Abstract
Shewanella algae is a rod-shaped Gram-negative marine bacterium frequently found in nonhuman sources such as aquatic ecosystems and has been shown to be the pathogenic agent in various clinical cases due to the ingestion of raw seafood. The results of this study showed that S. algae was present in approximately one in four samples, including water and shellfish samples. Positive reactions (API systems) in S. algae strains were seen for gelatinase (gelatin); however, negative reactions were found for indole production (tryptophan). S. algae is adapted to a wide range of temperatures (4°C, 25°C, 37°C, and 42°C) and salinity. Temperature is a key parameter in the pathogenicity of S. algae as it appears to induce hemolysis at 25°C and 37°C. S. algae exhibits pathogenic characteristics at widely varying temperatures, which suggests that it may have the ability to adapt to climate change.
1. Introduction
Recent studies indicate that climate change is driving ocean systems to recent increases in sea temperatures, with an associated risk of bacterial pathogens activity [1]. Shewanella algae has been identified as a new bacterial species, Shewanella spp., from clinical samples [2]. It is a rare human pathogen and symptoms of infection are often misidentified as Vibrio spp. [3]. It can be isolated from a wide range of environments, including fresh water, estuary, and the deep sea [4]. Risk factors associated with S. algae infections include chronic skin ulcer, chronic liver disease, and immune system disorders [5–7]. It appears to be more virulent in comparison with other Shewanella species [8–10].
Reports of infection with S. algae species in human cases are increasing, especially during the summer months and in tropical areas, such as India, China, and Taiwan [11–14]. In general, S. algae can be considered an opportunistic pathogen in humans exposed to a marine environment when it infects people via an existing soft tissue ulcer [15–22]. They have also been implicated in ear infection [23], eye infection, infective arthritis, osteomyelitis, bacteremia [24], infective endocarditis, and peritonitis in clinics [7]. Furthermore, S. algae infection tends to be associated with the ingestion of raw seafood, especially in individuals with hepatobiliary disease [3, 6, 13, 25]. This is a particular concern in some Asian regions in which there is a high demand for a wide variety of raw seafood. However, to date, there are few detailed data on S. algae with respect to its biochemical profiles and sources of infection in aquaculture.
In light of these questions, we conducted a study to analyze aquaculture and diverse water sources in order to determine the distribution of S. algae. Furthermore, we determined the profiles of samples obtained from diverse ranges of salinity and temperature. These results may serve as the basis of further study and could shed light on the ability of this pathogen to adapt to climate change.
2. Materials and Methods
2.1. Sample Collection and Preparation
Aquaculture and water samples were randomly collected from commercial oyster seedbeds along the west coast of Taiwan, fish markets, fishing ports, commercial abalone farms on the east coast, and some estuaries. The aquaculture samples were placed in sterile plastic bags, and water samples were collected in transportation tubes.
All samples were transported in refrigerated containers immediately after being collected. A total of 109 samples (water isolates (n=25) and aquaculture isolates (n=84) collected from 2012 to 2013 were investigated in this study (Figure 1).
Figure 1.
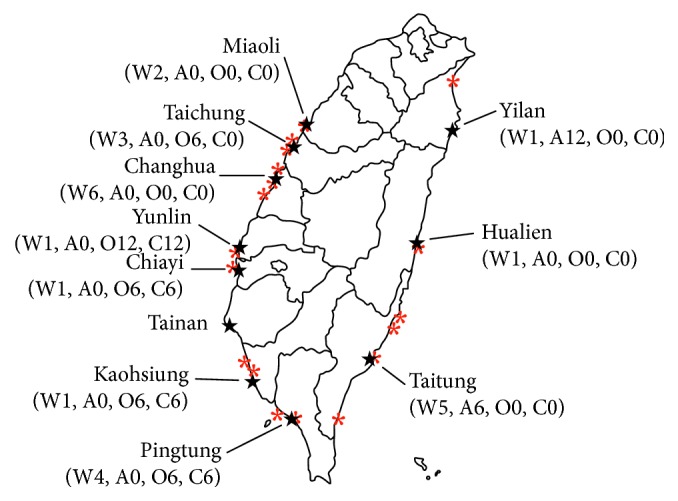
Location of sampling points of the aquaculture samples (★; A: Abalone; C: Clams; O: Oyster) and water samples (∗; W: water samples) in this study.
Each isolate from the digestive glands of oysters, abalone, clams, and water samples were prepared on marine broth 2216 (MB; BD) as tenfold dilutions [26].
Secondary enrichment incubation was applied for 48 hours, and then 2 μL of culture media was taken by loop and directly placed on the surface of marine agar 2216 (MA; BD). Colonies on marine agar were 2.0–2.5 mm in diameter, circular, convex with entire margins, and smooth after 2 days' incubation at 30°C. Orange-yellow or pink colonies on marine agar (BD) were identified as Gram-negative by Gram staining.
2.2. Biochemical and Nucleotide Sequence Analyses
The isolates were identified to species level by 16S rDNA sequence nucleotide sequence analyses. Each of the isolates identified by PCR analyses tested positive for 16S rDNA. Biochemical testing for phenotype was performed using an API20 NE (bioMérieux). All tests were performed according to the manufacturers' instructions. PCR-mediated amplification of the 16S rDNA was performed for confirmation of species identity. For nucleotide sequence analyses, genomic DNA was purified from overnight cultures of the isolates after growth on marine agar. The nucleotide was purified by using a QIAquick PCR purification kit (Qiagen). The extracted DNA was stored at −20°C until processing.
