Aluminum tolerance in European barley is affected by retrotransposon insertion in the upstream sequence of the major aluminum tolerance gene HvAACT1 and its DNA methylation status.
Abstract
Aluminum (Al) toxicity is a major stress factor limiting crop productivity in acid soil. Although there is great genotypic variation in tolerance to Al toxicity, the underlying molecular mechanisms are poorly understood. Here, we report that, in barley (Hordeum vulgare), the fourth largest cereal crop produced in the world, both retrotransposon insertion and DNA methylation are involved in regulating differential Al tolerance. HvAACT1 is a major gene responsible for citrate secretion from the roots for external detoxification of Al. A multiretrotransposon-like (MRL) sequence insertion at least 15.3 kb in length was detected in the upstream genomic region of HvAACT1 that displayed promoter activity and significantly enhanced HvAACT1 expression, especially in the root tips of Al-tolerant accessions. Furthermore, in a number of accessions with low levels of HvAACT1 expression, this MRL insertion was present but highly methylated. Geographical analysis showed that accessions with this MRL insertion are distributed mainly in European areas with acid soils. Two wild barley accessions were found to possess this MRL insertion, but with a high degree of methylation. These results indicate that the MRL insertion and its degree of DNA methylation influence HvAACT1 expression and that demethylation of this MRL insertion, which facilitates adaptation to acid soils, occurred following barley domestication. Moreover, our results indicate that barley accessions in East Asia and Europe have developed independent but equivalent strategies to withstand Al toxicity in acid soils.
Barley (Hordeum vulgare), the fourth largest cereal crop produced in the world after wheat (Triticum aestivum), maize (Zea mays), and rice (Oryza sativa), is an important crop for human consumption, malt in the brewing and distilling industry, and animal feed (Bartlett et al., 2008). Barley is one of the most highly adapted crops and is cultivated in a wide range of climates, in areas ranging from subarctic to subtropical (Jana and Pietrzak, 1988; Leff et al., 2004). This wide geographic distribution of barley is attributed to its high tolerance of cold, drought, alkalinity, and salinity (Schulte et al., 2009). However, barley production has been limited in many acid soil areas worldwide due to its high sensitivity to aluminum (Al) toxicity (Samac and Tesfaye, 2003; Bian et al., 2013).
Al toxicity is the major factor limiting crop production on acid soil, which comprises approximately 30% to 40% of arable land in the world (Von Uexküll and Mutert, 1995). Ionic Al rapidly inhibits root elongation and the uptake of water and nutrients (Ma, 2007; Kochian et al., 2015), leading to reduced crop yield. Barley is one of the most Al-sensitive cereal crops. Most Al-sensitive barley accessions either die within a few weeks after germination on acidic soils or yield very poorly (Mugwira et al., 1976; Minella and Sorrells, 1992). Therefore, Al toxicity has limited the cultivation of barley in acid soil areas worldwide (Minella and Sorrells, 1992). However, several studies have reported that there is wide genotypic variation in Al tolerance among barley accessions (Minella and Sorrells, 1992; Ma et al., 1997b; Zhou et al., 2016).
Different from rice, whose Al tolerance is controlled by multiple genes implicated in internal and external detoxification of Al (Ma et al., 2014), Al tolerance in barley is influenced by a single major gene on chromosome 4H, namely HvAACT1 (Al-activated citrate transporter1; Furukawa et al., 2007; Wang et al., 2007). HvAACT1, belonging to the multidrug and toxic compound extrusion (MATE) family, encodes a plasma membrane-localized transporter for citrate (Furukawa et al., 2007), which functions to mediate citrate release from the roots to detoxify Al externally in the rhizosphere. The expression of HvAACT1 is not affected by Al, but its expression level is constitutively higher in Al-tolerant accessions than in Al-sensitive accessions (Furukawa et al., 2007). There also is good correlation between HvAACT1 expression and citrate secretion as well as between HvAACT1 expression and Al tolerance (Furukawa et al., 2007; Fujii et al., 2012), indicating that HvAACT1 is a major gene associated with Al tolerance in barley.
The differential expression of HvAACT1 in different barley accessions has been linked to the insertion of a 1,023-bp CACTA-like transposon in the upstream genomic sequence of HvAACT1 in some Al-tolerant accessions in East Asia (Fujii et al., 2012). This 1-kb insertion promotes an enhanced expression level of HvAACT1 and also spatially alters the HvAACT1 expression pattern from the mature root region to the root tips, where it functions to release citrate to the xylem for iron (Fe) translocation from the roots to the shoots (Fujii et al., 2012). In addition, this 1-kb insertion was found only in cultivated barley accessions distributed in acid soil areas of Japan, Korea, and China, indicating that this insertion was gained during adaptation to acidic soils. Here, we report a novel mechanism regulating HvAACT1 expression in European barley accessions, which is different from that in East Asian accessions. A multiretrotransposon-like (MRL) sequence insertion of at least 15.3 kb was detected in the upstream genomic sequence of HvAACT1 in several European accessions. Furthermore, DNA demethylation of this MRL insertion was found to be involved in increasing the expression of HvAACT1 uniquely in Al-tolerant accessions. Geographic analysis of cultivated and wild barley accessions showed that the MRL insertion and its demethylation likely occurred after barley domestication.
RESULTS
Detection of a Sequence Insertion Upstream of HvAACT1
A previous study showed that a 1-kb insertion in the upstream genomic sequence of HvAACT1 functions as a promoter, which enhances the expression of HvAACT1 in the root tips of Al-tolerant barley accessions in East Asia (Fujii et al., 2012). To examine whether some Al-tolerant cultivars developed under acid soil conditions have the same insertion, we examined the four cultivars FM404 (Brazil), WB229 (Australia), Brindabella (Australia), and Dayton (United States; Read and Oram, 1995; Raman et al., 2002). Compared with the Al-sensitive accessions Morex (United States) and Golden Promise (United Kingdom), each of these four cultivars showed higher HvAACT1 expression and citrate secretion levels, similar to those in the Al-tolerant cultivar Murasakimochi (Japan) that possesses a 1-kb insertion in the upstream genomic sequence of HvAACT1 (Supplemental Fig. S1), indicating that the Al tolerance in these cultivars also relies on root citrate secretion. Alignment of HvAACT1 open reading frames (ORFs) showed that there are two single-nucleotide polymorphisms (SNPs) among different accessions (Supplemental Table S1), resulting in one amino acid change from Leu to Val at amino acid position 172. However, this substitution is not consistent with citrate secretion levels among Al-sensitive and -tolerant accessions (Supplemental Fig. S1; Supplemental Table S1). On the other hand, different from Murasakimochi and Dayton, the 1-kb insertion in the upstream genomic sequence of HvAACT1 was not detected in the three Al-tolerant cultivars FM404, WB229, and Brindabella (Supplemental Fig. S2). These results led us to hypothesize that a different mechanism regulating HvAACT1 expression is present in these Al-tolerant cultivars.
