Abstract
Background
Vertebral hemangioma is usually a benign and asymptomatic tumor of blood vessels, but can be aggressive (symptomatic) with expansion, pain, and spinal cord compression. The aim of this study was to review the effects of radiotherapy, surgery, and other treatment approaches in patients with aggressive vertebral hemangioma.
Material/Methods
Retrospective clinical review included 20 patients who underwent radiotherapy as their first-line treatment for aggressive vertebral hemangioma with mild or slowly developing neurological deficit. External radiation was divided into 20–25 fractions with a total dose of 40–50 Gy. Minimum clinical follow-up after treatment was 20 months.
Results
The 20 patients included eight men and 12 women (mean age, 46.6 years), with aggressive vertebral hemangioma located in the cervical, thoracic, and lumbar vertebrae in four, 14, and two patients, respectively. Following radiotherapy treatment, 65.0% of patients (13/20) were symptom-free, without recurrence or malignant transformation at the time of last clinical follow-up (average, 75.2 months). Due to minor post-radiation vertebral re-ossification, two of the 13 patients who were initially symptom-free after radiotherapy requested percutaneous vertebroplasty. A further seven patients required surgery after radiotherapy, due to increasing neurological deficit in three patients, and persistent neurological deficit in four patients. At the last follow-up (average, 63.6 months), six patients were symptom-free, and one patient still had slight residual symptoms.
Conclusions
Radiotherapy was a safe and effective treatment choice for aggressive vertebral hemangioma, but in case with severe spinal cord compression and neurological deficit, surgical intervention was required.
MeSH Keywords: Cementoplasty, Hemangioma, Orthopedic Procedures, Radiotherapy, Spine
Background
Vertebral hemangioma is a rare, benign vascular tumor of the vertebral body that is usually asymptomatic, but which can behave in a locally aggressive way by involving bone and local spread into the vertebral canal to cause spinal cord compression. Between 0.9–1.2% of vertebral hemangiomas are aggressive, or symptomatic [1–6], with aggressive vertebral hemangioma extending into the spinal canal and/or paravertebral space, leading to neurological deficit [2,4,7–12]. The choice of treatment for aggressive vertebral hemangioma is controversial, and in the literature, most previous reports have focussed on surgical management [2,4,7,8,11–14].
Few previous studies have investigated the effectiveness of radiotherapy in the treatment of aggressive vertebral hemangioma, and the outcome of radiation treatment [6,15–23]. There is also controversy regarding whether radiation therapy can induce malignant transformation in benign vertebral hemangioma [24,25].
In our clinical practice, radiotherapy is usually the first choice in patients with aggressive vertebral hemangioma who have mild or slowly developing neurological deficit. The aim of this retrospective study was to review the effects of radiotherapy, surgery, and other treatment approaches in patients with aggressive vertebral hemangioma.
Material and Methods
Patients
Sixty-one patients underwent treatment in the Orthopedic Department of our hospital from 2001 to 2016 for aggressive vertebral hemangioma, including 20 patients who underwent radiotherapy as their first-line treatment in our hospital. Patients with surgery as the first-line treatment have been previously described, and most of the patients treated before 2001 were lost during follow-up because of frequent changes to their contact details. This study was approved by the local Ethics Committee of our hospital, and the study was conducted according to the principles of the Declaration of Helsinki. Because this was a retrospective study, informed consent was waived.
Patient data
Patient data were acquired from office charts, pathology reports, and radiology reports. The clinical and demographic data collected included age, sex, symptoms, radiological features before and after treatment, radiotherapy treatment and results, surgical records, and treatment complications. Neurological function was assessed by the modified Frankel Scale. Pain was evaluated for each patient using a visual analog scale (VAS). The inclusion criteria were that radiotherapy was the initial treatment and the minimal follow-up was 18 months.
Imaging and biopsy
Radiological studies included posteroanterior and lateral spinal radiography, computed tomography (CT), and magnetic resonance imaging (MRI). Typical lesions of vertebral hemangioma had vertical trabeculation and a honeycomb appearance on CT and a ‘salt and pepper’ appearance on MRI (Figure 1). In cases with atypical images, CT-guided biopsy was indicated and performed by local interventional radiologists. The ratios of the vertebral canal encroachment of the aggressive vertebral hemangioma lesions were measured on the median sagittal plane on the MRI (Figure 2).
Figure 1.
