Abstract
Background
Laboratory criterion is needed for the classification of antiphospholipid syndrome (APS), which contain anticardiolipin antibodies (aCL) and anti-β2-glycoprotein 1 antibodies (aβ2GP1). They are commonly identified by enzyme-linked immunosorbent assay (ELISA), but lack standardized kits, resulting in substantial variations in the antibody positivity between different laboratories. The emergence of chemiluminescence automated BIO-FLASH may improve the situation.
Material/Methods
We selected 185 patients with APS, systemic lupus erythematosus (SLE), infertility, connective tissue disease (CTD), and other conditions in Peking University Third Hospital. We tested the aCL and aβ2GP1 levels by EUROIMMUN ELISA and 105 patients had at least one positive result for aCL and aβ2GP1, while the others had negative results. We retested them by chemiluminescence assay (CIA) and analyzed the result and compared the coincidence rate. The IgM levels were retested by AESKU ELISA. Data were analyzed using SPSS.
Results
Our result suggested that CIA had good performance for IgG isotype of aCL and aβ2GP1 in the coincidence rate. The positive coincidence rate of aCL IgM between CIA and EUROIMMUN ELISA was only 41.67%, but two ELISA kits showed good coincidence, CIA and AESKU ELISA had an obviously higher positive rate. CIA and AESKU had a higher coincidence than that of AESKU and EUROIMMUN in aβ2GP1-IgM.
Conclusions
The new automated CIA BIO-FLASH is suitable for detecting aCL and aβ2GP1 antibodies, especially IgG isotype, which may provide an alternative to time-consuming conventional ELISA method.
MeSH Keywords: Antibodies, Anticardiolipin; Antiphospholipid Syndrome; Luminescence
Background
The antiphospholipid syndrome (APS) is defined by the occurrence of venous or arterial thromboses, often multiple or recurrent fetal losses, frequently accompanied by a moderate thrombocytopenia in the presence of antiphospholipid antibodies (aPL). At least one clinical criterion (vascular thrombosis or pregnancy morbidity), and one laboratory criterion [anticardiolipin antibodies (aCL), lupus anticoagulant (LA), or anti-β2-glycoprotein 1 antibodies(aβ2GP1)], had to be met for the classification of APS [1]. The IgG and IgM of aβ2GP1 assays were added to the newer revised criteria [2]. IgA (aCL and aβ2GP1) are not currently included in the laboratory criteria for APS, but it has been suggested to consider them as “noncriteria” antibodies for patients who are seronegative but have clinical suspicion of APS [2,3].
Medium and high titers of aCL antibodies (IgG and/or IgM) associate with clinical manifestations of APS were selected as criteria in the Sapporo classification criteria. However, the threshold used to distinguish moderate-high levels from low levels had no standard [2], and routinely used assays in the clinical settings, particularly enzyme-linked immunosorbent assay (ELISA), lack standardized kits, resulting in substantial variations in the antibody positivity between different laboratories [4–7].
The chemiluminescence technology has been used for auto-antibody testing [8–10], and the fully automated HemosIL AcuStar aPL assay panel has shown similar performance to commercial ELISA kits commonly used by various laboratories to detect antiphospholipid antibodies [8]. Van Hoecke et al. evaluated the panels for aCL and aβ2GP1 antibodies of an automated chemiluminescence assay (CIA), which was used in the laboratory for diagnosis of APS [11]. Zhang et al. found that the novel CIA assay had good performance in measuring aβ2GP1 and aCL, especially in the detection of aβ2GP1 IgG, and could shed insight on the application of CIA in the laboratory diagnosis of APS in China [7].
Recurrent fetal loss is one clinical manifestations of APS, and recurrent early miscarriage and fetal death have been associated with aPL [12]. We aimed to study the availability of CIA in diagnosis of APS, especially for recurrent fetal loss; thus, the cohort of patients in our study represented mainly patients with recurrent fetal losses.
Material and Methods
Patient population
Our study included a total of 185 patients with APS, systemic lupus erythematosus (SLE), infertility, connective tissue disease (CTD), and other conditions in Peking University Third Hospital. There were 105 consecutive patients who had at least one positive result for aCL (IgG or IgM) and aβ2GP1(IgG or IgM), others had negative results. Study protocols were reviewed and approved by the Ethical Committee of Peking University Third Hospital and informed consents were obtained from all participants.
