Allelic variation of Pyrroline-5-carboxylate synthase1 underlies sequence divergence across the promoter in the cultivated and wild barley which modulates its drought-inducible transcriptional activity.
Abstract
Water scarcity is a critical threat to global crop production. Here, we used the natural diversity of barley (Hordeum vulgare) to dissect the genetic control of proline (Pro) mediated drought stress adaptation. Genetic mapping and positional cloning of a major drought-inducible quantitative trait locus (QPro.S42-1H) revealed unique allelic variation in pyrroline-5-carboxylate synthase (P5cs1) between the cultivated cultivar Scarlett (ssp. vulgare) and the wild barley accession ISR42-8 (ssp. spontaneum). The putative causative mutations were located in the promoter of P5cs1 across the DNA binding motifs for abscisic acid-responsive element binding transcription factors. Introgression line (IL) S42IL-143 carrying the wild allele of P5cs1 showed significant up-regulation of P5cs1 expression compared to Scarlett, which was consistent with variation in Pro accumulation under drought. Next, we transiently expressed promoter::reporter constructs of ISR42-8 and Scarlett alleles in Arabidopsis (Arabidopsis thaliana) mesophyll protoplasts. GUS expression analysis showed a significantly higher activation of the ISR42-8 promoter compared to Scarlett upon abscisic acid treatment. Notably, the ISR42-8 promoter activity was impaired in protoplasts isolated from the loss-of-function abf1abf2abf3abf4 quadruple mutant. A series of phenotypic evaluations demonstrated that S42IL-143 maintained leaf water content and photosynthetic activity longer than Scarlett under drought. These findings suggest that the ancestral variant of P5cs1 has the potential for drought tolerance and understanding drought physiology of barley and related crops.
Land plants evolved from algae approximately 500 million years ago and established themselves in a terrestrial habitat (Graham, 1993). This evolution was possible as plants developed multiple physiological and biochemical strategies to accomplish life under water-limiting conditions. The biosynthesis of osmotic compounds of low molecular weight, such as Pro, glycine, betaine, mannitol, and organic acids, seems to be an essential evolutionary event to conserve water in the terrestrial habitat (Blum, 2017). The effect of these evolutionary changes can be seen as unique adaptive strategies among the natural population (wild progenitors) of crop plants. Therefore, the genetic dissection of diverse genetic resources is essential to understand the drought physiology of crop plants as well as to develop drought-resilient cultivars.
Pro is a compatible solute and a highly soluble organic compound. It is located mainly in the cytosol, chloroplasts, and cytoplasmic compartments to protect cellular components from dehydration injury during osmotic stress (Szabados and Savouré, 2010). In addition, it contributes to stabilizing subcellular structures (e.g. membranes and proteins), scavenging free radicals and buffering cellular redox potential under stress conditions (Ashraf and Foolad, 2007). Thus, Pro is commonly referred to as an osmolyte or osmoprotectant. As an osmolyte, it has a role in plant responses to drought stress and climatic adaptation, including signal transduction, osmoregulation, and antioxidant systems (Kishor et al., 2005; Kesari et al., 2012).
Pro is synthesized primarily from glutamate (Glu) by the action of the pyrroline-5-carboxylate synthase (P5CS) and pyrroline-5-carboxylate reductase enzymes. In the first step, P5CS converts Glu to Glu-semialdehyde (Hu et al., 1992), which is subsequently reduced by pyrroline-5-carboxylate reductase to Pro (Szoke et al., 1992; Verbruggen et al., 1993). In many plant species, there exist two conserved P5CS genes, P5CS1 and P5CS2, which share approximately 82% nucleotide and 94% amino acid sequence identity. However, these genes are differentially regulated at the transcriptional level (Strizhov et al., 1997; Székely et al., 2008). In Arabidopsis (Arabidopsis thaliana), P5CS2 modulates housekeeping Pro biosynthesis in the cytosol, whereas up-regulation of P5CS1 in the chloroplast results in drought-inducible Pro accumulation. Arabidopsis P5CS1 is induced by osmotic and salt stresses and activated by the abscisic acid (ABA)-dependent regulatory pathway as well as H2O2-derived signals (Szabados and Savouré, 2010). Under ABA signals, ABA-responsive element (ABRE) binding proteins (AREB) or transcription factors (ABFs) like ABF1, ABF2/AREB1, ABF3, and ABF4/AREB2 regulate the expression of drought-inducible genes (Yoshida et al., 2015). ABFs are a group of basic Leu zipper (bZIP) domain transcription factors, which were discovered using ABREs as bait in yeast one-hybrid screens (Choi et al., 2000; Uno et al., 2000). It has been reported that ABREs require other copies of ABREs or the combination of an ABRE with one of several coupling elements (CEs) across the promoter region to form the ABA response complex (Shen et al., 1996). Hobo et al. (1999) showed that ABRE with ACGT core and CE3 are functionally equivalent and can be considered as a single class of cis-acting elements.
Although much is known about Pro metabolism in model plants, the genetics and utility are poorly understood as a trait in crops because of quantitative inheritance of Pro accumulation, especially under drought conditions (Kishor et al., 2005; Szabados and Savouré, 2010). This scenario demands quantitative analysis of drought-inducible Pro accumulation among the natural population of crop plants.
In this study, we employed a quantitative approach to dissect the genetic and molecular regulation of Pro accumulation under drought conditions in barley (Hordeum vulgare). For this, we employed a set of introgression lines (ILs) harboring genome-wide marker-defined chromosomal segments of an ancestral wild barley accession, ISR42-8 (ssp. spontaneum), in the background of spring barley cultivar Scarlett (ssp. vulgare). Here, we report the positional isolation and molecular analysis of a novel drought-inducible P5cs1 allele originated from wild barley accession ISR42-8.
RESULTS
P5cs1 Allelic Variation Is Associated with the Major Quantitative Trait Locus Effect for Drought-Inducible Pro Accumulation in Barley
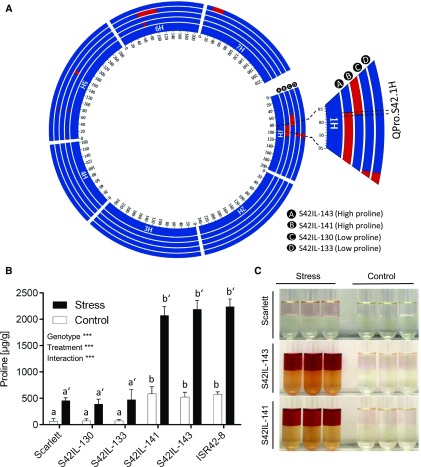
Initial screening of drought-inducible Pro accumulation was performed in an IL population designated as S42IL, which contains genome-wide wild barley ISR42-8 introgressions in the Scarlett background. This population exhibited a wide range of variation in Pro accumulation, especially under drought stress conditions (Supplemental Fig. S1). For mapping, we compared individual ILs with the recurrent parent Scarlett using the Dunnett test. This analysis identified a major drought-inducible quantitative trait locus (QTL) QPro.S42-1H on chromosome 1H, where two ILs, S42IL-143 and S42IL-141, sharing a common wild barley introgression resulted in significantly higher Pro accumulation (Fig. 1A). In the next step, we confirmed this QTL effect by rephenotyping the four selected ILs carrying wild and cultivated alleles at the target QTL region. Quantification of these ILs along with parental genotypes showed a significant increase in Pro accumulation in S42IL-143 and S42IL-141 up to 2,420 μg/g under drought stress (Fig. 1B). High concentrations of Pro produce a red color after reaction with ninhydrin. Thus, a dark red color indicated more Pro in the QTL allele harboring IL, S42IL-143, and S42IL-141 (Fig. 1C). Using a diagnostic marker (M-L), we confirmed that both ILs carried wild introgressions at the QPro.S42-1H region (Supplemental Fig. S2). Further, we validated this QTL effect and its segregation using 237 BC4S2 plants derived from S42IL-143 (Supplemental Fig. S3).