Computed tomography (CT) and magnetic resonance imaging (MRI) of aggressive vertebral hemangioma (VH). (A) The typical appearance of vertebral hemangioma (VH) on axial computed tomography (CT) reconstruction. (B) The typical appearance of vertebral hemangioma (VH) on sagittal CT reconstruction. (C) The typical appearance of vertebral hemangioma (VH) on axial magnetic resonance imaging (MRI). (D) The typical appearance of vertebral hemangioma (VH) on sagittal MRI.
Figure 2.

The measurement of spinal canal encroachment due to aggressive vertebral hemangioma (VH).
Treatment protocol for aggressive vertebral hemangioma
Radiotherapy was prioritized when aggressive vertebral hemangiomas were accompanied by mild or slowly developing neurological deficit (muscle strength grade greater than 3/5). Lesions with bony destruction were evaluated using the spine instability (SIN) scores [26], as either stable, suspected unstable requiring orthoses, or unstable requiring vertebroplasty or surgery. The benefits and risks of radiotherapy were discussed with the patients, and surgical treatment was provided as an alternative. For adolescent patients, the long-term risks of radiotherapy were discussed with the patients and their parents. Surgery was usually indicated for patients with severe or rapidly developing neurological deficits (muscle strength grade less than 3/5).
Radiotherapy was performed by local radiotherapy oncology specialists. The external radiation was divided into 20–25 fractions with a total dose of 40–50 Gy. Before 2012, three-dimensional conformal radiotherapy (3D-CRT) was used for the radiotherapy. Image-guided radiation therapy (IGRT) and intensity-modulated radiation therapy (IMRT) were used after 2012. The radiotherapy was delivered to each patient as an outpatient. During radiotherapy, steroids and mannitol were available for use, in case there was worsening of neurological deficit after the radiotherapy.
Surgical decompression was indicated if the neurological deficit worsened during or after radiotherapy, or neurological symptoms were not relieved at two-months follow-up. If radiology revealed no re-ossification after radiotherapy, percutaneous vertebroplasty was available, if indicated, to prevent vertebral fractures.
Clinical follow-up
X-ray images of the vertebra were obtained at three, six, and 12 months after the index procedure and then annually after that. MRI and CT were performed at the three-month follow-up and repeated annually. VAS and neurological recovery were recorded. If the patient had recurrent symptoms, immediate MRI and CT scans were performed. The minimum follow-up duration was 18 months.
Review of the literature: Search strategy
A brief review of the literature was performed of the current management of aggressive vertebral hemangioma in the PubMed, EBSCO, OVID, and Springer databases. The search criteria included publications within the last 20 years, using the following search terms: aggressive spinal or vertebral hemangioma, radiotherapy, radiosurgery, and associated treatment. Cases were selected based on a specific diagnosis of aggressive vertebral hemangioma, spinal cord compression, and neurological deficits with or without radiotherapy (Tables 1, 2).
Table 1.
A summary of the treatments for aggressive VHs based on the literature.
| Authors | No. of cases | Treatment |
|---|---|---|
| Fox et al. (1993) | 59 | Surgery (1 total resection, 10 subtotal resections, 2 preoperative embolizations radiotherapies, 5 adjuvant radiotherapies) |
| Pastushyn et al. (1998) | 86 | Surgery (64 laminectomies or extensive laminectomies with adjuvant radiotherapy for subtotal resection cases) |
| Doppman et al. (2000) | 11 | CT-guide injections of ethanol |
| Kato et al. (2010) | 5 | Surgery (5 preoperative embolizations and total excisions) |
| Acosta et al. (2011) | 10 | Surgery (10 preoperative embolizations and intralesional spondylectomies without adjuvant radiotherapy) |
| Singh et al. (2011) | 10 | Surgery (10 decompressions with intraoperative ethanol embolization) |
| Goldstein et al. (2015) | 68 | Surgery (33 preoperative embolizations; 17 palliative decompressions, including 3 with adjuvant radiotherapy; 37 intralesional spondylectomies, including 2 with radiotherapy; 7 en bloc spondylectomies including 1 with radiotherapy; 7 surgeries without details) |
| Asthana et al. (1990) | 17 | Radiotherapy with 35–40 Gy (17 patients with pain, including 9 paraplegic patients) |
| Sakata et al. (1997) | 14 | Radiotherapy with 36 Gy (13 patients with pain, including 2 with neurological deficits) |
| Beyzadeoglu et al. (2002) | 29 | Radiotherapy with 20 to 30 Gy (29 cases with pain) |
| Aksu et al. (2008) | 1 | Radiotherapy (1 case with spinal cord compression) |
| Grau et al. (2009) | 1 | Radiotherapy (1 case with a giant spinal hemangioma causing myelopathy) |
| Aich et al. (2010) | 7 | Radiotherapy (7 patients with pain and neurological deficits) |
| Bellomia et al. (2010) | 1 | Radiotherapy and embolization (1 patient with an aggressive and compressive hemangioma) |
| Hyde et al. (2010) | 84 | Radiotherapy with median 34 Gy (84 cases including 97.6% cases with pain and 28.6% neurological symptoms) |
| Tarantino et al. (2015) | 1 | Dissatisfying radiotherapy followed by surgery (1 case with neurological deficits |
| Sewell et al. (2016) | 1 | Radiotherapy with 40 Gy (1 case with spinal cord compression and neurological deficits) |
| Zhang et al. (2017) | 5 | Stereotactic radiosurgery (4 presented with a chief complaint of pain refractory to conservative measures. Three patients reported dysesthesias, and 2 reported upper-extremity weakness) |
Table 2.