The remaining PCR solution was prepared for sequencing to confirm species identity. A fragment of the 16S rDNA gene was PCR-amplified from each genomic preparation using forward primer 27F: 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R: 5′-TACGGCTACCTTGTTACGACTT-3ʹ. Reaction mixtures were incubated in an Eppendorf of Perkin-Elmerk GeneAmp 9600 PCR system. The reaction mix was put through the following temperatures with an initial denaturation for 1 min at 94°C, 1 min at 55°C, and 5 min at 72°C, for 30 cycles. The PCR products were thereafter cooled at 4°C. Sequences of these amplicons were completed by ABI 3730xl DNA Analyzer (Applied Biosystems). Reference sequences utilized in phylogenetic analysis were retrieved from NCBI's GenBank database. The 16S rDNA sequence data were compared with all currently available sequences of organisms belonging to the genus Shewanella.
Phenotypic characteristic assays included growth conditions (temperature and salinity tolerance). Assessment of biochemical features included measurement of oxidase, hydrogen sulfide, and indole production. Carbohydrate and fatty acids utilization, as well as hemolytic activity, was analyzed.
2.3. Phenotypic Characteristic Assays
Include growth conditions (temperature and salinity tolerance) and biochemical features (oxidase, hydrogen sulfide, and indole production; carbohydrate and fatty acids utilization; and hemolytic activity).
2.4. Cellular Characterization
The isolates were then grown in an overnight marine broth and the turbidity diluted to match a 0.5 MacFarland standard prior to inoculation at different temperatures (24 hrs∼7 days). All strains were tested for the ability to grow on MB and then placed into four separate incubators at 4°C, 25°C, 37°C, and 42°C for culturing (7 days). The growth of the isolates was routinely assessed indirectly by measuring the turbidity (OD600nm) using a UV-visible spectrophotometer (Tecan infinite 200, Switzerland). Growth was determined as an absorbance reading at or above 0.1.
2.5. Hemolysis Assay
To investigate the presence of potential virulence factors, we observed the hemolytic activity of S. algae on plates of 5% sheep blood agar (Commercialized Blood Agar Plate, Creative Co., Ltd., Taiwan) after incubation at two different temperatures (25°C and 37°C) and for two different times (24 hrs and 72 hrs).
2.6. Salinity Tolerance Assay
The salinity tolerance screening assay of the selected bacterial strains was carried out using tryptic soy broth (TSB, Difco) medium with 0–10% (w/v) concentration of NaCl. The flasks were inoculated with bacterial culture and incubated at 30°C on a rotator shaker (180 rpm) for 48 hrs. The bacterial growth assessment was carried out by measuring the turbidity (OD600nm) using a fluorescence spectrophotometer (Tecan infinite 200, Switzerland). The experiments were conducted in triplicate and the average values were recorded. The bacterial isolates were grown at 30°C for 7 days.
2.7. Statistical Analysis
Data were entered into Microsoft Excel 2017 (Microsoft Corporation, Redmond, USA) and analyzed.
3. Results
3.1. Quantity of Bacteria in Collected Samples
A total of 109 samples were collected. In total, 23% (19/84) of isolates from shellfishes and 28% (7/25) of water isolates were identified as Shewanella algae (Tables 1 and 2). We tested the significant differences in the isolation rates between water samples and shellfishes using Pearson's chi square with Yates' continuity correction. We found no significant difference between the two group (p=0.798 and R=0.022). The phenomenon suggests potential extensive water contamination which warrants continuous surveillance.
Table 1.
Incidence of Shewanella algae in aquaculture samples.
| Location | Sources of sample | Genus species | Total no. of samples | No. yielding Shewanella algae |
|---|---|---|---|---|
| TaiTung | Abalone (cultured) | Haliotis diversicolor | 6 | 2 |
| Yilan | Abalone (cultured) | Haliotis diversicolor | 12 | 0 |
| YunLin | Oyster (cultured) | Crassostrea angulate | 12 | 1 |
| ChiaYi | Oyster (cultured) | Crassostrea angulate | 6 | 2 |
| KaoHsiung | Oyster (cultured) | Crassostrea angulate | 6 | 2 |
| Taichung | Oyster (Fish market) | Crassostrea angulate | 6 | 2 |
| PingTung | Oyster (cultured) | Crassostrea angulate | 6 | 4 |
| YunLin | Clam (cultured) | Geloina erosa | 12 | 1 |
| KaoHsiung | Clam (cultured) | Meretrix lusoria | 6 | 3 |
| ChiaYi | Clam (cultured) | Meretrix lusoria | 6 | 0 |
| PingTung | Clam (cultured) | Perna viridis | 6 | 2 |
| Total number | 84 | 19 | ||
| Isolation rate (%) | 23 | |||
Table 2.