Serum antibodies determination
All sera were stored at −20°C until analysis. Serum aCL auto-antibodies (IgG, IgM) and aβ2GP1(IgG, IgM) were determined by both ELISA (EUROIMMUN, Germany) and CIA (QUANTA Flash® assays, INOVA Diagnostic, Inc.). The QUANTA Flash assays were performed on BIO-FLASH® instrument (Biokit S.A., Barcelona, Spain). The principle of the QUANTA Flash assay system was previously described by Mahler et al. [13] and Bentow et al. [14]. Serum aCL and aβ2GP1 IgM were retested using AESKU (AESKU.diagnostics, Germany) ELISA kits. All the methods were performed in accordance with the manufacturers’ recommended methodology. The cutoff values for positivity were set based on the recommendations of the respective manufacturer.
Statistical analysis
Data were analyzed using SPSS Statistics 19 (SPSS, Inc., Chicago, IL, USA). Comparison of CIA and ELISA was performed based on 2×2 contingency tables. Positive coincidence rate and total coincidence rate were calculated and expressed in percentage (%). Spearman correlation test were performed to analyze the qualitative and quantitative agreement between ELISA and CIA.
Results
The study population was divided into a serum positive group and a serum negative group depending on the EUROIMMUN ELISA results. The clinical characteristics are displayed in Table 1. All patients were female; pregnancy morbidity or infertility was seen in 71.4% and 65.7% of the seropositive and seronegative groups respectively.
Table 1.
Clinical characteristics.
| Group | APS | SLE | Infertility | CTD | Others | ||
|---|---|---|---|---|---|---|---|
| Pregnancy morbidity | Thrombosis | ||||||
| Serum positive | Number (all Female) | 31 | 12 | 7 | 44 | 4 | 7 |
| Median age (min, max) | 33 (24, 44) | 34 (25, 70) | 31 (23, 56) | 32 (22, 41) | 29.5 (29, 56) | 50 (27, 70) | |
| Serum negative | Number (all Female) | 34 | 1 | 0 | 36 | 2 | 7 |
| Median age (min,max) | 33 (26,38) | 28 | - | 34 (23, 44) | 34.5 (34, 35) | 30 (27, 35) | |
APS – antiphospholipid syndrome; SLE – systemic lupus erythematosus; CTD – connective tissue disease. “Serum positive group” have at least one positive result for the aCL autoantibodies (IgG, IgM) and aβ2GP1 (IgG, IgM).
There was at least one positive result for the aCL (IgG, IgM) and aβ2GP1 (IgG, IgM) in the positive serum group. We tested the same serum samples for aβ2GP1 and aCL (IgG/IgM) using CIA. Then, we compare the 2 methods. The results obtained by CIA and ELISA are reported in Table 2.
Table 2.
The comparison of ELISA and CIA.
| CIA | Total | Coincidence rate | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| aCL IgG | ELISA | Positive | 15 | 1 | 16 | 93.75% (15/16) |
| Negative | 10 | 79 | 89 | 88.76% (79/89) | ||
| Total | 25 | 80 | 105 | 89.52% (94/105) | ||
| aCL IgM | ELISA | Positive | 5 | 7 | 12 | 41.67% (5/12) |
| Negative | 21 | 72 | 93 | 77.42% (72/93) | ||
| Total | 26 | 79 | 105 | 73.33% (77/105) | ||
| aβ2GP1 IgG | ELISA | Positive | 17 | 1 | 18 | 94.44% (17/18) |
| Negative | 11 | 76 | 87 | 87.36% (76/87) | ||
| Total | 28 | 77 | 105 | 88.57% (93/105) | ||
| aβ2GP1 IgM | ELISA | Positive | 11 | 69 | 80 | 13.75% (11/80) |
| Negative | 2 | 23 | 25 | 92.00% (23/25) | ||
| Total | 13 | 92 | 105 | 32.38% (34/105) | ||
ELISA kit is EUROIMMUN
Our results suggested that CIA had good performance in the detection of IgG (aCL and aβ2GP1), the positive coincidence rate of IgG (aCL and aβ2GP1) between ELISA and CIA reached to 93.75% (15 out of 16) and 94.44% (17 out of 18), the total coincidence rate also reach to 89.52% (94 out of 105) and 88.57% (93 out of 105), and the Spearman coefficients rho (aCL IgG between ELISA and CIA, aβ2GP1 IgG between ELISA and CIA) was 0.799 and 0.941 respectively, and a significant correlation was observed between the results from the 2 assays (P<0.01, P<0.01) (Figures 1, 2). But the positive coincidence rate of IgM (aCL and aβ2GP1) between ELISA and CIA only had 41.67% (5 out of 12) and 13.75% (11 out of 80), the total coincidence rate was 73.33% (77 out of 105) and 32.38% (34 out of 105) respectively.