Reports summarizing radiotherapy for aggressive vertebral hemangiomas.
| Authors | No. of patinets | Treatment | Results | Further treatment after ineffective radiotherapy |
|---|---|---|---|---|
| Asthana et al. (1990) | 17 | Radiotherapy with 35–40 Gy (17 patients with pain, including 9 paraplegic patients) | All patients with pain and tenderness were relieved completely (87.5%) or partially (12.5%). Out of 9 paraplegic patients, 6 (66.6%) had recovered completely, 1 (11.2%) partially and 2 (22.2%) had no response | Not mentioned (−) |
| Sakata et al. (1997) | 14 | Radiotherapy with 36 Gy (13 patients with pain, including 2 with neurological deficits) | Thirteen patients with pain were completely or partially relieved. Two patients with neurological deficits treated with decompressive laminectomy followed by radiotherapy recovered completely after irradiatio | – |
| Beyzadeoglu et al. (2002) | 29 | Radiotherapy with 20 to 30 Gy (29 patients with pain) | Reasonable pain relief was obtained in all 29 patients | – |
| Aksu et al. (2008) | 1 | Radiotherapy (1 case with spinal cord compression and neurological deficits) | Patient’s symptoms improved significantly. | – |
| Grau et al. (2009) | 1 | Radiotherapy (1 case with a giant spinal hemangioma causing myelopathy) | The patient showed a complete regression of symptoms with stable condition after 3 months | – |
| Aich et al. (2010) | 7 | Radiotherapy (7 patients with pain and neurological deficits) | Pain relief with improvement of quality of life was obtained in all the patients | – |
| Bellomia et al. (2010) | 1 | Radiotherapy and embolization (1 patient with an aggressive and compressive hemangioma) | The patient experienced symptom relief | – |
| Hyde et al. (2010) | 84 | Radiotherapy with median 34 Gy (84 cases, including 82 cases with pain and 24 cases with neurological symptoms) | Complete symptom remission occurred in 61.9% of patients, 28.6% of patients had partial pain relief, and 9.5% of patients had no pain relief. Neurological symptoms other than pain completely resolved in 79.2% of patients, while a partial response was observed 20.8% of patients | – |
| Tarantino et al. (2015) | 1 | Dissatisfying radiotherapy followed by surgery (1 case with neurological deficits | The patient worsened at the 5th fraction. | Surgery (T6–T7 corporectomy with a titanium expandable cage implant) |
| Sewell et al. (2016) | 1 | Radiotherapy with 40 Gy (1 case with spinal cord compression and neurological deficits) | After 1 year, the patient had improved leg strength (MRC grade 4), was mobilized with walking aids for short distances, and had intact bladder and bowel function | – |
| Zhang et al. (2017) | 5 | Stereotactic radiosurgery (4 presented with a chief complaint of pain refractory to conservative measures. Three patients reported dysesthesias, and 2 reported upper-extremity weakness) | Three patients experienced complete relief. One had partial relief and 1 was stable | A secondary radiotherapy for the patient with partial relief. |
Results
Patient information
The retrospective clinical review included 20 patients who underwent radiotherapy as their first-line treatment for symptomatic vertebral hemangioma with mild or slowly developing neurological deficit, and included eight men and 12 women. The age at diagnosis ranged from 16–72 years, with a mean age of 46.6 years. Of the 20 patients treated at our hospital, three patients had undergone surgical decompression at other hospitals, with recurrence of the hemangiomas. The aggressive vertebral hemangiomas were located in the cervical, thoracic, and lumbar vertebrae in four, 14, and two patients, respectively. Five patients had multiple vertebral hemangiomas with only a single aggressive lesion. All aggressive vertebral hemangiomas included epidural extension on magnetic resonance imaging (MRI).