Figure 1.
Genetic mapping and validation of major QTLs for Pro accumulation. A, Circos plot showing the genetic map of four selected wild barley introgression lines carrying ISR42-8 and Scarlett alleles at the QTL region on chromosome1H. B, Quantification of Pro QTL QPro.S42-1H in four introgression lines. Bars represent the mean ± se (n = 5). Different letters indicate significant difference of genotypes and primes show differences between control and drought stress (P < 0.05) using Tukey’s HSD test. Pr (C) Free Pro reaction with ninhydrin. A darker color indicates more Pro accumulation in the samples.
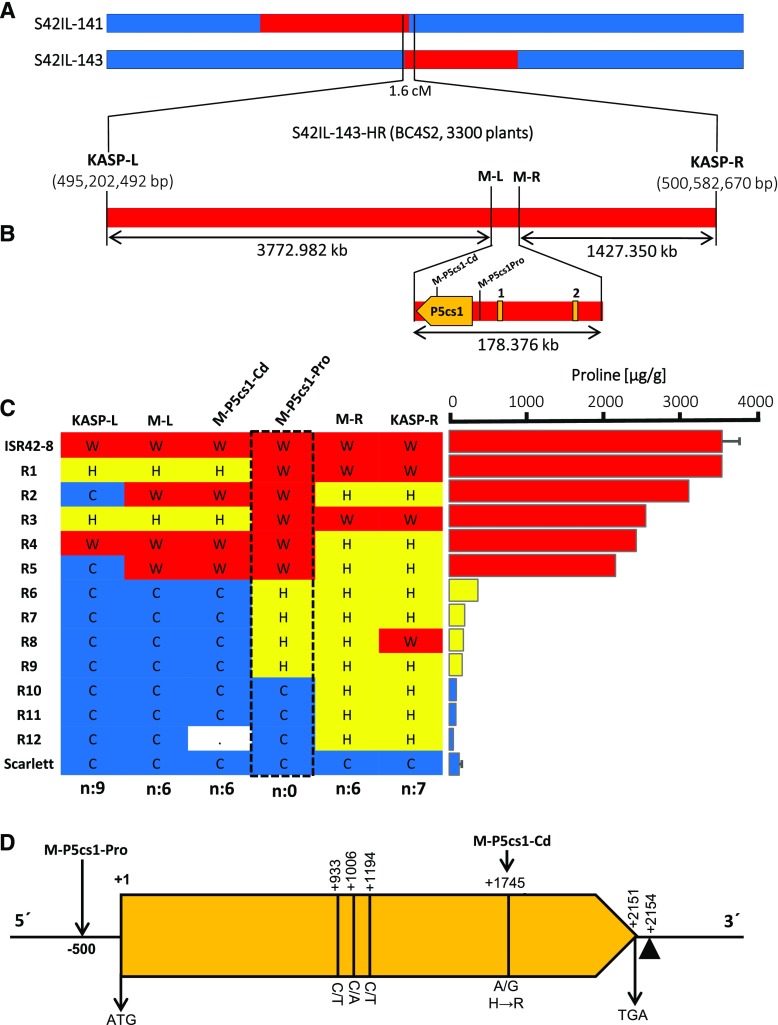
Positional cloning of QPro.S42-1H was conducted using 3,300 BC4S2 plants derived from S42IL-143. Initial mapping helped us to refine the targeted interval to 1.6 cM between left and right border KASP markers derived from SNP:TP59951 and SNP:TP3687 (Fig. 2A; Supplemental Table S1). By KASP genotyping, we identified 85 recombinants out of 3,300 plants that segregated for the targeted QTL region. Later, these recombinants were quantified for Pro accumulation under drought (Supplemental Fig. S4). Next, we incorporated two additional markers at the left (M-L) and right (M-R) border of the most promising candidate gene, P5cs1, which encodes a P5CS enzyme protein. By incorporating M-L and M-R, we established an interval that carried no additional high-confidence genes except P5cs1 (Fig. 2B; Supplemental Table S2). The genotyping of M-L and M-R markers in 12 segregants and their comparison with Pro accumulation data revealed six recombinants at each left and right border, indicating that the causal mutation influencing QPro.S42-1H lies within P5cs1. P5cs1 was oriented in the reverse strand and comprised of 20 exons encoding 716 amino acids. Notably, we found two high-Pro recombinants (R1, R3), which were heterozygous at the 3′ untranslated region (UTR) of P5cs1 but carried ISR42-8 homozygous alleles at the 5′ promoter of P5cs1. Next, we developed an additional marker, M-P5cs1-Pro, at the P5cs1 promoter region (Supplemental Table S2). Notably, at this marker, ISR42-8 and Scarlett alleles revealed 100% cosegregation with high and low Pro phenotypes, respectively. The heterozygous recombinants exhibited a marginal increase in Pro accumulation under drought stress conditions (Fig. 2C). Sequencing of P5cs1 in ISR42-8 and Scarlett revealed a single nucleotide polymorphism (SNP; A/G) in exon 15 that resulted in an amino acid substitution from His (ISR42-8) to Arg (Scarlett), along with three additional silent SNPs in exons 7 and 9 (Fig. 2D). To prove if the amino acid substitution was associated with Pro variation, we developed marker M-P5cs1-Cds at the substitution site via recombinant sequencing. Similar to M-L, this marker indicated the causal mutation upstream of the amino acid substitution toward the 5′ promoter region of P5cs1 (Fig. 2C). These results suggest that the allelic variation of P5cs1 controls the drought-inducible QTL (QPro.S42-1H) effect in ISR42-8 and Scarlett.
Figure 2.
