been remarkably reduced. However, treatment with AdipoRon significantly attenuated these reductions in the diabetic mice [59]. These results indicate that activation of AdipoRs up-regulates AC expression, which may explain the chronic mechanism by which adiponectin inhibited Panx1 channel expression in podocytes. Consistent with our findings, it has been reported that AdipoRs can be antagonized by a ceramidase inhibitor [60]. More recently, both AdipoR1 and AdipoR2 have been demonstrated to possess intrinsic basal ceramidase activity which is enhanced by adiponectin, although the ceramidase activity detected is low [61]. However, most previous studies support the hypothesis that adiponectin enhances acid ceramidase and/or neutral ceramidase in an AdipoR-dependent manner [41–43, 62]. To further confirm the role of AdipoRs in the inhibition of Panx1 channel by adiponectin, more studies will be performed in the future.
In summary, the present study demonstrated that Panx1 was an abundant form of pannexins in podocytes, and it could mediate anion conductance across plasma membrane to transport ATP. This Panx1 channel was found to be inhibited by adiponectin via AC activation and consequent production of S1P. These findings indicate that a tonic inhibition of Panx1 channel activity by adiponectin may be attenuated during obesity due to hypoadiponectinemia, which will lead to ATP release, inflammasome activation and consequent inflammatory response in local tissues such as glomeruli.
Supplementary Material
Highlights.
The pannexin-1 (Panx1) channel is highly enriched in murine podocytes and it conducts anion currents.
Adiponectin inhibits Panx1 channel activity.
Inhibition of the Panx1 channel by adiponectin is dependent on activation of acid ceramidase (AC).
Enhanced production of sphingosine-1-phosphate (S1P) due to activation of AC contributes to inhibition of Panx1 channel by adiponectin.
Acknowledgments
This study was supported by grants DK054927 and DK102539 from National Institutes of Health.
Abbreviations:
- Panx
pannexin
- CBX
carbenoxolone
- PBNC
probenecid
- Adip
adiponectin
- AR
AdipoRon
- AC
acid ceramidase
- NC
neutral ceramidase
- TNF-α
tumor necrosis factor-α
- IL-6
interleukin-6
- CKD
chronic kidney disease
- DAMP
damage-associated molecular pattern
- NLRP3
nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3
- AdipoR
adiponectin receptor
- AMPK
AMP-dependent kinase
- UT
Urtica dioica L
- SphK
sphingosine kinase
- PKD
polycystic kidney disease
- sPLA2
secretory phospholipase A2
- iNOS
inducible NO synthase
- NO
nitric oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors have conflict of interest.
References
- [1].Axelsson J, Heimburger O, Stenvinkel P, Adipose tissue and inflammation in chronic kidney disease, Contrib Nephrol, 151 (2006) 165–174. [DOI] [PubMed] [Google Scholar]
- [2].Briffa JF, McAinch AJ, Poronnik P, Hryciw DH, Adipokines as a link between obesity and chronic kidney disease, Am J Physiol Renal Physiol, 305 (2013) F1629–1636. [DOI] [PubMed] [Google Scholar]
- [3].Rotkegel S, Chudek J, Spiechowicz-Zaton U, Ficek R, Adamczak M, Wiecek A, The effect of sodium restricted diet on plasma visfatin levels in hypertensive patients with visceral obesity, Kidney Blood Press Res, 37 (2013) 124–131. [DOI] [PubMed] [Google Scholar]
- [4].Kang YS, Song HK, Lee MH, Ko GJ, Han JY, Han SY, Han KH, Kim HK, Cha DR, Visfatin is upregulated in type-2 diabetic rats and targets renal cells, Kidney Int, 78 (2010) 170–181. [DOI] [PubMed] [Google Scholar]
- [5].Kang YS, Cha DR, The role of visfatin in diabetic nephropathy, Chonnam Med J, 47 (2011) 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ouchi N, Walsh K, Adiponectin as an anti-inflammatory factor, Clin Chim Acta, 380 (2007) 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lara-Castro C, Fu Y, Chung BH, Garvey WT, Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease, Curr Opin Lipidol, 18 (2007) 263–270. [DOI] [PubMed] [Google Scholar]