The average duration of symptoms before presentation was 6.9 months (range, 1–17 months). Eighteen patients had Frankel D grade myelopathy, including five patients with local pain. The other two patients had Frankel E grade myelopathy with pain and radiculopathy. Two patients had a mild pathological vertebral fracture. All the spine lesions were stable or suspected unstable according to the spine instability (SIN) scores. Three illustrative cases out of the 20 cases in the study are presented in Figures 3–5.
Figure 3.
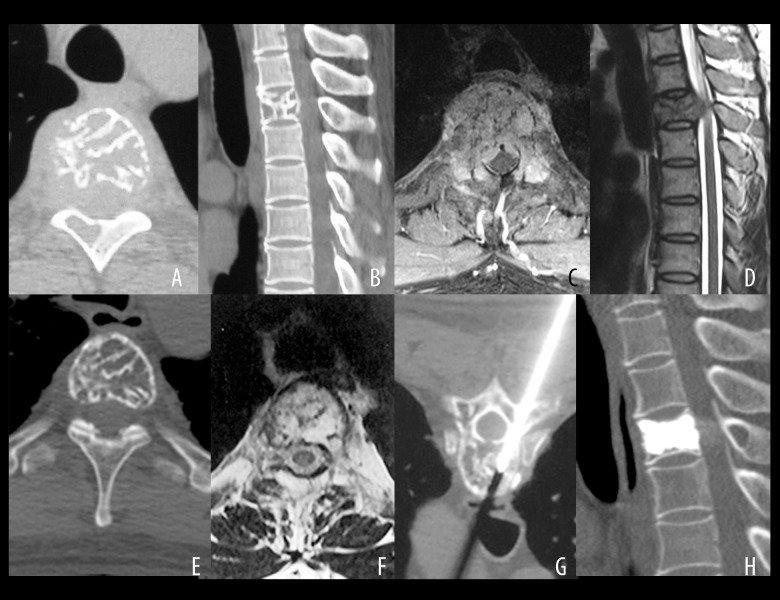
A case of a 43-year-old man with mild myelopathy and pain for four months (Frankel grade of D) showing vertebral computed tomography (CT) and magnetic resonance imaging (MRI) and histopathology following radiotherapy for aggressive vertebral hemangioma (VH). A 43-year-old man experienced mild myelopathy and pain for four months (Frankel grade D). After percutaneous biopsy, he underwent radiotherapy with 40 Gy. Complete neurological recovery was achieved one year after radiotherapy with minor re-ossification, requiring vertebroplasty 60 months after radiotherapy because of a suspicion of a possible pathological fracture. (A) Axial computed tomography (CT) scan of an osteolytic lesion in the vertebral body and right lamina of T4. (B) Sagittal CT scan of an osteolytic lesion in the vertebral body and right lamina of T4. (C) Axial magnetic resonance imaging (MRI) showing epidural compression with vertebral canal stenosis (33.4%). (D) Sagittal MRI shows epidural compression with vertebral canal stenosis (33.4%). (E) Slight re-ossification on disappearance of epidural compression 60 months after radiotherapy. (F) Slight re-ossification upon disappearance of epidural compression 60 months after radiotherapy. (G) Negative pathology results following percutaneous biopsy and vertebroplasty. (H) Axial CT scan at the 108 months of follow-up showing no recurrence of the hemangioma.
Figure 4.
A case of a 29-year-old man who experienced slowly progressive myelopathy and pain for five months (Frankel grade D) showing vertebral computed tomography (CT) and magnetic resonance imaging (MRI) and histopathology following radiotherapy for aggressive vertebral hemangioma (VH). A 29-year-old male patient underwent laminectomy with intraoperative vertebroplasty, two months after radiotherapy (40 Gy divided into 20 fractions) with no alleviation of neurological function. (A) Axial computed tomography (CT) image showing a lesion in T3 with bony mass in the mid-line of the lamina. (B) Sagittal CT image showing a lesion in T3 with bony mass in the mid-line of the lamina. (C) Axial magnetic resonance imaging (MRI) showing the rates of the vertebral canal encroachment at 43.7%. (D) Sagittal MRI showing rates of the vertebral canal encroachment at 43.7%. (E) Intraoperative injection of bone cement. (F) Intraoperative injection of bone cement. (G) X-radiograph at 51 months of follow-up. During this time, the patient was symptom-free without recurrence. (H) CT scan at 51 months of follow-up. During this time, the patient was symptom-free without recurrence.