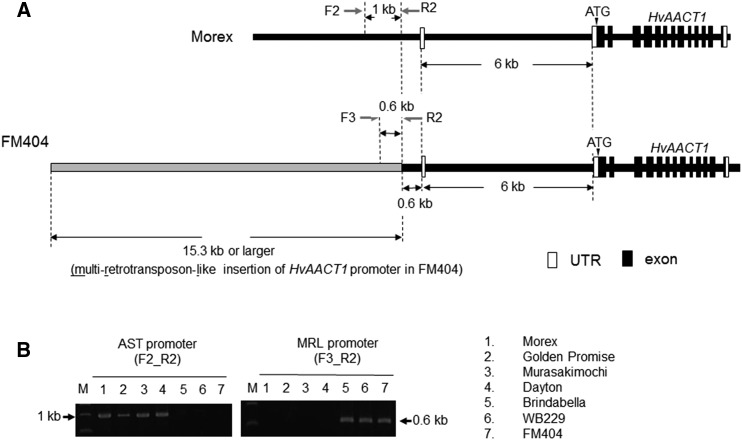
Therefore, we compared the upstream genomic sequence of HvAACT1 in FM404 and Morex. Using inverse PCR, we were able to detect an insertion of at least 15.3 kb in this genomic region in FM404 (Fig. 1A). This insertion did not show any similarity with the 1-kb insertion in the upstream genomic sequence of HvAACT1 reported before (Fujii et al., 2012). We failed to sequence farther upstream of this insertion by inverse PCR, and no information was available concerning the upstream sequences of this insertion in the recently released barley genome sequence (Mascher et al., 2017). A BLAST search showed that this insertion was an MRL sequence with long terminal repeats (LTRs; Supplemental Fig. S3). The LTR of Barley Retro Element belongs to copia, and BAGY belongs to the gypsy LTR retrotransposon group (Manninen and Schulman, 1993). The copia and gypsy genomic sequences are similar, although they differ in the order of component genes (Xiong and Eickbush, 1990). However, only the repeat region and no other constituent genes were detected in the MRL insertion (Supplemental Fig. S3). Furthermore, in the LTR regions, there were no regulatory motifs. The LTR retrotransposon includes promoter motifs for RNA synthesis and terminators (Kumar and Bennetzen, 1999). The LTR retrotransposon promoter can transcribe the downstream sequence of an inserted element in some cases (Suoniemi et al., 1996; Kumar and Bennetzen, 1999). In addition, since there was no target site duplication in these retrotransposons, it seems that this insertion may be a truncated element or nested by other LTR retrotransposons. By using specific primers for the MRL insertion, the same size band also was detected in Brindabella and WB229 (Fig. 1B), indicating that these three Al-tolerant cultivars have a similar insertion in the upstream genomic sequence of HvAACT1.
Figure 1.
Comparison of HvAACT1 genomic sequences in Al-sensitive (Morex) and Al-tolerant (FM404) barley cultivars. A, Schematic representation of the HvAACT1 genomic sequence in the Al-sensitive cultivar Morex and the Al-tolerant cultivar FM404. An insertion of at least 15.3 kb containing an MRL sequence (gray line) is located at 6.6 kb upstream of ATG in FM404. Black vertical boxes and black lines indicate exons and introns, respectively. White boxes indicate untranslated regions (UTRs). Gray arrows indicate the locations of the PCR primers used for different promoter amplification. B, Genome PCR of the HvAACT1 promoter in seven cultivars. The HvAACT1 AST (aluminum sensitive type) promoter and MRL insertion promoter were identified in different cultivars by PCR using the primers shown in A. Lanes are as follows: 1, Morex; 2, Golden Promise; 3, Murasakimochi; 4, Dayton; 5, Brindabella; 6, WB229; 7, FM404; M, Marker.
We also compared the downstream genomic sequence of HvAACT1 (up to 2.2 kb) of Al-tolerant and -sensitive accessions. Although we detected some SNPs, in contrast to the upstream sequence, there was no consistent trend between SNPs and Al tolerance.
Detection of the Transcriptional Start Site
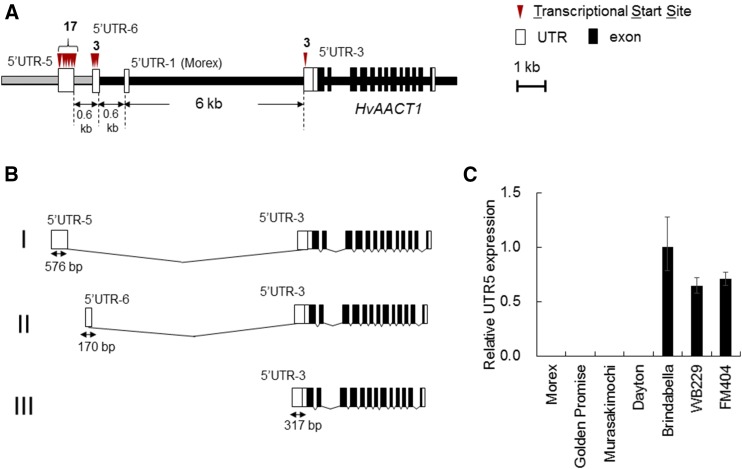
To identify the HvAACT1 promoter in FM404, the transcriptional start site (TSS) of HvAACT1 was investigated by 5′-RACE. As a result, multiple TSSs were detected at the MRL insertion region, specifically 6.6 and 7.2 kb upstream from the translational start site of HvAACT1 in FM404 (Fig. 2A), yielding three different types of transcript variants (Fig. 2B) with type I as a major form. By using specific primers (5′UTR-5) for this 5′-UTR of HvAACT1 in FM404, similar expression was demonstrated in FM404, Brindabella, and WB229 (Fig. 2C).
Figure 2.
Detection of the HvAACT1 TSS in Al-tolerant barley cultivar FM404. A, Schematic representation of the HvAACT1 5′-UTR in FM404. Red triangles indicate multiple HvAACT1 TSSs, and the number indicates the TSSs detected by cloning. B, Schematic representation of three HvAACT1 mRNA splicing variants in FM404. C, Expression of HvAACT1 5′UTR-5 in root tips (0–2 cm) of seven cultivars, normalized to that in Brindabella. Reverse transcription-quantitative PCR (RT-qPCR) was performed using primers for 5′UTR-5. Data are means ± sd (n = 3).
Promoter Activity Assay of the MRL Insertion
To investigate whether the MRL insertion had promoter activity, we performed a transient promoter assay by introducing GFP reporter plasmids into onion (Allium cepa) epidermal cells. GFP was placed under the control of either Putative promoter 1 (1 kb from just after the common TSS upstream region of Morex) or Putative promoter 2 (2.3 kb from 5′UTR-5 of the MRL insertion region of FM404; Supplemental Fig. S4A). Similar to cells expressing 35S-GFP (positive control), fluorescence signal was observed in cells expressing both Putative promoter 1 and 2 GFP reporter plasmids (Supplemental Fig. S4B). These results indicate that the MRL insertion functions as a promoter.
Spatial and Tissue-Specific Expression of HvAACT1 in FM404
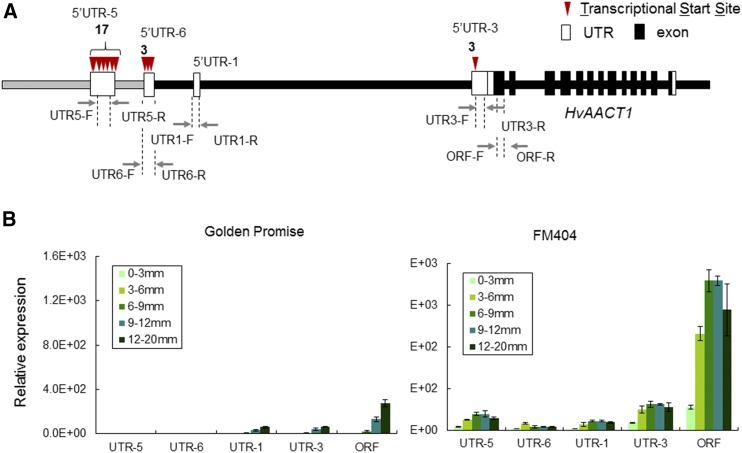
To examine the role of the MRL insertion in regulating HvAACT1 expression, the HvAACT1 expression pattern in root tissue of FM404 and Golden Promise was analyzed using primers targeting different spatial positions in the HvAACT1 upstream genomic sequence (5′-UTRs) as well as in its ORF (Fig. 3A). Similar to Morex, Golden Promise also is an Al-sensitive cultivar and does not have either the 1- or 15.3-kb insertion in the HvAACT1 upstream genomic sequence. Furthermore, the ORF sequence of HvAACT1 also was identical between Golden Promise and Morex (Supplemental Table S1). Absolute RT-qPCR analysis showed that the expression of all 5′-UTRs was much higher in FM404 than in Golden Promise in all root tissues (Fig. 3B). In particular, no transcript was detected in the root tips (0–9 mm from the root tip) of Golden Promise.