Figure 1.
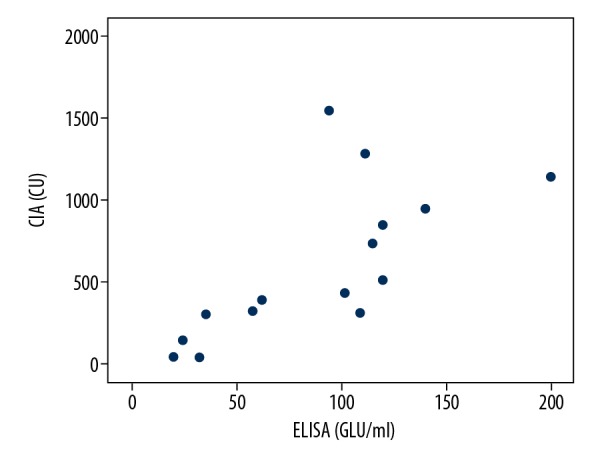
The positive aCL IgG correlation of ELISA and CIA. (Spearman rho=0.799), P<0.01. ELISA – enzyme-linked immunosorbent assay; CIA – chemiluminescence assay.
Figure 2.
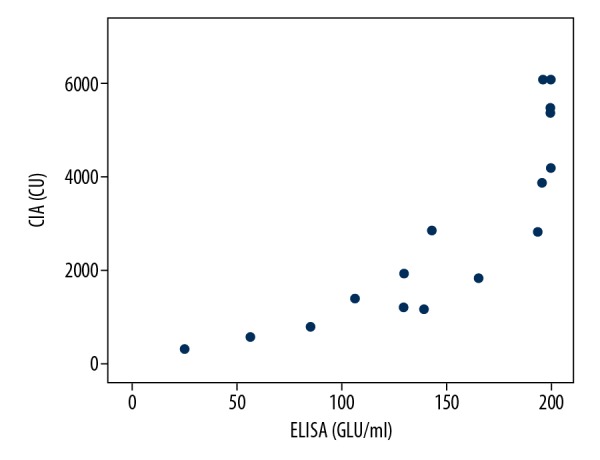
The positive aβ2GP1 IgG correlation of ELISA and CIA (Spearman rho=0.941), P<0.01. ELIS – enzyme-linked immunosorbent assay; CIA – chemiluminescence assay.
The ELISA positive rate of the seropositive group was between 11.43% to 17.14% for IgG (aCL and aβ2GP1) and aCL IgM, while the aβ2GP1 IgM reached 76.19%. The CIA positive rate of the seronegative group was between 23.81% and 26.67% for the IgG (aCL and aβ2GP1) and aCL IgM, which was significantly higher than the ELISA results, while the rate of aβ2GP1 IgM was significantly lower than the ELSIA results (Table 3). We retested the IgM levels using another ELISA kit, AESKU (AESKU.diagnostics, Germany), for which the aCL IgM or aβ2GP1 IgM was positive, and then we analyzed the coincidence rate and positive rate. We found that the positive coincidence rate of IgM (aCL and aβ2GP1) between ELISA (EUROIMMUN vs. AESKU) was 87.5% and 46.84%, the total coincidence rate is 74.71% and 45.98% respectively. The positive coincidence rate of that between CIA and AESKU was 68% and 84.62%, the total coincidence rate was 78.16% and 62.07% respectively (Tables 4, 5).
Table 3.
