In C4 Flaveria species, roots play an important role in the control of sulfur homeostasis and glutathione concentration in the leaf.
Abstract
The evolution of C4 photosynthesis led to an increase in carbon assimilation rates and plant growth compared to C3 photosynthetic plants. This enhanced plant growth, in turn, affects the requirement for soil-derived mineral nutrients. However, mineral plant nutrition has scarcely been considered in connection with C4 photosynthesis. Sulfur is crucial for plant growth and development, and preliminary studies in the genus Flaveria suggested metabolic differences in sulfate assimilation along the C4 evolutionary trajectory. Here, we show that in controlled conditions, foliar accumulation of the reduced sulfur compounds Cys and glutathione (GSH) increased with progressing establishment of the C4 photosynthetic cycle in different Flaveria species. An enhanced demand for reduced sulfur in C4 Flaveria species is reflected in high rates of [35S]sulfate incorporation into GSH upon sulfate deprivation and increased GSH turnover as a reaction to the inhibition of GSH synthesis. Expression analyses indicate that the γ-glutamyl cycle is crucial for the recycling of GSH in C4 species. Sulfate reduction and GSH synthesis seems to be preferentially localized in the roots of C4 species, which might be linked to its colocalization with the phosphorylated pathway of Ser biosynthesis. Interspecies grafting experiments of F. robusta (C3) and F. bidentis (C4) revealed that the root system primarily controls sulfate acquisition, GSH synthesis, and sulfate and metabolite allocation in C3 and C4 plants. This study thus shows that evolution of C4 photosynthesis resulted in a wide range of adaptations of sulfur metabolism and points out the need for broader studies on importance of mineral nutrition for C4 plants.
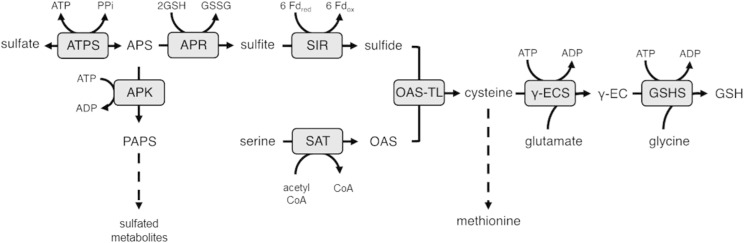
Sulfur (S) possesses a wide variety of essential functions for cell structure and metabolism. Incorporated into the amino acids Cys and Met, S is an important component of proteins. Cys is further a constituent of the tripeptide glutathione (GSH), which maintains cellular redox balance and is involved in cell signaling and xenobiotic and heavy metal detoxification (Rouhier et al., 2008). Furthermore, S is an important component of prosthetic groups, such as iron-sulfur clusters, lipoic acid, or coenzyme A, and a range of secondary metabolites (Takahashi et al., 2011). Plants acquire S from the soil as its inorganic anion, sulfate. Sulfate uptake and distribution within the organism is facilitated by sulfate transporters. For assimilation, the inert and stable sulfate is activated by ATP sulfurylase (ATPS) by transferring it onto an α-phosphate residue of ATP and yielding in adenosine-5′-phosphosulfate (APS; Fig. 1). APS, as a branch point intermediate, can be phosphorylated by APS kinase to form the donor of sulfate groups in secondary metabolism, 3′-phosphoadenosine 5′-phosphosulfate. The majority of APS, however, follows the reduction pathway and is reduced by APS reductase (APR). APR uses the reducing capacity of GSH to convert APS into sulfite. Subsequently, the ferredoxin-dependent reduction of sulfite by sulfite reductase (SIR) produces sulfide, which can further be incorporated into the amino acid backbone of O-acetyl-Ser (OAS), a Ser derivative produced by Ser acetyltransferase. OAS (thiol)lyase (OAS-TL) converts sulfide and OAS into Cys (Takahashi et al., 2011). Cys can serve as the initial substrate of Met biosynthesis or as a constituent amino acid of proteins and peptides, such as GSH. GSH biosynthesis is mediated by two ATP-consuming catalytic reactions by γ-glutamyl-Cys synthetase (γECS) and GSH synthetase, which join its constituent amino acids Glu, Cys, and Gly (Fig. 1) (Rouhier et al., 2008). Sulfate assimilation seems to belong to the core metabolism of most plant cells, with a notable exception of plants with C4 photosynthesis.
Figure 1.
Sulfate assimilation pathway in plants. ATPS, ATP sulfurylase; APS, adenosine-5′-phosphosulfate; APK, APS kinase; PAPS, 3′-phosphoadenosine-5′ phosphosulfate; APR, APS reductase; SIR, sulfite reductase; SAT, Ser acetyltransferase; OAS, O-acetyl-Ser; OAS-TL, OAS (thiol)lyase; γ-EC, γ-glutamyl-Cys; γ-ECS, γ-EC synthetase; GSH, red. glutathione; GSHS, GSH synthetase; GSSG, ox. glutathione; Fd, ferredoxin.
C4 photosynthesis minimizes the oxygenation reaction of Rubisco and increases the efficiency of photosynthesis under normal conditions. This is achieved by changes in leaf anatomy and strict cell type-specific enzyme localization, facilitating the distribution of carbon fixation and assimilation across two distinct cell types, mesophyll cells (MCs) and bundle sheath cells (BSCs; Sage et al., 2012). CO2 is initially converted in MCs into bicarbonate by carbonic anhydrase. Bicarbonate serves as a substrate for the carboxylation of 2-phosphoenolpyruvate (PEP) to oxaloacetate by PEP carboxylase (PEPCase). Oxaloacetate is subsequently reduced to C4 acids, malate, or Asp, which diffuse into BSC. In BSC the C4 acids are decarboxylated, and the released CO2 is assimilated by Rubisco in the Calvin cycle (Slack et al., 1969). Thus, the CO2 concentration in BSC is increased, and the oxygenase reaction of Rubisco and consequently photorespiration is reduced. These characteristics lead to high photosynthetic efficiency even at low CO2 intracellular concentration, which is particularly advantageous at warm and dry climates (Sage et al., 2012). C4 photosynthesis evolved independently more than 60 times in both monocot and dicot lineages in a repeatable and predictable evolutionary process (Sage et al., 2012; Heckmann et al., 2013; Williams et al., 2013). Intermediates of such evolutionary processes have been identified in a number of plant genera with partial characteristics of C4 plants and named as C3-C4 (Kennedy and Laetsch, 1974). Particularly important for studies of C4 photosynthesis are C3-C4 plants in genera also containing C3 and C4 species, the best example being Flaveria (Ku et al., 1991; Schulze et al., 2013). Flaveria is a small genus with 23 species native mostly to North America, particularly the south of the USA and northern part of Mexico (Powell, 1978; McKown et al., 2005). Phylogenetic analyses showed that the C3-C4 species are true evolutionary intermediates in the evolution of C4 photosynthesis, which in Flaveria evolved independently twice (Kopriva et al., 1996; McKown et al., 2005). In addition to C3-C4 and C4 species, three Flaveria species are classified as C4-like, because despite having highly active C4 photosynthetic enzymes and pronounced Kranz anatomy, some carbon fixation still occurs directly through the C3 cycle, and photosynthesis is sensitive to oxygen (Cheng et al., 1988; Ku et al., 1991). Interestingly, not only photosynthetic enzymes show cell-specific expression between MCs and BSCs in C4 plants, but also proteins involved in nitrate and sulfate assimilation (Kopriva, 2011).
Sulfur research in C4 plants was initiated by showing that 90% of ATPS activity is confined to the bundle sheath strand in Digitaria sanguinalis (Gerwick and Black, 1979). These results were later confirmed in 17 additional C4 species (Gerwick et al., 1980). Further studies showed that APR is also largely restricted to the BSC, whereas SIR and OAS-TL activity was equally distributed across MCs and BSCs (Schmutz and Brunold, 1984; Kopriva and Koprivova, 2005). However, in the C4 dicot Flaveria trinervia, no differences in the distribution of APR between MCs and BSCs were observed (Koprivova et al., 2001). On the other hand, analysis of Arabidopsis (Arabidopsis thaliana) BSC translatome revealed that transcripts of genes for sulfate assimilation are highly and coordinately enriched in BSCs of this C3 species. Therefore, BSC specificity of sulfur metabolism has been discussed as a species-dependent characteristic (Aubry et al., 2014; Weckopp and Kopriva, 2015). However, the analysis of S metabolism in Flaveria species of different photosynthesis types indicated another intriguing connection between C4 photosynthesis and sulfate reduction. The leaves of C4 species F. trinervia and F. australasica showed a higher abundance of Cys and GSH as well as higher APR activity when compared to C3 species (Koprivova et al., 2001). Accumulation of Cys and GSH is demand driven, and their synthesis rate is under the control of APR (Vauclare et al., 2002). Thus, these results indicate a complex link between C4 photosynthesis and S metabolism.
Here, we address the connection between S homeostasis and the evolution of C4 photosynthesis by comparative analysis of Flaveria species with different photosynthetic properties in nutrient-controlled environments. We show that, under controlled conditions with both normal and low S supply, C4 Flaveria species accumulate more Cys and GSH. The accumulation is a consequence of increased flux through sulfate assimilation in C4 species and driven by increased GSH turnover in C4 leaves. In addition, analysis of interspecies grafts between C3 F. robusta and C4 F. bidentis revealed that, in C4 Flaveria species, GSH is preferentially synthesized in the root and that the root controls the allocation of S between roots and shoots. Sulfate assimilation in C4 plants seems to be tightly linked with the synthesis of Ser by the phosphorylated pathway of Ser biosynthesis (PPSB).
RESULTS
Regulation of Sulfate Assimilation by Sulfur Deficiency in Flaveria
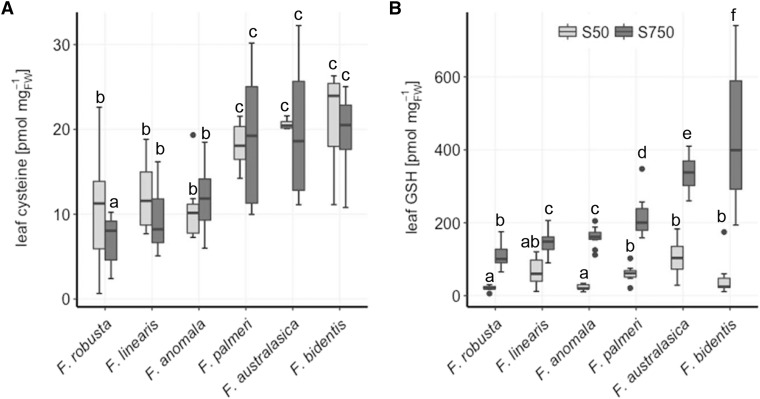
To understand the mechanisms of higher accumulation of Cys and GSH in C4 compared to C3 Flaveria species (Koprivova et al., 2001), a new cultivation system for Flaveria plants was established, allowing to control the nutrient concentration. This cultivation system was used to assess the effect of S deficiency on Flaveria species of different photosynthesis mechanisms, as this treatment has been used in the past to study the regulation of sulfate assimilation (Nikiforova et al., 2004). Under sufficient S, C4 Flaveria species consistently showed higher levels of low Mr thiols, Cys, and GSH in the leaves compared to their C3 ancestors (Fig. 2). The concentrations of the thiols correlated negatively with previously reported CO2 compensation points used as a quantitative measure for the establishment of a C4 cycle (Supplemental Fig. S1). Interestingly, Cys concentration in leaves of all species was not affected by S deficiency, indicating a tight and evolutionary conserved regulation of this metabolite (Fig. 2A). On the other hand, leaf GSH concentration was significantly reduced in S-deficient plants, to approximately the same level despite photosynthetic type (Fig. 2B). The sulfate content of the leaves was strongly reduced under S deficiency. However, sulfate content did not show a correlation with photosynthesis type (Supplemental Fig. S2A). The same was true for OAS concentration in the leaves, which accumulated more in S-deficient plants compared to the controls, but there was no correlation between photosynthesis type and the absolute levels or ratios between concentrations at the two S conditions (Supplemental Fig. S2B).