Figure 3.
Expression pattern of different spatial positions in the HvAACT1 genomic sequence in Al-sensitive (Golden Promise) and Al-tolerant (FM404) barley cultivars. A, Schematic representation of the HvAACT1 genomic sequence indicating primer positioning for RT-qPCR analysis. Black vertical boxes and black lines indicate exons and introns, respectively. White boxes indicate UTRs. The gray line indicates the MRL insertion. Gray arrows indicate the positions of PCR primers for different 5′-UTR and ORF targets. B, Expression pattern of different spatial positions in the HvAACT1 ORF and 5′-UTR. Expression levels were determined by RT-qPCR in different root segments. Data are means ± sd (n = 3).
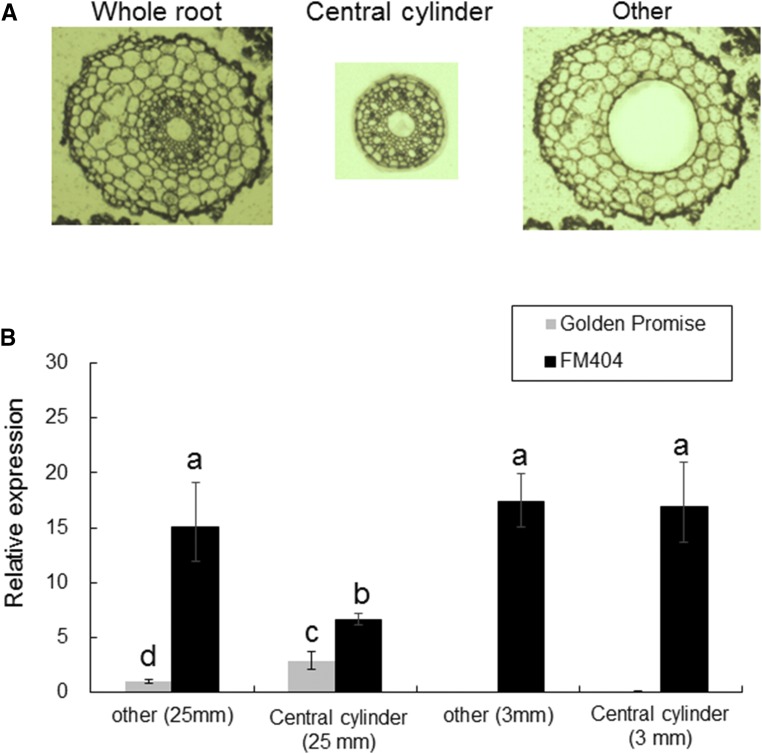
To investigate the tissue-specific expression of HvAACT1 in the roots, root tissue segments at around 3 and 25 mm from the root tip were divided into the central cylinder and other tissues by laser microdissection (Fig. 4B). At the 3-mm position, HvAACT1 expression in both the central cylinder and other tissues was minimal in Golden Promise but was considerably higher in FM404 (Fig. 4B). At the 25-mm position, the HvAACT1 expression level in other tissues was much higher in FM404 than in Golden Promise (Fig. 4B), but there was little difference in the HvAACT1 expression level in central cylinder tissue at this root position between the two cultivars. These results further indicate that high expression of HvAACT1 in the root tips of FM404 is caused primarily by the MRL insertion.
Figure 4.
Tissue-specific expression of HvAACT1 in Al-sensitive (Golden Promise) and Al-tolerant (FM404) barley cultivars. A, Representative images of different root tissues as separated by laser microdissection. The root segments at 3 and 25 mm were separated into two parts: central cylinder and other. B, Tissue specificity of HvAACT1 expression. Expression levels were determined by RT-qPCR and normalized to that in other tissue of the 25-mm root segment from Golden Promise. Data are means ± sd (n = 3). Statistical comparison was performed by Tukey’s multiple comparison tests using other tissue of the 25-mm root segment from Golden Promise as a control. Different letters indicate that the means are significantly different (P < 0.05).
Geographical Distribution of Barley Accessions with the MRL Insertion
To investigate the geographical distribution of barley accessions with the MRL insertion, we performed PCR using specific primers for the MRL insertion in 289 wild barley (H. vulgare ssp. spontaneum) and 274 cultivated barley (H. vulgare ssp. vulgare) collections from different regions of the world. Among the wild barley accessions, the MRL insertion was detected in the two accessions OUH601 from Southwest Asia and BCSP127 (ICWB181646) from Fertile Crescent/East Mediterranean (Table 1). Among the cultivated barley accessions, 26 accessions possessed the MRL insertion (Supplemental Table S2). Furthermore, these accessions were distributed in European areas only (Table 1).
Table 1. Geographic distribution of barley accessions with novel MRL insertions.
–, Absence of the MRL insertion; +, presence of the MRL insertion; Low, low expression of HvAACT1; High, high expression of HvAACT1.
| Taxon | Geographical Group | MRL Insertion | Total | ||
|---|---|---|---|---|---|
| – | + | ||||
| HvAACT1 Expression | |||||
| Low | High | ||||
| H. spontaneum | Mediterranean | 8 | |||
| Fertile Crescent, west | 50 | ||||
| Fertile Crescent, central | 117 | ||||
| Fertile Crescent, east | 25 | 1 | |||
| Trans Caucasus | 21 | ||||
| Southwest Asia | 61 | 1 | |||
| Unknown | 5 | ||||
| Total | 287 | 2 | 289 | ||
| H. vulgare | North America | 4 | 1 | ||
| North Europe | 19 | 15 | 8 | ||
| South Europe | 15 | 2 | |||
| North Africa | 11 | ||||
| Ethiopia | 37 | ||||
| Turkey | 18 | ||||
| Near East | 25 | ||||
| Trans Caucasus | 9 | ||||
| Southwest Asia | 10 | ||||
| Himalaya | 23 | ||||
| China | 22 | ||||
| Korea | 30 | ||||
| Japan | 25 | ||||
| Total | 248 | 17 | 9 | 274 | |
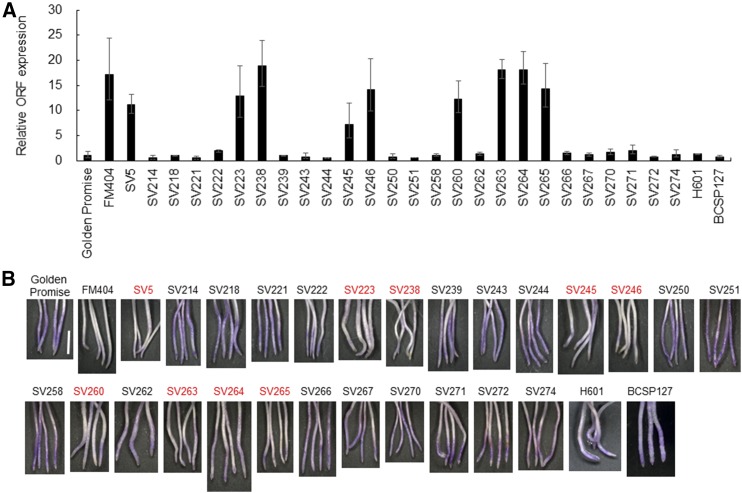
To link the MRL insertion to HvAACT1 expression level and Al tolerance in these accessions, we determined their HvAACT1 expression level and Al tolerance, the latter of which was evaluated by Eriochrome cyanine staining. Surprisingly, not all accessions with the MRL insertion showed high-level expression of HvAACT1 (Fig. 5A). Among 26 cultivated accessions and two wild barley accessions with the MRL insertion, only nine cultivated accessions showed high-level HvAACT1 expression comparable to that in FM404 (Fig. 5A). Eriochrome cyanine staining results were consistent with HvAACT1 expression (Fig. 5B), in that accessions with high-level HvAACT1 expression showed high Al tolerance (less staining). These results suggest that, in addition to the MRL insertion, other mechanisms are involved in regulating HvAACT1 expression.