Figure 5.
A case of a 16-year-old woman who underwent an incomplete vertebrectomy in another hospital for aggressive vertebral hemangioma (VH) presented with mild neurological deficit (Frankel grade D) in T10 without adjuvant radiotherapy treatment who had recurrent myelopathy and pain five months after primary surgery. A 16-year-old woman who underwent an incomplete vertebrectomy in another hospital for aggressive vertebral hemangioma was referred to our hospital 11 months after the index surgery, presenting with mild neurological deficit graded as Frankel D. Her height was 168cm and the long-term risks of radiotherapy were discussed with her and her parents. (A) Posterior-anterior (PA) X-radiography showing vertebral body osteolysis in T10 with implants. (B) Axial computed tomography (CT) showing vertebral body osteolysis in T10 with implants. (C) Sagittal CT showing vertebral body osteolysis in T10 with implants. (D) Axial magnetic resonance imaging (MRI) at the beginning of radiotherapy shows the epidural soft tissue mass. (E) Sagittal MRI at the beginning of radiotherapy shows the epidural soft tissue mass. The patients underwent radiotherapy (50 Gy, 20 fractions). (F–I) CT and MRI at four-year follow-up. The patient was symptom-free, and the soft tissue mass disappeared with re-ossification.
Radiotherapy
All 20 patients had radiotherapy as their primary treatment. Most of the radiotherapy was delivered as outpatient therapy. Following treatment with 40–50 Gy of radiotherapy, 65.0% of patients (13/20) were symptom-free, without recurrence or malignant transformation at the last clinical follow-up, which occurred at an average of 75.2 months (range, 18–193 months).
Re-ossification after radiotherapy
Sixteen patients had vertebral destruction due to vertebral hemangioma and of these, only 37.5% (6/16) had significant re-ossification at an average of 68.3 months following radiotherapy (Figure 5). Due to minor re-ossification, two of the 13 patients who were symptom-free after radiotherapy asked for percutaneous biopsy and vertebroplasty because they were concerned about the possibility of pathological fracture at 24 months and 60 months, respectively, following radiotherapy (Figure 3). The histopathology results of the biopsies were negative for malignancy. Following vertebroplasty, these patients were symptom-free without recurrence at 60 months and 108 months follow-up, respectively.
Surgery after radiotherapy
Seven other patients required surgery after radiotherapy. Preoperative vascular embolization was not used in any patient in the study. Neurological deficit persisted in four patients and worsened in three patients, even after conservative treatment with mannitol and steroids (Table 3). In these seven patients, neurological function was graded as Frankel D before radiotherapy and worsened to Frankel B and C after radiotherapy in one and two patients, respectively. Cervical spine lesions were present in one patient while six patients had thoracic spine lesions. All lesions involved the vertebral body and bilateral pedicles with epidural extension, including two cases with paravertebral involvements. The average duration between radiotherapy and surgery was 1.4 months (range, 1–2 months). Repeat MRI showed no progression of the tumor masses following radiotherapy.
Table 3.
General information of the patients who got surgery after radiotherapy.
| No | Gender | Age | Symptoms | Site | Dural compression | Frankel before Ra | Frankel after Ra | Duration between Ra and surgery (months) | Surgery | Follow-up (months) | Frankel at follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 41 | Mye+pain | T4 | Soft | D | E | 72 | PVP | 108 | E |
| 2 | Male | 38 | Mye | C2 | Soft | D | E | 24 | PVP | 60 | E |
| 3 | Male | 29 | Mye+pain | T3 | Bony+soft | D | D | 1 | De+VP | 51 | E |
| 4 | Male | 55 | Mye | T4 | Bony | D | D | 1 | De+VP | 120 | E |
| 5 | Female | 66 | Mye+pain | T12 | Bony | D | D | 2 | De | 72 | E |
| 6 | Female | 25 | Mye | T5 | Soft | D | C | 1 | De+VP | 20 | E |
| 7 | Male | 35 | Mye | T3 | Soft | D | B | 2 | De+VP | 33 | D |
| 8 | Female | 28 | Mye | C2–C4 | Soft+bony | D | C | 1 | De | 24 | E |
| 9 | Female | 63 | Mye | T11 | Bony | D | D | 60 | De+VP | 55 | E |
Ra – radiotherapy; Mye – myelopathy; PVP – percutaneous vertebroplasty; De – decompression; VP – vertebroplasty.