Positional cloning of the major QTL QPro.S42-1H. A, Chromosomal map of wild barley introgression lines S42IL-141 and S42IL-143 overlapping at the QTL region. B, Fine mapping of the QTL region using high-resolution segregating population S42IL-143HR. The refined QTL interval represents an approximately 178-kb region between the M-L and M-R harboring a single high-confidence gene, P5cs1. The numbers 1 and 2 represent a low-confidence gene and a putative transposon element having no functional annotation, respectively. The physical positions are according to Morex assembly HV_IBSC_PGSB_v2. C, Comparison of genotype and phenotype data among the recombinants segregating at the targeted QTL interval. The cultivated, wild, and heterozygous alleles are represented with letters C, W, and H, respectively. n = number of recombinants. Bars represent the mean ± se (n = 5). D, Coding sequence of P5cs1 showing mutations between ISR42-8 and Scarlett. Indicated SNPs represent substitutions from ISR42-8 to Scarlett. Black triangle represents 44-bp insertion in the 3′ UTR of Scarlett. Marker positions upstream and downstream of ATG are indicated by − and +, respectively.
Drought-Inducible Pro Accumulation in S42IL-143 Involves P5cs1 Induction
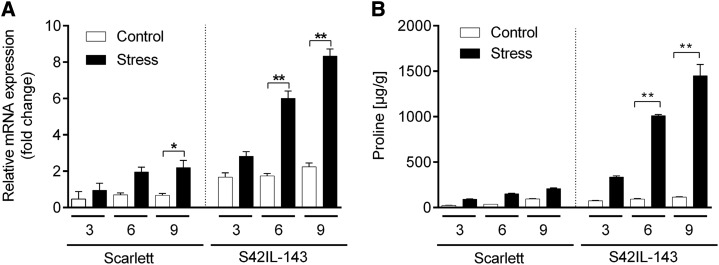
Pro accumulation upon water stress is preceded by increased expression of P5cs1 (Hu et al., 1992). Therefore, we hypothesized that the differential accumulation of Pro between Scarlett and S42IL-143 during water stress might also involve changes in the expression of P5cs1. To investigate this, we analyzed the expression of P5cs1 in Scarlett and S42IL-143 under varying water stress conditions. The leaf samples were collected at three different time points after drought stress: 3 days after stress (DAS), 6 DAS, and 9 DAS. RNA was extracted and analyzed for the expression of P5cs1 via quantitative reverse transcription-PCR (qRT-PCR). There were no significant changes in the expression of P5cs1 mRNA between Scarlett and S42IL-143 under control conditions. However, a significant increase in P5cs1 expression was observed in S42IL-143 at 6 DAS and 9 DAS, whereas a significant increase was only observed 9 DAS in Scarlett (Fig. 3A). Next, we tested whether this significant increase in P5cs1 expression was proportional to Pro accumulation. For this, we quantified the Pro content in the same leaf samples used for expression analysis. Intriguingly, we observed that the Pro accumulation in S42IL-143 and Scarlett followed the same trend as P5cs1 mRNA expression in response to water stress (Fig. 3B). These data suggested that the QTL bearing IL S42IL-143 showed a drought-inducible up-regulation of P5cs1, followed by a proportional increase in Pro content.
Figure 3.
Expression analyses of P5cs1 and Pro accumulation. A, Quantification of mRNA levels in leaves of Scarlett and S42IL-143 under control and drought stress conditions via qRT-PCR. The numbers on the x axis represent days after stress (DAS). B, Pro accumulation in leaves of Scarlett and S42IL-143 under control and drought stress conditions. Bars represent the mean ± SE (n = 3). Asterisks indicate significant difference between control and drought (*P < 0.05, **P < 0.01) using pairwise t tests.
Promoter Divergence at the ABF Binding Sites Putatively Determines P5cs1 Allelic Variation
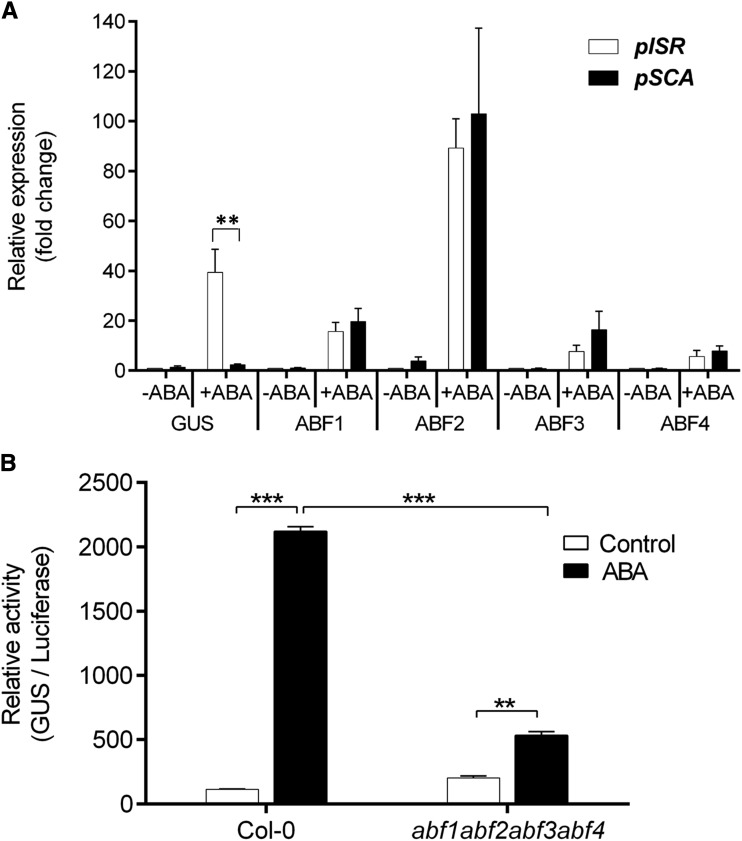
Recombination and gene expression analyses suggested that the causal mutations controlling drought-inducible Pro accumulation may lie in the promoter of P5cs1. Therefore, we sequenced an approximately 1.5-kb region upstream of ATG in ISR42-8 and Scarlett. The sequence comparison revealed mutations across putative ABREs and related CE3 motifs between Scarlett and ISR42-8 (Fig. 4). Based on this, we assumed that these mutations may influence the DNA binding of ABFs in the P5cs1 promoter. To investigate this, we established promoter::reporter constructs for ISR42-8 (pISR::eGFP-GUS) and Scarlett (pSCA::eGFP-GUS). Next, we transiently expressed the pISR::eGFP-GUS or pSCA::eGFP-GUS constructs in protoplasts isolated from Arabidopsis leaf mesophyll cells. Transfection efficiency was estimated using the GFP reporter (Supplemental Fig. S5). We then incubated transfected protoplasts of Col-0 ecotype in the presence of ABA (50 μm) for 4 h and analyzed the expression of GUS, ABF1, ABF2, ABF3, and ABF4 via qRT-PCR. The expression of all four ABFs was substantially induced upon ABA treatment in both pISR::eGFP-GUS- and pSCA::eGFP-GUS-transfected protoplasts. Similarly, expression of the GUS gene was strongly and significantly induced upon ABA treatment in pISR::eGFP-GUS transfected protoplasts; however, only modest up-regulation in GUS expression was observed in pSCA::eGFP-GUS-transfected protoplasts (Fig. 5A). To determine whether up-regulation of GUS expression in pISR::eGFP-GUS-transfected protoplasts was mediated by ABFs, we transfected this construct into mesophyll protoplasts isolated from the wild-type Arabidopsis (Col-0) and loss-of-function abf1abf2abf3abf4 quadruple mutant. Notably, we found that the up-regulation of GUS activity upon ABA treatment was significantly impaired in abf1abf2abf3abf4 as compared to the wild type (Fig. 5B). Taken together, these data suggest significantly different promoter activity of Scarlett and ISR42-8 alleles might be regulated in an ABF-dependent manner.