Figure 5.
HvAACT1 expression levels and Al tolerance in European barley accessions with the MRL insertion. A, HvAACT1 expression level in European barley accessions with the MRL insertion. RNA was extracted from root tips (0–20 mm) of 4-d-old seedlings. Expression levels were determined by RT-qPCR and normalized to that in Golden Promise. Data are means ± sd (n = 3). B, Al tolerance test. Al tolerance was evaluated by staining in 0.1% (w/v) Eriochrome cyanine R after 4-d-old seedlings were exposed to 5 µm AlCl3 for 2 d. Bar = 0.5 cm.
Methylation Analysis of the MRL Insertion Region
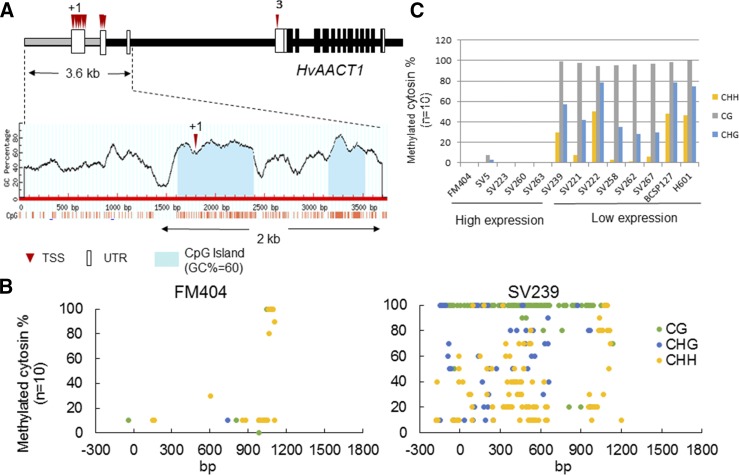
To investigate the novel mechanism regulating HvAACT1 expression, we compared the DNA methylation level of its promoter region (−300 to 1,800 kb) in FM404 and SV239, which contain the MRL insertion but show high- and low-level HvAACT1 expression, respectively. The GC-rich region was predicted to be located upstream of the 5′UTR-5 region in FM404 (Fig. 6A), specifically 2 kb from the common promoter. Comparison of DNA methylation in these regions indicated that 97.8% of whole cytosine was unmethylated in FM404 (Fig. 6B), whereas SV239 showed a much higher rate of methylation (Fig. 6, B and C). To further confirm the correlation between DNA methylation and HvAACT1 expression level, we randomly selected nine cultivated barley accessions and two wild barley accessions (OUH601 and ICWB181646 [BCSP127]) for DNA methylation analysis. We observed that all accessions with low-level HvAACT1 expression showed a much higher degree of DNA methylation, including CG, CHG, and CHH methylation, compared with accessions with high-level HvAACT1 expression (Fig. 6, B and C).
Figure 6.
Methylation of the MRL insertion in European barley accessions with high and low HvAACT1 expression. A, Schematic representation of CpG islands in the HvAACT1 promoter in FM404. The blue areas indicate 60% CpG islands. The CpG island prediction software (MethPrimer; http://www.urogene.org/cgibin/methprimer/methprimer.cgi) was used. Black vertical boxes and black lines indicate exons and introns, respectively. B, Extent of DNA methylation in the HvAACT1 promoter of Al-tolerant cultivar FM404 and Al-sensitive cultivar SV239. DNA methylation was determined by bisulfite sequencing analysis. Each bisulfite PCR product was cloned into the TA vector, and the sequence of 10 colonies was determined. C, Ratio of DNA methylation determined using the bisulfite primer 2 region of the HvAACT1 promoter in 13 different barley accessions with either high or low HvAACT1 expression.
Phylogenetic Analysis of Barley Accessions in Relation to MRL Insertion and Methylation
By using the information of 1,189 SNPs located in the transcript regions distributed over all barley chromosomes, we performed phylogenetic analysis of 274 barley accessions. Accessions with the MRL insertion from European regions formed a different group from those with the 1-kb insertion from Asian areas (Supplemental Fig. S5). Furthermore, accessions with the MRL insertion also were divided into different groups according to their HvAACT1 expression levels (Supplemental Fig. S6). These analyses suggest that the MRL insertion and the 1-kb insertion in the HvAACT1 upstream genomic sequence were independent events and that demethylation occurred following sequence insertion.
Transformation of the MRL Insertion Al-Sensitive Barley
To further confirm the role of the MRL insertion in HvAACT1 expression level and subsequent Al tolerance, we used the MRL insertion (2.9 kb) fused with the HvAACT1 ORF from Morex to transform the Al-sensitive cultivar Golden Promise (Supplemental Fig. S7A). Five independent transgenic lines carrying the MRL insertion were obtained (Supplemental Fig. S7, A and B). Analysis of these lines showed that HvAACT1 expression was not enhanced in plants of the T2 generation (Supplemental Fig. S7C). We then investigated the DNA methylation level of the introduced MRL insertion and found that a high degree of methylation was present in the transgenic barley lines (Supplemental Fig. S8, A and B). These results suggest that low-level HvAACT1 expression in the transgenic barley was the result of DNA methylation in the introduced promoter.
DISCUSSION
A Novel Retrotransposon-Like Insertion Is Involved in Enhancing HvAACT1 Expression in Al-Tolerant European Barley
Although Al is the most abundant metal in the Earth’s crust, it is highly toxic to plants when solubilized to ionic form (Al3+) under acid conditions. However, some plant species and cultivars within a species have evolved mechanisms to detoxify Al both externally and internally (Ma, 2007; Kochian et al., 2015). One of the most common Al detoxification mechanisms in both dicots and monocots is the secretion of organic acid anions from the roots, such as citrate, oxalate, and malate (Delhaize et al., 1993; Ma et al., 1997a, 2001; Ma, 2000; Ryan et al., 2001). These organic acid anions are able to chelate Al in the rhizosphere, thus detoxifying Al externally. Genes encoding transporters for the Al-induced secretion of malate (ALMT) and citrate (MATE/AACT) have been identified in a number of plant species (Sasaki et al., 2004; Furukawa et al., 2007; Magalhaes et al., 2007). Furthermore, genotypic differences in the Al tolerance of some species have been attributed to the differential expression of these genes (Delhaize et al., 2012; Ma et al., 2014).
Several studies have revealed mechanisms underlying genotypic differences in gene expression, including increased tandem repeated elements, increased copy number, alteration of cis-acting elements, and transposon insertion (Delhaize et al., 2012; Ma et al., 2014). For example, the high-level expression of ALMT1 in Al-tolerant wheat accessions is associated with tandem repeated elements in its promoter region (Sasaki et al., 2006; Ryan et al., 2010), whereas in sorghum (Sorghum bicolor), the repeat number of MITEs (tourist-like miniature inverted repeat transposable elements) in the upstream genomic sequence of SbMATE is broadly associated with its expression level (Magalhaes et al., 2007). The high-level expression of ScALMT1 and ZmMATE1 in rye (Secale cereale) and maize, respectively, is attributed to their increased copy number in the genome (Collins et al., 2008; Maron et al., 2013). In an accession of Yorkshire fog (Holcus lanatus) that is well adapted to acid soil, the high-level expression of HlALMT1 is facilitated by an increased number of cis-acting elements associated with ART1, a transcription factor for Al tolerance (Chen et al., 2013).