The surgery performed was posterior decompression with laminectomy, including five intraoperative vertebroplasties (Figure 4). Preoperative embolization was not mandatory. The postoperative pathological diagnosis remained that of benign hemangioma. The average surgery time was 245 min (range, 180–330 min). The average estimated blood loss was 801.4 mL (range, 260–2200 mL). At the last follow-up (average, 63.6 months; range, 20–120 months), six patients were symptom-free, and one patient still had mild symptoms (lower limb weakness) and was assigned a Frankel grade of D3.
Vertebral canal encroachment of epidural hemangioma
For the 13 patients with successful radiotherapy, the average ratio of the vertebral canal encroachment was 27.5% (range, 4.0–39.4%) on MRI at diagnosis. The spinal cord compression consisted of a soft tumor mass in 10 patients with additional small, bony compression in three patients. In the three patients who experienced neurological deterioration after radiotherapy, the rates of vertebral canal encroachment were 67.7%, 53.8%, and 63.2% (mean 61.6%), respectively. Two compressions consisted of soft tumor mass and one of combined soft tissue and bone. Also, the four patients showing no neurological improvement after radiotherapy mainly had bony compression, including one patient with vertebral fracture before radiotherapy and kyphosis due to fracture. The rates of the vertebral canal invasion in the four patients were 43.2%, 43.7%, 47.9%, and 52.1% (mean, 46.7%) (Figure 4).
Discussion
The treatment for aggressive (symptomatic) vertebral body hemangioma, including radiotherapy, ethanol injections, surgical decompression, intralesional vertebrectomy and en-bloc resection, have previously been reported to have good results (Table 1) [2–4,6–8,13–21,23]. According to the literature, the primary treatment method for aggressive vertebral hemangioma is surgery, while radiotherapy is usually suggested as adjuvant treatment. Minimally invasive procedures have been reported to be successful only in small lesions [13].
There have previously been few reports describing radiotherapy alone as the treatment of aggressive vertebral hemangioma, especially in cases with neurological deficit, and most of these previous publications were small case series or single case reports (Table 2) [15–23]. Hyde and coauthors [6] from seven major German institutions reviewed over 39 years of radiotherapy for 84 patients with symptomatic lesions of vertebral hemangioma, including 24 patients presenting with neurological symptoms, and showed an overall patient response rate of 90.5% after a median of 68 months of follow-up.
Although radiotherapy is usually safe and effective for aggressive vertebral hemangioma, it has some inherent drawbacks. First, the recovery of radiotherapy is relatively lower than that of surgery, which is reported to be almost 100%. In a report from Asthana et al. [15], 6 out of 9 patients who suffered neurological deficits caused by vertebral hemangioma were completely relieved of their symptoms (66.7%). In the 24 patients with vertebral hemangioma with neurologic symptoms, reported by Heyd et al. [6], neurological symptoms were completely resolved in 79.2% of patients. In a retrospective study of 137 patients with vertebral hemangioma experiencing pain (neurological deficits were not mentioned), Miszczyk et al. reported that 78.4% showed relief 18 months after radiotherapy [5].
Also, the recovery of neurological function is usually slow following treatment of vertebral hemangioma [10,19]. In a study by Aich et al. [20], radiotherapy was effective in six patients with vertebral hemangioma with neurological deficit. However, patients did not completely recover and their muscle strength took more than two years to improve. Zhang et al. studied the use of CyberKnife stereotactic radiosurgery for the treatment of vertebral hemangioma at Stanford University, and only four of five patients showed improvement during a mean period of one year [23].
Finally, the condition of some patients may worsen following radiotherapy. To our knowledge, the present study is one of the few to discuss this particular outcome. The reason for slow or unsatisfactory neurological recovery might be explained by the morphological response of vertebral hemangioma following radiotherapy. In the five cases reported from Stanford University, even with CyberKnife stereotactic radiosurgery, two patients had only between 20–40% reduction in lesion size in the most responsive dimension, as noted on imaging [23].
In the present study, seven out of the 20 cases experienced worse symptoms or did not experience symptom relief after radiotherapy. Patients experiencing severe neurologic compression (over 40%, especially with bony compression) most likely had unsatisfactory results after radiotherapy. The possible reason was assumed to be the edema associated with the vertebral hemangioma lesion after radiotherapy, which might lead to neurologic deterioration, potentially requiring surgical intervention.