Figure 4.
Promoter sequence analysis. P5cs1 promoter sequence alignment between wild barley ISR42-8 and cultivar Scarlett. SNP mutations are indicated in red bold letters. Core sequence of DNA motifs are underlined with bold letters. ABRE, abscisic acid-responsive element; CE3, coupling element 3.
Figure 5.
P5cs1 promoter activity analysis in Arabidopsis protoplasts upon ABA treatment. A, Relative expression of GUS, ABF1, ABF2, ABF3, and ABF4 in Arabidopsis Col-0 protoplasts transfected with P5cs1 promoter::reporter constructs of ISR42-8 (pISR::eGFP-GUS) and Scarlett (pSCA::eGFP-GUS). For untreated samples (−ABA), data represent relative expression of the indicated genes with the value of pISR::eGFP-GUS (Col-0) set to 1. For treated samples (+ABA), data represent relative expression of the indicated genes with the value of untreated protoplasts set to 1. Bars represent the mean ± se (n = 3). Asterisks indicate significance difference between pISR (pISR::eGFP-GUS) and pSCA (pSCA::eGFP-GUS) (**P < 0.01) using pairwise t tests. B, GUS activity of pISR::eGFP-GUS in Arabidopsis mesophyll protoplasts of Col-0 and abf1abf2abf3abf4 quadruple mutant. Bars represent the mean ± se (n = 3). Asterisks indicate significance difference between genotypes under −ABA (control) and +ABA (**P < 0.01, ***P < 0.001) using pairwise t tests.
S42IL-143 Maintains Higher Leaf Water Status and Photosynthetic Activity under Drought Stress Compared to Scarlett
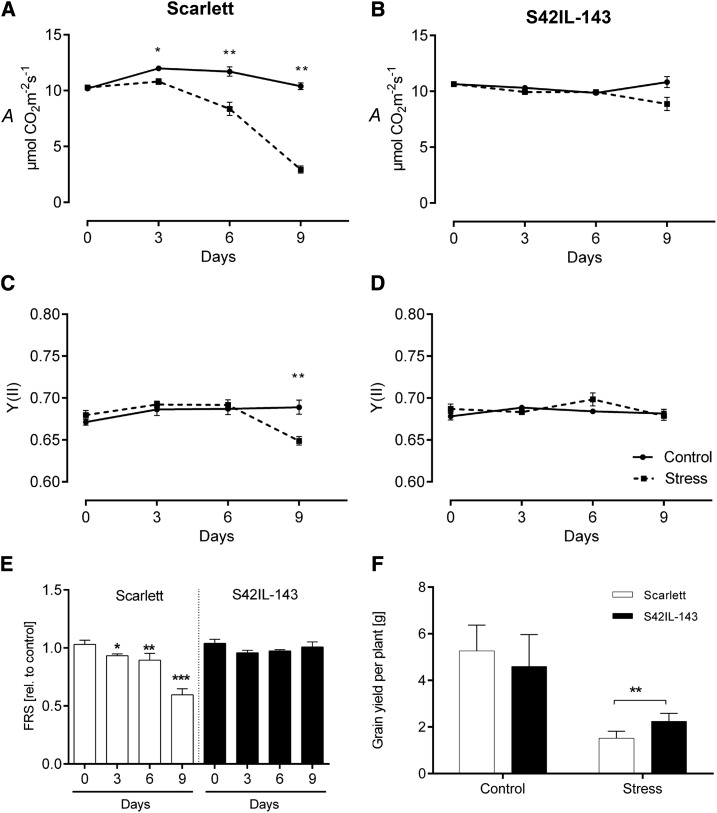
Plants affected by drought stress undergo changes in physiological processes involved in photosynthesis, such as stomatal conductance (gs), transpiration rate (E), and intercellular CO2 concentration (Ci). We investigated these parameters nondestructively using an infrared gas exchange analyzer (LI-6400XT, LI-COR). We found a significant reduction in gs in Scarlett under drought as compared to control conditions (Supplemental Fig. S6A). S42IL-143 maintained similar gs in both conditions at 3 DAS and 6 DAS, but drought reduced gs significantly at 9 DAS (Supplemental Fig. S6B). The effects of drought on E were similar to those of gs in both Scarlett and S42IL-143 (Supplemental Fig. S6, C and D). The influx of CO2, which is essential for carbon assimilation and photosynthetic activity, is directly affected by gs. We found that the internal Ci remained constant for both genotypes under control conditions until 6 DAS. However, the slope of Ci decline in Scarlett was 40% lower than that of S42IL-143 at 9 DAS (Supplemental Fig. S6, E and F). Consequently, we measured the photosynthetic rate (A), which was significantly reduced by drought in Scarlett at 3, 6, and 9 DAS (Fig. 6A). No significant difference in A was observed in S42IL-143 at 3 and 6 DAS, but a marginal reduction due to drought was observed at 9 DAS. A in S42IL-143 was 3-fold higher than in Scarlett under extreme drought conditions (Fig. 6B). In addition, we measured the effective quantum yield of PSII (YII) at steady-state photosynthesis under light using a MINI-PAM-II to confirm photosynthetic activity. YII in Scarlett was significantly reduced by drought at 9 DAS, whereas no significant difference was observed in S42IL-143 (Fig. 6, C and D). These data suggest an increased photosynthetic activity due to the QTL allele bearing-IL S42IL-143 under drought stress conditions. In addition, we assessed the dynamics of the water status of Scarlett and S42IL-143 leaves under control and drought stress conditions using a microwave sensor. According to Dadshani et al. (2015), the microwave parameter resonant frequency shift (FRS) strongly correlates with the amount of water stored in leaf tissues. Under drought, Scarlett showed a significant reduction in FRS value relative to control as compared to S42IL-143 (Fig. 6E). To test if these differences influenced grain yield of Scarlett and S42IL-143 under drought, we performed a pot experiment in the greenhouse under control and drought stress conditions. Grain weight per plant was significantly lower in Scarlett compared to S42IL-143 under drought stress conditions (Fig. 6F). These data indicate that drought-inducible Pro accumulation has a role in modulating physiological tolerance in IL S42IL-143.
Figure 6.