On the other hand, transposon insertion was found to alter the expression level of MATE/AACT genes in barley, some wheat cultivars, and rice. A 1-kb CACTA-like transposon insertion in barley, an 11.1-kb transposon-like element (a Sukkula-like transposon) in wheat, and a 1.2-kb retrotransposon in rice in upstream genomic regions enhanced the expression of HvAACT1, TaMATE1B, and OsFRDL4, respectively (Fujii et al., 2012; Tovkach et al., 2013; Yokosho et al., 2016). In this study, we identified a novel MRL sequence insertion (Fig. 1A). For simplicity, this sequence containing multiple retrotransposons is called an insertion in this study. However, due to our inability to sequence the entire upstream genomic region of HvAACT1, it is unclear whether the presence of this MRL insertion in certain accessions is the result of one or more insertions or whether the absence of this MRL insertion in other accessions is the result of deletion of this fragment. This MRL insertion does not resemble those identified so far as described above. The MRL insertion functions as a promoter, which greatly enhances the expression of HvAACT1 (Fig. 3B). In particular, the expression of HvAACT1 in the root tips (0–9 mm) was greatly enhanced (Fig. 3B), indicating that the MRL insertion also alters the tissue specificity of HvAACT1 expression. The root tip is particularly affected by Al toxicity (Ryan et al., 1993); therefore, enhanced expression of HvAACT1 in the root tip, resulting in more citrate secretion to the rhizosphere for Al detoxification, would confer greater Al tolerance.
Tissue specificity analysis of HvAACT1 expression showed that expression levels were comparable between Morex and FM404 in central cylinder tissue of the mature root zone compared with other tissues (Fig. 4B). HvAACT1 expression in the basal root region is responsible for the release of citrate to the xylem for root-to-shoot Fe translocation (Fujii et al., 2012). This means that the role of HvAACT1 in Fe translocation is not altered by the MRL insertion.
Barley in East Asia and Europe Developed Independent But Equivalent Strategies for Adaptation to Acid Soil
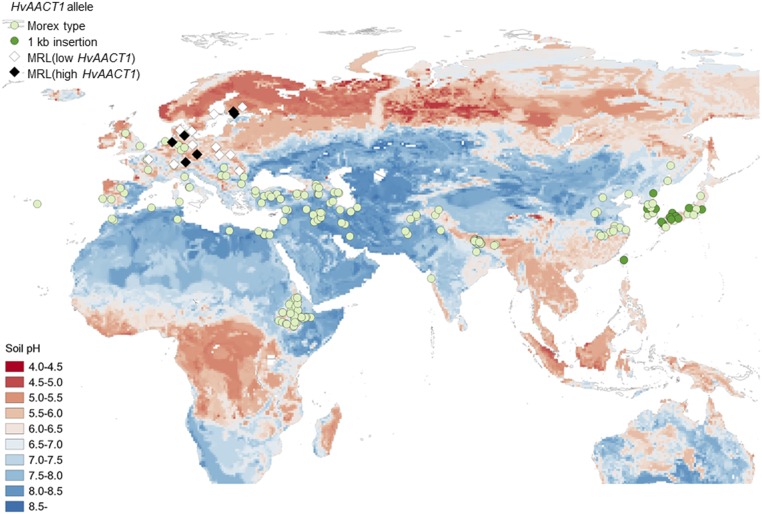
A previous study detected the 1-kb insertion in the HvAACT1 upstream genomic sequence uniquely in a number of barley accessions cultivated in acid soil areas, including those in Japan, China, and Korea, but not in wild barley (Fujii et al., 2012). By contrast, the distribution of the MRL insertion accessions identified in this study is confined to Europe (Fig. 7). Furthermore, the MRL insertion also was detected in two wild barley accessions (Table 1). Barley was domesticated from its wild ancestor H. vulgare ssp. spontaneum in the Near East approximately 10,000 years ago (Fujii et al., 2012). Soils in this region are predominantly alkaline, where there is no threat of Al-toxicity stress. Barley cultivation expanded toward the east 8,000 years ago and independently toward Europe approximately 6,000 years ago (von Bothmer et al., 2003), where the crop started to encounter Al-toxicity stress on acid soils. Our results indicate that independent adaptive events occurred in East Asian and European barley to cope with Al toxicity in acid soil, and phylogenetic analysis supports this hypothesis (Supplemental Figs. S5 and S6). In East Asia, the Al tolerance of cultivated barley has been acquired through a 1-kb insertion in the upstream HvAACT1 genomic sequence, whereas, in Europe, Al tolerance has been achieved by the MRL insertion and its demethylation, as discussed below.
Figure 7.
Geographic distribution of barley accessions with novel MRL insertions. Genotyping of the MRL insertion was performed by PCR using primer pair 3 (Supplemental Table S3) in 274 cultivated barley accessions. Accessions with the MRL insertion and either high or low HvAACT1 expression are indicated with black and white diamonds, respectively. Accessions with or without the 1-kb insertion, the latter being Morex type, are indicated with dark-green and light-green circles, respectively.
The presence of the MRL insertion in two wild barley accessions from Iran and Afghanistan suggests that some European barley accessions with the MRL insertion are closely related to these wild barley accessions. However, there is no evidence showing that these wild barley accessions are direct ancestors of domesticated barley. There is a possibility that a natural hybridization between wild barley and domesticated barley occurred, resulting in the transfer of the MRL insertion to the domesticated barley accessions.
In contrast to the homologs of HvAACT1 in other cereal crops such as OsFRDL4 in rice, the expression of HvAACT1 is not induced by Al. This suggests that the original role of HvAACT1 was not involved in Al tolerance but rather in the root-to-shoot translocation of Fe by releasing citrate to the xylem vessel in the basal region of the roots (Fujii et al., 2012). It seems that this gene for Al tolerance was reacquired in the Al-tolerant barley accessions through transposon insertion during postdomestication spread into acid soils.
Demethylation Probably Is Required for Adaptation to Acid Soils in European Barley
Gene expression is regulated by chromatin condensation, DNA methylation, transcriptional initiation, alternative splicing of RNA, mRNA stability, and other mechanisms (Wray et al., 2003). In addition to the MRL insertion, we found that DNA methylation also is involved in the regulation of HvAACT1 expression. In contrast to barley in East Asia (Fujii et al., 2012), in which the 1-kb insertion in the HvAACT1 upstream genomic sequence is able to enhance HvAACT1 expression, the MRL insertion identified in this study did not enhance the expression of HvAACT1 and Al tolerance when introduced into a number of Al-sensitive European barley accessions (Fig. 5). This was due to the extent of DNA methylation of the insertion (Fig. 6), as a higher degree of methylation was found in those barley accessions with the MRL insertion but low expression of HvAACT1 (Fig. 6; Supplemental Table S2). This means that demethylation is required to enhance HvAACT1 expression in these accessions.
Among three Al-tolerant cultivars developed under acid soil conditions, WB229 in Australia is derived from O’Connor/Kaniere, whereas Brindabella in Australia is derived from Weeah/CI71 (Furukawa et al., 2007)/HCB27/3Jadar II/4/Cantala (Park et al., 2009). The immediate parent of FM404 in Brazil was derived from pedigrees of Kenia and CIho 959 (Minella and Sorrells, 2002). According to pedigree using the U.S. National Plant Germplasm System (http://www.ars-grin.gov/npgs/index.html), WB229, Brindabella, and FM404 were developed originally using Binder (SV265) from Denmark, which has the MRL insertion and higher HvAACT1 expression (Supplemental Table S2; Supplemental Figs. S9–S11). Therefore, it seems that this trait of Al tolerance was selected in the process of breeding.
In conclusion, European barley has developed a novel strategy to cope with Al toxicity, namely the insertion of retrotransposon-like sequences in the HvAACT1 upstream genomic region and demethylation, which enhance HvAACT1 expression in the root tips and, subsequently, Al tolerance. These processes likely contributed to the postdomestication spread of European barley into acid soil areas.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Barley (Hordeum vulgare) seeds were soaked in deionized water for 2 h, and then the moist seeds on the paper filter in a petri dish were geminated overnight at 20°C in darkness. The germinated seeds were transferred to nets floating on a 0.5 mm CaCl2 solution (pH 5.6, aerated) in a 1.5-L plastic container. After growth at 20°C for 3 to 4 d, the seedlings were transferred to 3.5-L plastic pots containing one-fifth-strength Hoagland solution as described previously (Wu et al., 2016).