Figure 2.
Influence of S deficiency on leaf Cys and GSH concentration in Flaveria species. Leaf Cys (A) and GSH (B) was analyzed in 23-d-old seedlings of six Flaveria species. The plants were exposed to low sulfate (50 µm S; S50) or adequate sulfate (750 µm S; S750) conditions for 16 d. Data (n = 12 for S750, n = 4 for S50) are shown as box plot (25–75%); the line represents median; the whiskers represent 1.5 interquartile range (IQR). Different letters represent values significantly different at P < 0.05 (Student’s t test). F. robusta, C3; F. linearis and F. anomala, C3-C4; F. palmeri, C4-like; F. australasica and F. bidentis, C4.
A higher accumulation of Cys and GSH in the shoots of C4 plants appears to indicate higher demand for reduced S compounds with increasing C4 properties. To test whether C4 plants are more sensitive to S deficiency than C3 plants, the loss in shoot weight of all six species and the photosynthetic performance of the C3 species F. robusta and the C4 species F. australasica and F. bidentis were determined in response to S deprivation (Supplemental Fig. S3). No correlation was observed between the effect of S deficiency on shoot biomass and photosynthesis type. Photosynthetic performance was tested by measuring gas exchange in plants cultivated in an adapted cultivation system (unsterile pot culture, 1:1 sand-vermiculite mix, fertilized with full nutrient medium or 50 µm S nutrient solution). No significant effect of S deficiency was detected on the CO2 compensation points, CO2 assimilation rate at 40 Pa CO2, or photosynthetic efficiency (initial slope of a photosynthetic CO2 response curve) of any species (Supplemental Fig. S3B). The relatively mild S deficiency, therefore, seems not to affect the photosynthetic performance, regardless of the photosynthetic mechanism. Hence, the increasing accumulation of GSH following the establishment of a C4 cycle seems not to be necessary for the maintenance of the photosynthetic activity under steady-state conditions but can be connected to broader metabolic adaptions associated with the evolution toward C4 photosynthesis or possibly to the maintenance of photosynthesis under non-steady-state conditions.
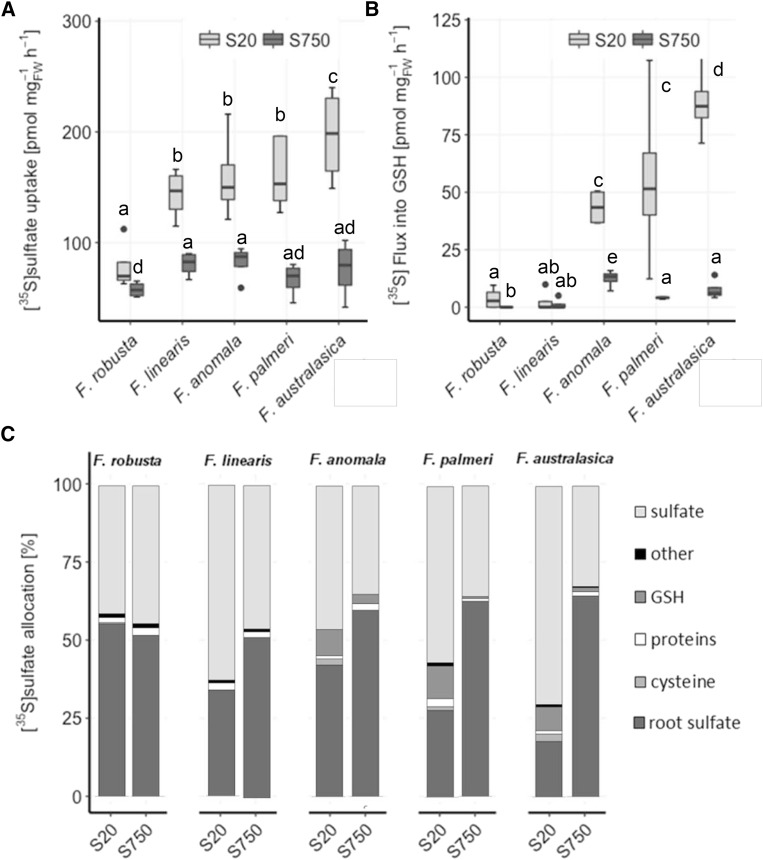
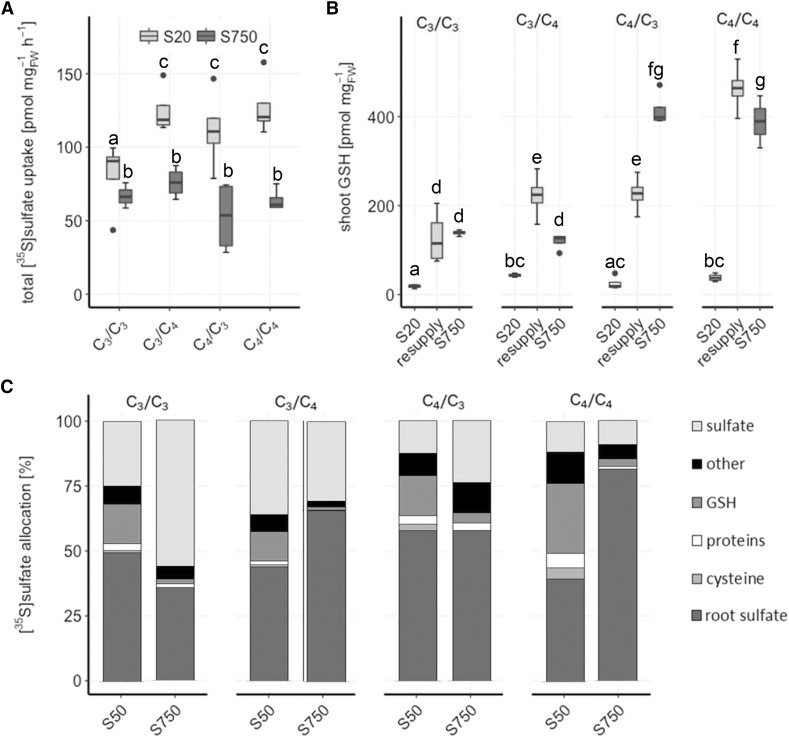
To address how the higher accumulation of Cys and GSH in C4 species is achieved, a [35S]sulfate feeding experiment was performed in five Flaveria species under fully nourished (S750) and S-deficient (S20) conditions (Fig. 3). At full sulfate supply, sulfate uptake rates did not vary significantly between C3 and C4 species (Fig. 3A). In S-deficient plants, uptake was increased to a higher degree in C4 species, whereas F. robusta only showed a slight but still significant increase in sulfate uptake (Fig. 3A). In vivo flux analysis revealed that the incorporation of the [35S]sulfate into GSH was significantly higher in leaves of F. anomala, F. palmeri, and F. australasica compared to F. robusta and F. linearis at full nutrition, but the difference increased highly under S deficiency (Fig. 3B). In F. robusta and F. linearis, the flux was not affected by S deficiency. Furthermore, with exception of F. robusta, S-deficient plants of other Flaveria species allocated more sulfur to the leaves than fully nourished plants (Fig. 3C). Particularly in S-deficient C4 F. australasica, more than 80% of the sulfur was translocated to the shoots, and about 10% was used for GSH synthesis. This result suggests that intermediate and C4 species prioritize the restoring of GSH pools in the shoots. In the roots of all species tested, the only [35S]-labeled compound detected after resupplying for 4 h was inorganic sulfate, confirming the higher metabolic capacity and demand of the shoots over the roots.
Figure 3.
[35S]sulfate uptake and partitioning in Flaveria species. Twenty-day-old seedlings of five Flaveria species were resupplied for 4 h with 0.2 mm [35S]sulfate nutrient solution after 6 d exposure to low sulfate (20 µm sulfate; S20) or adequate sulfate (750 µm sulfate; S750). Sulfate uptake (A) and root-to-shoot translocation (B) were determined by scintillation counting after tissue solubilization. C, Incorporation of [35S]sulfate into sulfate, GSH, Cys, and other compounds was determined by HPLC analysis. Relative [35S] partitioning into shoot sulfate, GSH, Cys, proteins, and other compounds after 4 h of resupply is shown, n = 4. Different letters represent values significantly different at P < 0.05 (Student’s t test).
C4 Flaveria Species Show Increased Turnover of GSH
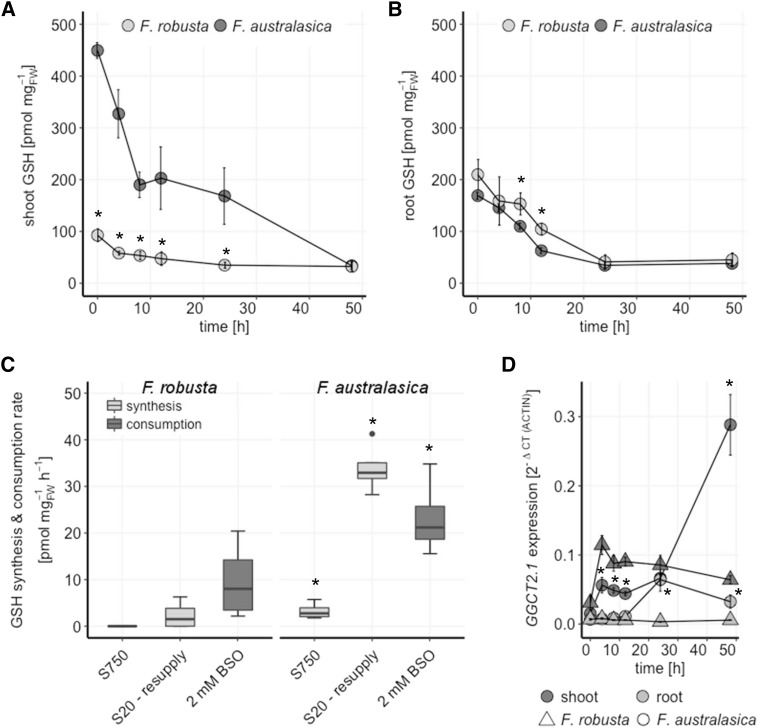
Our results indicate that C4 Flaveria species have a higher demand for GSH, supplied by higher flux into GSH as well as a higher storage capability for this metabolite. To test for differences in GSH turnover, seedlings of C3 F. robusta and C4 F. australasica were grown under full nutrition and exposed to buthionine-(S,R)-sulfoximine (BSO), a specific and potent inhibitor of γECS (Griffith and Meister, 1979), for 48 h. Exposure to BSO led to an expected decline in GSH concentrations in both species but with different kinetics in the shoots versus the roots (Fig. 4). Within the first 4 h of BSO supplementation, GSH concentrations in shoots of F. robusta decreased from 93 pmol mg FW−1 to 58 pmol mg FW−1, i.e. a rate of 9 pmol mg FW−1 h−1, reaching a final concentration of 35 pmol mg FW−1 after 24 h. The GSH concentration in C4 F. australasica shoots decreased in the first 8 h after exposure to BSO from 450 pmol mg FW−1 to 190 pmol mg FW−1, i.e. a rate of 32.5 pmol mg FW−1 h−1. In the following 16 h, GSH degradation rather stagnated, with a rate of GSH loss of only about 2 pmol mg FW−1 h−1, followed by another phase of rapid GSH consumption until reaching a final concentration of 34 pmol mg FW−1 after 48 h. Despite the different absolute GSH consumption rates, the relative rates (% of the initial concentration) were similar in the first 24 h. After 48 h, both species retained the same concentration of GSH, which represented 43% of the initial GSH pool in F. robusta but only 7.5% in F. australasica. The changes in accumulation of GSH in the roots of both species followed the same pattern. Within 24 h, the amount of GSH was reduced from approximately 200 pmol mg FW−1 to a minimum level of roughly 40 pmol mg FW−1. This concentration was retained in the roots of both species with further exposure to BSO (Fig. 4B). Thus, in the shoots as well as the roots of both species, a GSH concentration between 30 and 40 pmol mg FW−1 seems to be indispensable for the plants. The large margin between the maintained GSH pool and the minimum level in shoots of F. australasica underlines the importance and metabolic involvement of GSH for this C4 species.