Positive and negative results obtained from seropositive patients and seronegative patients using ELISA and CIA methods.
| Seropositive patients | Seronegative patients | |||||
|---|---|---|---|---|---|---|
| N | % | n | % | |||
| ELISA | aCL IgG | Positive | 16 | 15.24 | 0 | 0.00 |
| Negative | 89 | 84.76 | 78 | 100.00 | ||
| aCL IgM | Positive | 12 | 11.43 | 0 | 0.00 | |
| Negative | 93 | 88.57 | 78 | 100.00 | ||
| aβ2GP1 IgG | Positive | 18 | 17.14 | 0 | 0.00 | |
| Negative | 87 | 82.86 | 78 | 100.00 | ||
| aβ2GP1 IgM | Positive | 80 | 76.19 | 0 | 0.00 | |
| Negative | 25 | 23.81 | 78 | 100.00 | ||
| CIA | aCL IgG | Positive | 25 | 23.81 | 2 | 2.56 |
| Negative | 80 | 76.19 | 76 | 97.44 | ||
| aCL IgM | Positive | 26 | 24.76 | 0 | 0.00 | |
| Negative | 79 | 75.24 | 78 | 100.00 | ||
| aβ2GP1 IgG | Positive | 28 | 26.67 | 1 | 1.28 | |
| Negative | 77 | 73.33 | 77 | 98.72 | ||
| aβ2GP1 IgM | Positive | 13 | 12.38 | 0 | 0.00 | |
| Negative | 92 | 87.62 | 78 | 100.00 | ||
ELISA kits is EUROIMMUN
Table 4.
The comparison of ELISA (EUROIMMUN vs. AESKU).
| AESKU | Total | Coincidence rate | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| aCL IgM | EUROIMMUN | Positive | 7 | 1 | 8 | 87.50% (7/8) |
| Negative | 21 | 58 | 79 | 73.42% (58/79) | ||
| Total | 28 | 59 | 87 | 74.71% (65/87) | ||
| aβ2GP1 IgM | EUROIMMUN | Positive | 37 | 42 | 79 | 46.84% (37/79) |
| Negative | 5 | 3 | 8 | 37.50% (3/8) | ||
| Total | 42 | 45 | 87 | 45.98% (40/87) | ||
Table 5.
The comparison of ELISA (AESKU) and CIA.
| AESKU | Total | Coincidence rate | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| aCL IgM | EUROIMMUN | Positive | 17 | 8 | 25 | 68.00% (17/25) |
| Negative | 11 | 51 | 62 | 82.26% (51/62) | ||
| Total | 28 | 59 | 87 | 78.16% (68/87) | ||
| aβ2GP1 IgM | EUROIMMUN | Positive | 11 | 2 | 13 | 84.62% (11/13) |
| Negative | 31 | 43 | 74 | 58.11% (43/74) | ||
| Total | 42 | 45 | 87 | 62.07% (54/87) | ||
Discussion
ELISA is commonly used to identify aCL and aβ2GP1 antibodies, but it is time-consuming, laborious, and has poor repeatability. Whereas, it may take only about 30 minutes to complete a test with CIA, a fully automated technique, which might reduce operator handling and save time. Automation can also improve the reproducibility of results and reduce inter-laboratory and intra-laboratory variability [10,15]. The performance of CIA has been evaluated in several studies [3,7,10].
There are some patients who have negative laboratory criteria for APS but present with typical clinical manifestations of APS, and these patients are called seronegative patients [16,17]. They are not included in APS criteria, which might partly be due to the poor performance of tests that result in negative laboratory results including ELISA assays [6,16,17]. Therefore, it is very important to standardize ELISA testing; and many attempts have been made including several published recommendations [10,18–25], but there is no standardized ELISA methodology available, and the variability of inter- and intra-laboratory testing is still high [26–28].
Our results suggested that CIA could show good results for IgG isotype of aCL and aβ2GP1 for positive coincidence rate and total coincidence rate. However, the performance of aCL IgM and aβ2GP1 IgM were not as good, thus, we retested the IgM (aCL and aβ2GP1) using another ELISA kit, the AESKU kit (Tables 4, 5). We found that AESKU and EUROIMMUN kits showed good coincidence in aCL IgM, with the positive coincidence rate and the total coincidence rate of 87.50% (7 out of 8) and 74.71% (65 out of 87) respectively, and between AESKU and CIA the rates were 68.00% (17 out of 25) and 78.16% (68 out of 87) respectively. Furthermore, we observed that the positive rates of aCL IgM for AESKU and CIA were higher than the rates for EUROIMMUN, which may indicate better sensitivity. There were differences in the commercial ELISA kits: the ELISA plate of the EUROIMMUN kit was coated with cardiolipin and the important cofactor β2GP1 was added into the sample diluent, whereas other kits were coated with a compound of β2GP1 and cardiolipin, which may explain some of the differences in results.