Genomic Sequence Analysis
Inverse PCR was performed to identify unknown genome sequence of HvAACT1 in FM404. Genomic DNA was extracted from the leaves. The genomic DNA was digested by restriction enzymes (BglI, BglII, DraI, NcoI, NheI, SfiI, XbaI, and XhoI) and then ligated to itself. The circular DNA was amplified with KOD FX Neo (Toyobo) using a step-down cycle by gene-specific primers for PCR. The primers were designed using known sequence at restriction enzyme sites, and then the PCR product was sequenced using the BigDye Terminator version 3.1 cycle sequencing kit on an ABI PRISM 310 genetic analyzer.
To examine the presence of insertions in different barley accessions, genome PCR was performed using Quick taq HS DyeMix (Toyobo). A fragment of a 1-kb insertion was amplified using primer pair 1 (all primers are shown in Supplemental Table S3). The PCR conditions were as follows: 94°C for 2 min, followed by 30 cycles at 98°C for 10 s and the annealing and elongation temperature of 68°C for 2 min. The MRL insertion was amplified using primer pair 3. The Morex-type promoter sequence was amplified using primer pair 2. The PCR conditions were as follows: 94°C for 2 min, followed by 30 cycles at 98°C for 10 s and the annealing temperature of 68°C for 2 min. The PCR products were separated by electrophoresis on a 1% (w/v) agarose gel using 0.5× Tris-borate/EDTA buffer stained with ethidium bromide.
Identification of the TSS
Total RNA was extracted from the roots (0–20 mm from the tip) of 4-d-old seedlings using an RNeasy Plant Mini Kit (Qiagen). To determine the TSS of HvAACT1 in FM404, 5′-RACE was performed with a GeneRacer kit (Thermo Fisher Scientific) using 5 μg of total RNA. The RNA was treated by CIP to remove the 5′ phosphates. The dephosphorylated RNA was removed from the 5′ cap structure by tobacco (Nicotiana tabacum) acid pyrophosphatase. Treated RNA was ligated to the GeneRacer RNA oligo adapter 5′-CGACUGGAGCACGAGGACACUGACAUGGACUGAAGGAGUAGAAA-3′. The ligated mRNA with RNA oligo adapter was used to synthesize first-strand cDNA using SuperScript III RT and random primers in a reverse transcription reaction. The 5′ ends of HvAACT1 cDNA were amplified with primer pairs 4 and 5. The forward primer was conformed to the GeneRacer kit. PCR products were amplified by TaKaRa Ex taq DNA polymerase. The PCR conditions were as follows: 94°C for 2 min, followed by 30 cycles at 96°C for 20 s, the annealing temperature of 60°C for 30 s, and the elongation temperature of 72°C for 1 min. PCR products were gel purified with the QIAquick Gel Extraction Kit (Qiagen) and cloned into pGEM-T vector (Promega) for sequencing. Cloned plasmid was sequenced by the BigDye Terminator version 3.1 cycle sequencing kit and ABI PRISM 310 genetic analyzer (Thermo Fisher Scientific).
RT-qPCR Analysis
The tissue-dependent expression of HvAACT1 ORF and 5′-UTR in different root segments (Golden Promise and FM404) was determined by absolute RT-qPCR using the standard curve method. The standard curve was generated by using PCR-cloned plasmid from 5′-RACE of FM404 and dilution of the plasmid (0.1 ng to 1 fg) as a standard template for calculating gene copy number. Different root segments (0–3, 3–6, 6–9, 9–12, and 12–20 mm) were excised with a razor from 4-d-old seedlings and subjected to RNA extraction as described above. The cDNA was synthesized from the extracted RNA with the SuperScript III First-Strand Synthesis System for RT-qPCR (Thermo Fisher Scientific). The PCR mixture contained 1 μL of cDNA template, 5 μL of SsoFast EvaGreen Supermix (Bio-Rad), with 0.25 μm of the primer pairs 6 and 8 to 11. Reactions were run on the CFX Connect Real-Time PCR Detection System (Bio-Rad). The PCR conditions were as follows: 98°C for 30 s, followed by 40 cycles at 98°C for 5 s and the annealing temperature of 60°C for 5 s.
To compare HvAACT1 expression in different barley accessions, total RNA was extracted from 0- to 20-mm root segments of 4-d-old seedlings as described above. The cDNA was synthesized from the extracted RNA with the SuperScript II First-Strand Synthesis System for RT-qPCR (Thermo Fisher Scientific) or ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo). The PCR mixture contained 1 μL of cDNA template, 5 μL of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), with 0.25 μm of the primer pairs 6 and 7. Reactions were run on the CFX384 Touch (Bio-Rad). The PCR conditions were as follows: 95°C for 30 s, followed by 40 cycles at 98°C for 10 s and the annealing temperature of 60°C for 15 s.
To determine HvAACT1 expression in transgenic barley, RNA were extracted and synthesized to cDNA in the same way as above using KOD SYBR qPCR Mix (Toyobo). Reactions were run on the CFX Connect Real-Time PCR Detection System (Bio-Rad). The PCR conditions were as follows: 94°C for 2 min, followed by 40 cycles at 98°C for 10 s, the annealing temperature of 58°C for 10 s, and the elongation temperature of 68°C for 30 s.
Laser Microdissection Tissue Preparation
Barley roots (Golden Promise and FM404) were dissected at 0.5 to 5.5 mm and 22 to 27 mm from the root tips. The root segments were immersed immediately in the fixation solution (ethanol:acetic acid, 3:2) in a glass vial, followed by gentle triplicate centrifugation at 500g, 4°C, for 2 min with fresh fixation solution according to Takahashi et al. (2010). The samples were kept in the fixatives overnight at 4°C. The root segments at 3 and 25 mm were separated into central cylinder and other tissue by laser microdissection. Total RNA was extracted as described above. The cDNA was synthesized from extracted RNA with the SuperScript III First-Strand Synthesis System for RT-PCR (Thermo Fisher Scientific). The cDNA was used for the RT-qPCR analysis of gene expression with KOD SYBR qPCR Mix (Toyobo). The PCR mixture contained 1 μL of cDNA template, 5 μL of KOD SYBR qPCR Mix (Toyobo), with 0.25 μm of the primer pairs 6 and 7. Reactions were run on the CFX Connect Real-Time PCR Detection System (Bio-Rad). The PCR conditions were as follows: 94°C for 2 min, followed by 40 cycles at 98°C for 10 s, the annealing temperature of 58°C for 10 s, and the elongation temperature of 68°C for 30 s.
Transient Promoter Activity Assay
To examine the promoter activity of the MRL insertion, two putative promoters were placed upstream of GFP. Putative promoter 1 amplified from Morex as a control was located at 1 kb from just after the common TSS (Fujii et al., 2012), whereas Putative promoter 2 from FM404 was located at 2.3 kb from just after 5′UTR-5 (Supplemental Fig. S4A), which was amplified using primer pair 12 using KOD-FX Neo (Toyobo). The fragment was introduced into the pGEM-T (Easy) Vector System (Promega). This plasmid was digested by SphI and SalI, and the insert was gel purified with the QIAquick Gel Extraction Kit (Qiagen). The fragment was then introduced into the GFP-NOS terminator plasmid that had the native promoter deleted. The plasmid containing the 35S promoter upstream of GFP was used as a positive control. These constructs were introduced into onion (Allium cepa) epidermal cells by the Helios Gene Gun system (Bio-Rad). After overnight incubation, GFP fluorescence was observed with a confocal laser scanning microscope (LSM700; Carl Zeiss).