Radiotherapy might partially repair bony destruction otherwise, secondary vertebral fracture is possible [22]. In the report by Heyd et al., the re-ossification rate was only 26.2% [6]. Miszczyk et al. reported that the re-ossification rate and/or fatty conversion rate was 33.3% [5]. Sakata et al. reported that there were no signs of re-ossification five years after radiotherapy in 14 cases of vertebral hemangioma [16]. In the present study, the re-ossification rate was 37.5%. In the report by Cloran et al., a single vertebral hemangioma case experienced pain and recurrence after radiotherapy because of a second vertebral fracture, which was corrected by percutaneous vertebroplasty [24]. In the present study, percutaneous vertebroplasty was provided for two patients.
This study had several limitations. This was a retrospective study that included a small number of patients from a single center. The evaluation of larger patient numbers and more detailed analysis of treatment approaches for patients with aggressive vertebral hemangioma is needed. Controlled, multicenter, large-scale clinical studies are required in future to provide the evidence base for the optimum treatment for patients with aggressive (symptomatic) vertebral hemangioma.
Conclusions
This retrospective clinical study included 20 patients with aggressive (symptomatic) vertebral hemangioma treated at a single center, which showed that first-line treatment with radiotherapy was a safe and effective treatment choice. In cases with severe spinal cord compression, characterized by a vertebral canal encroachment ratio >40%, neurologic deterioration might be found after radiotherapy, which requires further surgical intervention. Percutaneous vertebroplasty might be indicated after radiotherapy for vertebral hemangioma with severe bony destruction without re-ossification.
Footnotes
Source of support: This study was funded by the Peking University Third Hospital (Ref: Y71508-01)
Conflict of interest
Author Liang Jiang has received grants from Peking University Third Hospital. There is no other conflict of interest.
References
- 1.Slon V, Stein D, Cohen H, et al. Vertebral hemangiomas: their demographical characteristics, location along the spine and position within the vertebral body. Eur Spine J. 2015;24(10):2189–95. doi: 10.1007/s00586-015-4022-y. [DOI] [PubMed] [Google Scholar]
- 2.Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78(1):36–45. doi: 10.3171/jns.1993.78.1.0036. [DOI] [PubMed] [Google Scholar]
- 3.Pastushyn AI, Slin’ko EI, Mirzoyeva GM. Vertebral hemangiomas: diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol. 1998;50(6):535–47. doi: 10.1016/s0090-3019(98)00007-x. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein CL, Varga PP, Gokaslan ZL, et al. Spinal hemangiomas: results of surgical management for local recurrence and mortality in a multicenter study. Spine (Phila Pa 1976) 2015;40(9):656–64. doi: 10.1097/BRS.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 5.Miszczyk L, Tukiendorf A. Radiotherapy of painful vertebral hemangiomas: The single center retrospective analysis of 137 cases. Int J Radiat Oncol Biol Phys. 2012;82(2):e173–80. doi: 10.1016/j.ijrobp.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Heyd R, Seegenschmiedt MH, Rades D, et al. Radiotherapy for symptomatic vertebral hemangiomas: Results of a multicenter study and literature review. Int J Radiat Oncol Biol Phys. 2010;77(1):217–25. doi: 10.1016/j.ijrobp.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, Kawahara N, Murakami H, et al. Surgical management of aggressive vertebral hemangiomas causing spinal cord compression: Long-term clinical follow-up of five cases. J Orthop Sci. 2010;15(3):350–56. doi: 10.1007/s00776-010-1483-z. [DOI] [PubMed] [Google Scholar]
- 8.Acosta FL, Jr, Sanai N, Cloyd J, et al. Treatment of Enneking stage 3 aggressive vertebral hemangiomas with intralesional spondylectomy: Report of 10 cases and review of the literature. J Spinal Disord Tech. 2011;24(4):268–75. doi: 10.1097/BSD.0b013e3181efe0a4. [DOI] [PubMed] [Google Scholar]
