Esculin, used as a sucrose mimic, shows that the velocity of phloem transport is regulated by environmental cues, changes in sucrose levels, and the expression of the sucrose transporter AtSUC2.
Abstract
The study of phloem transport and its vital roles in long-distance communication and carbon allocation have been hampered by a lack of suitable tools that allow high-throughput, real-time studies. Esculin, a fluorescent coumarin glucoside, is recognized by Suc transporters, including AtSUC2, which loads it into the phloem for translocation to sink tissues. These properties make it an ideal tool for use in live-imaging experiments, where it acts as a surrogate for Suc. Here, we show that esculin is translocated with a similar efficiency to Suc and, because of its ease of application and detection, demonstrate that it is an ideal tool for in vivo studies of phloem transport. We used esculin to determine the effect of different environmental cues on the velocity of phloem transport. We provide evidence that fluctuations in cotyledon Suc levels influence phloem velocity rapidly, supporting the pressure-flow model of phloem transport. Under acute changes in light levels, the phloem velocity mirrored changes in the expression of AtSUC2. This observation suggests that under certain environmental conditions, transcriptional regulation may affect the abundance of AtSUC2 and thus regulate the phloem transport velocity.
The phloem of higher plants consists of a highly developed network of specialized enucleate cells known as sieve elements (SEs), connected to their adjacent metabolically supportive companion cells (CCs) by specialized plasmodesmata called pore plasmodesmata units (van Bel, 1996; Oparka and Turgeon, 1999; Heo et al., 2014). Sieve elements are connected end-to-end by perforated sieve plates, allowing long-distance translocation of photosynthetically derived assimilates and a wide range of solutes, hormones, proteins, and RNAs (Wardlaw, 1990; Molnar et al., 2010; Bishopp et al., 2011; Liu et al., 2012; Paultre et al., 2016). The phloem network of plants thus performs key roles in carbon allocation and in the long-distance movement of systemic macromolecules.
The flow of the Suc-rich sap in the phloem is thought to occur by mass flow, as originally envisaged by Münch (1930). Sugars, such as Suc, are loaded into the SEs of the phloem in photosynthetically active tissues (sources). The high concentration of Suc in these phloem cells osmotically attracts water from the xylem, increasing the hydrostatic pressure within SEs and driving flow from source to sink regions of the plant where the Suc is unloaded and used in metabolism and growth. The removal of solutes and water from the sites of phloem unloading maintains an osmotic gradient along the SE files (sieve tubes) and creates the pressure differential required to drive long-distance flow (Knoblauch et al., 2016; Ross-Elliott et al., 2017).
Suc can be loaded into the phloem either symplastically or apoplastically. In symplastic loading, Suc reaches the companion cells through multiple plasmodesmata that connect them with the surrounding bundle sheath and parenchyma cells. It is either transported into the phloem by simple passive diffusion (diffusive loading) or converted into high-Mr polymers such as stachyose and raffinose (polymer trapping), with subsequent movement through the large-diameter pore plasmodesmata units between CC and SE; (van Bel, 1996; Rennie and Turgeon, 2009). Apoplastic loaders, such as Arabidopsis (Arabidopsis thaliana), use active proton-mediated transport via SUC TRANSPORTERS (SUTs) to load Suc into the CC from the apoplast against a concentration gradient (Sauer, 2007; Rennie and Turgeon, 2009). There are a number of SUTs and other sugar transporters present in Arabidopsis, but SUC TRANSPORTER2 (AtSUC2) is expressed specifically in CCs and is responsible for Suc loading into the collection phloem in the minor veins of leaves (Truernit and Sauer, 1995).
Recently, the simplicity of the Münch pressure-flow hypothesis has been questioned, one argument being that the magnitude of hydrostatic pressure gradients in large trees may be too low to drive the observed rates of flow (Turgeon, 2010). However, newer experimental methods, incorporating mathematical modeling, have provided data in support of the original Münch model (Jensen et al., 2011; Knoblauch et al., 2016).
Despite the fundamental importance of the phloem in assimilate distribution, basic questions remain as to how phloem transport responds to environmental changes. Indeed, considering the extensive literature on carbon partitioning in plants, there have been very few studies in which phloem transport velocity has been measured in planta. Since the 1970s, several studies have used 14C or 11C isotopes to measure rates of phloem transport in large plants. This was usually achieved by placing two or more Geiger-Muller tubes along the phloem pathway to track the movement of the radioactive solute front or by freeze-drying and exposing the tissue to autoradiographs (Christy and Fisher, 1978; Madore and Lucas, 1987; Minchin and Thorpe, 2003). However, these studies had limited resolution and were not suitable for use on very small plants or seedlings, such as Arabidopsis. More recently, phloem transport has been investigated using MRI techniques or refining the use of radioactive tracers such as 11C, for example, with the use of specialized hydroponic root chambers (Köckenburger et al., 1997; Peuke et al., 2001; Windt et al., 2006; Mullendore et al., 2010; Gould et al., 2012). However, these methods are expensive, time consuming, and lack the resolution needed to study phloem transport at the level of the SE (Gould et al., 2012; Ohmae et al., 2013; Kölling et al., 2015). Fluorescent tracers, such as carboxyfluorescein diacetate, which translocate in the phloem, were first described over 20 years ago (Grignon et al., 1989), but they have only rarely been used to measure the velocity of phloem transport and are more often used to confirm that phloem transport has simply occurred (Oparka et al., 1994; Wright and Oparka, 1996; Jensen et al., 2011; Savage et al., 2013).
We recently described a range of fluorescent probes that are translocated in the phloem of Arabidopsis and which allow in planta analysis of phloem transport (Knoblauch et al., 2015; Ross-Elliott et al., 2017). One of these, the coumarin glucoside esculin, is loaded into the Arabidopsis phloem specifically by the AtSUC2 transporter and does not enter the phloem in atsuc2 knockout seedlings, making it a potential surrogate for Suc in phloem transport studies (Reinders et al., 2012; Knoblauch et al., 2015; De Moliner et al., 2018). Here, we describe the use of esculin to measure the phloem transport velocity (PTV) in response to differing environmental conditions. We show that PTV can be measured rapidly in intact seedlings in a high-throughput manner. Our results provide evidence that fluctuations in leaf Suc levels may influence PTV, supporting the pressure-flow model of phloem transport. Our data also show that under acute changes in the light environment, the rate of phloem transport mirrors the transcriptional level of AtSUC2 in leaves, suggesting that AtSUC2 expression in CCs may regulate the PTV under certain environmental conditions.
RESULTS
Esculin Is Translocated with Similar Efficiency to Suc
We previously described the specific phloem translocation of esculin, a naturally occurring fluorescent coumarin glucoside, by AtSUC2 and detailed how the glucoside moiety of esculin is required for recognition by the Suc symporter (Knoblauch et al., 2015). We have also recently described the structural requirements of esculin for binding by AtSUC2 (De Moliner et al., 2018). Esculin does not enter the phloem in detectable amounts without AtSUC2 (Knoblauch et al., 2015; De Moliner et al., 2018). However, it was not known whether this probe was translocated as efficiently as Suc. We tested this by comparing the translocation of esculin in intact seedlings over time with the translocation of 14C-labeled Suc. Seedlings were tested at 7 d after germination (dag), and 0.3 μL of the adjuvant Adigor was added to each cotyledon shortly after dawn to facilitate loading through the cotyledons (Knoblauch et al., 2015). After 1 h, either 0.3 μL of esculin or 14C Suc was loaded onto each cotyledon. The relative percentage of probe that was washed off the cotyledon, remained within the cotyledon, or had been translocated to the rest of the seedling was measured at 4 h postloading (Fig. 1) using either scintillation counting (14C Suc) or fluorescence readings calibrated against a standard curve (esculin). While Suc is converted into insoluble fractions, esculin enters vacuoles and is degraded over time (Knoblauch et al., 2015), making longer-term comparisons between the two tracers unrealistic.