Occurrence of Shewanella algae in different water-sampling sites.
| Location | Sources of sample | No. of cultured | No. yielding Shewanella algae |
|---|---|---|---|
| Miaoli County (Houlong Township) | Mariculture | 2 | 1 |
| Changhua County (Yuanlin Township) | Sea gate | 3 | 1 |
| Changhua County (Fishing port) | Sea water | 3 | 0 |
| Taichung City (Dali Dist.) | Fresh water | 1 | 0 |
| Taichung City (Wuqi Dist.) | Sea water | 2 | 0 |
| Yunlin County (Kouhu Township) | Mariculture | 1 | 0 |
| Chiayi County (Budai Township) | Sea water | 1 | 0 |
| Kaohsiung City (Ziguan Dist.) | Sea water | 1 | 0 |
| Pingtung County (Fangliao Township) | Mariculture | 4 | 2 |
| Taitung County | Sea water | 4 | 2 |
| Taitung County (Donghe Township) | Fresh water | 1 | 0 |
| Hualien County (Fengbin Township) | Sea water | 1 | 1 |
| Yilan County (Toucheng Township) | Mariculture | 1 | 0 |
| Total numbers | 25 | 7 | |
| Isolation rate (%) | 28 | ||
The bivalve mussels were identified by the Department of Life Sciences, National Chung Hsing University. The mussels were confirmed to be related to Crassostrea angulata, Meretrix lusoria, Perna viridis, Geloina erosa, and Haliotis diversicolor. Among these, the isolation rates of Shewanella algae were 2/18 in abalone (Haliotis diversicolor), 11/36 in oyster (Crassostrea angulata), and 6/30 in clams including Meretrix lusoria, Perna viridis, and Geloina erosa (Table 1). In addition, the locations with the greatest prevalence of Shewanella algae in water samples were commercial aquaculture farms on the west coast, with an isolation rate of 37.5% (3/8), followed by fish markets in fishing ports on the east coast, with a rate of 26.7% (4/15) (Table 2).
3.2. Characterization of Shewanella Strains
S. algae isolates were cultured at four different temperatures to establish reference data for future research on possible adaptation to global warming. The results showed S. algae isolates grew at three temperatures within the linear range (25, 37, and 42°C), but grew poorly at 4°C (Table 3). The growth curves of S. algae under different temperature are shown in Figure 2.
Table 3.
Phenotypic characteristics of Shewanella algae isolates.
| Reaction | Values are positive percentages | |
|---|---|---|
| Aquaculture isolates (n=19) (%) | Water isolates (n=7) (%) | |
| Growth at | ||
| 4°C on MB | 0 | 14 |
| 25°C on MB | 100 | 100 |
| 37°C on MB | 100 | 100 |
| 42°C on MB | 74 | 100 |
| 30°C in LB with 0% NaCl | 100 | 100 |
| 30°C in LB with 2% NaCl | 100 | 100 |
| 30°C in LB with 6% NaCl | 100 | 100 |
| 30°C in LB with 10% NaCl | 0 | 0 |
|
| ||
| Hemolysis of blood agar plate at | ||
| 37°C (24 hrs) | 89 | 100 |
| 25°C (24 hrs) | 0 | 0 |
| 37°C (72 hrs) | 100 | 100 |
| 25°C (72 hrs) | 53 | 57 |
|
| ||
| Reactions/enzymes (API20NE) | ||
| Reduction of nitrates to nitrites | 100 | 100 |
| Indole production | 0 | 0 |
| Glucose fermentation | 0 | 0 |
| Arginine dihydrolase | 5 | 0 |
| Urease | 32 | 29 |
| β-Glucosidase | 53 | 43 |
| Gelatinase | 95 | 100 |
| β-Galactosidase | 11 | 0 |
|
| ||
| Assimilation (API20NE) | ||
| Glucose | 0 | 14 |
| Arabinose | 0 | 0 |
| Mannose | 0 | 0 |
| Mannitol | 0 | 0 |
| N-Acetyl-glucosamine | 95 | 100 |
| Maltose | 16 | 14 |
| Potassium gluconate | 0 | 14 |
| Capric acid | 79 | 71 |
| Adipic acid | 0 | 0 |
| Malic acid | 100 | 86 |
| Trisodium citrate | 11 | 29 |
| Phenylacetic acid | 0 | 0 |
|
| ||
| Others | ||
| Oxidase | 100 | 100 |
| H2S-production (TSIA) | 100 | 100 |
Figure 2.
Growth curve of Shewanella algae isolates. (a) Aquaculture-origin, laboratory ID: O12. (b) Water-origin, laboratory ID: E-W1. Shewanella algae isolates were cultivated in LB broth at 12, 25, 37, and 42°C with shaking at 200 rpm. The optical density (OD600) was measured every 3 hours from zero point until 48 hours.
The biochemical profiles showed that all of the strains were unable to utilize some carbohydrates, but produced hydrogen sulfide (H2S). Positive reactions in S. algae strains were seen for H2S (from sodium thiosulfate) and cytochrome oxidase (oxidase test) and gelatinase (gelatin); however, negative results were found for indole production (tryptophan) and carbohydrates utilization, including arabinose, mannose, mannitol, adipic acid, and phenylacetic acid (Table 3).
Some S. algae isolates produced urease, which were related to positive urea reaction. Furthermore, most S. algae strains shared the ability to react with N-acetyl-glucosamine (NAG) as membrane substrates and reduction of nitrate to nitrite (potassium nitrate), and few S. algae isolates were able to assimilate maltose. Moreover, the majority of S. algae isolates assimilated capric acid and malic acid.