- 9.Blecher R, Smorgick Y, Anekstein Y, et al. Management of symptomatic vertebral hemangioma: Follow-up of 6 patients. J Spinal Disord Tech. 2011;24(3):196–201. doi: 10.1097/BSD.0b013e3181e489df. [DOI] [PubMed] [Google Scholar]
- 10.Sewell MD, Dbeis R, Bliss P, et al. Radiotherapy for acute, high-grade spinal cord compression caused by vertebral hemangioma. Spine J. 2016;16(3):e195–96. doi: 10.1016/j.spinee.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L, Liu XG, Yuan HS, et al. Diagnosis and treatment of vertebral hemangiomas with neurologic deficit: A report of 29 cases and literature review. Spine J. 2014;14(6):944–54. doi: 10.1016/j.spinee.2013.07.450. [DOI] [PubMed] [Google Scholar]
- 12.Vasudeva VS, Chi JH, Groff MW. Surgical treatment of aggressive vertebral hemangiomas. Neurosurg Focus. 2016;41(2):E7. doi: 10.3171/2016.5.FOCUS16169. [DOI] [PubMed] [Google Scholar]
- 13.Doppman JL, Oldfield EH, Heiss JD. Symptomatic vertebral hemangiomas: Treatment by means of direct intralesional injection of ethanol. Radiology. 2000;214(2):341–48. doi: 10.1148/radiology.214.2.r00fe46341. [DOI] [PubMed] [Google Scholar]
- 14.Singh P, Mishra NK, Dash HH, et al. Treatment of vertebral hemangiomas with absolute alcohol (ethanol) embolization, cord decompression, and single level instrumentation: A pilot study. Neurosurgery. 2011;68(1):78–84. doi: 10.1227/NEU.0b013e3181fc60e9. discussion 84. [DOI] [PubMed] [Google Scholar]
- 15.Asthana AK, Tandon SC, Pant GC, et al. Radiation therapy for symptomatic vertebral haemangioma. Clin Oncol (R Coll Radiol) 1990;2(3):159–62. doi: 10.1016/s0936-6555(05)80151-7. [DOI] [PubMed] [Google Scholar]
- 16.Sakata K, Hareyama M, Oouchi A, et al. Radiotherapy of vertebral hemangiomas. Acta Oncol. 1997;36(7):719–24. doi: 10.3109/02841869709001344. [DOI] [PubMed] [Google Scholar]
- 17.Beyzadeoglu M, Dirican B, Oysul K, et al. Evaluation of radiation carcinogenesis risk in vertebral hemangioma treated by radiotherapy. Neoplasma. 2002;49(5):338–41. [PubMed] [Google Scholar]
- 18.Aksu G, Fayda M, Saynak M, Karadeniz A. Spinal cord compression due to vertebral hemangioma. Orthopedics. 2008;31(2):169. doi: 10.3928/01477447-20080201-02. [DOI] [PubMed] [Google Scholar]
- 19.Grau SJ, Holtmannspoetter M, Seelos K, et al. Giant multilevel thoracic hemangioma with spinal cord compression in a patient with Klippel-Weber-Trenaunay syndrome: Case report. Spine (Phila Pa 1976) 2009;34(14):E498–500. doi: 10.1097/BRS.0b013e3181a4e4b8. [DOI] [PubMed] [Google Scholar]
- 20.Aich RK, Deb AR, Banerjee A, et al. Symptomatic vertebral hemangioma: Treatment with radiotherapy. J Cancer Res Ther. 2010;6(2):199–203. doi: 10.4103/0973-1482.65248. [DOI] [PubMed] [Google Scholar]
- 21.Bellomia D, Viglianesi A, Messina M, et al. Vertebral aggressive hemangioma. A case report and literature review. Neuroradiol J. 2010;23(5):629–32. doi: 10.1177/197140091002300514. [DOI] [PubMed] [Google Scholar]
- 22.Tarantino R, Donnarumma P, Nigro L, Delfini R. Surgery in extensive vertebral hemangioma: Case report, literature review and a new algorithm proposal. Neurosurg Rev. 2015;38(3):585–92. doi: 10.1007/s10143-015-0616-4. discussion 592. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Chen YR, Chang SD, Veeravagu A. CyberKnife stereotactic radiosurgery for the treatment of symptomatic vertebral hemangiomas: A single-institution experience. Neurosurg Focus. 2017;42(1):E13. doi: 10.3171/2016.9.FOCUS16372. [DOI] [PubMed] [Google Scholar]
- 24.Cloran FJ, Pukenas BA, Loevner LA, et al. Aggressive spinal haemangiomas: Imaging correlates to clinical presentation with analysis of treatment algorithm and clinical outcomes. Br J Radiol. 2015;88(1055):20140771. doi: 10.1259/bjr.20140771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazonakis M, Tzedakis A, Lyraraki E, Damilakis J. Radiation dose and cancer risk to out-of-field and partially in-field organs from radiotherapy for symptomatic vertebral hemangiomas. Med Phys. 2016;43(4):1841. doi: 10.1118/1.4944422. [DOI] [PubMed] [Google Scholar]
- 26.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35(22):E1221–29. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]