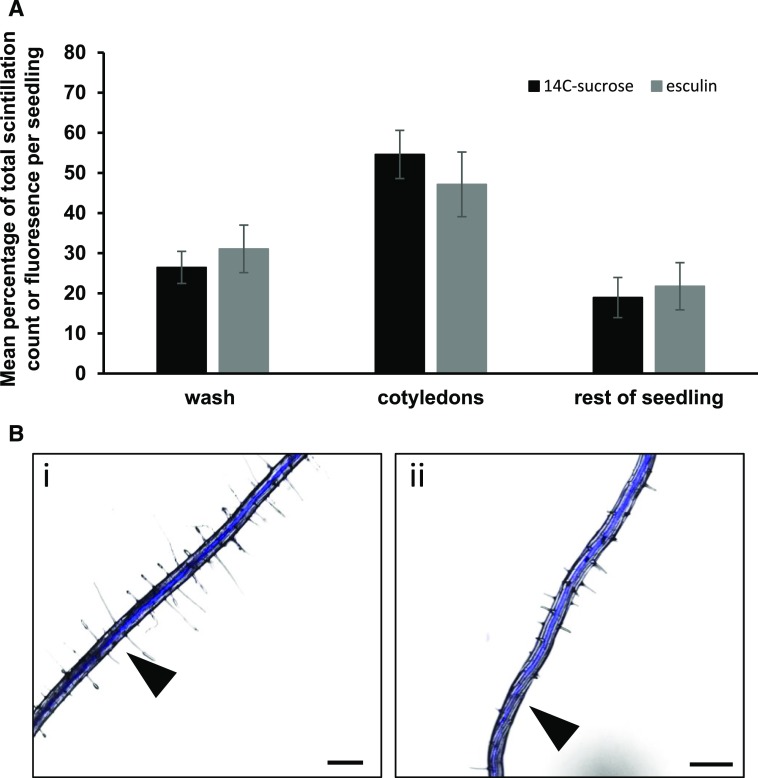
Figure 1.
Comparison of the translocation of 14C-Suc and the phloem-mobile fluorescent probe esculin. A, Black bars, 14C-Suc; gray bars, esculin. Mean percentage of total scintillation counts per seedling following application of 14C-Suc or mean percentage of total fluorescence per seedling following application of esculin. Both measurements taken 4 h after application to cotyledons. Each bar represents a minimum of 10 seedlings across two independent experiments. Error bars, se. B, Esculin translocating in the root of a 7-d-old Arabidopsis seedling following application to the cotyledons (i) early in the phloem, first point marked and time noted for velocity measurements, and (ii) moving toward the root tip in the phloem, second point marked. Bar, 0.5 mm.
After 4 h, the highest concentration of both 14C-Suc and esculin was present in the cotyledons (Fig. 1) and was similar for both probes (50%), as was the amount that remained on the surface of the cotyledon and that could be washed off. For both tracers, close to 20% of the total added to the leaf was translocated to the rest of the seedling by 4 h. This demonstrated that esculin was translocated in Arabidopsis seedlings as efficiently as Suc.
Influence of Environmental Conditions on PTV
As the major solute carried within the phloem is Suc, it has long been used as a proxy for describing the velocity of phloem transport. Based on the pressure-flow hypothesis, it is a reasonable assumption that factors affecting the rate of photosynthesis, and thus the amounts of Suc produced, could affect the velocity of phloem transport. However, few studies have been carried out on the direct impact of environmental changes on PTV. Those that have been conducted used relatively mature plants and complex methods for measurement, which reduce the opportunity for larger datasets and the testing of multiple experimental parameters (Mullendore et al., 2010; Savage et al., 2013; Knoblauch et al., 2016).
Young Arabidopsis seedlings provide an uncomplicated model to study PTV. At 7 dag, the architecture is simple, consisting of two expanded cotyledons (source), a hypocotyl (path), and a primary root tip (sink) that functions as the recipient of assimilates. Furthermore, the phloem is arranged in two distinct poles, adjacent to the xylem poles, allowing for easy identification and monitoring of flow in the translucent roots. Esculin was used to measure PTV in live seedlings. The probe was applied to both the cotyledons of seedlings as described above, and the plants kept under standardized environmental conditions. The roots were then monitored with an epifluorescence microscope for the arrival of esculin in the phloem (Fig. 1B). The position of the fluorescent front was marked on the back of the plate, and the seedlings were returned to the same growth conditions for a further 10–20 min. The seedlings were then reimaged, and the new esculin front was marked on the plate. Marks were checked for the accuracy of their position immediately after being made, and exact times were recorded. The basipetal phloem velocity in the root was then calculated as distance/time (v = s/t). This method measures only the visible front, and there may be undetectable levels of esculin ahead of this front. However, as the aim was to compare an estimate of PTV rapidly across large numbers of live seedlings, the accuracy is more than sufficient to allow these relative comparisons, rather than the absolute measurements provided by, for example, photobleaching methods (Jensen et al., 2011; Savage et al., 2013), and also avoids the effects of exposure to excess light.
Exogenous Suc Application to Roots Inhibits PTV
The pressure-flow hypothesis requires a pressure differential between photosynthetic source tissues and sink tissues, where the assimilates are unloaded and utilized for growth. Thus, increasing the source strength, by increasing Suc availability, should in theory increase the pressure differential and increase PTV. This has been tested previously by feeding Suc to excised leaves of several species (Vaughn et al., 2002; Lobo et al., 2015). The reverse should also be true, i.e. a reduction in sink strength by providing exogenous Suc to the root, should lead to a reduction in PTV by decreasing sink strength. We tested this by growing seedlings on media containing either 0%, 1% (30 mm), or 2% (60 mm) Suc, the latter two being standard concentrations used in growth media for Arabidopsis seedlings. Reducing the sink strength by supplying 30 mm Suc to the root significantly reduced the PTV by more than 2-fold, as measured by esculin transport, compared to growth on media containing no Suc (Fig. 2A). Growth on media containing 60 mm Suc did not have a further effect, suggesting that the response to exogenous Suc was saturated. Growth on the same concentrations of the nonmetabolized osmolyte, mannitol, showed no effect at 30 mm and a slight increase in PTV at the higher concentration of 60 mm, demonstrating that the reduction in PTV is likely due to the exogenous Suc, not changes in osmotic potential (Supplemental Fig. S1). However, the PTV is not altered in seedlings transiently exposed to increased Suc concentrations for short periods (Supplemental Fig. S2).
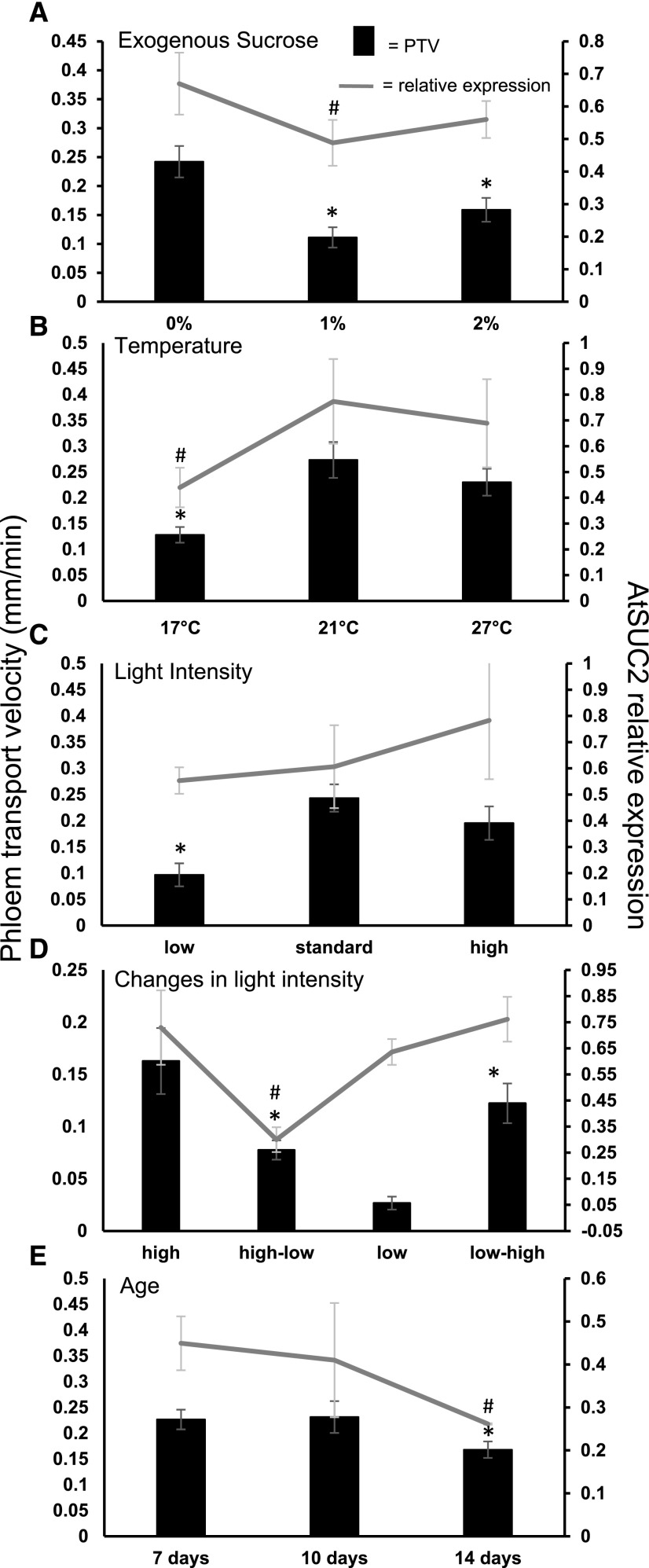
Figure 2.