3.3. Effect of Salinity and Hemolysis In Vitro
The cultures were incubated at 30°C and all of them grew in the presence of a wide range of NaCl concentrations from 0, 2%, 6%, and 10% (w/v) (Table 3). Comparing the growth effects in different conditions, the isolates from aquaculture and water samples were favored by 0%, 2%, and 6% salinity. However, no bacterial growth was found at 10% NaCl.
Hemolysis occurred in sheep blood agar after incubation at two different temperatures (25°C and 37°C) (Table 3). One hundred percent hemolysis was found in S. algae from both aquaculture and water isolates at 37°C (after 72 hours). Compared with the 25°C group, only 50% hemolysis occurred after 72 hours.
4. Discussion
In this study, we investigated the prevalence of S. algae in a variety of environments around Taiwan. We collected 109 samples and identified S. algae in 23% (19/84) of isolates from shellfishes and in 28% (7/25) of water isolates (Tables 1 and 2).
S. algae can be found in samples from coastal areas, aquaculture farms, and aquaculture products [27]. S. algae is frequently found in the marine environment and is widely distributed in nature. Reports of infections with this opportunistic pathogen in humans are rare, although they are on the rise. In many clinical reports of hepatobiliary disease involving S. algae infection, there was a history of raw seafood ingestion [3, 25]. However, reports of S. algae infection in aquatic animals are rare. In view of this, we searched for articles on relevant cases in the ScienceDirect, PubMed, and Google Scholar databases using the following terms: “Aquaculture disease or Aquaculture products or Environment” in conjunction with “Shewanella algae or Shewanella alga.” The collected studies included research articles and case reports, as well as retrospective and series studies. Table 4 shows a list of results for S. algae infections in aquaculture animals from 1999 to 2017 in different countries [15, 16, 28–40]. The results showed that S. algae is endemic in Asia. This finding is consistent with a number of studies conducted in areas with warm climates, largely in Asia [9, 41, 42]. A literature review of the period 1999 to 2017 showed that over 64% (9/14) of infection cases in aquatic animals were in Asia, including China, Japan, Malaysia, and Iran, as shown in Table 4. In addition, sea water was the predominant source of contamination and some cases were without disease symptoms.
Table 4.
Distribution of Shewanella algae in environment samples and aquaculture animals (1999–2017).
| Year | Region | Sampling location | Host | Disease symptoms | References |
|---|---|---|---|---|---|
| 1999 | Denmark | Sea water | Environment samples | No | Gram et al., [28] |
| 2000 | Denmark | Sea water | Environment samples | No | Vogel et al., [15] |
| 2002 | China | Sea water | Scinenops ocellata | Ulcer disease | Chang et al., [16] |
| 2006 | China | Pond water | Abalone | Whitening, shrunken muscles | Cai et al., [29] |
| 2008 | USA | Sea water | Shellfish | Nonavailable | Richards et al., [30] |
| 2009 | Japan | Sea water | Sea cucumber | Nonavailable | Beleneva et al., [31] |
| 2010 | Malaysia | Tank water | Shrimp | Healthy post larvae | Zadeh et al., [32] |
| 2010 | Japan | Tank water | Pufferfish | Healthy fish | Sugita et al., [33] |
| 2011 | USA | Sea water | Sediments | No | Cummings et al., [34] |
| 2012 | China | Sea water | Environment samples | No | Zhao and Dang, [35] |
| 2013 | China | Sea water | Marine culture | No | Liu et al., [25, 36, 37] |
| 2013 | China | Sea water | Deep-sea sediments | No | Jiang et al., [36] |
| 2013 | Portuguese | Sea water | Deep sea | No | Martins et al., [38] |
| 2015 | Iran | Sea water | Mussels/sediment | No | Bayat et al., [39] |
| 2017 | China | Sea water | Fish | Noticeable histological lesions | Z. Han et al., [40] |
In many reports of human infection with S. algae, the patient had a previous history of hepatobiliary disease or hemochromatosis and had recently consumed raw seafood [25, 28]. It is well understood that patients with hereditary hemochromatosis or hepatobiliary disease are prone to iron overload [43, 44]. S. algae could be tolerant to bile salts and may produce tetrodotoxin [42], exoenzymes, or siderophores [8], which are considered virulence factors. Furthermore, iron (Fe) serves as a terminal electron acceptor when Shewanella spp. are exposed to anoxic conditions [45, 46].
Our results showed S. algae strains are capable of growing in the presence of 0∼6% NaCl. Shewanella spp. are commonly found in marine environments and are believed to be halophilic bacteria [47, 48]. The traditional methods of processing seafood often take advantage of the preservative properties of salt, which permit long-term storage. The high salinity in the seafood product may influence the osmotic pressure and physiological properties of any bacteria that may be present. High salinity can result in the loss of microbial activity and cell plasmolysis. However, moderately salt-tolerant bacteria can resist or reduce the damaging effects of salt concentrations of up to 5-20% salinity [49]. Therefore, these salt-tolerant bacteria are potential food-borne pathogens. In our results, growth of S. algae was observed in a wide range of salinities (Table 3). Surprisingly, S. algae was also found in fresh water and nonmarine environments, and thus did not appear to require Na+, as shown in Table 3.