Evaluation of physiological parameters in Scarlett and S42IL-143. A and B, Comparison of photosynthetic rate (A) in Scarlett and S42IL-143 under control and different drought stress levels (0, 3, 6, 9 d after stress). C and D, Effective quantum yield of PSII (YII) in Scarlett and S42IL-143 under control and different drought stress levels (0, 3, 6, and 9 d after stress). E, FRS value of Scarlett and S42IL-143 relative to control at different drought stress levels (0, 3, 6, and 9 d after stress). F, Grain yield per plant in Scarlett and S42IL-143 under control and drought stress conditions. Data points and bars represent the mean ± se (n = 5). Asterisks indicate significance difference (*P < 0.05, **P < 0.01, ***P < 0.001) using pairwise t tests.
DISCUSSION
In this study, we focused on the major QTL QPro.S42-1H located on chromosome 1H, as it yields the strongest drought-inducible effect on Pro accumulation in a library of wild barley ILs. Two independent ILs, S42IL-143 and S42IL-141, complemented this QTL effect due to the introgression of a wild P5cs1 allele in the Scarlett background. S42IL-143 and S42IL-141 carried wild introgressions that shared a small common segment at the P5cs1 gene locus. This arrangement of introgressions was advantageous to refine the target QTL interval and to exclude the background effects of additional genes as the extent of QTL QPro.S42-1H was almost similar in both ILs. Also, the near isogenic genetic background of these ILs appeared more effective for the reproducibility of this drought-inducible QTL during positional cloning. By this, we showed single gene resolution of P5cs1 at the QTL region via high-resolution recombination analysis using six recombinants at each left and right borders of this gene. These findings are also in line with the previous studies of Pro metabolism in the model plant Arabidopsis and related higher plants, which suggest a vital role of enzyme-encoding gene P5CS1 for Pro accumulation under drought stress conditions (Liang et al., 2013).
In the next step, we investigated sequence polymorphisms underlying genetic and molecular regulation of the drought-inducible P5cs1 allele of ISR42-8. Intriguingly, we found mutations in the promoters of Scarlett and ISR42-8 across the predicted binding motifs of the ABF transcription factors. Previous reports have suggested that ABFs are transcription factors that bind to ABREs and regulate ABA-responsive gene expression (Choi et al., 2000; Uno et al., 2000). The ABF gene family is expressed in vegetative tissues in response to ABA and osmotic stress in Arabidopsis, suggesting a fundamental role in ABA-mediated drought stress tolerance (Fujita et al., 2011). All ABF transcription factors carry four conserved domains in addition to the bZIP domain (Fujita et al., 2011, 2013). Transcription factors having a bZIP domain target DNA duplex sites as homo- or heterodimers and bind to related but distinct palindromic sequences (Ellenberger, 1994; Hurst, 1995). Shen et al. (1996) shed interesting insight on the ABRE cis-elements; they found that these elements require other copies of ABREs or the combination of an ABRE with one of several CEs across the promoter region. These researchers also claimed that a single copy of an ABRE was insufficient to activate ABA-responsive genes (Riley et al., 2008). The role of multiple transcription factors binding sites (TFBSs) is well documented in other systems, e.g. G-box factor, in substantiating transcriptional up-regulation in plants (Schulze-Lefert et al., 1989; Toniatti et al., 1990), which suggests that the active role of multiple TFBSs in gene up-regulation depends primarily on targeting transcription factor itself, the inter-TFB distance and the adjoining sequence of the TFBS. In light of these reports, we proposed that the sequence polymorphisms in ABREs and adjacent CEs across the promoter region of ISR42-8 and Scarlett may influence the action of ABF transcription factors under ABA-modulated drought stress cascades. In support of this hypothesis, we performed transient expression of Scarlett and ISR42-8 promoter alleles in Arabidopsis (Col-0) protoplasts with or without ABA treatment. We found significantly higher GUS expression in pISR::eGFP-GUS upon ABA treatment. However, a significantly impaired GUS expression was observed in the protoplasts transfected with pSCA::eGFP-GUS. To test the putative role of ABFs, which bind on ABRE motifs, we expressed pISR::eGFP-GUS in the protoplasts of Col-0 and the abf1abf2abf3abf4 quadruple mutant. Notably, GUS activity was strongly impaired in abf1abf2abf3abf4 as compared to Col-0. All together, these data suggest that P5cs1 allelic polymorphism of ABRE motifs putatively regulate differential Pro accumulation in ISR42-8 and Scarlett under drought stress conditions.
Finally, we tested whether drought-inducible Pro accumulation has a role in mediating drought stress tolerance in S42IL-143. Several physiological measurements, such as leaf water status, photosynthetic parameters, and efficiency of photochemistry, have been widely used as markers for evaluating drought stress tolerance in various plant species (Souza et al., 2004; Chaves et al., 2009; Liu et al., 2015). Hence, we measured these parameters in S42IL-143 and Scarlett under control conditions and drought stress. Water status, measured through the microwave parameter FRS, demonstrated that S42IL-143 was able to maintain higher tissue water status compared to Scarlett under drought conditions. Dadshani et al. (2015) also found that FRS correlates positively with water status in barley leaves. Gas exchange and photosynthetic rates are considered important parameters in the determination of drought stress tolerance for rain-fed agriculture. It is logical that reduced gs may result in the reduction of A in plants (Lawlor and Tezara, 2009; Brestic and Zivcak, 2013; Hossain et al., 2015). However, several reports confirm that drought-tolerant genotypes maintain open stomata and active photosynthesis, even under dehydration conditions as compared to drought-sensitive genotypes (Benešová et al., 2012; Hossain et al., 2015). Our data showed significant reductions in gs and A occurring early under drought stress as compared to control conditions in Scarlett, whereas significant reductions under drought in S42IL-143 occurred only in more severe instances of drought. These parameters are direct indicators to characterize the efficiency of photochemistry under varying environmental conditions (Rascher et al., 2000). According to Yuan et al. (2016), plants that maintain YII under drought are recognized as stress-tolerant. Our results also showed that the S42IL-143 maintained YII similar to control under drought, unlike Scarlett. These data suggest that increased Pro accumulation modulates physiological parameters and drought tolerance in S42IL-143 due to the introgression of a novel P5cs1 allele from wild barley.
In conclusion, this study successfully demonstrated the isolation of a new P5cs1 allele of wild origin. This QTL allele reveals promoter variation associated to transcriptional induction of P5cs1, which was associated with subsequent Pro accumulation, thus improving drought tolerance. Future research will be focused to prove a direct interaction between the causative DNA and regulatory proteins, which will help to understand the molecular regulation of the ABA-driven Pro cascade in mediating drought tolerance. Further, we are refining the QTL allele bearing IL S42IL-143 to fix the P5cs1 allele in the isogenic background of cultivated barley to test the broader significance of Pro accumulation for yield and yield sustainability traits in barley under field conditions.
MATERIALS AND METHODS
Initial Screening and Mapping of Drought-Inducible Pro Accumulation
Initial genetic screening of Pro accumulation was carried out in a population (S42IL) of 72 wild barley ILs. The S42IL lines (BC3S4:10) were developed from an initial cross between the German spring cultivar Scarlett (Hordeum vulgare ssp. vulgare) and the Israeli wild barley accession ISR42-8 (H. vulgare ssp. spontaneum), followed by three rounds of backcrossing. The cultivar Scarlett was used as the recurrent parent for subsequent backcrossing, whereas ISR42-8 was utilized as the donor of the drought-related traits. The development of the S42IL population was described previously by Schmalenbach et al. (2008).