Variations in environmental conditions affect phloem velocity and partially regulate AtSUC2 expression. A, Exogenous Suc concentration. B, Growth temperature. C, Light intensity. D, Dynamic response to changes in light intensity. E, Seedling age (days after germination). Primary y axis is phloem transport velocity; error bars, se, n = minimum of 25 across minimum of three independent biological replicates. Secondary y axis is relative expression of AtSUC2. Error bars, se from four independent biological replicates. * or #P ≤ 0.05 determined by t test, for PTV or AtSUC2 expression, respectively, compared with the relevant control.
Temperature Affects PTV
Seedlings grown at low temperatures are generally smaller and yet are often as photosynthetically active as those grown at higher temperatures (Strand et al., 1999). Low temperatures have also been shown to induce adaptations for acclimation, including an increase in CC and SE numbers among the collection phloem of leaves (Cohu et al., 2013). When plants were exposed to cool temperatures, while light intensity remained at more optimal levels, Suc accumulated in the leaves, suggesting that demand falls below production (Pollock and Lloyd, 1987). Short-term drops in the daytime temperature reduce the rate of photosynthesis (Pyl et al., 2012). This lowers Suc production and results in a weaker source strength. To test the effect of weakening or strengthening the source via temperature, we measured the PTV of plants grown at low and high temperatures (Fig. 2B). To ameliorate some of the potential effects of plant size on the resultant PTV, seedlings were grown for 4 d at 21°C constant temperature and were then transferred to either 17°C or 27°C for 3 d. Seedlings grown at 17°C showed more than a 2-fold reduction in PTV (0.13 ± 0.019 mm min−1) compared to those at the standard temperature of 21°C (0.28 ± 0.039 mm min−1; Fig. 2B). Interestingly, there was no significant increase in the PTV of seedlings grown at 27°C, despite a 2-fold increase in seedling biomass (Fig. 2B; Supplemental Fig. S3).
Light Intensity Alters PTV
Generally, photosynthetic output increases with light intensity, until an optimum or capacity is reached. Photosynthetic capacity is significantly greater in leaves of apoplastic loading plants grown at high light compared with those grown under low-light conditions (Amiard et al., 2005). This means that Suc production is reduced under low light.
At low-light levels (<10 μmol m−2 s−1), the PTV in seedlings was 2-fold slower than in those grown under our standard light conditions (0.097 ± 0.022 versus 0.24 ± 0.026 mm min−1), supporting the idea that a reduction in Suc production, and thus source strength, reduces the overall pressure gradient in the phloem. Growth under high light resulted in a PTV similar to that under standard conditions (Fig. 2C).
Several apoplastic loaders undergo physical changes when switched from low-light to high-light conditions. Among these are alterations in plasma membrane surface area, which are thought to increase phloem-loading capacity (Amiard et al., 2005). Such physical acclimation takes place over several days. We tested whether PTV could respond more dynamically to a short-term change in light intensity. Plants were grown under low-, standard-, or high-light conditions (9, 100, and 190 µmol m−2 s−1, respectively). At dawn, prior to measurement, the seedlings were transferred from either low light (LL) to high light (HL), or vice versa, for 2 h. The PTV displayed a clear response to the change in light intensities (Fig. 2D). There was a significant drop in PTV when plants were switched from HL to LL compared with those that remained at HL (0.078 ± 0.009 from 0.16 ± 0.032 mm min−1). The reverse scenario, moving from LL to HL, increased the PTV from 0.027 ± 0.0062 to 0.12 ± 0.019 mm min−1. This provides evidence that PTV responds, within relatively short time frames, to metabolic or photosynthetic changes that affect source strength.
PTV in Seedlings of Different Ages
As seedlings develop, new leaves undergo the sink-source transition before they are fully expanded (Wright et al., 2003; Fitzgibbon et al., 2013). Once the first true leaves become carbon sources, the cotyledons are not required to produce Suc to the same extent as before. We measured the PTV in seedlings grown for 7, 10, and 14 dag under our standard conditions (Fig. 2E). There was no difference in PTV between 7 and 10 dag, but a significant drop in PTV at 14 dag (0.23 ± 0.019 versus 0.17 ± 0.015). This decline may be due to decreased export from the cotyledons. However, by 14 dag, a number of lateral roots have developed, potentially diluting the sink strength of the primary root tip. We attempted to discriminate between these confounding factors by comparing the PTV in 14-dag seedlings by loading esculin onto either the cotyledons or the true leaves (Supplemental Fig. S4). There was no significant difference in velocity between the two, although both were slower than the cotyledon-derived PTV seen at 7 or 10 dag, suggesting that the true leaves had indeed taken over some of the export duties.
AtSUC2 Expression Is Regulated in Response to Environmental Cues
AtSUC2 is expressed specifically in the CCs of the source phloem (Truernit and Sauer, 1995). The expression of AtSUC2 has been shown previously to be regulated by leaf developmental stage and abiotic stresses (Truernit and Sauer, 1995; Gong et al., 2015; Durand et al., 2016). Such changes in expression were linked directly to Suc levels in the sugar beet (Beta vulgaris) homolog, BvSUT1 (Vaughn et al., 2002), where both the levels of BvSUT1 protein and carbon export were reduced in leaves supplied with exogenous Suc. The tomato (Solanum lycopersicum) homolog, LeSUT1 also shows transcriptional regulation by light (Kühn et al., 1997). Additionally, Atsuc2-1 mutants are severely restricted in their growth and development but their phenotype can be partially rescued by growth on media supplemented with Suc (Gottwald et al., 2000). As the primary role of AtSUC2 is to load Suc into the CCs, it is a clear candidate for regulating PTV, although this has not been directly demonstrated. We therefore examined the expression of AtSUC2 by reverse transcription-quantitative PCR (RT-qPCR) under the same environmental conditions that induced alterations in the PTV. To allow easy comparison with the PTV response, we plotted AtSUC2 relative expression on a secondary y axis along with the PTV results for each environmental condition (Fig. 2).
Expression of AtSUC2 was reduced in seedlings grown on media containing Suc (Fig. 2A). Relative expression in seedlings grown without exogenous Suc was 0.67 ± 0.095, reducing to 0.49 ± 0.07 in seedlings grown on 1% Suc. There was no significant change in AtSUC2 expression between plants grown on 1% and 2% Suc, mirroring the results seen for PTV (Fig. 2A).
AtSUC2 expression levels increased with temperature, from 0.44 ± 0.08 in seedlings grown at 17°C to 0.77 ± 0.16 at 21°C and 0.69 ± 0.17 at 27°C (Fig. 1B). This was a similar trend to the PTV results where the difference between 21°C and 27°C was also not significant (Fig. 2B).
The expression of AtSUC2 under different light intensities was very variable across the biological replicates and, despite the mean levels following a general increase in expression from low light to high light, there was no significant difference between the light conditions (low light, 0.55 ± 0.05; standard light, 0.61 ± 0.16; high light, 0.78 ± 0.22; Fig. 2C). Interestingly, despite clear effects on the PTV, dynamic changes in light intensity only produced a significant change in expression levels of AtSUC2 following a change from HL to LL (Fig. 2D).
The expression of AtSUC2 also altered with plant developmental stage (Fig. 1E). There was no significant difference between 7 and 10 dag, but by 14 dag the relative expression had decreased from 0.41 ± 0.13 to 0.26 ± 0.0015 (Fig. 1E).