Bisulfite Sequencing
Genomic DNA from FM404, SV5, SV221, SV222, SV223, SV239, SV258, SV260, SV262, SV263, SV267, and two wild barley (OUH601 and ICWB181646 [BCSP127]) accessions were treated with 10 μg mL−1 RNaseA (Sigma) at 37°C for 1 h to remove RNA, followed by purification by phenol/chloroform and ethanol precipitation. The genomic DNA (2 µg) was then treated with EpiTect bisulfite (Qiagen), followed by incubation at 95°C for 5 min, 60°C for 20 min, 95°C for 5 min, 60°C for 85 min, 95°C for 5 min, 60°C for 125 min, 95°C for 5 min, 80°C for 120 min, and 20°C overnight. The DNA was column purified according to the manufacturer’s protocol and amplified by PCR with Quick taq HS DyeMix (Toyobo) using bisulfite primer pairs 13 to 16 for FM404 and SV239 and primer pair 14 for other accessions (Supplemental Table S3). The PCR conditions were as follows: 94°C for 20 s, 51°C for 30 s, and 68°C for 1 min, 44 cycles. The PCR products were cloned by TA cloning, and 10 independent colonies were sequenced as described above.
Generation of Transgenic Barley
To isolate the promoter region from FM404, three fragments were amplified by PCR with KOD-FX Neo (Toyobo) using cloning primer pairs 17 to 19. The pALP-1 and pALP-2 PCR products were cloned into pCR2 vector by the TOPO XL PCR Kit (Thermo Fisher Scientific). These plasmids and the pALP-3 PCR fragment were treated each with restriction enzymes ScaI and NotI (pALP-1), ScaI and NdeI (pALP-2), NdeI and NotI (pALP-3), followed by gel purification with the QIAquick Gel Extraction Kit (Qiagen). These pALP-2 and pALP-3 fragments were ligated by T4 DNA ligase (New England Biolabs) into pCR2-pALP-1 (pCR2-pALP-123). The HvAACT1 ORF was amplified from Morex cDNA by PCR with KOD-FX Neo (Toyobo) using primer pair 20. This fragment was cloned into pGEM vector (Promega). The ORF was inserted with the NOS terminator into the pGEM vector. The plasmids of the cloned promoter and ORF and pCR-ALP-123 were digested by NotI and BamHI, KpnI and NotI, followed by gel purification. The purified fragments were ligated into pPZP2H-lac vector by KpnI and BamHI and transformed into Agrobacterium tumefaciens strain EHA101 (Fuse et al., 2001). Transformation into immature embryos of barley (Golden Promise) was conducted as described previously (Hensel et al., 2008). The T2 generation was used for phenotypic analysis as described below.
Phenotypic Analysis of Transgenic Barley
Transgenic lines carrying the FM404 promoter fused with HvAACT1 were selected by PCR using MRL insertion primer sets (Supplemental Fig. S6A). To determine HvAACT1 expression in transgenic barley lines, the root segments (0–20 mm) were excised from 4-d-old seedlings and plants were exposed to a 0.5 mm CaCl2 solution for 4 d and then subjected to RNA extraction as described above. The relative expression of HvAACT1 was measured by RT-qPCR.
Geographic Analysis of Barley Accessions
A panel of 274 domesticated barley representatives of global barley diversity and 289 wild barley accessions were used to investigate the presence of the MRL insertion. Genomic DNA was extracted from each accession and used as a template for PCR amplification using primer pair 3 (Supplemental Table S3). The two-step PCR described above was run using KOD-FX Neo (Toyobo).
Phylogenetic Analysis
The genotypes of 274 barley lines were determined using the Illumina GoldenGate platform from the barley oligonucleotide pooled assay 1 (Close et al., 2009). Among the SNPs derived from the transcript regions that were genetically mapped on seven barley chromosomes, 1,189 SNPs without missing data among 274 barley lines were used for phylogenic analysis. The similarities of 274 haplotypes were calculated by the neighbor-joining method with default parameters, and the phylogenetic tree was built with test values for 1,000 bootstrap replications by MEGA6 (Tamura et al., 2013).
Evaluation of Al Tolerance
The Al tolerance of different barley accessions was evaluated by Eriochrome cyanine staining (Ma et al., 1997b). Seedlings (4 d old) were exposed to a 1 mm CaCl2 solution with 5 µm Al (pH 5) for 48 h, followed by staining in 0.1% (v/w) Eriochrome cyanine R solution for 15 min. The roots were washed with deionized water for 10 min and then photographed (Ma et al., 1997b).
Root Exudate Collection
To collect root exudates, seedlings (3 weeks old) of seven barley cultivars were exposed to a 1 mm CaCl2 solution containing 10 µm AlCl3 for 18 h. Other procedures for purification and concentration of the root exudates were as described previously (Furukawa et al., 2007). Citrate in the root exudates was determined by an enzymatic method (Delhaize et al., 1993).
Accession Numbers
The nucleotide sequence data have been deposited in the GenBank/DDBJ/EMBL nucleotide sequence database under accession codes LC330943 (FM404) and LC330944 (SV239).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Comparison of HvAACT1 expression and citrate secretion in different barley accessions.
Supplemental Figure S2. Detection of the 1-kb insertion in the upstream sequence of HvAACT1 in different barley accessions.
Supplemental Figure S3. Structure of the MRL insertion in the upstream sequence of HvAACT1 in FM404.
Supplemental Figure S4. Promoter activity assay of the MRL insertion.
Supplemental Figure S5. Molecular relationships of 1,189 SNP haplotypes for 274 accessions of cultivated barley.
Supplemental Figure S6. Molecular relationships of 1,189 SNP haplotypes for selected accessions of cultivated barley.
Supplemental Figure S7. Transformation of barley with the MRL promoter.
Supplemental Figure S8. Detection of DNA methylation in transgenic barley lines.
Supplemental Figure S9. Pedigree of Brindabella.
Supplemental Figure S10. Pedigree of WB229.
Supplemental Figure S11. Pedigree of FM404.
Supplemental Table S1. Sequence alignment of HvAACT1 in different barley accessions.
Supplemental Table S2. List of barley accessions with the MRL insertion in the upstream sequence of HvAACT1.
Supplemental Table S3. List of primers used in this study.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Dr. Akio Miyao for discussion and the National Bioresource Project of Barley, MEXT, of Japan for providing barley seeds.
Footnotes
This work was supported by a Grant-in-Aid for Specially Promoted Research (Japan Society for the Promotion of Science KAKENHI Grant 16H06296 to J.F.M.).
Articles can be viewed without a subscription.