Figure 4.
GSH turnover in F. robusta and F. australasica. GSH concentrations in shoots (A) and roots (B) of 20-d-old seedlings of F. robusta (C3) and F. australasica (C4) in a time course of 48 h after transfer to medium supplemented with 2 mm BSO. Data are presented as means and se, n = 4. C, GSH synthesis was analyzed in 20-d-old seedlings exposed to low sulfate (20 µm sulfate; S20) or adequate sulfate (750 µm sulfate; S750) for 6 d by resupply with 0.2 mm [35S]sulfate solution for 4 h. GSH consumption rate is calculated from A at 4 h after treatment with 2 mm BSO. Data are shown as box plot (25%–75%); the line represents median, and the whiskers represent 1.5 IQR, n = 4. D Transcript levels of GGCT2.1 in shoots and roots of 20-d-old seedlings in a time course of 48 h after transfer to medium supplemented with 2 mm BSO. Data are presented as means and SEM, n = 4. Asterisks represent significant differences between F. robusta and F. australasica at P < 0.05 (Student’s t test).
The importance of the GSH pool in F. australasica is further supported by the comparison of de novo GSH synthesis and its consumption rate in F. robusta and F. australasica (Fig. 4C). De novo GSH synthesis was determined by feeding fully nourished and sulfur-starved seedlings with [35S]sulfate for 4 h. The GSH consumption rate in F. australasica was almost 3-fold higher in comparison to F. robusta. At full nutrient conditions, shoot GSH synthesis could not be detected in F. robusta and was low in F. australasica. This result points to a high turnover of GSH as it is synthesized. Analysis of GSH synthesis in S-deficient plants revealed that the investment of newly acquired sulfate in restoring the GSH pool was of great importance for F. australasica. The rate of GSH synthesis was 34 pmol mg FW−1 h−1 higher than the determined consumption rate. Hence, this C4 species invests largely in restoring and maintaining the internal GSH pool as turnover proceeds. The higher GSH consumption rate in C4 Flaveria leaves was corroborated by increased transcript levels of γ-glutamyl cyclotransferase 2.1 (GGCT2.1), a crucial factor in the maintenance of GSH homeostasis, which degrades GSH to 5-oxo-Pro and Cys-Gly peptide (Paulose et al., 2013). GGCT2.1 expression levels in the shoots of F. robusta and F. australasica were higher than in the roots. Moreover, while initially at comparable levels, in the course of BSO exposure the expression in C4 species was induced to a higher degree than in the C3 species (Fig. 4D). Similarly to our analysis of F. robusta and F. bidentis, data mining from comparative transcriptomic data of two species from the Cleomaceae family, Tarenaya hassleriana (C3) and Gynandropsis gynandra (C4; Külahoglu et al., 2014), revealed higher GGCT2.1 transcript levels in leaves of C4 compared to C3 species (Supplemental Fig. S4). Thus, GGCT2.1 seems to be a crucial regulator of GSH homeostasis in C4 species beyond Flaveria.
Inhibition of GSH synthesis by BSO often results in the accumulation of Cys (Vauclare et al., 2002; Hartmann et al., 2004). Interestingly, shoot Cys concentrations did not change over the course of 48 h of BSO treatment in both F. robusta and F. australasica (Supplemental Fig. S5A). On the other hand, Cys accumulated slightly but significantly in the roots of F. robusta, from 7 pmol mg FW−1 to 18 pmol mg FW−1 and significantly and more profoundly in the roots of F. australasica, from 8 pmol mg FW−1 to 55 pmol mg FW−1 (Supplemental Fig. S5B). This 7-fold increase in root Cys indicates a high activity of GSH synthesis in root tissues of the C4 species. Indeed, transcript levels of γECS were higher in roots than in shoots of both species and roots of C4 Flaveria compared to C3 roots, and the same is true for T. hassleriana and G. gynandra (Supplemental Fig. S6). Molecular adaptions of the root system leading to higher GSH synthesis are therefore likely to be involved in the adjustment of S supply and GSH homeostasis of C4 plants.
Partitioning of S in Shoots and Roots of Flaveria Species
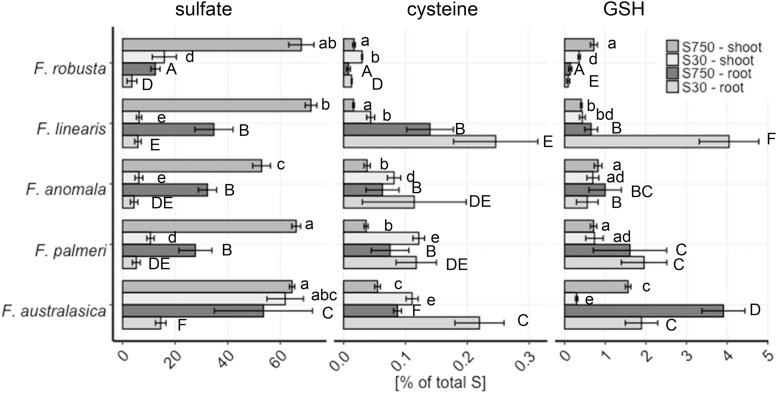
To test the significance of the root for S metabolism in the context of the evolution of C4 photosynthesis, the five species were grown under full nutrient and low S conditions. Total S, sulfate and low Mr thiols were determined in shoots and roots (Supplemental Fig. S7). Whereas total S and sulfate did not show any clear patterns relative to photosynthetic type, Cys, and GSH were again more abundant in leaves of C4 species at full nutrition. Cys was also more abundant under S deficiency (Supplemental Fig. S7, E and G). In the roots, GSH but not Cys accumulated more in the C4 species at full nutrition. To better understand the partitioning of S in the different species, the relative portions of total S in sulfate, Cys, and GSH were calculated (Fig. 5). In the shoots of fully nourished Flaveria species, the fraction of total S occupied by inorganic sulfate was relatively stable at 50%–70%. However, in the roots, the fraction of inorganic sulfate was higher in the C4 species. Exposure to S deficiency reduced the sulfate pool in the shoots and roots of F. robusta, F. linearis, F. anomala, and F. palmeri to 3.5%–16%. The C4 species F. australasica suffered little loss of relative sulfate pool in shoots, but showed a strong decrease in roots. The increase in GSH fractions of total S in shoots and roots of all species along the evolutionary gradient resembles the gradient observed in shoot GSH concentration (Figs. 2 and 5; Supplemental Fig. S7). The gradient is more pronounced in root tissue, ranging from 0.14% in F. robusta to 3.9% in F. australasica. F. linearis showed a root Cys and GSH relative pattern similar to C4 species, due to the fact that total S concentrations in F. linearis roots were exceptionally low (Fig. 5; Supplemental Fig. S7). The highest impact of sulfur deficiency on relative GSH pools was observed in F. australasica (Fig. 5). Cys fractions in S-deficient plants increased, since its levels were not affected by S deficiency, but total S content decreased (Fig. 5; Supplemental Fig. S7).
Figure 5.
Partitioning of sulfur in shoots and roots of Flaveria species. Total sulfur, sulfate, Cys, and GSH were determined in shoots and roots of 23-d-old seedlings of five Flaveria species grown in low sulfate (30 µm sulfate; S30) or adequate sulfate (750 µm sulfate; S750) for 16 d. Sulfate, Cys, and GSH concentrations were calculated relative to total sulfur concentrations. Data are presented as mean % of total sulfur and se, n = 4. Different letters represent values significantly different at P < 0.05 (Student´s T-test).
Roots Control S Distribution between Plant Organs in Flaveria
The analyses so far showed fundamental differences between C3 and C4 Flaveria species in the partitioning of S metabolites. To find out whether these changes are controlled by the root or by the shoot, we established cross-species grafting with a C3 F. robusta and C4 F. bidentis. Reciprocal grafted plants and homografted control plants were exposed to full nutrition conditions (750 µm sulfate) or S starvation (20 µm sulfate) for 6 d and resupplied with 200 µm [35S]sulfate solution. As observed before, no difference in sulfate uptake under full nutrition was detected (Fig. 6A). After S deficiency, which induced uptake in all plants, all grafted plants containing at least one C4 organ, scion (shoot), or stock (root) showed higher rates of sulfate uptake than the C3/C3 control (Fig. 6A). Under full nutrition, the scion GSH concentration corresponded to the scion identity, i.e. high in C4 scions, and was unaffected by the individual stock, showing the importance of high GSH for the metabolic processes in C4 leaves (Fig. 6B). When the plants were exposed to S starvation, GSH pools were depleted. Resupply with 200 µm [35S]sulfate solution for 4 h was sufficient for the control C3/C3 (scion/stock) and C4/C4 plants to fully restore their scion GSH pools to the levels at full S supply. However, C4/C3 grafted plants failed to restore their GSH pools and accumulated only half as much GSH when compared to plants under full nutrition and the C4/C4 controls. On the other hand, C3/C4 plants accumulated almost twice the amount of GSH in their scions compared to plants under full nutrition (Fig. 6B). Hence, maintenance of GSH homeostasis in the C4 shoot seems to be dependent on the root.
Figure 6.
[35S]sulfate uptake and partitioning in interspecies grafts of F. robusta and F. bidentis in dependence on the nutrient regime. Seedlings of F. robusta (C3) and F. bidentis (C4) were grafted 5 d after germination. 35 d after grafting, the grafted plants were resupplied for 4 h with 0.2 mm [35S]sulfate nutrient solution after 6 d exposure to low sulfate (20 µm; S20) or adequate sulfate (750 µm; S750). A, Total [35S]sulfate uptake. B, Total shoot GSH concentration in S-starved (S20), resupplied, and fully nourished (S750) grafts. Data are shown as box plot (25–75%); the line represents median, and the whiskers represent 1.5 IQR, n = 4. C, Relative [35S]sulfate partitioning into shoot sulfate, GSH, Cys, proteins, and other compounds after 3 h of resupply. Different letters represent values significantly different at P < 0.05 (Student’s t test), n = 4.
The partitioning of [35S]-labeled metabolites was also quantified in the grafted plants. Compared to full nutrition, S deficiency resulted in increased [35S] allocation to the scions in grafted plants with C4 rootstocks but not in those with C3 stock (Fig. 6C). The sulfate pools in scions of grafted plants supplied by a C4 stock did not change between the nutrient regimes, but the increase in scion [35S]sulfur was due to higher amounts of GSH. In contrast, in grafts supplied by a C3 stock, the scion sulfate pool was diminished. The large influence of the root system corroborates the hypothesis of enhanced GSH synthesis and translocation in the roots of C4 Flaveria species.