PTV Varies Diurnally
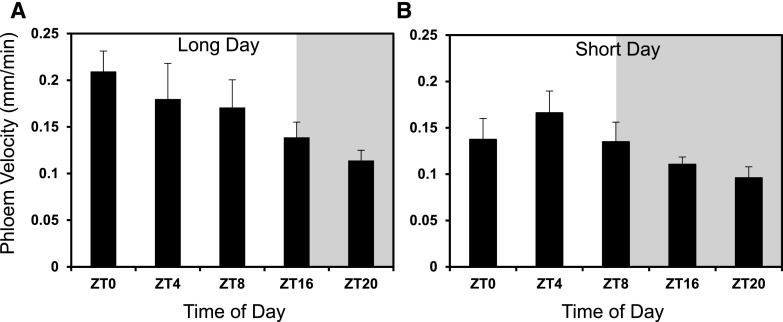
Photosynthetic rate is inextricably linked to light perception and the circadian clock, and plants adapt their physiological and metabolic processes in order to optimize growth under different photoperiods (Sulpice et al., 2014). We investigated whether PTV varied diurnally, mirroring the differences seen in Suc production under different photoperiods (Sulpice et al., 2014). Under long-day conditions (16 h light:8 h dark) the PTV peaked around dawn (ZT0; Zeitgeber time 0) at 0.21 (±0.022) mm min−1 and gradually decreased over the day to its lowest rate of 0.11 mm min−1 (±0.011) at ZT20 in the dark (Fig. 3A). A decrease in PTV from a daytime peak at ZT4 was also observed under short days (8 h light:16 h dark), but this plateaued in the dark from ZT16 onwards (Fig. 3B).
Figure 3.
Phloem transport velocity varies throughout the day. Under long-day conditions (A; 16 h light:8 h dark) and short-day conditions (B; 8 h light:16 h dark). ZT0 = dawn. Shaded areas represent the relevant period of dark. Error bars, se, n = minimum of 25 seedlings across a minimum of three independent biological replicates.
As the PTV varied diurnally, AtSUC2 expression was tested for a circadian clock-linked expression profile (Supplemental Fig. S5). The expression profile showed expression peaking in the dark period between ZT20 and ZT24 (Supplemental Fig. S5A). However, the expression of AtSUC2 was also strongly linked to the transitions between light and dark, suggesting that other factors may be involved in controlling the expression levels. In order to test whether the changes in expression were driven by light or the circadian clock, we tested the expression levels in seedlings entrained under the same long-day period but then grown for a further 2 d in constant light. The peaks of expression were reduced when the seedlings were switched to constant light, suggesting that there is a strong element of light regulation in the expression of AtSUC2 (Supplemental Fig. S5B).
Accumulation of Suc in the Cotyledons Varies Under Differing Environmental Conditions
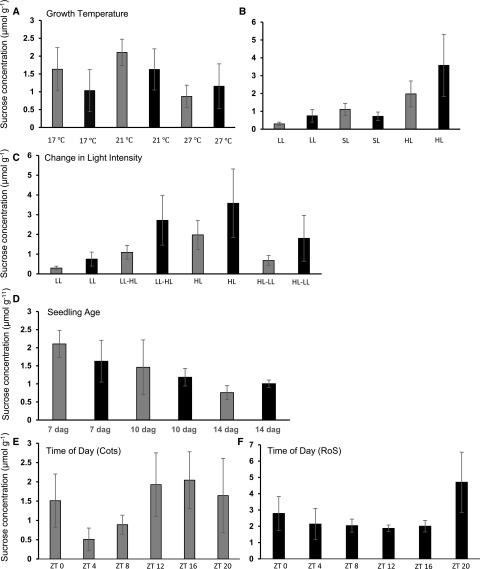
To verify whether the control of PTV or the expression of AtSUC2 was linked to changes in source and sink strength, we determined the amount of Suc accumulated in the cotyledons and the amount present in the rest of the seedling under the range of environmental conditions used to examine PTV (Fig. 4, A–F). At 17°C, Suc was present in the cotyledons at similar levels to seedlings grown under 21°C. Seedlings grown under 27°C had significantly less Suc per gram fresh weight in their cotyledons (0.87 ± 0.32 versus 2.1 ± 0.37), yet similar amounts were present in the rest of the seedling (Fig. 4A). Of course, measurement of Suc concentration in the rest of the seedling only provides a partial indication of the exported Suc. It does not provide a complete measurement, as it only accounts for Suc that has not yet been metabolized or utilized for increases in biomass, the rate of which is also likely to vary under different environmental conditions.
Figure 4.
Suc concentration in both the source and sink tissues varies under different environmental conditions. Suc concentration was measured in an enzymatic assay from the cotyledons (Cots, gray bars) and the rest of the seedling (RoS, black bars) grown under a range of environmental conditions. A, Growth temperature. B, Light intensity. LL, Low light; SL, standard light; HL, high light. C, Dynamic changes in light intensity; seedlings grown under one light intensity were switched to the opposite at dawn and harvested after 2 h. D, Seedling age. E, Time of day, cotyledons only. ZT, Zeitgeber time; ZT0 is defined as time of lights on. F, Time of day, RoS only. Error bars represent se, n = 4 across three independent replicates. *P ≤ 0.05 for Suc concentration compared with the relevant control calculated by t test on log-transformed data.
Under low-light conditions, very little Suc was present in the cotyledons, although similar amounts were present in the rest of the seedlings compared with those grown under standard light (Fig. 4B). Under high light, more Suc was present than under low light, but not significantly more than under standard light intensity in the rest of the seedling (Fig. 4B). This suggests that lower light intensity results in lower rates of photosynthesis, potentially resulting in lower concentrations of Suc exported. After just 2 h in high light, seedlings previously grown at low light showed an increase in the mean amount of Suc, compared with those maintained at low light (1.09 ± 0.35 versus 0.3 ± 0.1), and much of this had already been exported to the rest of the seedling (Fig. 4C) at levels comparable with seedlings grown under high light. The opposite was also true. Seedlings grown at high light and transferred to low light for 2 h at dawn displayed lower levels of Suc in their cotyledons than those at continuous high light, although still higher than those grown continuously under low light (Fig. 4C; 0.68 ± 0.25 versus 0.3 ± 0.1).
Seedling age had a significant impact on the concentration of available Suc (Fig. 4D), with a reduction in concentration found in the cotyledons at 14 dag, suggesting that the first leaves are beginning to take over the role of carbon sources. There were some differences in Suc concentration across the day in the cotyledons, with the peak concentration occurring toward the end of the day at ZT12 and during the night and reducing through the night to the lowest levels by early morning (ZT4; Fig. 4, E and F).
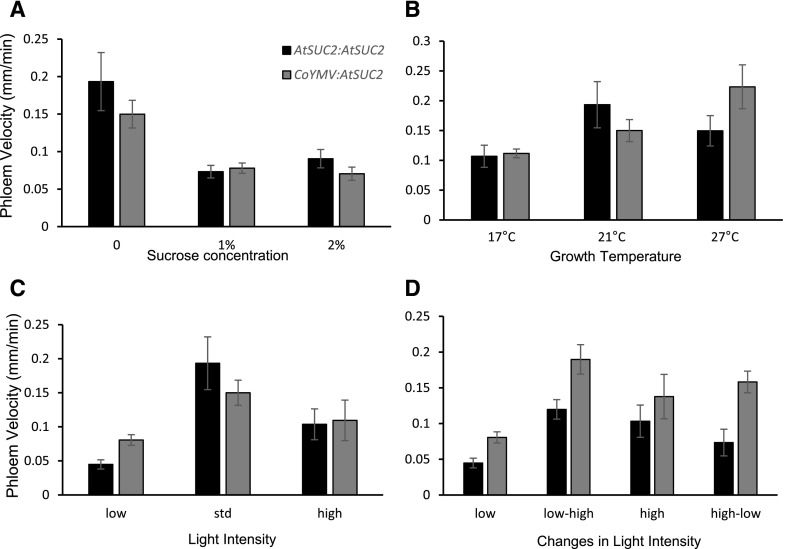
PTV Remains Sensitive to Environmental Changes When AtSUC2 Is Expressed from an Exotic Promoter
Suc has been shown to negatively regulate AtSUC2 expression (Dasgupta et al., 2014); however, our results were not always conclusive regarding AtSUC2 expression level and the corresponding PTV (Fig. 2). When AtSUC2 is expressed from a CC-localized promoter from Commelina Yellow Mottle Virus (CoYMV), it rescues the phenotype of atsuc2 knockout (ko) plants, indicating that it works as a functional replacement for native AtSUC2 (Srivastava et al., 2009). Therefore, we used CoYMV:AtSUC2 lines to examine whether the regulation of PTV via AtSUC2 required the AtSUC2 promoter. Under each set of environmental conditions, CoYMV:AtSUC2 seedlings behaved similarly to AtSUC2:AtSUC2 lines, suggesting that, despite the effects seen on the abundance of AtSUC2 transcripts, PTV regulation does not depend entirely on transcriptional regulation from the AtSUC2 promoter (Fig. 5, A–D). However, some minor differences were noted, in particular the PTV did not respond to low light nor acute changes in light as dynamically in CoYMV:AtSUC2 seedlings compared to AtSUC2:AtSUC2 (Fig. 5D; LL = 0.045 ± 0.0068 versus 0.081 ± 0.0078, LL-HL = 0.12 ± 0.014 versus 0.19 ± 0.021) or as described previously for wild type (Fig. 2D). This suggests that the AtSUC2 promoter may be required to regulate the PTV under acute changes in light intensity.