- [8].Fantuzzi G, Adiponectin and inflammation: consensus and controversy, J Allergy Clin Immunol, 121 (2008) 326–330. [DOI] [PubMed] [Google Scholar]
- [9].Wang H, Yan WJ, Zhang JL, Zhang FY, Gao C, Wang YJ, Bond Law W, Tao L, Adiponectin partially rescues high glucose/high fat-induced impairment of mitochondrial biogenesis and function in a PGC-1alpha dependent manner, Eur Rev Med Pharmacol Sci, 21 (2017) 590–599. [PubMed] [Google Scholar]
- [10].He Y, Zou L, Zhou Y, Hu H, Yao R, Jiang Y, Lau WB, Yuan T, Huang W, Zeng Z, Cao Y, Adiponectin ameliorates the apoptotic effects of paraquat on alveolar type cells via improvements in mitochondrial function, Mol Med Rep, 14 (2016) 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS, Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis, Nature, 467 (2010) 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA, Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response, J Immunol, 168 (2002) 6436–6445. [DOI] [PubMed] [Google Scholar]
- [13].Pelegrin P, Barroso-Gutierrez C, Surprenant A, P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage, J Immunol, 180 (2008) 7147–7157. [DOI] [PubMed] [Google Scholar]
- [14].Boini KM, Xia M, Abais JM, Li G, Pitzer AL, Gehr TW, Zhang Y, Li PL, Activation of inflammasomes in podocyte injury of mice on the high fat diet: Effects of ASC gene deletion and silencing, Biochim Biophys Acta, 1843 (2014) 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boini KM, Xia M, Koka S, Gehr TW, Li PL, Instigation of NLRP3 inflammasome activation and glomerular injury in mice on the high fat diet: role of acid sphingomyelinase gene, Oncotarget, 7 (2016) 19031–19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ, Adiponectin regulates albuminuria and podocyte function in mice, J Clin Invest, 118 (2008) 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sharma K, The link between obesity and albuminuria: adiponectin and podocyte dysfunction, Kidney Int, 76 (2009) 145–148. [DOI] [PubMed] [Google Scholar]
- [18].Cammisotto PG, Bendayan M, Adiponectin stimulates phosphorylation of AMP-activated protein kinase alpha in renal glomeruli, J Mol Histol, 39 (2008) 579–584. [DOI] [PubMed] [Google Scholar]
- [19].Mortimer L, Moreau F, Cornick S, Chadee K, The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive Entamoeba histolytica via Activation of alpha5beta1 Integrin at the Macrophage-Amebae Intercellular Junction, PLoS Pathog, 11 (2015) e1004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Albalawi F, Lu W, Beckel JM, Lim JC, McCaughey SA, Mitchell CH, The P2X7 Receptor Primes IL-1beta and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain, Front Cell Neurosci, 11 (2017) 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G, The pannexin 1 channel activates the inflammasome in neurons and astrocytes, J Biol Chem, 284 (2009) 18143–18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kelmanson IV, Shagin DA, Usman N, Matz MV, Lukyanov SA, Panchin YV, Altering electrical connections in the nervous system of the pteropod mollusc Clione limacina by neuronal injections of gap junction mRNA, Eur J Neurosci, 16 (2002) 2475–2476. [DOI] [PubMed] [Google Scholar]
- [23].Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE, Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other, J Biol Chem, 285 (2010) 24420–24431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kar R, Batra N, Riquelme MA, Jiang JX, Biological role of connexin intercellular channels and hemichannels, Arch Biochem Biophys, 524 (2012) 2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cea LA, Riquelme MA, Vargas AA, Urrutia C, Saez JC, Pannexin 1 channels in skeletal muscles, Front Physiol, 5 (2014) 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, Ivanov DV, Skryma R, Prevarskaya N, Functional implications of calcium permeability of the channel formed by pannexin 1, J Cell Biol, 174 (2006) 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Orellana JA, Montero TD, von Bernhardi R, Astrocytes inhibit nitric oxide-dependent Ca(2+) dynamics in activated microglia: involvement of ATP released via pannexin 1 channels, Glia, 61 (2013) 2023–2037. [DOI] [PubMed] [Google Scholar]