AESKU and CIA had a higher coincidence rate than AESKU and EUROIMMUN in aβ2GP1 IgM; the positive coincidence numbers for EUROIMMUN, AESKU, and CIA were 79, 42, and 13, respectively. The positive coincidence numbers for AESKU and EUROIMMUN were obviously more than that of AESKU and CIA, suggesting that these assays might lead to poor coincidence rate. Significant inter-laboratory variability with the performance of the aβ2GP1 ELISA might reside in the inconsistent exposure of residues 40 and 43 on domain I when β2GP1 is coated onto various commercial ELISA plates [29,30]. aβ2GP1 may specifically bind to the domain IV/V of β2GP1 or targeted domain I [31–33], while different commercial kits may differ in targeting different domains, thus leading to different results.
However, IgM is less often associated with thrombosis than IgG [34]. Some studies have demonstrated a significant correlation of antibody titers and agreement between the ELISA and CIA and found that the correlation coefficient for aCL IgG and aβ2GP1 IgG was higher than those of “M” isotype antibodies. Thus, agreement of the IgG isotype between the 2 methods would be expected to be superior, and, in fact, the IgG isotype is generally considered more clinically relevant for APS [10,35–37]. Our results indicated that CIA was suitable for detecting aCL and aβ2GP1 antibodies, especially for the IgG isotype. CIA may provide an alternative to time-consuming conventional ELISA method.
An appropriate low/medium antibody threshold is important for clinical administration to APS patients. Lakos et al. described a clinical approach for establishing the low/medium antibody threshold for aPL antibody assays, and successfully employed it to define 95 and 31 CU respectively (i.e., the equivalent of 40 GPL and MPL units), as the low/medium cutoff point for QUANTA Flash aCL IgG and IgM results [38]. However, the 99th percentile often defines values which are significantly different from the recommended 40 GPL or MPL units. The 99th percentile cutoff level seems more sensitive than the >40 GPL value for APS classification, as it includes patients with aCL positivity alone as well as patients with pregnancy morbidity [5]. However, the variability from different kits will make the cutoff difficult to standardized, thus we need more effort in developing standardization.
Conclusions
The new automated chemiluminescent immunoassay is suitable for detecting aCL and aβ2GP1 antibodies, especially the IgG isotype; and it may provide an alternative to time-consuming conventional ELISA method.
Footnotes
Source of support: This work was supported by National Natural Science Foundation of China (grant numbers 61221077)
Conflict of interest
None.
References
- 1.Gomez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun. 2014;48–49:20–25. doi: 10.1016/j.jaut.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertolaccini ML, Amengual O, Atsumi T, et al. ‘Non-criteria’ aPL tests: Report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;20:191–205. doi: 10.1177/0961203310397082. [DOI] [PubMed] [Google Scholar]
- 4.Devreese KM. Standardization of antiphospholipid antibody assays. Where do we stand? Lupus. 2012;21:718–21. doi: 10.1177/0961203312439335. [DOI] [PubMed] [Google Scholar]
- 5.Ruffatti A, Olivieri S, Tonello M, et al. Influence of different IgG anticardiolipin antibody cut-off values on antiphospholipid syndrome classification. J Thromb Haemost. 2008;6:1693–96. doi: 10.1111/j.1538-7836.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 6.Forastiero R, Papalardo E, Watkins M, et al. Evaluation of different immunoassays for the detection of antiphospholipid antibodies: Report of a wet workshop during the 13th International Congress on Antiphospholipid Antibodies. Clin Chim Acta. 2014;428:99–105. doi: 10.1016/j.cca.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Wu Z, Li P, et al. Evaluation of the clinical performance of a novel chemiluminescent immunoassay for detection of anticardiolipin and anti-beta2-Glycoprotein 1 antibodies in the diagnosis of antiphospholipid syndrome. Medicine. 2015;94:e2059. doi: 10.1097/MD.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Moerloose P, Reber G, Musial J, Arnout J. Analytical and clinical performance of a new, automated assay panel for the diagnosis of antiphospholipid syndrome. J Thromb Haemost. 2010;8:1540–46. doi: 10.1111/j.1538-7836.2010.03857.x. [DOI] [PubMed] [Google Scholar]