Figure 5.
Phloem velocity remains responsive to environmental conditions when AtSUC2 is expressed under a phloem-specific foreign promoter. Esculin translocation was used to measure phloem velocity under a range of environmental conditions in 7-d-old atsuc2-1 seedlings expressing either AtSUC2pro:AtSUC2 or CoYMVpro:AtSUC2. A, Exogenous Suc concentration. B, Growth temperature. C, Light intensity. D, Dynamic response to changes in light intensity. Error bars represent se, n = minimum of 10 seedlings across three replicates.
DISCUSSION
Esculin Facilitates High-Throughput Measurement of PTV in Young Seedlings
The fluorescence of esculin and its recognition by AtSUC2 provides a straightforward mechanism for monitoring phloem transport in planta. This method allows relatively high-throughput studies to be conducted in live seedlings, where growth on agar plates in environmentally controlled growth chambers allows perturbation of environmental conditions and measurement of the effects of such changes on PTV. We have shown that esculin is an excellent proxy for Suc in this type of study, as it is translocated from the cotyledon surface to the rest of the seedling with the same efficiency as radiolabeled Suc (Fig. 1). Furthermore, esculin only enters the phloem in Arabidopsis in detectable amounts using the AtSUC2 symporter, the key Suc loader in Arabidopsis (Knoblauch et al., 2015; De Moliner et al., 2018).
PTV has been measured in a number of different species, but previous studies focused on large, mature plants due to the technical limitations of the available methods. The reported PTV, measured by MRI or fluorescent tracer methods, varies across species, tissues, and developmental stages, ranging from 0.18 mm min−1 to 102 mm min−1. The majority of species that have been measured show a PTV of around 15 mm min−1 (Windt et al., 2006; Jensen et al., 2011). The results presented here indicate that the velocity (around 0.25 mm min−1) in young Arabidopsis seedling roots, measured under normal growth conditions, is toward the slower end of this scale. A velocity of 1.5 mm min−1 was reported for individual cells in the region where the metaphloem transfers assimilate to the protophloem, slowing to 0.3 mm min−1 in cells in the protophloem unloading zone (Ross-Elliott et al., 2017). Our measurements were made in roots before esculin had reached the unloading zone, but ultimately represent an average of the cells within the measured root region, rather than the velocity within a single sieve element. Although measuring PTV at the single-cell level is possible with esculin, it is much more time consuming and thus does not lend itself to the high-throughput method described here. Our method allowed us to rapidly monitor the effects of environmental variation on the PTV and could be easily adapted for use in older plants and other species. The simplicity of this method does not account for esculin moving ahead of the visible front, nor does it compensate for any lateral loss of esculin along the phloem pathway; thus, the relative PTV measurements presented here are likely to be slower than the absolute PTV for Suc. Further, as any lateral loss from the phloem is undetected, we cannot determine whether any environmental variables induce changes in the rate of lateral loss, which would contribute to the effect seen on the PTV. However, our method is a significant advantage over the use of radiolabeled Suc, which does not permit the level of resolution we have reported here.
Environmental Conditions Affect PTV
While it has generally been assumed that environmental conditions, particularly those likely to affect the production or metabolism of Suc, could have a substantial effect on the PTV, actual data has been limited to a relatively small number of studies, one of which showed that developmental stage in cucurbits affected PTV (Savage et al., 2013) and another which detailed the effects of developmental stage and osmotic stress in Arabidopsis (Durand et al., 2018). Other studies suggest that, despite fluctuations in carbon export over the day and night, PTV remains more or less constant in the stems of several species (Windt et al., 2006). These authors argued that the PTV was likely to be regulated to a consistent velocity in order to allow constant transmission of long-distance molecular signals through the phloem. Our results contrast with this, showing that PTV in Arabidopsis varies markedly in response to a variety of stimuli.
Growth at low temperatures, low light, and acute changes from high to low light, resulted in significant reductions in PTV (Fig. 2). Furthermore, in contrast to the results obtained for three out of four species tested by Windt et al. (2006), there was a distinct diurnal variation in PTV in Arabidopsis, with the lowest velocity recorded late in the night (Fig. 3). These are all environmental conditions that affect the rate of photosynthesis and thus Suc production. This indicates that PTV may be related to the amount of Suc available for export.
Is PTV Regulated by Complex Signals or Simply by Source/Sink Strength?
The Münch hypothesis states that the phloem flow is created by the disparity between the concentration of Suc in the source and sink tissues. The stronger the demand for carbon in the sink tissues, the more Suc is required to be loaded into the collection phloem in the source tissues. However, what happens if the sink strength is reduced? We tested this using exogenously applied Suc to the roots. This resulted in PTV being reduced by around half (Fig. 2).
Many researchers grow Arabidopsis seedlings on media supplemented with Suc, despite the fact that Suc can change the expression of more than 797 genes and regulates many of the elements of the circadian clock (Gonzali et al., 2006; Dalchau et al., 2011). Unfortunately, much of the experimental data publicly available has been produced using seedlings grown on Suc. Clearly, care must be taken when interpreting such data, especially expression data for Suc-related genes.
It has been shown that a reduction in sink strength leads to Suc accumulation in source leaves, which in turn inhibits photosynthesis, reducing the Suc available for transport (Paul and Pellny, 2003). Therefore, conditions that naturally reduce the efficiency of photosynthesis should also reduce the amount of Suc available for transport. Our results corroborate this model, as under low light (<10 μmol m−2 s−1) PTV is reduced and so is the Suc concentration (Figs. 2, C and D, and 4, B and C). Equally, as the seedling develops, the cotyledons begin to senesce and therefore become less important, as the first true leaves take on a more dominant role in carbon export. In our experiments, this could be seen as a drop in PTV in seedlings at 14 dag (Fig. 2E), attributed at least in part to reduced export from the cotyledons and increased export from the true leaves (Supplemental Fig. S4), coupled to a corresponding reduction in Suc levels in the cotyledons (Fig. 4D). However, it has been postulated that decreased PTV, caused by reduced sink strength, would not be sufficient to alter the concentration of Suc in the mesophyll in active apoplastic loaders such as Arabidopsis, as the proton motive force that drives the Suc symporters enables phloem loading at even very high apoplastic Suc concentrations (Ainsworth and Bush, 2011). Further, there is some evidence to suggest that K+ ions have a role in maintaining the hydraulic pressure gradient of the phloem pathway, playing a role in reloading leaked Suc (Deeken et al., 2002). Therefore, this would suggest that phloem loading must be down-regulated to allow Suc to build up in the mesophyll cells. We asked whether this response could occur through a feedback signal on the transcription rates of the AtSUC2 gene, thus reducing the number of transporters available. This would provide a natural “brake” on the amount of Suc being loaded into the phloem, reducing flow. Indeed, there was a slight reduction in the expression of the main Suc transporter, AtSUC2, when seedlings were grown on exogenous Suc, suggesting that the PTV may, at least in part, be linked to the transcriptional regulation of the transporter. A homolog of AtSUC2, the sugar beet BvSUT1, is transcriptionally repressed by exogenous Suc applied to leaf discs (Vaughn et al., 2002), and Suc transport activity and mRNA abundance were decreased in leaves fed exogenous Suc via the xylem (Chiou and Bush, 1998). This is not simply an osmotic stress effect, as seedlings grown on mannitol did not show a decrease in PTV (Supplemental Figs. S1 and S2). In fact, at 60 mm, mannitol induced an increase in PTV. Such opposing effects have been previously reported in pea seedlings, although the increase was triggered at even lower concentrations of mannitol (Schulz, 1994). Schulz (1994) suggests that the increase in PTV following mannitol treatments serves to counteract the effects of osmotic stress, where higher amounts of solutes are drawn from the phloem in response to the low apoplasmic potential. However, in pea seedlings, as in our Arabidopsis seedlings, low concentrations of Suc (<75 mm) result in an inhibition of phloem transport. One potential explanation for this is that the uptake of Suc into the sink cells would reduce the exosmosis of water (Schulz, 1994). Such effects are usually associated with short-term osmotic stress, with equilibrium being reached in the root tip after a few hours (Schulz, 1994), yet our seedlings were grown on media from germination. Interestingly, the observed effects may not be solely an osmotic response, as Suc and mannitol have previously been shown to have opposite effects on the activity of the AtSUC2 promoter, (Dasgupta et al., 2014). Equally, it is not possible to rule out from these experiments that the Suc effect on PTV is not partially caused by physical changes in the phloem and a general difference in biomass caused by growth on exogenous Suc (Supplemental Fig. S3). When seedlings were transferred from media without any Suc or mannitol to media containing either 30 mm or 60 mm of either Suc or mannitol for 2 h, no reduction in PTV was seen, although the increase in PTV caused by a high concentration mannitol was replicated (Supplemental Fig. S2). This may mean that the Suc effect on PTV does require structural changes to the phloem or, as the reduction in import from the phloem was seen following similarly short timescales in pea seedlings, it may suggest that 2 h is not sufficient for the uptake into the roots from solid media, as opposed to root tips submerged in liquid media (Schulz, 1994). Equally, the timescale may be too short for a potential feedback signal from sink to source to have a significant effect on the PTV.