To understand the influence of roots and shoots on GSH metabolism and sulfate assimilation, transcript levels of genes involved in both pathways were analyzed in scion and stock tissue of interspecific C3-C4 grafts grown under full nutrition (Supplemental Fig. S8). The transcript levels of γECS in the grafted plants corresponded to the levels in ungrafted F. robusta and F. bidentis, i.e. they were higher in C4 scions and stocks compared to their C3 counterparts and higher in C4 rootstocks than C4 scions. Interestingly, the GGCT2.1 mRNA accumulated to higher levels in scions of C4/C4 homografts than of C4/C3 grafted plants (Supplemental Fig. S8B). This result points to diminished GSH turnover when the C4 scion is provided by a C3 stock. Transcript levels of SULTR2.1, a low-affinity sulfate transporter facilitating sulfate translocation to shoots (Takahashi et al., 2000), were higher in both scions and stocks of C3/C3 than C4/C4 grafts (Supplemental Fig. S8C). When a C4 scion was grafted onto a C3 stock, SULTR2.1 mRNA levels in the stock were lower than in the C3/C3 homograft. Hence, the scion seems to play a role in the down-regulation of SULTR2.1 in the stock tissue. The expression analysis showed that C4 stocks have lower SULTR2.1 transcript levels than C3 stocks, which should result in lower transport of sulfate into the shoots. Since the transport of S into the shoot is unchanged and even increased at low S, and the transcript levels of γECS are high in C4 roots, GSH is likely to be the form of S translocated to shoots in this C4 species.
Links between S Metabolism and Amino Acid Biosynthesis in C3 and C4 Flaveria
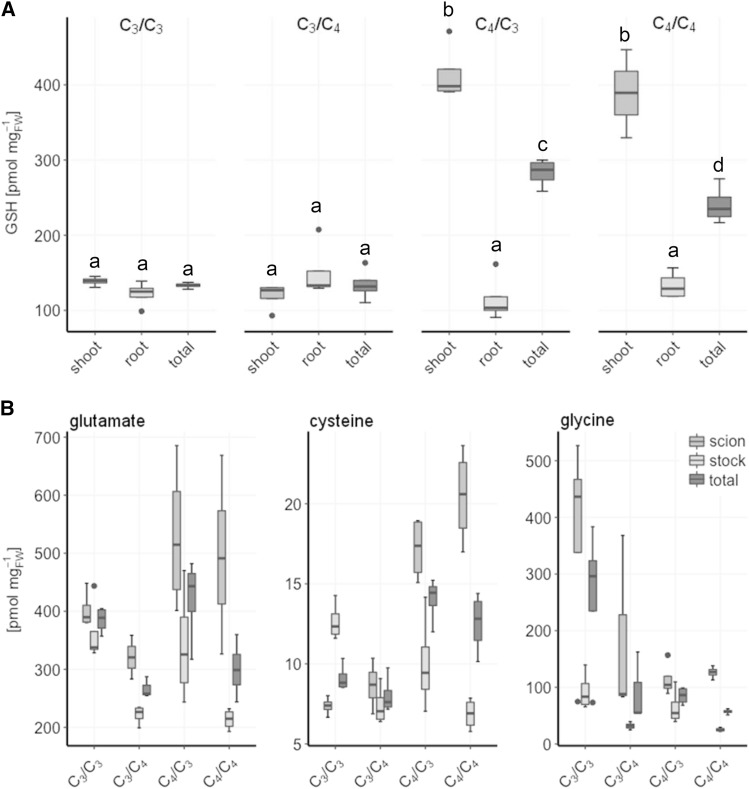
Should GSH be the transport metabolite in C4 plants, the metabolism of its constituting amino acids Glu and Gly, as well as Ser, the precursor of Cys, should be coordinated with sulfate assimilation. In C3 plants, GSH synthesis is usually limited by Cys availability (Takahashi et al., 2011; Noctor et al., 2012). Indeed, in full nutrition, Cys allocation in the grafted plants largely reflects the allocation of GSH (Fig. 7). Glu, the most abundant amino acid, shows higher concentrations in the scion tissues compared to the root tissues. C4 scions and C3 stocks accumulate more Glu compared to C3 scions and C4 stocks, respectively. Remarkably, the C3 scion Glu concentration is reduced when supplied by a C4 stock. The highest Gly concentration is found in stocks of C3/C3 homografts, and Gly is high in the C3 scion of the C3/C4 grafted plants (Fig. 7B). In C3 rootstocks, Gly levels are higher than in C4 stocks. This result points to photorespiration being the main pathway for Gly synthesis.
Figure 7.
Root and shoot concentrations of GSH and its composing amino acids in interspecies grafts of F. robusta and F. bidentis. Seedlings of F. robusta (C3) and F. bidentis (C4) were grafted 5 d after germination and analyzed 35 d after grafting. A, Steady-state levels of GSH. B, Steady-state concentrations of the GSH-composing amino acids Glu, Cys, and Gly. Data are shown as box plot (25–75%); the line represents median, and the whiskers represent 1.5 IQR, n = 4. Different letters represent values significantly different at P < 0.05 (Student’s t test).
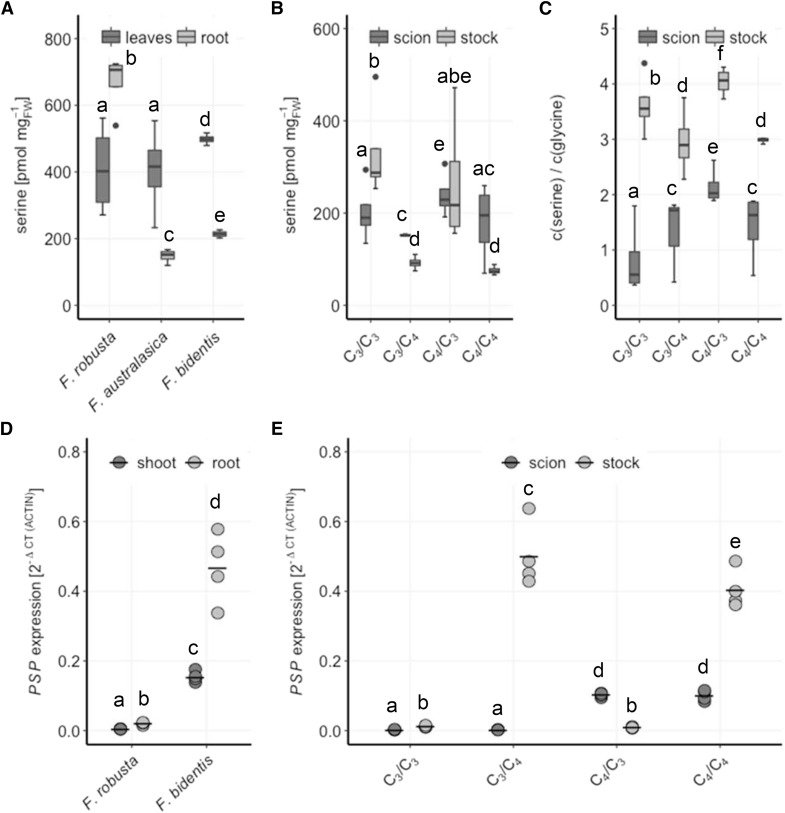
In the C4 species F. australasica and F. bidentis, as well as in the C4/C4 homografts, Ser showed a higher abundance in the scion tissue compared to the stocks. This allocation pattern was reversed in the C3 species F. robusta and the C3/C3 homografts. Additionally, the Ser concentration in the total plant was higher in C3 compared to C4 species (Fig. 8A). The C3/C4 grafted plants resemble the C4/C4 homografts, whereas in the C4/C3 grafted plants, Ser concentration in the scion and stock are similar, since Ser concentrations in the tissues follow those in homografted controls (Fig. 8A). Due to the photorespiratory conversion of Gly to Ser in C3 plants, the Ser-to-Gly ratio is often used as a marker for photosynthesis type, with higher values for C4 species. The Ser-Gly ratio was higher in C3 stocks compared to C4 stocks (Fig. 8C). The Ser-Gly ratio in scions of C3/C4 grafted plants, however, increased to values of C4 scions with the highest value in C4/C3 grafts. Hence, C4 roots are important for the manifestation of C4 characteristic Ser-Gly ratio, suggesting that these ratios may not be controlled only by photorespiration.
Figure 8.
Ser biosynthesis in Flaveria species and interspecies grafts of F. robusta and F. bidentis. A, Leaf and root Ser concentrations were analyzed in 23-d-old seedlings of F. robusta (C3), F. australasica (C4), and F. bidentis (C4). B, Seedlings of F. robusta and F. bidentis were grafted 5 d after germination and analyzed 35 d after grafting for steady-state Ser concentrations. C, Ser-Gly ratio in scions and stocks of the grafted plants in 7B and 8B were calculated. Data are shown as box plot (25–75%), the line represents median, the whiskers represent 1.5 IQR, n = 4. D PSP transcript levels relative to ACTIN in shoots and roots in 20-d-old seedlings of F. robusta (C3) and F. bidentis (C4). E, Seedlings of F. robusta and F. bidentis were grafted 5 d after germination and analyzed 35 d after grafting. Transcript levels of PSP relative to ACTIN are shown. Crossbar, mean; n = 4. Different letters represent values significantly different at P < 0.05 (Student’s t test).
Photorespiration, however, is not the only source of Ser biosynthesis. Heterotrophic tissue synthesizes Ser by the PPSB (Ho et al., 1999; Ros et al., 2014). Therefore, transcript levels of phospho-Ser phosphatase (PSP), which is involved in this pathway, were analyzed in shoots and roots of F. robusta and F. bidentis, as well as scions and stocks of grafted plants (Fig. 8B). PSP transcript levels were low and comparable in F. robusta shoots and roots and C3 organs of the grafted plants. Higher levels of PSP mRNA were detected in the C4 species F. bidentis, with approximately 3-fold higher levels in the roots than in the leaves, which were retained in the grafted plants. The accumulation of PSP transcripts in C4 species points to the importance of the PPSB for Ser supply in C4 plants, particularly in the root. Increased Ser biosynthesis in the roots of C4 plants might, therefore, facilitate the conversion of Ser to Cys and subsequently GSH in the same tissue.
DISCUSSION
The Gradient in GSH Accumulation toward C4 Species
In recent years, substantial progress in the understanding of C4 photosynthesis and its evolution has been achieved (Heckmann et al., 2013; Schulze et al., 2013; Williams et al., 2013; Schuler et al., 2016). However, knowledge about nutritional aspects associated with the enhanced photosynthetic efficiency of C4 plants remains limited. This study addresses a significant gap in knowledge and shows that nutrient homeostasis has to be taken into account to understand the full complexity of the evolution of C4 plants. The initial interest in the connection between S metabolism and C4 photosynthesis came from the localization of the enzymes of sulfate assimilation (Gerwick et al., 1980; Koprivova et al., 2001; Kopriva and Koprivova, 2005; Weckopp and Kopriva, 2015). However, Koprivova et al. (2001) indicated further adaptations in sulfur metabolism dependent on the photosynthesis type, showing increasing leaf Cys and GSH content with the progression of a C4 cycle in Flaveria. Here, these results were confirmed under strictly controlled nutrient conditions in another set of Flaveria and, at least for Cys, also at two levels of sulfur supply. Interestingly, this work reveals that the increasing leaf Cys and GSH concentration does not correlate with leaf inorganic sulfate (Supplemental Figs. S2A and S7), despite sulfate comprising the largest plant sulfur pool (Fig. 5). This result emphasizes the importance of the regulation of Cys and GSH levels in C4 Flaveria species. In addition, OAS, which is an important regulator of sulfate assimilation in Arabidopsis (Hubberten et al., 2012), does not seem to underlie the regulation in Flaveria. So what is the biological driver for this high GSH accumulation in C4 plants?