- [28].Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I, ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation, Cell Death Dis, 3 (2012) e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Adamson SE, Meher AK, Chiu YH, Sandilos JK, Oberholtzer NP, Walker NN, Hargett SR, Seaman SA, Peirce-Cottler SM, Isakson BE, McNamara CA, Keller SR, Harris TE, Bayliss DA, Leitinger N, Pannexin 1 is required for full activation of insulin-stimulated glucose uptake in adipocytes, Mol Metab, 4 (2015) 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Odegaard JI, Chawla A, Mechanisms of macrophage activation in obesity-induced insulin resistance, Nat Clin Pract Endocrinol Metab, 4 (2008) 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Iwabu M, Okada-Iwabu M, Yamauchi T, Kadowaki T, Adiponectin/adiponectin receptor in disease and aging, NPJ Aging Mech Dis, 1 (2015) 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ren S, Babelova A, Moreth K, Xin C, Eberhardt W, Doller A, Pavenstadt H, Schaefer L, Pfeilschifter J, Huwiler A, Transforming growth factor-beta2 upregulates sphingosine kinase-1 activity, which in turn attenuates the fibrotic response to TGF-beta2 by impeding CTGF expression, Kidney Int, 76 (2009) 857–867. [DOI] [PubMed] [Google Scholar]
- [33].Natoli TA, Husson H, Rogers KA, Smith LA, Wang B, Budman Y, Bukanov NO, Ledbetter SR, Klinger KW, Leonard JP, Ibraghimov-Beskrovnaya O, Loss of GM3 synthase gene, but not sphingosine kinase 1, is protective against murine nephronophthisis-related polycystic kidney disease, Hum Mol Genet, 21 (2012) 3397–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Abais JM, Zhang C, Xia M, Liu Q, Gehr TW, Boini KM, Li PL, NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia, Antioxid Redox Signal, 18 (2013) 1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bruggemann A, George M, Klau M, Beckler M, Steindl J, Behrends JC, Fertig N, High quality ion channel analysis on a chip with the NPC technology, Assay Drug Dev Technol, 1 (2003) 665–673. [DOI] [PubMed] [Google Scholar]
- [36].Li CX, Xia M, Han WQ, Li XX, Zhang C, Boini KM, Liu XC, Li PL, Reversal by growth hormone of homocysteine-induced epithelial-to-mesenchymal transition through membrane raft-redox signaling in podocytes, Cell Physiol Biochem, 27 (2011) 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang C, Boini KM, Xia M, Abais JM, Li X, Liu Q, Li PL, Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia, Hypertension, 60 (2012) 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maciel W, Lopes WD, Cruz B, Teixeira W, Felippelli G, Sakamoto CA, Favero FC, Buzzulini C, Soares V, Gomes LV, Bichuette M, da Costa AJ, Effects of Haematobia irritans infestation on weight gain of Nelore calves assessed with different antiparasitic treatment schemes, Prev Vet Med, 118 (2015) 182–186. [DOI] [PubMed] [Google Scholar]
- [39].Xia M, Conley SM, Li G, Li PL, Boini KM, Inhibition of hyperhomocysteinemia-induced inflammasome activation and glomerular sclerosis by NLRP3 gene deletion, Cell Physiol Biochem, 34 (2014) 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang J, Ambrosi C, Qiu F, Jackson DG, Sosinsky G, Dahl G, The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation, Sci Signal, 7 (2014) ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE, Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin, Nat Med, 17 (2011) 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang Y, Wang X, Lau WB, Yuan Y, Booth D, Li JJ, Scalia R, Preston K, Gao E, Koch W, Ma XL, Adiponectin inhibits tumor necrosis factor-alpha-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation, Circ Res, 114 (2014) 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Obanda DN, Zhao P, Richard AJ, Ribnicky D, Cefalu WT, Stephens JM, Stinging Nettle (Urtica dioica L.) Attenuates FFA Induced Ceramide Accumulation in 3T3-L1 Adipocytes in an Adiponectin Dependent Manner, PLoS ONE, 11 (2016) e0150252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Taylor KA, Wright JR, Mahaut-Smith MP, Regulation of Pannexin-1 channel activity, Biochem Soc Trans, 43 (2015) 502–507. [DOI] [PubMed] [Google Scholar]