Furthermore, the seasonal growth and infection rate of S. algae peak during the summer. Based on the growth curves in our study, S. algae adapt to a wide range of temperatures, with optimal growth temperatures ranging at room temperature; under experimental conditions, the optimal temperatures for bacterial growth were 25°C and 37°C (Table 3). Comparing the two isolates of the curves based on optical density, the curves of growth rate versus temperature are in direct proportion as drawn in Figure 2. This probably explains why reports of S. algae infection are more common in warm water areas or tropical regions during the summer than in cold-water environments [8]. Prior study revealed that S. algae grows under the condition of temperature 26–34°C, pH 5–9 [40]. The data provide further support of the capacity of survival of S. algae under ocean acidification caused by global warming.
Earlier research has suggested that hemolysis may be used as a marker to predict potentially virulent strains of S. algae [3]. We previously reported that a substantial number of S. algae strains were capable of growing on sheep blood agar. S. algae strains from all isolates exhibited hemolysis on sheep blood agar. In Table 3, it can be seen hemolytic activity on sheep blood agar was high (90% to 100%) at 37°C. In contrast, there was no obvious hemolysis on the first day of incubation at 25°C; however, it was clearly evident on the second day. This finding implies that Shewanella species exert various forms of hemolysis. Our results are also consistent with epidemiologic studies in Denmark [28] and Taiwan [25], which showed the infection rates of S. algae were correlated with temperature fluctuation. In general, most S. alga strains exhibited hemolysis after prolonged incubation (48 to 72 h) and the area of hemolysis was clear.
The major characteristics in all S. algae isolates in this study include the ability to exert a strong hemolytic effect: an inability to utilize carbohydrates, although a few isolates were able to use maltose from some water samples. Other studies previously found that a few Shewanella species could utilize L-arabinose and glucose [9, 50]; however, we were not able to confirm these results.
S. algae utilizes few carbohydrates as the sole carbon source according to the results of this study. In principle, bacteria are quite diverse in terms of nutrient utilization and metabolic requirements in a specific environment, owing to their biosynthetic capabilities. Previous biochemical characterization studies have suggested that while some Shewanella species are able to metabolize different sugars for growth, they might be limited to biosynthetic purposes such as cell wall synthesis or as a storage molecule, rather than as a carbon source [51, 52]. This could explain the apparent inconsistencies among several experimental observations. In our results, only 14% of S. algae from diverse water sources and 16% of aquaculture isolates utilized maltose (Table 3). From these, we may infer S. algae adapt to environmental changes via different biosynthetic pathways. Shewanella spp. possess a number of mechanisms to assimilate carbohydrates from the environment. It has been proposed that Shewanella oneidensis MR-1 uses the formaldehyde produced from pyruvate during growth under anaerobic or oxygen-limited conditions [53].
In addition, the results of indole production were all negative for S. algae isolates. Indole can act as an extracellular signal to regulate biofilm-promoting factors and the expression of adhesion molecules [54]. Bacteria which give negative results for the indole test include some Aeromonas species and Vibrio spp. [55]. Recent studies showing that non-indole-producing bacteria generate various oxygenases which may degrade indole or interfere with indole signaling [56, 57]. Many oxygenase and reductase reactions may be involved in metal ion facilitation in bacteria [58]. The ability of S. oneidensis to reduce oxidized metals or nitrate effectively has been identified as an important intrinsic activity of Shewanella species [59–62]. These results all indicate that S. algae is capable of adapting to environmental changes. Further studies are needed to clarify the role of regulating the intrinsic activity of S. algae.
There were several limitations in this study. First, continuous multisite surveillance is needed to demonstrate seasonal variation and long-term effect of global warming on S. algae population. Second, the lack of discrimination by the API systems for Shewanella bacteria is understandable since the isolates show diverse results in biochemical testing. It is necessary to improve identification schemes to identify pathogenic and nonpathogenic strains of S. algae in the natural environment.
In summary, our study identified the presence of S. algae in water and aquaculture products in Taiwan. We further identified hemolytic activity in all isolates, indicating that this species of bacteria possesses a pathogenic potential. We found high levels of S. algae isolates contained in diverse sources (oysters, abalone, clam, and water samples). The ability of Shewanella strains to tolerate a wide range of temperatures and salinities in experimental challenges may be due to the expression or repression of genes, but further research is needed to explore the potential underlying mechanisms involved. These results suggest that monitoring the levels of pathogenic species and strains should be continued in Taiwan and expanded to other tropical and subtropical zones in Asia.
Data Availability
The data used to support the findings of this study are currently under embargo while the research findings are published. Requests for data, 6 months after publication of this article, will be considered by the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Shu-Ying Tseng wrote the manuscript. Zong-Yen Wu and Po-Yu Liu conducted the data analysis and contributed to the microbiological analysis. Yi-Hsuan Lee, Ching-Chang Cheng, and Chiu-Chen Huang collected samples from different areas. Chiu-Chen Huang repeated the growth curves of S. algae under different temperature. Kwong-Chung Tung revised the manuscript. All authors contributed to data analysis, drafting, and critically revising the paper, read and approved the final manuscript, and agreed to be accountable for all aspects of the work.