- 9.Chung Y, Kim JE, Lim HS, Kim HK. Clinical performance of anticardiolipin and antibeta2 glycoprotein I antibodies using a new automated chemiluminescent assay: Superior thrombotic prediction of combined results measured by two different methods. Blood Coagul Fibrinolysis. 2014;25:10–15. doi: 10.1097/MBC.0b013e32836466b5. [DOI] [PubMed] [Google Scholar]
- 10.Meneghel L, Ruffatti A, Gavasso S, et al. The clinical performance of a chemiluminescent immunoassay in detecting anti-cardiolipin and anti-beta2 glycoprotein I antibodies. A comparison with a homemade ELISA method. Clin Chem Lab Med. 2015;53:1083–89. doi: 10.1515/cclm-2014-0925. [DOI] [PubMed] [Google Scholar]
- 11.Van Hoecke F, Persijn L, et al. Performance of two new, automated chemiluminescence assay panels for anticardiolipin and anti-beta2-glycoprotein I antibodies in the laboratory diagnosis of the antiphospholipid syndrome. Int J Lab Hematol. 2012;34:630–40. doi: 10.1111/j.1751-553X.2012.01448.x. [DOI] [PubMed] [Google Scholar]
- 12.Levy RA, Dos Santos FC, de Jesus GR, de Jesus NR. Antiphospholipid antibodies and antiphospholipid syndrome during pregnancy: Diagnostic concepts. Front Immunol. 2015;6:205. doi: 10.3389/fimmu.2015.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahler M, Radice A, Yang W, et al. Development and performance evaluation of novel chemiluminescence assays for detection of anti-PR3 and anti-MPO antibodies. Clin Chim Acta. 2012;413:719–26. doi: 10.1016/j.cca.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Bentow C, Swart A, Wu J, et al. Clinical performance evaluation of a novel rapid response chemiluminescent immunoassay for the detection of autoantibodies to extractable nuclear antigens. Clin Chim Acta. 2013;424:141–47. doi: 10.1016/j.cca.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Andreoli L, Rizzini S, Allegri F, et al. Are the current attempts at standardization of antiphospholipid antibodies still useful? Emerging technologies signal a shift in direction. Semin Thromb Hemost. 2008;34:356–60. doi: 10.1055/s-0028-1085478. [DOI] [PubMed] [Google Scholar]
- 16.Conti F, Capozzi A, Truglia S, et al. The mosaic of “seronegative” antiphospholipid syndrome. J Immunol Res. 2014;2014 doi: 10.1155/2014/389601. 389601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes GR, Khamashta MA. Seronegative antiphospholipid syndrome. Ann Rheum Dis. 2003;62:1127. doi: 10.1136/ard.2003.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favaloro EJ, Plebani M, Lippi G. Standardization and harmonization of antiphospholipid antibody assays. Semin Thromb Hemost. 2014;40:161–62. doi: 10.1055/s-0033-1364184. [DOI] [PubMed] [Google Scholar]
- 19.Reber G, Tincani A, Sanmarco M, et al. Proposals for the measurement of anti-beta2-glycoprotein I antibodies. Standardization group of the European Forum on Antiphospholipid Antibodies. J Thromb Haemost. 2004;2:1860–62. doi: 10.1111/j.1538-7836.2004.00910.x. [DOI] [PubMed] [Google Scholar]
- 20.Tincani A, Allegri F, Balestrieri G, et al. Minimal requirements for antiphospholipid antibodies ELISAs proposed by the European Forum on antiphospholipid antibodies. Thromb Res. 2004;114:553–58. doi: 10.1016/j.thromres.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 21.Wong RC, Gillis D, Adelstein S, et al. Consensus guidelines on anti-cardiolipin antibody testing and reporting. Pathology. 2004;36:63–68. doi: 10.1080/00313020310001643615. [DOI] [PubMed] [Google Scholar]
- 22.Wong RC, Favaloro EJ, Adelstein S, et al. Consensus guidelines on anti-beta 2 glycoprotein I testing and reporting. Pathology. 2008;40:58–63. doi: 10.1080/00313020701717720. [DOI] [PubMed] [Google Scholar]