References
- Bartlett JG, Alves SC, Smedley M, Snape JW, Harwood WA (2008) High-throughput Agrobacterium-mediated barley transformation. Plant Methods 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian M, Waters I, Broughton S, Zhang XQ, Zhou M, Lance R, Sun D, Li C (2013) Development of gene-specific markers for acid soil/aluminum tolerance in barley Hordeum vulgare L. Mol Breed 32: 155–164 [Google Scholar]
- Chen ZC, Yokosho K, Kashino M, Zhao FJ, Yamaji N, Ma JF (2013) Adaptation to acidic soil is achieved by increased cis-acting element numbers regulating ALMT1 expression in Holcus lanatus. Plant J 76: 10–23 [DOI] [PubMed] [Google Scholar]
- Close TJ, Bhat PR, Lonardi S, Wu Y, Rostoks N, Ramsay L, Druka A, Stein N, Svensson JT, Wanamaker S, et al. (2009) Development and implementation of high-throughput SNP genotyping in barley. BMC Genomics 10: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NC, Shirley NJ, Saeed M, Pallotta M, Gustafson JP (2008) An ALMT1 gene cluster controlling aluminum tolerance at the Alt4 locus of rye (Secale cereale L.). Genetics 179: 669–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ma JF, Ryan PR (2012) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17: 341–348 [DOI] [PubMed] [Google Scholar]
- Fujii M, Yokosho K, Yamaji N, Saisho D, Yamane M, Takahashi H, Sato K, Nakazono M, Ma JF (2012) Acquisition of aluminium tolerance by modification of a single gene in barley. Nat Commun 3: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48: 1081–1091 [DOI] [PubMed] [Google Scholar]
- Fuse T, Sasaki T, Yano M (2001) Ti-plasmid vectors useful for functional analysis of rice genes. Plant Biotechnol 18: 219–222 [Google Scholar]
- Hensel G, Valkov V, Middlefell-Williams J, Kumlehn J (2008) Efficient generation of transgenic barley: the way forward to modulate plant-microbe interactions. J Plant Physiol 165: 71–82 [DOI] [PubMed] [Google Scholar]
- Jana S, Pietrzak LN (1988) Comparative assessment of genetic diversity in wild and primitive cultivated barley in a center of diversity. Genetics 119: 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Piñeros MA, Liu J, Magalhaes JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66: 571–598 [DOI] [PubMed] [Google Scholar]
- Kumar A, Bennetzen JL (1999) Plant retrotransposons. Annu Rev Genet 33: 479–532 [DOI] [PubMed] [Google Scholar]
- Leff B, Ramankutty N, Foley JA (2004) Geographic distribution of major crops across the world. Global Biogeochem Cycles 18: 10.1029/2003GB002108 [Google Scholar]
- Ma JF. (2000) Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol 41: 383–390 [DOI] [PubMed] [Google Scholar]
- Ma JF. (2007) Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol 264: 225–252 [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Hiradate S, Matsumoto H (1997a) Detoxifying aluminum with buckwheat. Nature 390: 569–5709403684 [Google Scholar]
- Ma JF, Zheng SJ, Li XF, Takeda K, Matsumoto H (1997b) A rapid hydroponic screening system for aluminum tolerance in barley. Plant Soil 191: 133–137 [Google Scholar]
- Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6: 273–278 [DOI] [PubMed] [Google Scholar]
- Ma JF, Chen ZC, Shen RF (2014) Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 381: 1–12 [Google Scholar]
- Magalhaes JV, Liu J, Guimarães CT, Lana UG, Alves VM, Wang YH, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, et al. (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39: 1156–1161 [DOI] [PubMed] [Google Scholar]
- Manninen I, Schulman AH (1993) BARE-1, a copia-like retroelement in barley (Hordeum vulgare L.). Plant Mol Biol 22: 829–846 [DOI] [PubMed] [Google Scholar]
- Maron LG, Guimarães CT, Kirst M, Albert PS, Birchler JA, Bradbury PJ, Buckler ES, Coluccio AE, Danilova TV, Kudrna D, et al. (2013) Aluminum tolerance in maize is associated with higher MATE1 gene copy number. Proc Natl Acad Sci USA 110: 5241–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M, Gundlach H, Himmelbach A, Beier S, Twardziok SO, Wicker T, Radchuk V, Dockter C, Hedley PE, Russell J, et al. (2017) A chromosome conformation capture ordered sequence of the barley genome. Nature 544: 427–433 [DOI] [PubMed] [Google Scholar]
- Minella E, Sorrells ME (1992) Aluminum tolerance in barley: genetic relationships among genotypes of diverse origin. Crop Sci 32: 593–598 [Google Scholar]
- Minella E, Sorrells ME (2002) Genetic analysis of aluminum tolerance in Brazilian barleys. Pesq Agropec Bras Brazilia 37: 1099–1103 [Google Scholar]
- Mugwira LM, Elgawhary SN, Patel KI (1976) Differential tolerances of triticale, wheat, rye and barley to aluminum in nutrient solution. Agron J 68: 782–787 [Google Scholar]
- Park R, Wellings C, Bariana H, Bansal U (2009) Australia Cereal Cultivar Pedigree and Seedling Rust Genotype Information. Cereal Rust Report, Vol 7, Issue 2 Australian Cereal Rust Control Program, University of Sydney, Sydney [Google Scholar]
- Raman H, Moroni S, Sato K, Read J, Scott J (2002) Identification of AFLP and microsatellite markers linked with an aluminium tolerance gene in barley ( Hordeum vulgare L.). Theor Appl Genet 105: 458–464 [DOI] [PubMed] [Google Scholar]
- Read BJ, Oram RN (1995) Hordeum vulgare (barley) cv. Brindabella. Aust J Exp Agric 35: 425 [Google Scholar]
- Ryan PR, Ditomaso JM, Kochian LV (1993) Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot 44: 437–446 [Google Scholar]
- Ryan P, Delhaize E, Jones D (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52: 527–560 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Raman H, Gupta S, Sasaki T, Yamamoto Y, Delhaize E (2010) The multiple origins of aluminium resistance in hexaploid wheat include Aegilops tauschii and more recent cis mutations to TaALMT1. Plant J 64: 446–455 [DOI] [PubMed] [Google Scholar]
- Samac DA, Tesfaye M (2003) Plant improvement for tolerance to aluminum in acid soils: a review. Plant Cell Tissue Organ Cult 75: 189–207 [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37: 645–653 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ryan PR, Delhaize E, Hebb DM, Ogihara Y, Kawaura K, Noda K, Kojima T, Toyoda A, Matsumoto H, et al. (2006) Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant Cell Physiol 47: 1343–1354 [DOI] [PubMed] [Google Scholar]
- Schulte D, Close TJ, Graner A, Langridge P, Matsumoto T, Muehlbauer G, Sato K, Schulman AH, Waugh R, Wise RP, et al. (2009) The International Barley Sequencing Consortium: at the threshold of efficient access to the barley genome. Plant Physiol 149: 142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suoniemi A, Narvanto A, Schulman AH (1996) The BARE-1 retrotransposon is transcribed in barley from an LTR promoter active in transient assays. Plant Mol Biol 31: 295–306 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kamakura H, Sato Y, Shiono K, Abiko T, Tsutsumi N, Nagamura Y, Nishizawa NK, Nakazono M (2010) A method for obtaining high quality RNA from paraffin sections of plant tissues by laser microdissection. J Plant Res 123: 807–813 [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovkach A, Ryan PR, Richardson AE, Lewis DC, Rathjen TM, Ramesh S, Tyerman SD, Delhaize E (2013) Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol 161: 880–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bothmer R, Sato K, Komatsuda T, Yasuda S, Fischbeck G (2003) The domestication of cultivated barley. In von Bothmer R, van Hintum T, Knüpffer H, Sato K, eds, Diversity in Barley (Hordeum vulgare). Elsevier Science, Amsterdam, pp 9–27 [Google Scholar]
- Von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171: 1–15 [Google Scholar]
- Wang J, Raman H, Zhou M, Ryan PR, Delhaize E, Hebb DM, Coombes N, Mendham N (2007) High-resolution mapping of the Alp locus and identification of a candidate gene HvMATE controlling aluminium tolerance in barley (Hordeum vulgare L.). Theor Appl Genet 115: 265–276 [DOI] [PubMed] [Google Scholar]
- Wray GA, Hahn MW, Abouheif E, Balhoff JP, Pizer M, Rockman MV, Romano LA (2003) The evolution of transcriptional regulation in eukaryotes. Mol Biol Evol 20: 1377–1419 [DOI] [PubMed] [Google Scholar]
- Wu D, Yamaji N, Yamane M, Kashino-Fujii M, Sato K, Ma JF (2016) The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol 172: 1899–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Eickbush TH (1990) Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J 9: 3353–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Fujii-Kashino M, Ma JF (2016) Retrotransposon-mediated aluminum tolerance through enhanced expression of the citrate transporter OsFRDL4. Plant Physiol 172: 2327–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Broughton S, Zhang XQ, Ma Y, Zhou M, Li C (2016) Genome-wide association mapping of acid soil resistance in barley (Hordeum vulgare L.). Front Plant Sci 7: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]