GSH is the most abundant low-molecular-weight thiol in plant cells. It is present in its reduced (GSH) and oxidized form (GSSG), acting as a cellular redox buffer. Its redox buffering capacities are dependent on the GSH/GSSG ratio as well as the concentration of GSH in the cells (Schafer and Buettner, 2001; Pfannschmidt, 2003). Thus, enhanced concentrations of GSH, as observed along the evolutionary gradient of Flaveria and in the C4 scions of grafted plants, could be an indication for adapted redox buffering capacities. Correspondingly, S-starved intermediate and C4 species showed significantly higher translocation of S and flux into shoot GSH (Fig. 3). The large investment of newly acquired sulfate into GSH could, therefore, serve the purpose of restoring the full GSH redox potential. Drastic changes in the cellular redox potential evoked by S starvation and the reduction in GSH pools could have a direct impact on the general plant performance and photosynthetic efficiency. However, neither the reduction in shoot biomass nor the photosynthetic performance showed a photosynthesis type-dependent reaction to S starvation (Supplemental Fig. S3). As S deprivation did not influence the photosynthetic performance of F. robusta, F. australasica, and F. bidentis, it is possible that minimum levels of leaf GSH are maintained to secure redox reactions in primary metabolism by adapting the GSH/GSSG ratio. Therefore, the high accumulation of GSH in fully nourished C4 Flaveria species is likely to serve other metabolic and regulatory purposes. Alternatively, the redox buffering capacity could become important during non-steady-state conditions, such as light transients when redox demand fluctuates rapidly as leaf C3 and C4 cycles are coordinated (Slattery et al., 2018).
One of the possible functions of high GSH accumulation is scavenging of reactive oxygen species (ROS). GSH-dependent ROS detoxification can occur within the ascorbate-glutathione pathway, by glutaredoxin-dependent peroxiredoxins or GST peroxidase activity (Foyer and Noctor, 2009; Noctor et al., 2012). An increase in oxidative stress can harm the function of the photosynthetic apparatus and might act as a collateral selective pressure to evoke higher storage capacities for GSH (Wise and Naylor, 1987; Krause, 1988). Indeed, C4 species of the genus Cleome were reported to have a higher tolerance to ROS formation than their C3 relatives (Uzilday et al., 2012). This higher tolerance may become particularly important during fluctuating light intensities (Slattery et al., 2018). However, comparison of leaf transcriptomes of Flaveria species revealed that some genes involved in redox regulation have increased expression in C4 species but others in C3 species (Gowik et al., 2011). A thorough monitoring of the redox state in the C3 and C4 Flaveria species, e.g. by rhoGFP, would thus be necessary but is not feasible since no transformation protocols are available for the C3 species.
GSH is also involved in the control of plant development, tissue differentiation, and cell cycle regulation (Diaz-Vivancos et al., 2015). GSH is indispensable to maintain cell division in the root apical meristem and to preserve the unimpaired development of the floral meristem (Vernoux et al., 2000; Reichheld et al., 2007; Bashandy et al., 2010). The interaction of GSH and auxin plays a crucial role in these processes. GSH regulates the expression and distribution of PIN proteins and, hence, the directed distribution of auxin as well as the interaction of auxin and strigolactones (Vernoux et al., 2000; Bashandy et al., 2010; Koprivova et al., 2010; Marquez-Garcia et al., 2014; Schnaubelt et al., 2015). Additionally, recruitment of GSH into the nucleus and fluctuations in the cellular GSH concentrations are crucial for the progression of the cell cycle (Pellny et al., 2009; Diaz Vivancos et al., 2010). Besides increased growth rates, one major characteristic of this complex trait is the establishment of the Kranz anatomy, associated with differentiation of the BSC and increase in vascular tissue density (Laetsch, 1974; Sage, 2004; Atkinson et al., 2016). Though the underlying mechanism is not yet understood, an increase in cell number and differentiation connected to auxin metabolism has been discussed (Aubry et al., 2014). Therefore, GSH may be involved in the evolution of Kranz anatomy, as a regulator of the cell cycle and vascular tissue differentiation. As with the cell-specific localization of the pathway in C4 leaves, the role of high GSH remains an open question.
High GSH Turnover in C4 Flaveria
Unlike Cys pools, leaf GSH pools decreased in response to S deficiency in all Flaveria species analyzed and did not correlate with the establishment of C4 photosynthesis (Fig. 2). GSH is the main storage compound for reduced S (Kopriva and Rennenberg, 2004; Noctor et al., 2011). Hence, the observed depletion of the GSH pool could be a consequence of GSH breakdown to serve the demand for steady Cys levels. GSH is metabolized in the γ-glutamyl cycle, in which GGCT catalyses the γ-glutamyl dipeptide degradation producing 5-oxo-Pro, which is further converted to l-Glu by 5-oxoprolinase (Meister, 1974). In Arabidopsis, GGCT2.1 has been identified as the major γ-glutamyl cyclotransferase involved in GSH degradation (Paulose et al., 2013). Correspondingly, GGCT2.1 transcript levels are induced by S deficiency (Nikiforova et al., 2003). The Flaveria GGCT2.1 homologs showed higher transcript abundance in shoots of the C4 species F. bidentis compared to the C3 species F. robusta, pointing to a higher GSH turnover in C4 species. Interestingly, similar differences were observed in transcriptomic data of T. hassleriana and G. gynandra (Külahoglu et al., 2014), closely related C3 and C4 species of the Cleomaceae family. With two examples of independently emerged C4 photosynthesis, this indicates that GSH turnover in the leaf is important for the establishment of a C4 cycle.
This hypothesis is strengthened by the results obtained during the treatment of F. robusta and F. australasica with BSO. Upon BSO treatment, synthesis of GSH is impaired, whereas sulfate assimilation into Cys is not affected. Therefore, Cys often accumulates in BSO-treated plants (Hartmann et al., 2004). Remarkably, the GSH consumption rate in leaves of F. australasica was 2-fold higher in the first 4 h of exposure to BSO compared to F. robusta. The decline in shoot GSH concentrations in F. australasica correlates with the transcript levels of GGCT2.1. An increase in GGCT2.1 transcript indicates active GSH turnover upon BSO treatment. The reasons for high GSH turnover in C4 leaves, even under unstressed conditions, remain elusive so far.
Interestingly, upon exposure to BSO, shoot Cys concentrations in F. robusta and F. australasica remained stable. Only the root of C4 F. australasica accumulated Cys, indicating a high activity of GSH synthesis, as described, e.g. in Hartmann et al. (2004) (Supplemental Fig. S5). Transcript analysis of γECS supports this hypothesis, since γECS transcript increases more strongly in roots of F. australasica than F. robusta upon BSO treatment (Supplemental Fig. S6). In contrast, shoot γECS transcript levels react only slightly to BSO. Additionally, unstressed roots of C4 F. bidentis and G. gynandra show significantly higher amounts of γECS transcript compared to shoot tissue and roots of their C3 relatives, F. robusta and T. hassleriana (Supplemental Fig. S6). Taken together, the increased expression of γECS in C4 roots and the accumulation of Cys in the roots of F. australasica upon BSO treatment indicate higher GSH synthesis rates in the roots than in the shoots of C4 plants. Indeed, 2-fold higher enzymatic activity of γECS was detected in roots of 7- and 11-d-old seedlings of maize compared to the activity in shoots (Ruegsegger and Brunold, 1993). The enhanced GSH synthesis in the roots thus seems to be a general feature in C4 plants and might be linked to the establishment of C4 photosynthesis.
The partitioning of S into different pools in shoots and roots also supports the hypothesis of high GSH synthesis in C4 roots. GSH fractions of shoots and roots were steadily increasing along the evolutionary axis (except for F. linearis roots, which accumulated exceptionally little S in the roots), and were particularly increased by S deprivation in F. palmeri roots as well as shoots and roots of F. australasica. Interestingly, in C4 F. australasica the sulfate pool in the shoots was not depleted by S deficiency as strongly as in other species and remained at approximately 60% of total sulfur (Fig. 5). Sulfate is retained in vacuoles of mutants of the vacuolar sulfate transporters sultr4;1 and sultr4;2 (Kataoka et al., 2004); therefore, these transporters need to be identified and analyzed in detail in F. australasica and other C4 species. Since GSH decreased upon S deficiency, it is possible that only little of the shoot sulfate pool is invested into GSH synthesis in C4 F. australasica. Considering the increased GSH synthesis rates in the roots and GSH accumulation in the shoots of unstressed C4 Flaveria, transport of GSH or its precursor γEC from the root into the shoot is likely. Correspondingly, after feeding with [35S]sulfate, no labeled Cys or GSH was detected in the roots, indicating that the reduced compounds are rapidly translocated.
Roots Control of Sulfur Homeostasis in C4 Flaveria
To determine whether the different allocation of S metabolites in C3 and C4 plants is driven by the root or the shoot, we performed reciprocal grafting between the C3 F. robusta and C4 F. bidentis. Grafting is extensively applied to gain knowledge on systemic signaling in plants (Tsutsui and Notaguchi, 2017). The grafted plants were analyzed 35 d after grafting, when the roots and shoots are well developed, and were used to dissect the interaction of root and shoot in nutrient and metabolite transport connected to S metabolism. Total [35S] uptake and translocation rates indicate that, under unstressed conditions, S root-to-shoot translocation is mainly controlled by the root system, particularly the C4 root (Fig. 6). Correspondingly, SULTR2.1 shows higher transcript abundance in scions and stocks of C3/C3 homografts compared to C4/C4 homografts. In C3/C4 grafted plants, SULTR2.1 is expressed according to the individual tissue identity, but C4/C3 grafts show a significant decrease of SULTR2.1 in scions and stocks. The C4 rootstock thus seems to be adapted to lower rates of inorganic sulfate translocation into the shoot. The down-regulation of SULTR2.1 in the C3 stocks of C4/C3 grafts could be dependent on systemic signals excreted by the C4 scion to maintain shoot S homeostasis. The signal might be GSH, which is known to down-regulate SULTR2.1 transcript levels (Lappartient et al., 1999), and its high levels present in unstressed C4 scions could influence the expression of SULTR2.1 in C3 stocks of C4/C3 grafted plants (Figs. 6 and S8).
In S-deficient plants, only grafts supplied by a C4 stock showed increased translocation of newly acquired S into their scions, regardless of the scion identity (Fig. 6). The up-regulation of sulfate uptake and assimilation was sufficient to fully restore GSH pool in the C3/C3 and C4/C4 homografts after 4 h feeding. Remarkably, at the same time, C4/C3 grafted plants only accumulated about 50% of their control GSH pool, whereas the C3/C4 plants reached almost twice as much GSH in their scions compared to fully nourished plants. This result indicates that C4 roots produce large amounts of GSH that is transported into the shoots via the xylem. Similarly, increased translocation of GSH was seen in poplars overexpressing γECS, which showed 5-fold higher GSH concentration in the xylem than wild-type poplars (Herschbach et al., 2000). GSH turnover is, however, slower in C3 scions, due to lower expression of GGCT2.1, causing overaccumulation of GSH.