- 23.Harris EN, Pierangeli SS. Revisiting the anticardiolipin test and its standardization. Lupus. 2002;11:269–75. doi: 10.1191/0961203302lu202cr. [DOI] [PubMed] [Google Scholar]
- 24.Lakos G, Favaloro EJ, Harris EN, et al. International consensus guidelines on anticardiolipin and anti-beta2-glycoprotein I testing: Report from the 13th International Congress on Antiphospholipid Antibodies. Arthritis Rheum. 2012;64:1–10. doi: 10.1002/art.33349. [DOI] [PubMed] [Google Scholar]
- 25.Pierangeli SS, Favaloro EJ, Lakos G, et al. Standards and reference materials for the anticardiolipin and anti-beta2glycoprotein I assays: A report of recommendations from the APL Task Force at the 13th International Congress on Antiphospholipid Antibodies. Clin Chim Acta. 2012;413:358–60. doi: 10.1016/j.cca.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 26.Pierangeli SS, Harris EN. A quarter of a century in anticardiolipin antibody testing and attempted standardization has led us to here, which is? Semin Thromb Hemost. 2008;34:313–28. doi: 10.1055/s-0028-1085473. [DOI] [PubMed] [Google Scholar]
- 27.Reber G, Boehlen F, de Moerloose P. Technical aspects in laboratory testing for antiphospholipid antibodies: Is standardization an impossible dream? Semin Thromb Hemost. 2008;34:340–46. doi: 10.1055/s-0028-1085476. [DOI] [PubMed] [Google Scholar]
- 28.Wong R, Favaloro E, Pollock W, et al. A multi-centre evaluation of the intra-assay and inter-assay variation of commercial and in-house anti-cardiolipin antibody assays. Pathology. 2004;36:182–92. doi: 10.1080/00313020410001672037. [DOI] [PubMed] [Google Scholar]
- 29.Pelkmans L, Kelchtermans H, de Groot PG, et al. Variability in exposure of epitope G40-R43 of domain i in commercial anti-beta2-glycoprotein I IgG ELISAs. PLoS One. 2013;8:e71402. doi: 10.1371/journal.pone.0071402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krilis SA, Giannakopoulos B. Laboratory methods to detect antiphospholipid antibodies. Hematology Am Soc Hematol Educ Program. 2014;2014:321–28. doi: 10.1182/asheducation-2014.1.321. [DOI] [PubMed] [Google Scholar]
- 31.Andreoli L, Nalli C, Motta M, et al. Anti-beta(2)-glycoprotein I IgG antibodies from 1-year-old healthy children born to mothers with systemic autoimmune diseases preferentially target domain 4/5: Might it be the reason for their ‘innocent’ profile? Ann Rheum Dis. 2011;70:380–83. doi: 10.1136/ard.2010.137281. [DOI] [PubMed] [Google Scholar]
- 32.de Laat B, Derksen RH, van Lummel M, et al. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood. 2006;107:1916–24. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 33.Fickentscher C, Magorivska I, Janko C, et al. The Pathogenicity of Anti-beta2GP1-IgG Autoantibodies Depends on Fc Glycosylation. J Immunol Res. 2015;2015 doi: 10.1155/2015/638129. 638129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devreese KM. Antiphospholipid antibody testing and standardization. Int J Lab Hematol. 2014;36:352–63. doi: 10.1111/ijlh.12234. [DOI] [PubMed] [Google Scholar]
- 35.Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003;101:1827–32. doi: 10.1182/blood-2002-02-0441. [DOI] [PubMed] [Google Scholar]
- 36.Galli M, Reber G, de Moerloose P, de Groot PG. Invitation to a debate on the serological criteria that define the antiphospholipid syndrome. J Thromb Haemost. 2008;6:399–401. doi: 10.1111/j.1538-7836.2008.02862.x. [DOI] [PubMed] [Google Scholar]
- 37.Galli M, Luciani D, Bertolini G, Barbui T. Anti-beta 2-glycoprotein I, antiprothrombin antibodies, and the risk of thrombosis in the antiphospholipid syndrome. Blood. 2003;102:2717–23. doi: 10.1182/blood-2002-11-3334. [DOI] [PubMed] [Google Scholar]
- 38.Lakos G, Bentow C, Mahler M. A clinical approach for defining the threshold between low and medium anti-cardiolipin antibody levels for QUANTA flash assays. Antibodies. 2016:5. doi: 10.3390/antib5020014. [DOI] [PMC free article] [PubMed] [Google Scholar]