References
- 1.Bally M., Garrabou J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: a new case of emerging disease linked to climate change. Global Change Biology. 2007;13(10):2078–2088. doi: 10.1111/j.1365-2486.2007.01423.x. [DOI] [Google Scholar]
- 2.Nozue H., Hayashi T., Hashimoto Y., et al. Isolation and characterization of Shewanella alga from human clinical specimens and emendation of the description of S. alga Simidu et al., 1990, 335. International Journal of Systematic Bacteriology. 1992;42(4):628–634. doi: 10.1099/00207713-42-4-628. [DOI] [PubMed] [Google Scholar]
- 3.Myung D. S., Jung Y.-S., Kang S.-J., et al. Primary Shewanella algae bacteremia mimicking Vibrio septicemia. Journal of Korean Medical Science. 2009;24(6):1192–1194. doi: 10.3346/jkms.2009.24.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu X., Wang D., Yin X., Du P., Kan B. Time course transcriptome changes in Shewanella algae in response to salt stress. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096001.e96001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domínguez H., Vogel B. F., Gram L., Hoffmann S., Schaebel S. Shewanella alga bacteremia in two patients with lower leg ulcers. Clinical Infectious Diseases. 1996;22(6):1036–1039. doi: 10.1093/clinids/22.6.1036. [DOI] [PubMed] [Google Scholar]
- 6.Otsuka T., Noda T., Noguchi A., Nakamura H., Ibaraki K., Yamaoka K. Shewanella infection in decompensated liver disease: a septic case. Journal of Gastroenterology. 2007;42(1):87–90. doi: 10.1007/s00535-006-1957-0. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas J., Pillai M., Vinod V., Dinesh R. K. Skin and soft tissue infections due to Shewanella algae: an emerging pathogen. Journal of Clinical and Diagnostic Research. 2015;9(2):DC16–DC20. doi: 10.7860/JCDR/2015/12152.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khashe S., Janda J. M. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens. Journal of Clinical Microbiology. 1998;36(3):783–787. doi: 10.1128/jcm.36.3.783-787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt H. M., Gahrn-Hansen B., Bruun B. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clinical Microbiology and Infection. 2005;11(5):347–352. doi: 10.1111/j.1469-0691.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 10.Janda J. M. Shewanella: a marine pathogen as an emerging cause of human disease. Clinical Microbiology Newsletter. 2014;36(4):25–29. doi: 10.1016/j.clinmicnews.2014.01.006. [DOI] [Google Scholar]
- 11.Tsai M.-S., You H.-L., Tang Y.-F., Liu J.-W. Shewanella soft tissue infection: case report and literature review. International Journal of Infectious Diseases. 2008;12(6):e119–e124. doi: 10.1016/j.ijid.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Liu P.-Y., Shi Z. Y., Lin C. F., et al. Shewanella infection of snake bites: a twelve-year retrospective study. Clinics. 2012;67(5):431–435. doi: 10.6061/clinics/2012(05)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim B. K., Cho S.-Y., Kang B., et al. A case of spontaneous bacterial peritonitis with bacteremia caused by Shewanella algae. Infection & Chemotherapy. 2014;46(4):264–268. doi: 10.3947/ic.2014.46.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumathi B. G., Kumarswamy S. R., Amritam U., Arjunan R. Shewanella algae: first case report of the fast emerging marine pathogen from squamous cell carcinoma patient in India. South Asian Journal of Cancer. 2014;3(3):188–189. doi: 10.4103/2278-330x.136819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel B. F., Holt H. M., Gerner-Smidt P., Bundvad A., Søgaard P., Gram L. Homogeneity of danish environmental and clinical isolates of Shewanella algae. Applied and Environmental Microbiology. 2000;66(1):443–448. doi: 10.1128/aem.66.1.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang C., Chaoqun H., Xiaoyan C., Luping Z. Identification and characterization of Shewanella algae as a novel pathogen of ulcer disease of fish Scinenops ocellata. Oceanologia et Limnologia Sinica. 2002;34(1):1–8. [Google Scholar]
- 17.Wang T. Model of life expectancy of chronic hepatitis B carriers in an endemic region. Journal of Epidemiology. 2009;19(6):311–318. doi: 10.2188/jea.je20090039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma K. K., Kalawat U. Emerging infections: Shewanella—a series of five cases. Journal of Laboratory Physicians. 2010;2(2):61–65. doi: 10.4103/0974-2727.72150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath R., Choudhury G., Saikia L., Das P. Isolation of Shewanella algae from rectal swabs of patients with bloody diarrhoea. Indian Journal of Medical Microbiology. 2011;29(4):p. 428. doi: 10.4103/0255-0857.90186. [DOI] [PubMed] [Google Scholar]
- 20.Wait S., Chen D.-S. Towards the eradication of hepatitis B in Taiwan. Kaohsiung Journal of Medical Sciences. 2012;28(1):1–9. doi: 10.1016/j.kjms.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner N., Otto L., Podda M., Schmitt Y., Tappe D. Travel-related chronic hemorrhagic leg ulcer infection by Shewanella algae. Journal of Travel Medicine. 2013;20(4):262–264. doi: 10.1111/jtm.12037. [DOI] [PubMed] [Google Scholar]