C4 Flaveria species are adapted to higher GSH concentrations in their shoots than C3 species. However, high concentrations of GSH have been reported to have negative effects on plant development (Creissen et al., 1999; Herschbach et al., 2010; Ivanova et al., 2011). The stocks of C4/C3 grafts are confronted with 4-fold increased GSH concentrations in their scions. Acropetal transport of GSH in these grafts might, therefore, evoke a physiological response in the stock tissue. Indeed, SULTR2;1 and γECS transcript levels were decreased in C3 stocks of C4/C3 grafts and point to long-distance feedback regulation of sulfate translocation and GSH synthesis in the roots (Supplemental Figs. S6 and S8). Although the stocks show transcriptional effects in γECS, feedback inhibition of γECS occurs largely posttranslationally in animals and plants (Richman and Meister, 1975; Hell and Bergmann, 1990; Noctor et al., 2002). Since we were not able to measure GSH synthesis rates in the roots, enzyme activity assays of γECS and GSH synthase in the interspecific grafts could provide important insight into the systemic regulation of GSH biosynthesis.
We conclude that the increased GSH accumulation in C4 shoots is caused by increased GSH synthesis in the roots because (1) γECS is more highly expressed in roots than in shoots and C4 roots compared to C3 roots (Supplemental Figs. S6 and S8) and is also induced by BSO to higher degree in C4 roots than in C3 roots (Supplemental Fig. S6); (2) in the BSO experiment, Cys not used for GSH synthesis accumulates in roots and not in shoots (Supplemental Fig. S5), and the accumulation is higher in C4 than in C3 roots; (3) C4 shoots supplied by C4 roots accumulate more 35S in GSH than when supplied by C3 roots. Since GSH synthesis in the shoots should be the same, the difference strongly indicates GSH transport from roots; (4) transcript levels of SULTR2;1, necessary for translocation of sulfate (as an alternative to GSH), are lower in C4 than in C3 shoots; (5) in C3 shoots of C3/C4 grafted plants, GSH is not increased compared to C3/C3 because SULTR2;1 is highly expressed in the C3 shoots and facilitate sulfate translocation, driving the S from GSH synthesis in the root.
Ser Biosynthesis as a Possible Driving Force for the Allocation of GSH Synthesis in C3 and C4 Flaveria
GSH synthesis has been allocated to roots in C4 plants (Fig. 6B). The accumulation of GSH-constituent amino acids, however, does not show correspondingly higher levels in C4 roots. Cys and Gly require Ser for their synthesis. In C3 plants, Ser is mainly derived from photorespiration in photoautotrophic tissues (Bauwe et al., 2010; Maurino and Peterhansel, 2010). In heterotrophic tissues, the PPSB constitutes the main source of Ser (Ho and Saito, 2001; Benstein et al., 2013). Up-regulation of genes involved in the PPSB in response to darkness and elevated CO2 concentrations indicates that the PPSB is important when photorespiration is minimized (Benstein et al., 2013; Toujani et al., 2013). Therefore, PPSB is probably responsible for Ser production in C4 plants with low rates of photorespiration (Bräutigam and Gowik, 2016).
Analysis of steady-state Ser levels in roots and shoots of F. robusta, F. australasica, and F. bidentis showed the same shoot-root allocation as detected for Cys in C3/C3 and C4/C4 autografts of F. robusta and F. bidentis. As observed for Cys, scion-stock allocation of Ser in interspecies grafts was rather C4-like. C4/C3 grafts accumulated comparable amounts of Ser in scions and stocks. When supplied by a C4 stock, Ser concentrations decreased in C3 scions. Again, this indicates a complex, photosynthesis type-dependent interplay of shoot and root involved in Ser homeostasis. The signals, transport processes, and mechanisms driving this interplay remain to be elucidated. The regulation is potentially under the control of the stock tissue, as C3/C4 grafts revealed scion and stock ratios reflecting the C4 control.
PSP showed similar expression levels in various organs of Arabidopsis, and only little sensitivity to environmental influences (Cascales-Miñana et al., 2013). The mRNA levels of PSP were higher in F. bidentis compared to F. robusta and showed 3-fold higher transcript levels in the roots of the C4 species, which was maintained in interspecies grafts. High PSP mRNA levels in C4 shoots indicate the importance of the PPSB pathway in C4 plants. This has been corroborated by a transcriptomics analysis of Cleomaceae species of different photosynthesis types, with the C4 Gynandropsis gynandra showing substantially higher PSP transcript levels in roots than in leaves and in C3 Tarenaya hassleriana (Külahoglu et al., 2014), which corresponds well with the reduced importance of photorespiration. It seems that in C4 plants the PPSB largely substitutes photorespiratory Ser biosynthesis. Therefore, Ser biosynthesis is relocated to the root. Hence, the relocation of GSH metabolism in C4 plants could be the evolutionary answer to changes in Ser biosynthesis caused by decreased rates of photorespiration.
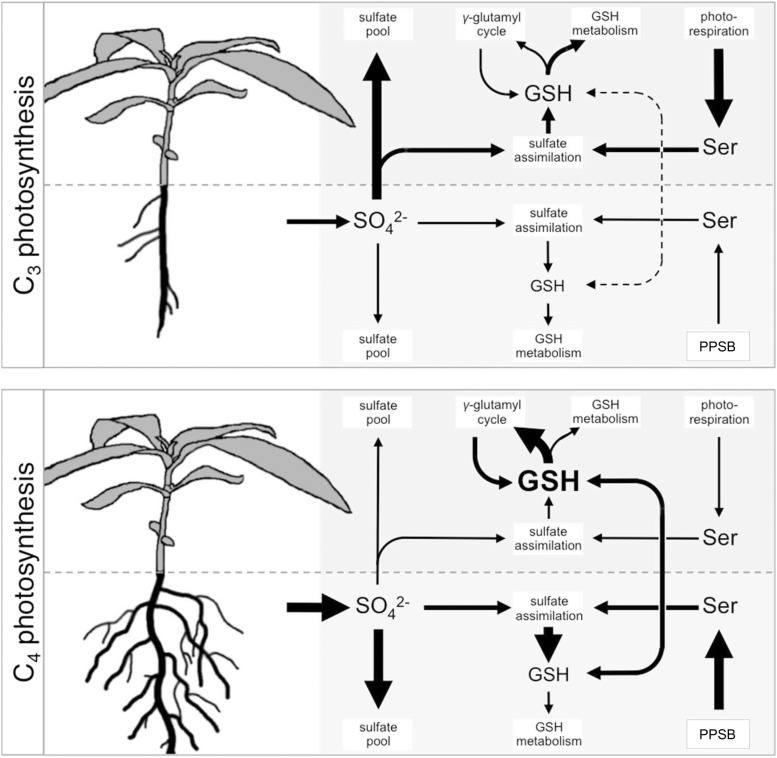
The following model for sulfur metabolism in C3 and C4 Flaveria is proposed based on the presented data (Fig. 9). C4 species show higher uptake capacity for inorganic sulfate compared to C3 species. C3 species transport large amounts of sulfate to the shoot, where the main sulfate pool is located. The major part of Ser, required for sulfate assimilation into Cys, is derived from photorespiration. GSH pools are kept at low levels with de novo synthesis in leaves meeting the instant demand. C4 species, on the other hand, store considerable amounts of newly acquired sulfate in their roots and show low sulfate translocation rates. Sulfate assimilation into GSH is enhanced in the roots, connected to Ser synthesis by the PPSB. GSH is transported into the shoot, supplementing the shoot GSH pool. Large amounts of shoot GSH enters the γ-glutamyl cycle. The significance of the vast accumulation of GSH and its high turnover and degradation rates in C4 plants, however, remains elusive.
Figure 9.
Differences in organization of sulfate uptake, assimilation, and GSH metabolism between C3 and C4 Flaveria species. Arrow sizes indicate differences in transport and flux between C3 and C4 Flaveria. GSH, glutathione; PPSB, phosphorylated pathway of Ser biosynthesis.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Six Flaveria species were selected by their CO2 compensation points to provide a gradient in C4 photosynthesis manifestation (Ku et al., 1991; Supplemental Table S1). Seeds were kindly provided by Udo Gowik (Institute for Biology and Environmental Sciences, Carl von Ossietzky University, Oldenburg). Flaveria seeds were surface sterilized by chlorine gas sterilization for 4 h. Sterilized seeds were placed on 0.5× Murashige and Skoog agar plates with sterile toothpicks and stratified for 3 d at 4°C in the dark. The plates were subsequently incubated in a Percival phyto chamber under long-day conditions (16 h light at 100 µE, 21°C, 50% humidity) for 7 d. The seedlings were transferred to sterile 500-mL WECK mold jars in which 80 mL Long Ashton nutrient solution containing 0.65% agarose was poured. The jars were closed with two clamps each and transferred to a high-light growth chamber (Johnson Control) with long-day conditions (day 16 h, light 500– 600 µE, 21°C constant, 50% humidity) for 16 d. This allowed the control of nutrient supply, which was adjusted to 750 µm MgSO4 in full supply and 20 to 50 µm MgSO4 (supplemented with MgCl2 to obtain 750 µm Mg2+). The sulfate levels were adjusted according to growth conditions and length of treatment to achieve S deficiency, which was tested in preliminary experiments. Alternatively, for the [35S]sulfate feeding experiments, 10-d-old seedlings were transferred onto large square petri dishes (245 mm × 245 mm × 25 mm) filled with 250 mL of Long Ashton medium with 0.65% agarose. Low sulfur conditions were adjusted to 20 µm MgSO4 × 7 H2O and substituted with 730 µm MgCl2 × 6 H2O. The plates were incubated in a Johnson high-light growth chamber for 7 d. For BSO treatment, the control (750 µm sulfate) plates with seedlings of F. robusta and F. australasica were incubated for 8 d. The seedlings were transferred to another large square petri dishes filled with solidified (0.65% agarose) Long Ashton medium supplemented with 2 mm BSO. Transfer and subsequent incubation was conducted in a high-light growth chamber, and samples were collected at 0, 4, 8, 12, 24, and 48 h after transfer.
To conduct gas exchange measurements, 21-d-old plants were initially transferred to 9-cm round pots with 1:1 mix of vermiculite and sterile sand as substrate. The plants were grown in the high-light chamber, watered from underneath, and fertilized every third day with 30 mL Long Ashton medium. After 20 d, the plants were transferred to 8-cm square pots and were grown at the same conditions.
For all experiments, each biological replicate was collected from plants grown in different glasses or plates. All experiments were performed independently at least twice with similar results.
Interspecies Micrografting of F. robusta and F. bidentis
Interspecies micrografting of F. robusta and F. bidentis was conducted using the modified collar-free micrografting protocol by Marsch-Martínez et al. (2013) with seedlings 5 d after germination under sterile conditions. The seedlings were transferred onto small square petri dishes (120 mm × 120 mm × 15 mm) containing a thin layer (2–4 mm) of liquid Long Ashton medium. Cotyledons were removed with a single cut with a thin, commercial razor blade. Scion and stock were separated by a single horizontal cut in the upper third of the hypocotyl. Subsequently, scion and stock were joined thoroughly as soon as possible on fresh small square petri dishes with Long Ashton medium, supplemented with 0.5% Suc. Cutting and graft union was performed with the help of a Hund SM33 binocular stereoscope (Hund). Fresh grafts were incubated in a Percival phyto chamber for 7 d with a slight tilt of the plates at 5 to 10 degrees and subsequently moved to high-light conditions. Twelve days after grafting, the grafts were transferred to large, square petri dishes to grant suitable space for scion and stock development. Exposure to different nutrient conditions was conducted 29 d after grafting. F. bidentis was used for the grafting, even though F. australasica has been analyzed in previous experiments, because grafting with F. australasica was not successful.