Not all environmental conditions that affected PTV and Suc accumulation caused a significant change in the expression levels of AtSUC2 (Fig. 1). Seedlings grown under low temperatures showed a decrease in AtSUC2 expression, but seedlings grown under low light, despite a significant change in PTV, did not (Fig. 1C). Intriguingly, a significant drop in expression was seen for seedlings grown under high light and then moved to low light for 2 h. This indicates that perhaps the direct control of expression is used to deal with dynamic short-term fluctuations in the environment.
Under consistent low light, there was essentially no change in AtSUC2 expression despite a significant reduction in PTV. Xu et al. (2018) found that Arabidopsis and other apoplastic loaders, grown under low-light conditions, did show reduced expression of a range of Suc transporters (Xu et al., 2018). However, their low-light conditions were at 40 μmol m−2 s−1, whereas in this study, they were significantly lower (10 μmol m−2 s−1). We did see a significant reduction of AtSUC2 expression in plants moved from high light to low light for 2 h, supporting the idea that there is dynamic regulation of the promoter under acute environmental changes. Further, we cannot rule out that there may be morphological alterations induced in the root phloem in seedlings grown under low-light or low-temperature conditions, which may well affect the PTV, although they were only grown under such conditions for 3 d in order to try to reduce such effects.
By expressing AtSUC2 in an atsuc2ko background using a phloem specific promoter from Commelina Yellow Mottle Virus (CoYMV), we were able to test whether the dynamic changes seen in PTV were the result of promoter-specific changes in AtSUC2 expression (Srivastava et al., 2009). Despite previous work showing that exogenous Suc inhibits promoter activity, and our RT-qPCR data showing mild effects on the expression levels of AtSUC2 under certain conditions, this was not the case; PTV responded to the different environmental cues in a similar way in both CoYMV:AtSUC2 seedlings and AtSUC2:AtSUC2 seedlings (Fig. 5). The main exceptions involved low light and acute changes in light intensity. Here, the CoYMV:AtSUC2 seedlings were less responsive to a change from high to low light, suggesting that under these conditions transcriptional repression of AtSUC2 from the promoter may indeed play a more important role.
It is likely that several factors converge on SUTs to fine-tune their transcriptional regulation. For example, blocking protein phosphatase activity in sugar beet resulted in a decrease in symporter transcript abundance, and ultimately symporter abundance (Ransom-Hodgkins et al., 2003). Further experiments suggested that there might be a phosphorylated protein that is a negative regulator of BvSUt1 transcription (Ransom-Hodgkins et al., 2003). Other factors have been shown to be involved in regulation of SUTs. For example, StSUT4 accumulates under far-red-light conditions (Liesche et al., 2011). However, it also accumulates following actinomycin D treatment, suggesting the accumulation is due to increased transcript stability, not increased transcription (Liesche et al., 2011). Indeed, further regulation is likely to occur at the posttranscriptional level, as most SUT mRNAs are relatively short lived, with half-lives ranging from 60 to 130 min (Vaughn et al., 2002; He et al., 2008: Liesche et al., 2011)
Control of transporter activity clearly also occurs at the posttranslational level, with many SUTs proving to be relatively unstable proteins; StSUT1 is degraded in <4 h and BvSUT1 in just 2.7 h (Vaughn et al., 2002). Xu et al. (2018) reported an increase in LeSUT1 abundance under high-light conditions, despite a lack of transcriptional change, suggesting that there is either posttranscriptional or translational regulation, such as an increase in protein stability. Some SUTs have also been shown to dimerize with different proteins. For SUT1, this is likely to be redox dependent, with oxidizing conditions favoring homodimerization and increased plasma membrane targeting (Reinders et al., 2002; Krügel et al., 2008). SUT4 has been suggested to act as an inhibitor of SUT1, thus inhibiting Suc transport directly (Liesche et al., 2011). It is possible that a similar situation could occur in Arabidopsis. Another potential layer of regulation could come simply from the availability of protons required for the active loading of Suc (Khadilkar et al., 2016).
CONCLUSION
Our data, using esculin as a proxy for Suc, suggest that the expression, activity, and stability of SUTs is dynamically regulated by a number of pathways. This fits with a physiological system that needs to be able to respond rapidly to sudden changes in environmental conditions that affect Suc production and thus source-sink relations.
MATERIALS AND METHODS
Plant Growth
Seeds of Arabidopsis (Arabidopsis thaliana), ecotype Col-0 were surface sterilized by immersion in 10% (v/v) bleach for 15 min, then rinsed in 70% v/v ethanol, followed by five rinses in sterile double-distilled water. Seeds were plated in two rows, with an average of 15 seeds per plate on 25 mL 0.5× Murashige and Skoog basal salt media (Duchefa; MO221), solidified with 2% (w/v) Phytoagar (Melford; P1003). Seeds were stratified at 4°C for 2 days before transfer to controlled environment growth chambers (Percival) within a climate controlled dark room. Standard conditions were 80 to 100 μmol m−2 s−1 white light, under long days (16 h light:8 h dark) at a constant 21°C. Seeds of atscu2-4 AtSUC2:AtSUC2 and CoYMV:AtSUC2 were the kind gift of Brian Ayre and have been previously described (Srivastava et al., 2009)
Phloem Transport Efficiency of Fluorescent Probes versus Radiolabeled Suc
Seedlings were treated at 7 dag. Both cotyledons were pretreated with 0.3 μL of a 2.5% (v/v) Adigor (Syngenta) solution for 1 h. Then, either 0.3 μL of 9 mg mL−1 Esculin, or 14C Suc (Perkin-Elmer) was added to each cotyledon. Seedlings were sampled at 4 h post-probe-application. Remnants of the probe solution were washed off by submerging the intact seedling’s cotyledons into 600 μL of ethanol. The cotyledons were then removed into a separate tube of 600 μL ethanol and the remainder of the seedling (root, hypocotyl, meristem, and emerging true leaves) into a third tube of 600 μL ethanol. All tubes were heated to 75°C for 1 h, chilled on ice briefly, and then centrifuged at full speed for 2 min. For measurement, 300 μL of the radiolabeled samples was added to 3 mL of scintillant in a scintillation vial and counted on for 2 min per sample with two repeats. Fluorescent samples were split into 200-μL portions and loaded into separate wells in a 96-well plate (Greiner) before being read on a Tecan M200 with excitation set at 405 nm and emission collected at 454 nm for esculin. Control samples from seedlings not treated with esculin were used to give background readings and subtracted from all samples. A minimum of five seedlings per treatment and time point were used, with two independent replicate experiments for each probe.
Measurement of Phloem Transport Velocity
Except where noted, seedlings were pretreated at ZT0/putative dawn (lights on) with 0.3 μL of 2.5% Adigor in double-distilled water for 1 h. The cotyledons were blotted lightly to remove excess Adigor solution and then 0.3 μL of esculin was added to each cotyledon. After 10 min, the seedlings were checked for the appearance of esculin in the phloem in the root. The fluorescent front was marked on the plate and time noted. Seedlings were rechecked, and the new front marked together with time. The distance moved was calculated by measuring the root length between the two marks using ImageJ software. The velocity was calculated as velocity = distance/time. A minimum of 25 seedlings spread over a minimum of three independent replicates was used for each condition. In order to minimize differences caused by variation in growth and biomass, seedlings for temperature and light intensity experiments were grown under standard conditions for 4 d, before being transferred to the appropriate condition. For acute change in light intensity, seedlings were transferred to the opposite light intensity at ZT0/putative dawn.