- 22.Wu T.-W., Lin H. H., Wang L.-Y. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology. 2013;57(1):37–45. doi: 10.1002/hep.25988. [DOI] [PubMed] [Google Scholar]
- 23.Holt H. M., Søgaard P., Gahrn-Hansen B. Ear infections with Shewanella alga: a bacteriologic, clinical and epidemiologic study of 67 cases. Clinical Microbiology and Infection. 1997;3(3):329–333. doi: 10.1111/j.1469-0691.1997.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 24.Iwata M., Tateda K., Matsumoto T., Furuya N., Mizuiri S., Yamaguchi K. Primary Shewanella alga Septicemia in a Patient on Hemodialysis. Journal of Clinical Microbiology. 1999;37(6):2104–2105. doi: 10.1128/jcm.37.6.2104-2105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P.-Y., Lin C.-F., Tung K.-C., et al. Clinical and microbiological features of Shewanella Bacteremia in patients with hepatobiliary disease. Internal Medicine. 2013;52(4):431–438. doi: 10.2169/internalmedicine.52.8152. [DOI] [PubMed] [Google Scholar]
- 26.Al-Harbi A. H., Naim Uddin M. Seasonal variation in the intestinal bacterial flora of hybrid tilapia (Oreochromis niloticus×Oreochromis aureus) cultured in earthen ponds in Saudi Arabia. Aquaculture. 2004;229(1–4):37–44. doi: 10.1016/s0044-8486(03)00388-0. [DOI] [Google Scholar]
- 27.Cabral J. P. S. Water microbiology. Bacterial pathogens and water. International Journal of Environmental Research. 2010;7(10):3657–3703. doi: 10.3390/ijerph7103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gram L., Bundvad A., Melchiorsen J., Johansen C., Vogel B. F. Occurrence of Shewanella algae in Danish coastal water and effects of water temperature and culture conditions on its survival. Applied and Environmental Microbiology. 1999;65(9):3896–3900. doi: 10.1128/aem.65.9.3896-3900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai J., Chen H., Thompson K. D., Li C. Isolation and identification of Shewanella alga and its pathogenic effects on post-larvae of abalone Haliotis diversicolor supertexta. Journal of Fish Diseases. 2006;29(8):505–508. doi: 10.1111/j.1365-2761.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 30.Richards G. P., Watson M. A., Crane E. J., Burt I. G., Bushek D. Shewanella and Photobacterium spp. in oysters and seawater from the Delaware Bay. Applied and Environmental Microbiology. 2008;74(11):3323–3327. doi: 10.1128/aem.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beleneva I. A., Magarlamov T. Y., Eliseikina M. G., Zhukova N. V. Biochemical and pathogenic properties of the natural isolate of Shewanella algae from peter the great bay, Sea of Japan. Journal of Invertebrate Pathology. 2009;102(3):250–255. doi: 10.1016/j.jip.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Zadeh S. S., Saad C. R., Christianus A., et al. Assessment of growth condition for a candidate probiotic, Shewanella algae, isolated from digestive system of a healthy juvenile Penaeus monodon. Aquaculture International. 2010;18(6):1017–1026. doi: 10.1007/s10499-010-9319-6. [DOI] [Google Scholar]
- 33.Sugita H., Sugiyama K., Itoi S. Culturable bacterial flora in the intestinal tract of Japanese Pufferfish Takifugu rubripes. Aquaculture Science. 2010;58(3):437–438. [Google Scholar]
- 34.Cummings D. E., Archer K. F., Arriola D. J., et al. Broad dissemination of plasmid-mediated quinolone resistance genes in sediments of two urban coastal wetlands. Environmental Science & Technology. 2011;45(2):447–454. doi: 10.1021/es1029206. [DOI] [PubMed] [Google Scholar]
- 35.Zhao J., Dang H. Coastal seawater bacteria harbor a large reservoir of plasmid-mediated quinolone resistance determinants in Jiaozhou Bay, China. Microbial Ecology. 2012;64(1):187–199. doi: 10.1007/s00248-012-0008-z. [DOI] [PubMed] [Google Scholar]
- 36.Jiang W., Xia B., Liu Z. A serine hydroxymethyltransferase from marine bacterium Shewanella algae: isolation, purification, characterization and l-serine production. Microbiological Research. 2013;168(8):477–484. doi: 10.1016/j.micres.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Liu G., Zhou J., Meng X., et al. Decolorization of azo dyes by marine Shewanella strains under saline conditions. Applied Microbiology and Biotechnology. 2013;97(9):4187–4197. doi: 10.1007/s00253-012-4216-8. [DOI] [PubMed] [Google Scholar]
- 38.Martins A., et al. Photoprotective bioactivity present in a unique marine bacteria collection from Portuguese deep sea hydrothermal vents. Marine Drugs. 2013;11(5):1506–1523. doi: 10.3390/md11051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayat Z., Hassanshahian M., Hesni M. A. Enrichment and isolation of crude oil degrading bacteria from some mussels collected from the Persian Gulf. Marine Pollution Bulletin. 2015;101(1):85–91. doi: 10.1016/j.marpolbul.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Han Z., Sun J., Lv A., et al. Isolation, identification and characterization of Shewanella algae from reared tongue sole, Cynoglossus semilaevis Günther. Aquaculture. 2017;468:356–362. doi: 10.1016/j.aquaculture.2016.10.038. [DOI] [Google Scholar]
- 41.Finkelstein R., Oren I. soft tissue infections caused by marine bacterial pathogens: epidemiology, diagnosis, and management. Current Infectious Disease Reports. 2011;13(5):470–477. doi: 10.1007/s11908-011-0199-3. [DOI] [PubMed] [Google Scholar]