Phenotypic evaluation of the S42IL population for Pro accumulation was made in a split-plot design in two biological replicates of individual ILs under control and drought conditions in a plastic tunnel. The treatments (control and drought) were assigned to the subplots, within which the lines were assigned randomly. Seeds of individual S42IL genotypes were sown in a plastic pot (22 × 22 × 26 cm) containing a mixture of topsoil (40%) and natural sand (60%) (Terrasoil; Cordel & Sohn). Plants were watered three times a day using a drip irrigation system (Netafilm). Echo2 sensors (Decagon) were used to determine the volumetric moisture content (VMC) digitally with the frequency domain technique. The drought stress treatment was performed 30 days after sowing by eliminating the water supply completely at plant development stage BBCH 29 to 31 (Lancashire et al., 1991). The plants were exposed to stress for 26 d until the VMC reached the maximum drought stress threshold that is close to the wilting point (VMC near 0%). The control block was kept under a continuous supply of irrigation. The first fully developed leaf of individual genotypes in each block was harvested and flash frozen in liquid nitrogen for drought and control conditions and stored at −80°C until Pro determination. Colorimetric method of Pro determination described by Bates et al. (1973) was adopted for Pro content measurement. Pro accumulation was quantified in µg/g of the harvested fresh leaf material.
The S42IL population was genotyped using an Illumina 1,536 SNP array and genotyping by sequencing approaches, which have been already described (Schmalenbach et al., 2011; Honsdorf et al., 2014). This SNP map was compared among the individual introgression lines to map the QTL region associated with drought-inducible Pro accumulation. ILs showing significant drought-inducible Pro accumulation were identified by comparing the S42IL population with the recurrent parent Scarlett under control and drought stress conditions using Dunnett test (Dunnett, 1955; Naz et al., 2014).
QTL Validation
The major QTL effect QPro.S42-1H was validated by quantifying two wild allele-bearing ILs, S42IL-143 and S42IL-141, in comparison to two cultivated allele-bearing ILs, S42IL-130 and S42IL-133, for Pro accumulation under control and drought stress conditions inside the growth chamber. For this, each IL was evaluated in five biological replicates along with the parental genotypes. The growth chamber was supplied with 12 h of artificial light at 22°C ± 2°C with 50% to 60% relative humidity. For Pro content measurement, 2-week-old seedlings were exposed to drought stress by eliminating water supply completely.
To test the segregation of QTL alleles, the experiment was performed in the S42IL-143HR population derived from S42IL-143. This population was developed via backcrossing of QTL bearing S42IL-143 with Scarlett and two further rounds of selfing (i.e. BC4S2) as described previously (Schmalenbach et al., 2008). Seeds of the S42IL-143HR population and parents were sown in climate chambers under control conditions. The pots were randomized after sowing a single seed per pot (10 × 10 × 12 cm). The growth chamber was supplied with 12 h of artificial light at 22°C ± 2°C with 50 to 60% relative humidity. Drought stress treatment was carried out 10 d after germination by eliminating the water supply completely. The treatment pots were kept under stress, and the first fully expanded leaf was harvested from drought and control conditions for Pro measurement. Pro content was measured 9 d after drought treatment by the colorimetric procedure as described previously (Bates et al., 1973). At least five independent biological replicates of parents were used for Pro content measurement. Higher Pro concentration was visualized by its reaction with ninhydrin.
For genotyping, a simple sequence length polymorphic (SSLP) marker M-L was developed from a 3′ UTR of the putative candidate gene P5cs1. This marker revealed 44-bp deletions in the ISR42-8 allele compared to the Scarlett allele. A total of 237 BC4S2 segregating progenies were genotyped using this diagnostic SSLP marker and phenotyped for Pro variation under drought stress conditions. The allelic polymorphism of Scarlett and ISR42-8 alleles was visualized on 2.5% standard agarose gel.
Positional Cloning of QTL QPro.S42-1H
For positional cloning, 3,300 BC4S2 seeds of S42IL-143HR were sown along with parental genotypes Scarlett and ISR42-8. In the next step, DNA was extracted from fully expanded leaves of 1-week old seedlings using the CTAB extraction method according to a protocol devised by VA Tech Small Grains Breeding. For genotyping, two SNP-derived KASP markers at the left (KASP-L) and right (KASP-R) border of the QTL region were established. The KASP genotyping was outsourced at TraitGenetics. All the markers used for KASP genotyping used in this study are listed in Supplemental Table S1. After KASP genotyping, recombinants were selected among the 3,300 BC4S2 progenies that showed recombination between KASP-L and KASP-R markers. These recombinants were then subjected to drought stress for 9 d, and Pro accumulation was measured as described previously (Bates et al., 1973). Later, two additional SSLP markers, M-L and M-R, were incorporated to refine the QTL region. These markers enabled refining the QTL region to a single candidate gene. Later, a gene-specific marker (M-P5cs1-Pro) was established to compare the cosegregation of Scarlett and ISR42-8 alleles with low and high Pro phenotypes under drought stress conditions, respectively. A list of markers and corresponding primers sequence information is given in Supplemental Table S2.
Expression Analysis of P5cs1 mRNA
Expression analysis of P5cs1 mRNA was performed in Scarlett and S42IL-143 under varying drought stress conditions inside the growth chamber. The experiment was designed in three biological replicates for each genotype in drought stress and control treatments. Drought stress treatment was introduced to 10-d-old seedlings. Later, leaf samples were harvested from each plant and flash frozen in liquid nitrogen and stored at −80°C until further processing. Samples were collected 3 , 6, and 9 DAS from both control and stress treatments. RNA extraction, purification, and quantification were performed using the TRIZOL RNA Isolation Protocol. The Thermo Fisher RT-qPCR kit was used for cDNA synthesis following the manufacturer’s instructions. qRT-PCR was performed in 96-well plates using a 7500 fast real-time PCR system and an SYBR green-based PCR assay using three technical replicates of each cDNA sample. Each reaction contained 3 μL cDNA, 10 μL Maxima SYBR green/ROX qPCR master mix, and each primer at 0.4 μm to a final volume of 20 μL. The reaction mix was subjected to the following conditions: 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 30 s. Melting curves were then analyzed at 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. In addition, a reverse transcription negative control was included to assess potential genomic DNA contamination. For each cDNA sample, three technical replicates were used. Elongation factor (Ef1-a) was used as an internal control gene. Relative expression of P5cs1 was calculated according to the 2−ΔΔCt method (Livak and Schmittgen, 2001). A list of primer sequences used for expression analysis is shown in Supplemental Table S3.