Gene Expression Measurements
Seedlings were grown as described above and 100 mg seedlings harvested per sample in duplicate, 1 h after dawn or 2 h following acute changes in light intensity. Time of day and circadian experiment seedlings were harvested at the time points described. Seedlings were harvested in 1 mL of RNAlater (Sigma-Aldrich) and kept in the dark overnight at 4°C before being transferred to −20°C until extraction. RNA was extracted using RNEasy Plant Mini Kit (Qiagen). In brief, tissue was ground in 450 μL RLT buffer with two steel beads using a Tissuelyser Mixer Mill (Qiagen) for 2 min at 27 s−1, the blocks were rotated and run again for 2 min. Samples were then incubated at 56°C for 3 min before being added to the shredder columns and manufacturer’s protocol was followed, including the DNAseI step. Samples were double eluted in 35 μL of RNase free water. One microgram RNA per sample was reverse transcribed using a RevertAid First Strand cDNA Synthesis kit (Thermo Scientific). RT-qPCRs were set up using Roche Lightcyler 480 SYBR Green 1 Master mix in technical triplicates and cycled in a LightCycler 480. The mRNA abundance was calculated using the Relative Quantification function. Oligos used were AtSUC2 F-GCAGACGGGTGAGTTAGA and AtSUC2 R-GGAGATTGGACCACAGAG (Durand et al., 2016) and for the reference gene, ACT7 F-TGAACAATCGATGCACCTGA and ACT7 R-CAGTGTCTGGATCGGAGGAT.
Extraction and Enzymatic Quantification of Suc
Seedlings were harvested, and cotyledons and the rest of the seedling tissue were split into separate tubes, 1 mL of 70% ethanol was added and then flash-frozen in liquid N2. For extraction, samples were boiled at 90°C for 10 min, then spun down and supernatant transferred to a fresh Eppendorf. Then 250 μL of double-distilled water was added and samples vortexed for 1 min before adding 250 μL ethanol and then boiled at 90°C again for 10 min. Samples were then spun down and supernatant transferred to a new tube. The total volume was brought to 1.6 mL with double-distilled water and stored at −20°C until quantification. In clear 96-well microtitre plates, 200 μL of assay cocktail (10 mg NADP, 33 mg ATP dissolved in 40 mL of 150 mm Tris and 5 mm MgCl2 [pH 8.1]) was added to each well, together with 45 μL double-distilled water and 5 μL of sample. Each sample was loaded in triplicate and mixed well. Absorbance at A340 (read A) was measured on a Fluostar Omega plate reader (BMG Labtech) and then 5 μL (0.5 units) of Hexokinase and Glc-6-phosphate dehydrogenase was added to each well, mixed thoroughly and incubated at room temperature for 30 min. Then read at A340 again (read B). The initial reading (A) was subtracted from the second (B) to give the Glc level. In a second plate, 40 μL of 100 mm trisodium citrate with 5 mm MgCl2 (pH 5) was added to each well, along with 5 μL of each sample and 4 μL of invertase and then incubated at room temperature for 10 min. Then, 200 μL of assay cocktail was added, mixed, and then the wells were read at A340 (read C), before adding 5 μL (0.5 units) of hexokinase/G6-PDH to each well and incubated at room temperature for 30 min before a final reading at A340 (read D). Suc dA340 = (D − C) − (B − A). Absorbency of NADPH is 6.22 so Suc concentration (μmol) was calculated as dA340/6.22 and then multiplied by 50 to account for the initial dilution. Each result was then divided by the fresh weight of the initial sample to give the concentrating in μmol g−1.
Accession Numbers
Sequence data for AtSUC2 can be found using accession number At1g22710.
Supplemental Data
The following supplemental materials are available:
Supplemental Figure S1. Comparison of the effects of growth on different concentrations of Suc and mannitol on PTV.
Supplemental Figure S2. Comparison of the effects of transient exposure to Suc or mannitol on PTV.
Supplemental Figure S3. Mean wet biomass for seedlings grown under a range of environmental conditions.
Supplemental Figure S4. Comparison of phloem velocity in 14-d-old seedlings following loading of cotyledons or true leaves.
Supplemental Figure S5. AtSUC2 expression is not tightly circadian but regulated by light signals.
Supplemental Figure S6. Root length of 7-d-old AtSUC2:AtSUC2 and CoYMV:AtSUC2 seedlings.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Dr. Marc Vendrell, Dr. Fabio De Moliner (both CIR, QMRI, University of Edinburgh), Dr. Tim Hawkes, and Dr. Ryan Ramsey (both Syngenta) for helpful discussions.
Footnotes
This work was supported by Biotechnology and Biological Sciences Research Council Grant BB/M025160/1.
References
- Ainsworth EA, Bush DR (2011) Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol 155: 64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiard V, Mueh KE, Demmig-Adams B, Ebbert V, Turgeon R, Adams WW III (2005) Anatomical and photosynthetic acclimation to the light environment in species with differing mechanisms of phloem loading. Proc Natl Acad Sci USA 102: 12968–12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp A, Lehesranta S, Vatén A, Help H, El-Showk S, Scheres B, Helariutta K, Mähönen AP, Sakakibara H, Helariutta Y (2011) Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Curr Biol 21: 927–932 [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95: 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy AL, Fisher DB (1978) Kinetics of C-photosynthate translocation in morning glory vines. Plant Physiol 61: 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohu CM, Muller O, Demmig-Adams B, Adams WW III (2013) Minor loading vein acclimation for three Arabidopsis thaliana ecotypes in response to growth under different temperature and light regimes. Front Plant Sci 4: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau N, Baek SJ, Briggs HM, Robertson FC, Dodd AN, Gardner MJ, Stancombe MA, Haydon MJ, Stan GB, Gonçalves JM, et al. (2011) The circadian oscillator gene GIGANTEA mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proc Natl Acad Sci USA 108: 5104–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta K, Khadilkar AS, Sulpice R, Pant B, Scheible WR, Fisahn J, Stitt M, Ayre BG (2014) Expression of sucrose transporter cDNAs specifically in companion cells enhances phloem loading and long-distance transport of sucrose but leads to an inhibition of growth and the perception of a phosphate limitation. Plant Physiol 165: 715–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeken R, Geiger D, Fromm J, Koroleva O, Ache P, Langenfeld-Heyser R, Sauer N, May ST, Hedrich R (2002) Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 216: 334–344 [DOI] [PubMed] [Google Scholar]
- De Moliner F, Knox K, Reinders A, Ward JM, McLaughlin PJ, Oparka K, Vendrell M (2018) Probing binding specificity of the sucrose transporter AtSUC2 with fluorescent coumarin glucosides. J Exp Bot 69: 2473–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand M, Porcheron B, Hennion N, Maurousset L, Lemoine R, Pourtau N (2016) Water deficit enhances C export to the roots in Arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol 170: 1460–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand M, Mainson D, Porcheron B, Maurousset L, Lemoine R, Pourtau N (2018) Carbon source-sink relationship in Arabidopsis thaliana: the role of sucrose transporters. Planta 247: 587–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon J, Beck M, Zhou J, Faulkner C, Robatzek S, Oparka K (2013) A developmental framework for complex plasmodesmata formation revealed by large-scale imaging of the Arabidopsis leaf epidermis. Plant Cell 25: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Liu M, Zhang L, Ruan Y, Ding R, Ji Y, Zhang N, Zhang S, Farmer J, Wang C (2015) Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol Plant 153: 119–136 [DOI] [PubMed] [Google Scholar]
- Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P (2006) Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res 119: 115–123 [DOI] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97: 13979–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould N, Thorpe MR, Pritchard J, Christeller JT, Williams LE, Roeb G, Schurr U, Minchin PE (2012) AtSUC2 has a role for sucrose retrieval along the phloem pathway: evidence from carbon-11 tracer studies. Plant Sci 188-189: 97–101 [DOI] [PubMed] [Google Scholar]
- Grignon N, Touraine B, Durand M (1989) 6(5) carboxyfluorescein as a tracer of phloem sap translocation. Am J Bot 76: 871–877 [Google Scholar]
- He H, Chincinska I, Hackel A, Grimm B, Kühn C (2008) Phloem mobility and stability of sucrose transporter transcripts. Open Plant Sci J 2: 1–14 [Google Scholar]
- Heo JO, Roszak P, Furuta KM, Helariutta Y (2014) Phloem development: current knowledge and future perspectives. Am J Bot 101: 1393–1402 [DOI] [PubMed] [Google Scholar]