Isolation of Total RNA and Expression Analysis
RNA was isolated with TRI Reagent (Merck; Chomczynski and Sacchi, 1987). Approximately 50 mg of plant material were homogenized and extracted in 500 µL TRI Reagent for 5 min. One hundred microliters chloroform were added and incubated for 15 min at room temperature. After centrifugation at 16,000g and 4°C for 15 min the upper aqueous phase was transferred into a new reaction tube, mixed thoroughly with 250 µL isopropanol and incubated on ice for 60 min. RNA was pelleted by centrifugation at 12,000g and 4°C for 10 min. The pellet was washed twice with 80% ethanol at room temperature and centrifuged for 5 min at full speed (4°C). Finally, the supernatant was removed, and the pellet was dried and resolved in 30 µL water at 55°C for 10 min. RNA samples were stored at −80°C.
The concentration of nucleic acids was measured spectrophotometrically, using Nanodrop (Nanodrop ND1000, Peqlab). For RT-qPCR a DNAse treatment and synthesis of first-strand cDNA was performed using QuantiTect Reverse Transcription Kit (Qiagen), according to the manufacturer´s instructions. The qPCR was performed using CFX96 Touch Real-Time PCR Detection System (Bio-Rad). Transcript levels were calculated relative to ACTIN transcript levels using the ΔCT method (Pfaffl, 2012). The primers (Supplemental Table S2) were designed on the 3′ end of cDNA sequences of de novo assembled contigs of F. robusta or F. pringlei and F. bidentis, kindly provided by Udo Gowik. The sequences are 70 to 75% identical with the cDNA sequences of the corresponding genes in Arabidopsis (Arabidopsis thaliana).
To compare expression of relevant genes in two species from the Cleomaceae family, Tarenaya hassleriana (C3) and Gynandropsis gynandra (C4), the data set available as Supplemental Data Set 1 in Külahoglu et al. (2014) was used.
Measurement of S Containing Metabolites, Sulfate Uptake, and Flux through Sulfate Assimilation
Sulfate was determined from approximately 50 mg lyophilized plant material by ion chromatography as described in Huang et al. (2016).
The low molecular weight thiols Cys and GSH were extracted and quantified as their monobromobimane-derivatized products from approximately 30 mg lyophilized plant material as described by Mugford et al. (2009).
Total S was determined by inductively coupled plasma mass spectrometry from 100 to 200 mg lyophilized root or shoot tissue by the Plant Metabolism and Metabolomics Laboratory, University of Cologne, using an Agilent 7700 ICP-MS (Agilent Technologies; Almario et al., 2017).
Sulfate uptake and flux through sulfate assimilation was determined in fully nourished and sulfur-deficient plants grown on large square plates essentially as described in Mugford et al. (2011). Two seedlings transferred to 12-well plates were incubated shortly in 2 mL nonradioactive Long Ashton nutrient solution containing 0.2 mm sulfate, which was subsequently replaced by 1 mL of the same solution supplemented with 12 µCi [35S]sulfuric acid. The plants were incubated in the hot solution in the light for 4 h. Roots and shoots were washed thoroughly, blotted dry, and stored in liquid nitrogen until further processing on the same day. Root and shoot samples were extracted in 10-fold volume of 0.1 m HCl. Ten microliters of extract were used to determine sulfate uptake and 50 µL aliquots of each extract were collected for quantification of [35S] incorporation into thiols and proteins exactly as in Kopriva et al. (1999)
Quantification of Amino Acids
Proteinogenic amino acids in ethanolic leaf extracts were detected and quantified by derivatization with ortho-phthaldialdehyde and RP-HPLC-based separation as described by Krueger et al. (2017) using approximately 50 mg lyophilized leaf material.
Gas Exchange
Gas exchange was determined in young, fully expanded leaves of 10-week-old plants grown in pots using a Li-Cor LI-6800 with small light source and 9-cm2 chamber (Li-Cor Biosciences). Steady-state photosynthesis parameters were measured at 40 Pa CO2, 1,500 µmol m−2 s−1 PAR with a leaf temperature maintained at 25°C and flow rate of 300 µmol s−1. The photosynthetic response to CO2 (A-Ci curves) was measured under 1,500 PAR, using CO2 partial pressures of the following order: 40, 40, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 10, 15, 20, 40, 40, 80, 160, 200, 120, 40 Pa. The photosynthetic carbon efficiency and the CO2 compensation point were determined from the slope and x-intercept of the initial linear portion of the A-Ci curve, respectively.
Accession Numbers
RNA sequence data underlying the expression analysis of G. gynandra and T. hassleriana can be found in NCBI GenBank under accession numbers SRP036637 and SRP036837 (Külahoglu et al., 2014). The read data underlying the assembled contigs of F. robusta or F. pringlei and F. bidentis used for primer design can be found in NCBI Short Read Archive under accession number SRP006166 (Gowik et al., 2011).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Leaf Cys and GSH content in Flaveria species in dependence on their CO2 compensation point.
Supplemental Figure S2. Influence of S deficiency on leaf sulfate and OAS concentration in Flaveria species.
Supplemental Figure S3. Influence of S deficiency on shoot biomass and photosynthetic performance in Flaveria species.
Supplemental Figure S4. GGCT2.1 expression in C3 and C4 species.
Supplemental Figure S5. Effect of BSO treatment on shoot and root Cys concentrations in F. robusta and F. australasica.
Supplemental Figure S6. Expression patterns of γECS in roots and shoots of C3 and C4 species.
Supplemental Figure S7. Concentration of total sulfur and other sulfur pools in shoots and roots of Flaveria species.
Supplemental Figure S8. Expression of γECS, GGCT2.1, and SULTR2.1 in scions and stocks of interspecies grafts of F. robusta and F. bidentis.
Supplemental Table S1. Species of the genus Flaveria used in this study.
Supplemental Table S2. Oligonucleotide primers used in this study.
Supplemental File S1. Transcript sequences of analyzed genes from F. robusta or F. pringlei and F. bidentis.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Dr. U. Gowik for seeds of Flaveria species, B. Welter for technical support, and the Biocenter MS Platform Cologne for the total sulfur measurements.
Footnotes
Research in S.Ko.’s lab is supported by the Deutsche Forschungsgemeinschaft (EXC 1028). S.Kr. is supported by the Deutsche Forschungsgemeinschaft (Grant Kr4245/2-1). B.J.W. has been funded through a postdoctoral research fellowship awarded by the Alexander von Humboldt Foundation.
Articles can be viewed without a subscription.
References
- Almario J, Jeena G, Wunder J, Langen G, Zuccaro A, Coupland G, Bucher M (2017) Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proc Natl Acad Sci USA 114: E9403–E9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RR, Mockford EJ, Bennett C, Christin PA, Spriggs EL, Freckleton RP, Thompson K, Rees M, Osborne CP (2016) C4 photosynthesis boosts growth by altering physiology, allocation and size. Nat Plants 2: 16038. [DOI] [PubMed] [Google Scholar]
- Aubry S, Smith-Unna RD, Boursnell CM, Kopriva S, Hibberd JM (2014) Transcript residency on ribosomes reveals a key role for the Arabidopsis thaliana bundle sheath in sulfur and glucosinolate metabolism. Plant J 78: 659–673 [DOI] [PubMed] [Google Scholar]
- Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP (2010) Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 22: 376–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15: 330–336 [DOI] [PubMed] [Google Scholar]
- Benstein RM, Ludewig K, Wulfert S, Wittek S, Gigolashvili T, Frerigmann H, Gierth M, Flügge UI, Krueger S (2013) Arabidopsis phosphoglycerate dehydrogenase1 of the phosphoserine pathway is essential for development and required for ammonium assimilation and tryptophan biosynthesis. Plant Cell 25: 5011–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Gowik U (2016) Photorespiration connects C3 and C4 photosynthesis. J Exp Bot 67: 2953–2962 [DOI] [PubMed] [Google Scholar]
- Cascales-Miñana B, Muñoz-Bertomeu J, Flores-Tornero M, Anoman AD, Pertusa J, Alaiz M, Osorio S, Fernie AR, Segura J, Ros R (2013) The phosphorylated pathway of serine biosynthesis is essential both for male gametophyte and embryo development and for root growth in Arabidopsis. Plant Cell 25: 2084–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Moore BD, Edwards GE, Ku MS (1988) Photosynthesis in Flaveria brownii, a C(4)-Like Species: Leaf Anatomy, Characteristics of CO(2) Exchange, Compartmentation of Photosynthetic Enzymes, and Metabolism of CO(2). Plant Physiol 87: 867–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Creissen G, Firmin J, Fryer M, Kular B, Leyland N, Reynolds H, Pastori G, Wellburn F, Baker N, Wellburn A, et al. (1999) Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11: 1277–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Vivancos P, Wolff T, Markovic J, Pallardó FV, Foyer CH (2010) A nuclear glutathione cycle within the cell cycle. Biochem J 431: 169–178 [DOI] [PubMed] [Google Scholar]
- Diaz-Vivancos P, de Simone A, Kiddle G, Foyer CH (2015) Glutathione—linking cell proliferation to oxidative stress. Free Radic Biol Med 89: 1154–1164 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11: 861–905 [DOI] [PubMed] [Google Scholar]
- Gerwick BC, Black CC (1979) Sulfur assimilation in c(4) plants: intercellular compartmentation of adenosine 5′-triphosphate sulfurylase in crabgrass leaves. Plant Physiol 64: 590–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwick BC, Ku SB, Black CC (1980) Initiation of sulfate activation: a variation in c4 photosynthesis plants. Science 209: 513–515 [DOI] [PubMed] [Google Scholar]
- Gowik U, Bräutigam A, Weber KL, Weber AP, Westhoff P (2011) Evolution of C4 photosynthesis in the genus Flaveria: how many and which genes does it take to make C4? Plant Cell 23: 2087–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254: 7558–7560 [PubMed] [Google Scholar]
- Hartmann T, Hönicke P, Wirtz M, Hell R, Rennenberg H, Kopriva S (2004) Regulation of sulphate assimilation by glutathione in poplars (Populus tremula x P. alba) of wild type and overexpressing gamma-glutamylcysteine synthetase in the cytosol. J Exp Bot 55: 837–845 [DOI] [PubMed] [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber AP, Lercher MJ (2013) Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153: 1579–1588 [DOI] [PubMed] [Google Scholar]
- Hell R, Bergmann L (1990) λ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta 180: 603–612 [DOI] [PubMed] [Google Scholar]
- Herschbach C, van Der Zalm E, Schneider A, Jouanin L, De Kok LJ, Rennenberg H (2000) Regulation of sulfur nutrition in wild-type and transgenic poplar over-expressing gamma-glutamylcysteine synthetase in the cytosol as affected by atmospheric H2S. Plant Physiol 124: 461–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschbach C, Rizzini L, Mult S, Hartmann T, Busch F, Peuke AD, Kopriva S, Ensminger I (2010) Over-expression of bacterial γ-glutamylcysteine synthetase (GSH1) in plastids affects photosynthesis, growth and sulphur metabolism in poplar (Populus tremula x Populus alba) dependent on the resulting γ-glutamylcysteine and glutathione levels. Plant Cell Environ 33: 1138–1151 [DOI] [PubMed] [Google Scholar]