Promoter Analysis
An approximately 1.5-kb region upstream from ATG of P5cs1 was sequenced for ISR42-8 and Scarlett. The promoter sequence was aligned using MAFFT alignment tools (Katoh et al., 2002). DNA binding motifs across the promoter were identified using MULAN analysis (Ovcharenko et al., 2005) and the plant cis-acting regulatory DNA elements (PLACE) database (Higo et al., 1999)
Transient Expression of ISR42-8 and Scarlett Promoter
Promoter regions upstream of the start codon of ISR42-8 (1,534 bp) and Scarlett (1,537 bp) were synthesized commercially (Invitrogen) and cloned in a Gateway cloning vector pDONR221 (Invitrogen) according to the manufacturer’s instructions. The verified promoters were fused with the eGFP-GUS tag in the expression vector pBGWFS7 (Yoo et al., 2007). Transient expression analysis using Arabidopsis (Arabidopsis thaliana) leaf mesophyll cells (treated with or without ABA) was performed as described previously (Yoshida et al., 2002, 2015; Yoo et al., 2007). A luciferase gene under the control of the 35S promoter (Karimi et al., 2005) was used as an internal control for GUS activity analysis between Col-0 and the abf1abf2abf3abf4 quadruple mutant. The luciferase activity was performed using the Luciferase Assay System (Promega; catalog no. E1500) following the manufacturer’s instructions. The abf1abf2abf3abf4 quadruple mutant was derived from abf1 (SALK_132819), areb1/abf2 (SALK_002984), abf3 (SALK_096965), and areb2/abf4 (SALK_069523) in Col-0 background (Yoshida et al., 2015). The seeds of the quadruple mutant were kindly provided by Dr. Yamaguchi-Shinozaki’s lab. Total RNA was extracted using an RNeasy plant mini kit (Qiagen) following the manufacturer’s instructions. Contaminating DNA was digested with DNase1 using a DNA-free DNA removal kit (Ambion), and the RNA was used to synthesize cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosynthesis) following the manufacturer’s instructions. qRT-qPCR was performed with the StepOne plus real-time PCR system (Applied Biosystems) as described recently (Mendy et al., 2017; Shah et al., 2017). The 18S ribosomal gene was used as an endogenous control. For this, cDNA was diluted 1:100 for 18S analysis. Data were analyzed using Pfaffl’s method (Pfaffl, 2001). A list of primer sequences used for expression analysis is shown in Supplemental Table S4.
Determination of Physiological and Photosynthetic Parameters
Various physiological parameters were analyzed to determine the effect of drought stress on Scarlett and the IL S42IL-143. For this purpose, a pot experiment was conducted in a climate chamber with a 12-h/12-h light/dark photoperiod at 22°C ± 2°C with 50 to 60% relative humidity. The seeds of S42IL-143 were sown in 10 replications each for control and treatment blocks in 1.2-liter pots (10 × 10 × 12 cm) in a completely randomized design. Drought stress treatment was performed at the three-leaf stage (10 d after germination) by eliminating the water supply completely. The treated pots were kept under stress for 3, 6, and 9 d. For all sensor measurements, the fully expanded third leaf was analyzed nondestructively to detect the moisture content of plant tissue (leaves) and parameters affecting photosynthetic activity. The EMISENS dual mode cavity microwave resonator (EMISENS) was used to nondestructively estimate the water status of barley leaves. During the assessment of a leaf with the microwave resonator, the change of the resonant frequency with respect to the empty resonator was recorded. As described previously by Dadshani et al. (2015), the microwave sensor parameter FRS, which is the negative relative frequency shift of resonant frequency, is highly correlated with the water content in tested plant material. Five measurements were taken from each leaf. The infrared gas exchange analyzer (LI-6400 XT; LI-COR) was used to measure the photosynthetic parameters, namely stomatal conductance (gs), transpiration rate (E), intercellular CO2 concentration (Ci), and photosynthesis rate (A). To take continuous measurements, the center of the third leaf attached to the sensor was positioned in a leaf chamber. The effective quantum yield of photosystem II (Y(II)) at steady-state photosynthesis under light conditions was measured using the MINI-PAM-II (Heinz Walz, Effeltrich, Germany) according to manufacturer's instructions (http://www.walz.com).
Grain Yield Evaluation of Scarlett and S42IL-143 under Drought Stress
Scarlett and S42IL-143 seeds were sown in a pot (22 × 22 × 26) containing a mixture of topsoil, silica sand, milled lava, and peat dust (Terrasoil; Cordel & Sohn). The experiment was conducted in a randomized complete block design with five replications for each genotype per treatment. Plants were watered three times a day using a drip irrigation system (Netafilm). Two drought stress treatments were applied, at tillering stage and before heading. Water supply was withheld for 2 weeks, and the pots were rewatered. Matured panicles and straw were harvested and oven dried at 37°C for 72 h. Grain weight per plant was evaluated under control and drought conditions.
Accession Numbers
Sequence data for P5cs1 can be found in the GenBank/EMBL data libraries under the following accession numbers: gene ID (HORVU1Hr1G072780) and protein ID (A0A287G756).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Variation in Pro accumulation in the S42IL population under control and drought stress conditions.
Supplemental Figure S2. Confirmation of common wild barley introgression in selected ILs.
Supplemental Figure S3. Segregation of QTL alleles for Pro accumulation in the S42IL-143-HR population.
Supplemental Figure S4. Variation in Pro accumulation in the S42IL-143-HR population under drought stress conditions.
Supplemental Figure S5. Transfection efficiency in Arabidopsis protoplasts.
Supplemental Figure S6. Gas exchange parameters of Scarlett and S42IL-143 under control and drought stress conditions.
Supplemental Table S1. Primers and sequences of KASP markers for fine mapping.
Supplemental Table S2. Primers used for positional cloning of P5cs1.
Supplemental Table S3. Primers used for expression analysis and sequencing.
Supplemental Table S4. Primers used for transient expression assays.
Acknowledgments
We thank Dr. Muhammad Ilyas for the guidelines in the protoplast transient expression assay; Mrs. Karin Woitol, Mr. Süleyman Gunal, and Ms. Annika Kortz for providing valuable support during phenotyping and genotyping; and Prof. Michael Frei, Ms. Inci Vogt, and Ms. Anna Backhaus for reading the manuscript. We also thank Dr. Yamaguchi-Shinozaki for providing seeds of the abf quadruple mutant. We are grateful to Prof. Dr. Florian M.W. Grundler (Molecular Phytomedicine, University of Bonn) for sharing experimental facilities.
Footnotes
S.M.’s PhD scholarship was supported by the Higher Education Commission of Pakistan. A.S. was supported by the German Research Society (DFG) graduate college (Grant GRK 2064).
Articles can be viewed without a subscription.