- Jensen KH, Lee J, Bohr T, Bruus H, Holbrook NM, Zwieniecki MA (2011) Optimality of the Münch mechanism for translocation of sugars in plants. J R Soc Interface 8: 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadilkar AS, Yadav UP, Salazar C, Shulaev V, Paez-Valencia J, Pizzio GA, Gaxiola RA, Ayre BG (2016) Constitutive and companion cell-specific overexpression of AVP1, encoding a proton-pumping pyrophosphatase, enhances biomass accumulation, phloem loading, and long-distance transport. Plant Physiol 170: 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M, Vendrell M, de Leau E, Paterlini A, Knox K, Ross-Elliot T, Reinders A, Brockman SA, Ward J, Oparka K (2015) Multispectral phloem-mobile probes: properties and applications. Plant Physiol 167: 1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M, Knoblauch J, Mullendore DL, Savage JA, Babst BA, Beecher SD, Dodgen AC, Jensen KH, Holbrook NM (2016) Testing the Münch hypothesis of long distance phloem transport in plants. eLife 5: e15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köckenburger W, Pope JM, Xia Y, Jeffrey KR, Komor E, Callaghan PT (1997) A non-invasive measurement of phloem and xylem water flow in castor bean seedlings by nuclear magnetic resonance microimaging. Planta 201: 53–63 [Google Scholar]
- Kölling K, Thalmann M, Müller A, Jenny C, Zeeman SC (2015) Carbon partitioning in Arabidopsis thaliana is a dynamic process controlled by the plants metabolic status and its circadian clock. Plant Cell Environ 38: 1965–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel U, Veenhoff LM, Langbein J, Wiederhold E, Liesche J, Friedrich T, Grimm B, Martinoia E, Poolman B, Kühn C (2008) Transport and sorting of the solanum tuberosum sucrose transporter SUT1 is affected by posttranslational modification. Plant Cell 20: 2497–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298–1300 [DOI] [PubMed] [Google Scholar]
- Liesche J, Krügel U, He H, Chincinska I, Hackel A, Kühn C (2011) Sucrose transporter regulation at the transcriptional, post-transcriptional and post-translational level. J Plant Physiol 168: 1426–1433 [DOI] [PubMed] [Google Scholar]
- Liu DD, Chao WM, Turgeon R (2012) Transport of sucrose, not hexose, in the phloem. J Exp Bot 63: 4315–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo AKM, de Oliveira Martins M, Lima Neto MC, Machado EC, Ribeiro RV, Silveira JA (2015) Exogenous sucrose supply changes sugar metabolism and reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity. J Plant Physiol 179: 113–121 [DOI] [PubMed] [Google Scholar]
- Madore MA, Lucas WJ (1987) Control of photoassimilate movement in source-leaf tissues of Ipomoea tricolor Cav. Planta 171: 197–204 [DOI] [PubMed] [Google Scholar]
- Minchin PEH, Thorpe MR (2003) Using the short-lived isotope 11C in mechanistic studies of photosynthate transport. Funct Plant Biol 30: 831–841 [DOI] [PubMed] [Google Scholar]
- Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC (2010) Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 328: 872–875 [DOI] [PubMed] [Google Scholar]
- Mullendore DL, Windt CW, Van As H, Knoblauch M (2010) Sieve tube geometry in relation to phloem flow. Plant Cell 22: 579–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch E. (1930) Material Flow in Plants. Translated 2003 by JA Millburn and KH Kreeb, Germany: University of Bremen. Gustav Fischer Verlag, Jena, Germany [Google Scholar]
- Ohmae Y, Hirose A, Sugita R, Tanoi K, Nakanishi TM (2013) Carbon-14 labelled sucrose transportation in an Arabidopsis thaliana using an imaging plate and real time imaging system. J Radioanal Nucl Chem 296: 413–416 [Google Scholar]
- Oparka KJ, Turgeon R (1999) Sieve elements and companion cells-traffic control centers of the phloem. Plant Cell 11: 739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Duckett CM, Prior DAM, Fisher DB (1994) Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J 6: 759–766 [Google Scholar]
- Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54: 539–547 [DOI] [PubMed] [Google Scholar]
- Paultre DSG, Gustin M-P, Molnar A, Oparka KJ (2016) Lost in transit: long-distance trafficking and phloem unloading of protein signals in Arabidopsis homografts. Plant Cell 28: 2016–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuke AD, Rokitta M, Zimmermann U, Schreiber L, Haase A (2001) Simultaneous measurement of water flow velocity and solute transport in xylem and phloem of adult plants of Ricinus communis over a daily time course by nuclear magnetic resonance spectrometry. Plant Cell Environ 24: 491–503 [Google Scholar]
- Pollock CJ, Lloyd EJ (1987) The effect of low temperature upon starch, sucrose and fructan synthesis in leaves. Ann Bot 60: 231–235 [Google Scholar]
- Pyl ET, Piques M, Ivakov A, Schulze W, Ishihara H, Stitt M, Sulpice R (2012) Metabolism and growth in Arabidopsis depend on the daytime temperature but are temperature-compensated against cool nights. Plant Cell 24: 2443–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom-Hodgkins WD, Vaughn MW, Bush DR (2003) Protein phosphorylation plays a key role in sucrose-mediated transcriptional regulation of a phloem-specific proton-sucrose symporter. Planta 217: 483–489 [DOI] [PubMed] [Google Scholar]
- Reinders A, Schulze W, Kühn C, Barker L, Schulz A, Ward JM, Frommer WB (2002) Protein-protein interactions between sucrose transporters of different affinities colocalized in the same enucleate sieve element. Plant Cell 14: 1567–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A, Sun Y, Karvonen KL, Ward JM (2012) Identification of amino acids important for substrate specificity in sucrose transporters using gene shuffling. J Biol Chem 287: 30296–30304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Turgeon R (2009) A comprehensive picture of phloem loading strategies. Proc Natl Acad Sci USA 106: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Elliott TJ, Jensen KH, Haaning KS, Wager BM, Knoblauch J, Howell AH, Mullendore DL, Monteith AG, Paultre D, Yan D, et al. (2017) Phloem unloading in Arabidopsis roots is convective and regulated by the phloem-pole pericycle. eLife 6: e24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N. (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581: 2309–2317 [DOI] [PubMed] [Google Scholar]
- Savage JA, Zwieniecki MA, Holbrook NM (2013) Phloem transport velocity varies over time and among vascular bundles during early cucumber seedling development. Plant Physiol 163: 1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A. (1994) Phloem transport and differential unloading in pea seedlings after source and sink manipulations. Planta 192: 239–248 [Google Scholar]
- Srivastava AC, Ganesan S, Ismail IO, Ayre BG (2009) Effective carbon partitioning driven by exotic phloem-specific regulatory elements fused to the Arabidopsis thaliana AtSUC2 sucrose-proton symporter gene. BMC Plant Biol 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand A, Hurry V, Henkes S, Huner N, Gustafsson P, Gardeström P, Stitt M (1999) Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol 119: 1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Flis A, Ivakov AA, Apelt F, Krohn N, Encke B, Abel C, Feil R, Lunn JE, Stitt M (2014) Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol Plant 7: 137–155 [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N (1995) The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta 196: 564–570 [DOI] [PubMed] [Google Scholar]
- Turgeon R. (2010) The puzzle of phloem pressure. Plant Physiol 154: 578–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel AJE. (1996) Interaction between sieve element and companion cell and the consequences for photoassimilate distribution. Two structural hardware frames with associated physiological software packages in dicotyledons? J Exp Bot 47: 1129–1140 [DOI] [PubMed] [Google Scholar]
- Vaughn MW, Harrington GN, Bush DR (2002) Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci USA 99: 10876–10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw IF. (1990) Tansley review no. 27: the control of carbon partitioning in plants. New Phytol 116: 341–381 [DOI] [PubMed] [Google Scholar]
- Windt CW, Vergeldt FJ, de Jager PA, van As H (2006) MRI of long-distance water transport: a comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant Cell Environ 29: 1715–1729 [DOI] [PubMed] [Google Scholar]
- Wright KM, Oparka KJ (1996) The fluorescent probe HPTS as a phloem-mobile, symplastic tracer: an evaluation using confocal laser scanning microscopy. J Exp Bot 47: 439–445 [Google Scholar]
- Wright KM, Roberts AG, Martens HJ, Sauer N, Oparka KJ (2003) Structural and functional vein maturation in developing tobacco leaves in relation to AtSUC2 promoter activity. Plant Physiol 131: 1555–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Chen S, Yunjuan R, Chen S, Liesche J (2018) Regulation of sucrose transporters and phloem loading in response to environmental cues. Plant Physiol 176: 930–945 [DOI] [PMC free article] [PubMed] [Google Scholar]