- Ho CL, Saito K (2001) Molecular biology of the plastidic phosphorylated serine biosynthetic pathway in Arabidopsis thaliana. Amino Acids 20: 243–259 [DOI] [PubMed] [Google Scholar]
- Ho CL, Noji M, Saito M, Saito K (1999) Regulation of serine biosynthesis in Arabidopsis. Crucial role of plastidic 3-phosphoglycerate dehydrogenase in non-photosynthetic tissues. J Biol Chem 274: 397–402 [DOI] [PubMed] [Google Scholar]
- Huang XY, Chao DY, Koprivova A, Danku J, Wirtz M, Müller S, Sandoval FJ, Bauwe H, Roje S, Dilkes B, et al. (2016) Nuclear localised MORE SULPHUR ACCUMULATION1 epigenetically regulates sulphur homeostasis in Arabidopsis thaliana. PLoS Genet 12: e1006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubberten HM, Klie S, Caldana C, Degenkolbe T, Willmitzer L, Hoefgen R (2012) Additional role of O-acetylserine as a sulfur status-independent regulator during plant growth. Plant J 70: 666–677 [DOI] [PubMed] [Google Scholar]
- Ivanova LA, Ronzhina DA, Ivanov LA, Stroukova LV, Peuke AD, Rennenberg H (2011) Over-expression of gsh1 in the cytosol affects the photosynthetic apparatus and improves the performance of transgenic poplars on heavy metal-contaminated soil. Plant Biol (Stuttg) 13: 649–659 [DOI] [PubMed] [Google Scholar]
- Kataoka T, Watanabe-Takahashi A, Hayashi N, Ohnishi M, Mimura T, Buchner P, Hawkesford MJ, Yamaya T, Takahashi H (2004) Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell 16: 2693–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RA, Laetsch WM (1974) Plant species intermediate for c3, c4 photosynthesis. Science 184: 1087–1089 [DOI] [PubMed] [Google Scholar]
- Kopriva S. (2011) Nitrogen and sulfur metabolism in C4 plants. In Raghavendra AS, Sage RF, eds, Advances in Photosynthesis and Respiration, Vol. 32, Springer, Dordrecht, The Netherlands, pp. 109–128 [Google Scholar]
- Kopriva S, Koprivova A (2005) Sulfate assimilation and glutathione synthesis in C4 plants. Photosynth Res 86: 363–372 [DOI] [PubMed] [Google Scholar]
- Kopriva S, Rennenberg H (2004) Control of sulphate assimilation and glutathione synthesis: interaction with N and C metabolism. J Exp Bot 55: 1831–1842 [DOI] [PubMed] [Google Scholar]
- Kopriva S, Chu C-C, Bauwe H (1996) Molecular phylogeny of Flaveria as deduced from the analysis of nucleotide sequences encoding the H-protein of the glycine cleavage system. Plant Cell Environ 19: 1028–1036 [Google Scholar]
- Kopriva S, Muheim R, Koprivova A, Trachsel N, Catalano C, Suter M, Brunold C (1999) Light regulation of assimilatory sulphate reduction in Arabidopsis thaliana. Plant J 20: 37–44 [DOI] [PubMed] [Google Scholar]
- Koprivova A, Melzer M, von Ballmoos P, Mandel T, Brunold C, Kopriva S (2001) Assimilatory sulfate reduction in C(3), C(3)-C(4), and C(4) species of Flaveria. Plant Physiol 127: 543–550 [PMC free article] [PubMed] [Google Scholar]
- Koprivova A, Mugford ST, Kopriva S (2010) Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Rep 29: 1157–1167 [DOI] [PubMed] [Google Scholar]
- Krause GH. (1988) Photoinhibition of photosynthesis: an evaluation of damaging and protective mechanisms. Physiol Plant 74: 566–574 [Google Scholar]
- Krueger S, Benstein RM, Wulfert S, Anoman AD, Flores-Tornero M, Ros R (2017) Studying the function of the phosphorylated pathway of serine biosynthesis in Arabidopsis thaliana. Methods Mol Biol 1653: 227–242 [DOI] [PubMed] [Google Scholar]
- Ku MS, Wu J, Dai Z, Scott RA, Chu C, Edwards GE (1991) Photosynthetic and photorespiratory characteristics of flaveria species. Plant Physiol 96: 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Külahoglu C, Denton AK, Sommer M, Maß J, Schliesky S, Wrobel TJ, Berckmans B, Gongora-Castillo E, Buell CR, Simon R, et al. (2014) Comparative transcriptome atlases reveal altered gene expression modules between two Cleomaceae C3 and C4 plant species. Plant Cell 26: 3243–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch WM. (1974) The C4 syndrome: a structural analysis. Annu Rev Plant Physiol 25: 27–52 [Google Scholar]
- Lappartient AG, Vidmar JJ, Leustek T, Glass AD, Touraine B (1999) Inter-organ signaling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound. Plant J 18: 89–95 [DOI] [PubMed] [Google Scholar]
- Marquez-Garcia B, Njo M, Beeckman T, Goormachtig S, Foyer CH (2014) A new role for glutathione in the regulation of root architecture linked to strigolactones. Plant Cell Environ 37: 488–498 [DOI] [PubMed] [Google Scholar]
- Marsch-Martínez N, Franken J, Gonzalez-Aguilera KL, de Folter S, Angenent G, Alvarez-Buylla ER (2013) An efficient flat-surface collar-free grafting method for Arabidopsis thaliana seedlings. Plant Methods 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurino VG, Peterhansel C (2010) Photorespiration: current status and approaches for metabolic engineering. Curr Opin Plant Biol 13: 249–256 [DOI] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG (2005) Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. Am J Bot 92: 1911–1928 [DOI] [PubMed] [Google Scholar]
- Meister A. (1974) Glutathione synthesis. In Boyer PD, ed, The Enzymes, Vol 10 Academic Press, San Diego, CA, pp 671–697 [Google Scholar]
- Mugford SG, Yoshimoto N, Reichelt M, Wirtz M, Hill L, Mugford ST, Nakazato Y, Noji M, Takahashi H, Kramell R, et al. (2009) Disruption of adenosine-5′-phosphosulfate kinase in Arabidopsis reduces levels of sulfated secondary metabolites. Plant Cell 21: 910–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford SG, Lee B-R, Koprivova A, Matthewman C, Kopriva S (2011) Control of sulfur partitioning between primary and secondary metabolism. Plant J 65: 96–105 [DOI] [PubMed] [Google Scholar]
- Nikiforova V, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R (2003) Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. Plant J 33: 633–650 [DOI] [PubMed] [Google Scholar]
- Nikiforova VJ, Gakière B, Kempa S, Adamik M, Willmitzer L, Hesse H, Hoefgen R (2004) Towards dissecting nutrient metabolism in plants: a systems biology case study on sulphur metabolism. J Exp Bot 55: 1861–1870 [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53: 1283–1304 [DOI] [PubMed] [Google Scholar]
- Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH (2011) Glutathione. Arabidopsis Book 9: e0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35: 454–484 [DOI] [PubMed] [Google Scholar]
- Paulose B, Chhikara S, Coomey J, Jung HI, Vatamaniuk O, Dhankher OP (2013) A γ-glutamyl cyclotransferase protects Arabidopsis plants from heavy metal toxicity by recycling glutamate to maintain glutathione homeostasis. Plant Cell 25: 4580–4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellny TK, Locato V, Vivancos PD, Markovic J, De Gara L, Pallardó FV, Foyer CH (2009) Pyridine nucleotide cycling and control of intracellular redox state in relation to poly (ADP-ribose) polymerase activity and nuclear localization of glutathione during exponential growth of Arabidopsis cells in culture. Mol Plant 2: 442–456 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2012) Quantification strategies in real-time polymerase chain reaction. In Filion M, ed, Quantitative Real-Time PCR in Applied Microbiology, Caister Academic Press, Poole, UK, pp 53–62 [Google Scholar]
- Pfannschmidt T. (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci 8: 33–41 [DOI] [PubMed] [Google Scholar]
- Powell AM. (1978) Systematics of Flaveria (Flaveriinae-Asteraceae). Ann Mo Bot Gard 65: 590–636 [Google Scholar]
- Reichheld JP, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y (2007) Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell 19: 1851–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman PG, Meister A (1975) Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem 250: 1422–1426 [PubMed] [Google Scholar]
- Ros R, Muñoz-Bertomeu J, Krueger S (2014) Serine in plants: biosynthesis, metabolism, and functions. Trends Plant Sci 19: 564–569 [DOI] [PubMed] [Google Scholar]
- Rouhier N, Lemaire SD, Jacquot JP (2008) The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol 59: 143–166 [DOI] [PubMed] [Google Scholar]
- Ruegsegger A, Brunold C (1993) Localization of [gamma]-glutamylcysteine synthetase and glutathione synthetase activity in maize seedlings. Plant Physiol 101: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. (2004) The evolution of C4 photosynthesis. New Phytol 161: 341–370 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63: 19–47 [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212 [DOI] [PubMed] [Google Scholar]
- Schmutz D, Brunold C (1984) Intercellular localization of assimilatory sulfate reduction in leaves of Zea mays and Triticum aestivum. Plant Physiol 74: 866–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaubelt D, Queval G, Dong Y, Diaz-Vivancos P, Makgopa ME, Howell G, De Simone A, Bai J, Hannah MA, Foyer CH (2015) Low glutathione regulates gene expression and the redox potentials of the nucleus and cytosol in Arabidopsis thaliana. Plant Cell Environ 38: 266–279 [DOI] [PubMed] [Google Scholar]
- Schuler ML, Mantegazza O, Weber AP (2016) Engineering C4 photosynthesis into C3 chassis in the synthetic biology age. Plant J 87: 51–65 [DOI] [PubMed] [Google Scholar]
- Schulze S, Mallmann J, Burscheidt J, Koczor M, Streubel M, Bauwe H, Gowik U, Westhoff P (2013) Evolution of C4 photosynthesis in the genus flaveria: establishment of a photorespiratory CO2 pump. Plant Cell 25: 2522–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack CR, Hatch MD, Goodchild DJ (1969) Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J 114: 489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery RA, Walker BJ, Weber APM, Ort DR (2018) The impacts of fluctuating light on crop performance. Plant Physiol 176: 990–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K (2000) The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J 23: 171–182 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62: 157–184 [DOI] [PubMed] [Google Scholar]
- Toujani W, Muñoz-Bertomeu J, Flores-Tornero M, Rosa-Téllez S, Anoman AD, Alseekh S, Fernie AR, Ros R (2013) Functional characterization of the plastidial 3-phosphoglycerate dehydrogenase family in Arabidopsis. Plant Physiol 163: 1164–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Notaguchi M (2017) The use of grafting to study systemic signaling in plants. Plant Cell Physiol 58: 1291–1301 [DOI] [PubMed] [Google Scholar]
- Uzilday B, Turkan I, Sekmen AH, Ozgur R, Karakaya HC (2012) Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci 182: 59–70 [DOI] [PubMed] [Google Scholar]
- Vauclare P, Kopriva S, Fell D, Suter M, Sticher L, von Ballmoos P, Krähenbühl U, den Camp RO, Brunold C (2002) Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5′-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols. Plant J 31: 729–740 [DOI] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, et al. (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos PD, Dong Y, Ziegler K, Markovic J, Pallardó FV, Pellny TK, Verrier PJ, Foyer CH (2010) Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J 64: 825–838 [DOI] [PubMed] [Google Scholar]
- Weckopp SC, Kopriva S (2015) Are changes in sulfate assimilation pathway needed for evolution of C4 photosynthesis? Front Plant Sci 5: 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Johnston IG, Covshoff S, Hibberd JM (2013) Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. eLife 2: e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 83: 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]