- [45].Bruzzone R, Barbe MT, Jakob NJ, Monyer H, Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes, J Neurochem, 92 (2005) 1033–1043. [DOI] [PubMed] [Google Scholar]
- [46].Taylor KA, Wright JR, Vial C, Evans RJ, Mahaut-Smith MP, Amplification of human platelet activation by surface pannexin-1 channels, J Thromb Haemost, 12 (2014) 987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pelegrin P, Surprenant A, Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor, EMBO J, 25 (2006) 5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI, Kolesnikov SS, The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable, J Cell Sci, 125 (2012) 5514–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bao L, Locovei S, Dahl G, Pannexin membrane channels are mechanosensitive conduits for ATP, FEBS Lett, 572 (2004) 65–68. [DOI] [PubMed] [Google Scholar]
- [50].Lohman AW, Weaver JL, Billaud M, Sandilos JK, Griffiths R, Straub AC, Penuela S, Leitinger N, Laird DW, Bayliss DA, Isakson BE, S-nitrosylation inhibits pannexin 1 channel function, J Biol Chem, 287 (2012) 39602–39612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG, Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse, Blood, 116 (2010) 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Forst AL, Olteanu VS, Mollet G, Wlodkowski T, Schaefer F, Dietrich A, Reiser J, Gudermann T, Mederos y Schnitzler M, Storch U, Podocyte Purinergic P2X4 Channels Are Mechanotransducers That Mediate Cytoskeletal Disorganization, J Am Soc Nephrol, 27 (2016) 848–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rutkowski JM, Wang ZV, Park AS, Zhang J, Zhang D, Hu MC, Moe OW, Susztak K, Scherer PE, Adiponectin promotes functional recovery after podocyte ablation, J Am Soc Nephrol, 24 (2013) 268–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM, Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation, J Immunol, 186 (2011) 6553–6561. [DOI] [PubMed] [Google Scholar]
- [55].Olivera A, Spiegel S, Sphingosine kinase: a mediator of vital cellular functions, Prostaglandins Other Lipid Mediat, 64 (2001) 123–134. [DOI] [PubMed] [Google Scholar]
- [56].Schwalm S, Pfeilschifter J, Huwiler A, Targeting the sphingosine kinase/sphingosine 1-phosphate pathway to treat chronic inflammatory kidney diseases, Basic Clin Pharmacol Toxicol, 114 (2014) 44–49. [DOI] [PubMed] [Google Scholar]
- [57].Nagahashi M, Takabe K, Terracina KP, Soma D, Hirose Y, Kobayashi T, Matsuda Y, Wakai T, Sphingosine-1-phosphate transporters as targets for cancer therapy, Biomed Res Int, 2014 (2014) 651727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pyne NJ, McNaughton M, Boomkamp S, MacRitchie N, Evangelisti C, Martelli AM, Jiang HR, Ubhi S, Pyne S, Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation, Adv Biol Regul, 60 (2016) 151–159. [DOI] [PubMed] [Google Scholar]
- [59].Choi SR, Lim JH, Kim MY, Kim EN, Kim Y, Choi BS, Kim YS, Kim HW, Lim KM, Kim MJ, Park CW, Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy, Metabolism, (2018). [DOI] [PubMed] [Google Scholar]
- [60].Kupchak BR, Garitaonandia I, Villa NY, Smith JL, Lyons TJ, Antagonism of human adiponectin receptors and their membrane progesterone receptor paralogs by TNFalpha and a ceramidase inhibitor, Biochemistry, 48 (2009) 5504–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vasiliauskaite-Brooks I, Sounier R, Rochaix P, Bellot G, Fortier M, Hoh F, De Colibus L, Bechara C, Saied EM, Arenz C, Leyrat C, Granier S, Structural insights into adiponectin receptors suggest ceramidase activity, Nature, 544 (2017) 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Holland WL, Xia JY, Johnson JA, Sun K, Pearson MJ, Sharma AX, Quittner-Strom E, Tippetts TS, Gordillo R, Scherer PE, Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis, Mol Metab, 6 (2017) 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.