- 42.Vignier N., Théodose R., Barreau M., et al. Human infection with Shewanella putrefaciens and S. algae: report of 16 cases in martinique and review of the literature. American Journal of Tropical Medicine and Hygiene. 2013;89(1):151–156. doi: 10.4269/ajtmh.13-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schamroth L., Edelstein W., Politzer W. M., Stevens N. Serum iron in the diagnosis of hepatobiliary disease. BMJ. 1956;1(4973):960–963. doi: 10.1136/bmj.1.4973.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pietrangelo A. Hereditary hemochromatosis: a new look at an old disease. New England Journal of Medicine. 2004;350(23):2383–2397. doi: 10.1056/nejmra031573. [DOI] [PubMed] [Google Scholar]
- 45.Straub K. L., Benz M., Schink B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiology Ecology. 2001;34(3):181–186. doi: 10.1111/j.1574-6941.2001.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 46.Weber K. A., Achenbach L. A., Coates J. D. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nature Reviews Microbiology. 2006;4(10):752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- 47.DeFrank J. J., Beaudry W. T., Cheng T.-C., Harvey S. P., Stroup A. N., Szafraniec L. L. Screening of halophilic bacteria and Alteromonas species for organophosphorus hydrolyzing enzyme activity. Chemico-Biological Interactions. 1993;87(1–3):141–148. doi: 10.1016/0009-2797(93)90035-w. [DOI] [PubMed] [Google Scholar]
- 48.Gram L., Huss H. H. Microbiological spoilage of fish and fish products. International Journal of Food Microbiology. 1996;33(1):121–137. doi: 10.1016/0168-1605(96)01134-8. [DOI] [PubMed] [Google Scholar]
- 49.Larsen H. Halophilic and halotolerant microorganisms-an overview and historical perspective. FEMS Microbiology Letters. 1986;39(1-2):3–7. doi: 10.1111/j.1574-6968.1986.tb01835.x. [DOI] [Google Scholar]
- 50.Vogel B. F., Jørgensen K., Christensen H., Olsen J. E., Gram L. Differentiation of Shewanella putrefaciens and Shewanella alga on the basis of whole-cell protein profiles, ribotyping, phenotypic characterization, and 16S rRNA gene sequence analysis. Applied and Environmental Microbiology. 1997;63(6):2189–2199. doi: 10.1128/aem.63.6.2189-2199.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venkateswaran K., Moser D. P., Dollhopf M. E., et al. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. International Journal of Systematic Bacteriology. 1999;49(2):705–724. doi: 10.1099/00207713-49-2-705. [DOI] [PubMed] [Google Scholar]
- 52.Heidelberg J. F., Paulsen I. T., Nelson K. E., et al. Genome sequence of the dissimilatory metal ion–reducing bacterium Shewanella oneidensis. Nature Biotechnology. 2002;20(11):1118–1123. doi: 10.1038/nbt749. [DOI] [PubMed] [Google Scholar]
- 53.Tang Y. J., Meadows A. L., Kirby J., Keasling J. D. Anaerobic central metabolic pathways in Shewanella oneidensis MR-1 reinterpreted in the light of isotopic metabolite labeling. Journal of Bacteriology. 2007;189(3):894–901. doi: 10.1128/jb.00926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martino P. D., Fursy R., Bret L., Sundararaju B., Phillips R. S. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Canadian Journal of Microbiology. 2003;49(7):443–449. doi: 10.1139/w03-056. [DOI] [PubMed] [Google Scholar]
- 55.Lee J.-H., Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiology Reviews. 2010;34(4):426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 56.Hu M., Zhang C., Mu Y., Shen Q., Feng Y. Indole Affects biofilm formation in bacteria. Indian Journal of Microbiology. 2010;50(4):362–368. doi: 10.1007/s12088-011-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han T. H., Lee J.-H., Cho M. H., Wood T. K., Lee J. Environmental factors affecting indole production in Escherichia coli. Research in Microbiology. 2011;162(2):108–116. doi: 10.1016/j.resmic.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varkey A. J., Dlamini M. D., Mansuetus A. B., Tiruneh A. T. Germicidal action of some metals/metal ions in combating E. coli bacteria in relation to their electro-chemical properties. Journal of Water Resource and Protection. 2013;5(12):1132–1143. doi: 10.4236/jwarp.2013.512119. [DOI] [Google Scholar]
- 59.Richardson D. J. Bacterial respiration: a flexible process for a changing environment. Microbiology. 2000;146(3):551–571. doi: 10.1099/00221287-146-3-551. [DOI] [PubMed] [Google Scholar]
- 60.DiChristina T. J., Moore C. M., Haller C. A. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. Journal of Bacteriology. 2002;184(1):142–151. doi: 10.1128/jb.184.1.142-151.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hau H. H., Gralnick J. A. Ecology and biotechnology of the genus Shewanella. Annual Review of Microbiology. 2007;61(1):237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- 62.Wen J., Zhou S., Chen J. Colorimetric detection of Shewanella oneidensis based on immunomagnetic capture and bacterial intrinsic peroxidase activity. Scientific Reports. 2014;4(1) doi: 10.1038/srep05191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are currently under embargo while the research findings are published. Requests for data, 6 months after publication of this article, will be considered by the corresponding author.