References
- Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59: 206–216 [Google Scholar]
- Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207 [Google Scholar]
- Benešová M, Holá D, Fischer L, Jedelský PL, Hnilička F, Wilhelmová N, Rothová O, Kočová M, Procházková D, Honnerová J, et al. (2012) The physiology and proteomics of drought tolerance in maize: early stomatal closure as a cause of lower tolerance to short-term dehydration? PLoS One 7: e38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. (2017) Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ 40: 4–10 [DOI] [PubMed] [Google Scholar]
- Brestic M, Zivcak M (2013) PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: protocols and applications. In Rout GR, Das AB, eds, Molecular Stress Physiology of Plants. Springer, Dordrecht, The Netherlands, pp 87–131 [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103: 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Hong J, Ha J, Kang J, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275: 1723–1730 [DOI] [PubMed] [Google Scholar]
- Dadshani S, Kurakin A, Amanov S, Hein B, Rongen H, Cranstone S, Blievernicht U, Menzel E, Léon J, Klein N, et al. (2015) Non-invasive assessment of leaf water status using a dual-mode microwave resonator. Plant Methods 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett CW. (1955) A Multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50: 1096–1121 [Google Scholar]
- Ellenberger T. (1994) Getting a grip on DNA recognition: structures of the basic region leucine zipper, and the basic region helix-loop-helix DNA-binding domains. Curr Opin Struct Biol 4: 12–21 [Google Scholar]
- Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124: 509–525 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Yoshida T, Yamaguchi-Shinozaki K (2013) Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol Plant 147: 15–27 [DOI] [PubMed] [Google Scholar]
- Graham LE. (1993) Origin of land plants. John Wiley & Sons, New York [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobo T, Asada M, Kowyama Y, Hattori T (1999) ACGT-containing abscisic acid response element (ABRE) and coupling element 3 (CE3) are functionally equivalent. Plant J 19: 679–689 [DOI] [PubMed] [Google Scholar]
- Honsdorf N, March TJ, Hecht A, Eglinton J, Pillen K (2014) Evaluation of juvenile drought stress tolerance and genotyping by sequencing with wild barley introgression lines. Mol Breed 34: 1475–1495 [Google Scholar]
- Hossain MM, Lam H-M, Zhang J (2015) Responses in gas exchange and water status between drought-tolerant and -susceptible soybean genotypes with ABA application. Crop J 3: 500–506 [Google Scholar]
- Hu CA, Delauney AJ, Verma DP (1992) A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci USA 89: 9354–9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst HC. (1995) Transcription factors 1: bZIP proteins. Protein Profile 2: 101–168 [PubMed] [Google Scholar]
- Karimi M, De Meyer B, Hilson P (2005) Modular cloning in plant cells. Trends Plant Sci 10: 103–105 [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30: 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesari R, Lasky JR, Villamor JG, Des Marais DL, Chen YJ, Liu TW, Lin W, Juenger TE, Verslues PE (2012) Intron-mediated alternative splicing of Arabidopsis P5CS1 and its association with natural variation in proline and climate adaptation. Proc Natl Acad Sci USA 109: 9197–9202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishor PK, Sangam S, Amrutha R, Laxmi PS, Naidu K, Rao K, Rao S, Reddy K, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88: 424–438 [Google Scholar]
- Lancashire PD, Bleiholder H, van den Boom T, Langelüddeke P, Stauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119: 561–601 [Google Scholar]
- Lawlor DW, Tezara W (2009) Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot 103: 561–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhang L, Natarajan SK, Becker DF (2013) Proline mechanisms of stress survival. Antioxid Redox Signal 19: 998–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang X, Tran H, Shan L, Kim J, Childs K, Ervin EH, Frazier T, Zhao B (2015) Assessment of drought tolerance of 49 switchgrass (Panicum virgatum) genotypes using physiological and morphological parameters. Biotechnol Biofuels 8: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2CT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Mendy B, Wang’ombe MW, Radakovic ZS, Holbein J, Ilyas M, Chopra D, Holton N, Zipfel C, Grundler FM, Siddique S (2017) Arabidopsis leucine-rich repeat receptor-like kinase NILR1 is required for induction of innate immunity to parasitic nematodes. PLoS Pathog 13: e1006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz AA, Arifuzzaman M, Muzammil S, Pillen K, Léon J (2014) Wild barley introgression lines revealed novel QTL alleles for root and related shoot traits in the cultivated barley (Hordeum vulgare L.). BMC Genet 15: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovcharenko I, Loots GG, Giardine BM, Hou M, Ma J, Hardison RC, Stubbs L, Miller W (2005) Mulan: multiple-sequence local alignment and visualization for studying function and evolution. Genome Res 15: 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascher U, Liebig M, Lüttge U (2000) Evaluation of instant light response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ 23: 1397–1405 [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A (2008) Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9: 402–412 [DOI] [PubMed] [Google Scholar]
- Schmalenbach I, Körber N, Pillen K (2008) Selecting a set of wild barley introgression lines and verification of QTL effects for resistance to powdery mildew and leaf rust. Theor Appl Genet 117: 1093–1106 [DOI] [PubMed] [Google Scholar]
- Schmalenbach I, March TJ, Bringezu T, Waugh R, Pillen K (2011) High-resolution genotyping of wild barley introgression lines and fine-mapping of the threshability locus thresh-1 using the Illumina GoldenGate assay. G3 (Bethesda) 1: 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P, Dangl JL, Becker-André M, Hahlbrock K, Schulz W (1989) Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J 8: 651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SJ, Anjam MS, Mendy B, Anwer MA, Habash SS, Lozano-Torres JL, Grundler FMW, Siddique S (2017) Damage-associated responses of the host contribute to defence against cyst nematodes but not root-knot nematodes. J Exp Bot 68: 5949–5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Zhang P, Ho TH (1996) Modular nature of abscisic acid (ABA) response complexes: composite promoter units that are necessary and sufficient for ABA induction of gene expression in barley. Plant Cell 8: 1107–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza RP, Machado EC, Silva JAB, Lagôa AMMA, Silveira JAG (2004) Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ Exp Bot 51: 45–56 [Google Scholar]
- Strizhov N, Abrahám E, Okrész L, Blickling S, Zilberstein A, Schell J, Koncz C, Szabados L (1997) Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J 12: 557–569 [DOI] [PubMed] [Google Scholar]
- Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15: 89–97 [DOI] [PubMed] [Google Scholar]
- Székely G, Abrahám E, Cséplo A, Rigó G, Zsigmond L, Csiszár J, Ayaydin F, Strizhov N, Jásik J, Schmelzer E, et al. (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53: 11–28 [DOI] [PubMed] [Google Scholar]
- Szoke A, Miao G-H, Hong Z, Verma DPS (1992) Subcellular location of delta-pyrroline-5-carboxylate reductase in root/nodule and leaf of soybean. Plant Physiol 99: 1642–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniatti C, Demartis A, Monaci P, Nicosia A, Ciliberto G (1990) Synergistic trans-activation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J 9: 4467–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Villarroel R, Van Montagu M (1993) Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiol 103: 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Maruyama K, Mogami J, Todaka D, Shinozaki K, Yamaguchi-Shinozaki K (2015) Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ 38: 35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Yang Z, Li Y, Liu Q, Han W (2016) Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 54: 28–39 [Google Scholar]