GmMEKK1 regulates cell death and defense responses independent of activation of GmMPK4 and possibly by differentially regulating GmMPK3 and GmMPK6 via distinct downstream GmMKKs in soybean.
Abstract
MAPK signaling pathways play critical roles in plant immunity. Here, we silenced multiple genes encoding MAPKs using virus-induced gene silencing mediated by Bean pod mottle virus to identify MAPK genes involved in soybean (Glycine max) immunity. Surprisingly, a strong hypersensitive response (HR) cell death was observed when soybean MAPK KINASE KINASE1 (GmMEKK1), a homolog of Arabidopsis (Arabidopsis thaliana) MEKK1, was silenced. The HR was accompanied by the overaccumulation of defense signaling molecules, salicylic acid (SA) and hydrogen peroxide. Genes involved in primary metabolism, translation/transcription, photosynthesis, and growth/development were down-regulated in GmMEKK1-silenced plants, while the expression of defense-related genes was activated. Accordingly, GmMEKK1-silenced plants were more resistant to downy mildew (Peronospora manshurica) and Soybean mosaic virus compared with control plants. Silencing GmMEKK1 reduced the activation of GmMPK6 but enhanced the activation of GmMPK3 in response to flg22 peptide. Unlike Arabidopsis MPK4, GmMPK4 was not activated by either flg22 or SA. Interestingly, transient overexpression of GmMEKK1 in Nicotiana benthamiana also induced HR. Our results indicate that GmMEKK1 plays both positive and negative roles in immunity and appears to differentially activate downstream MPKs by promoting GmMPK6 activation but suppressing GmMPK3 activation in response to flg22. The involvement of GmMPK4 kinase activity in cell death and in flg22- or SA-triggered defense responses in soybean requires further investigation.
MAPK cascades are evolutionarily conserved signaling hubs that regulate innate immune responses in both plants and animals (Meng and Zhang, 2013). The recognition of nonself by pattern recognition receptors on the cell surface activates a MAPK phosphorylation relay. Initial activation of a MAP kinase kinase kinase (MAP3K/MEKK) leads to the phosphorylation and activation of a downstream MAP kinase kinase (MAP2K/MKK), which subsequently phosphorylates a downstream MAPK. The activated MAPK then phosphorylates its target proteins, such as WRKY transcription factors, and ultimately reprograms gene expression (Pitzschke et al., 2009b; Rasmussen et al., 2012; Meng and Zhang, 2013; Smeets et al., 2013; Pitzschke, 2015).
The genome of Arabidopsis (Arabidopsis thaliana) encodes 60 MAP3Ks, 10 MAP2Ks, and 20 MAPKs (Ichimura et al., 2002), indicating that MAPK cascades are not simple linear MAP3K-MAP2K-MAPK modules. Instead, they form tightly regulated signaling networks that coordinate intracellular responses to external and internal stimuli. Arabidopsis MEKK1, the most extensively studied MAP3K in plants, has been implicated in biotic and abiotic stress responses, cytokinesis, and growth/development (Pitzschke et al., 2009b; Rasmussen et al., 2012; Xu and Chua, 2012; Meng and Zhang, 2013; Xu and Zhang, 2015). Using a truncated version of MEKK1 in protoplasts, a complete MAP kinase signaling module (MEKK1-MKK4/MKK5-MPK3/MPK6-WRKY22/WRKY29) was identified that acts downstream of flagellin receptor, the Flagellin Sensing Locus2 (Asai et al., 2002). Activation of this MAPK cascade confers resistance to both bacterial and fungal pathogens (Asai et al., 2002). However, genetic evidence indicates that, in planta, MEKK1 is dispensable for MPK3/MPK6 activation (Nakagami et al., 2006; Suarez-Rodriguez et al., 2007; Gao et al., 2008; Qiu et al., 2008).
MEKK1 also negatively regulates defense. The mekk1, mkk1/mkk2, and mpk4 mutants share similar phenotypes of constitutive defense responses, including dwarfed stature, increased accumulation of salicylic acid (SA) and hydrogen peroxide (H2O2), induction of pathogenesis-related (PR) genes, and enhanced pathogen resistance (Petersen et al., 2000; Ichimura et al., 2006; Mészáros et al., 2006; Nakagami et al., 2006; Suarez-Rodriguez et al., 2007; Gao et al., 2008; Qiu et al., 2008; Pitzschke et al., 2009a). Some phenotypes of these mutants can be rescued by the sid2 mutation or the expression of NahG, both of which cause SA deficiencies, indicating that the activation of defenses is partially SA dependent (Petersen et al., 2000; Suarez-Rodriguez et al., 2007; Gao et al., 2008). Constitutive defense responses and loss of flg22-induced MPK4 activation are seen only in mkk1/mkk2 double mutants but not in either single mutant, indicating that MKK1 and MKK2 have redundant functions in MPK4 signaling (Gao et al., 2008; Qiu et al., 2008). MKK1/MKK2 interact with both MEKK1 and MPK4 in vivo (Ichimura et al., 1998; Mizoguchi et al., 1998; Gao et al., 2008), and MEKK1 phosphorylates MKK1/MKK2 (Teige et al., 2004; Hadiarto et al., 2006). The activation of MPK4 by flg22 is abolished in mekk1 and mkk1/mkk2 mutants (Nakagami et al., 2006; Suarez-Rodriguez et al., 2007; Gao et al., 2008; Qiu et al., 2008). These data establish that MEKK1, MKK1/MKK2, and MPK4 form a linear signaling module to negatively regulate SA-dependent immune responses in Arabidopsis (Suarez-Rodriguez et al., 2007; Gao et al., 2008; Qiu et al., 2008; Pitzschke et al., 2009a).
Interestingly, MPK4 interacts directly with the N-terminal regulatory domain of MEKK1, suggesting that MEKK1 may serve as a scaffold, binding a MAP2K and a MAPK (Ichimura et al., 1998; Nakagami et al., 2006). In support of this idea, the kinase activity of MEKK1 may not be required for flg22-induced MPK4 activation, because a kinase-dead version of MEKK1 (K361M) can largely rescue the activation of MPK4 and the dwarf phenotype of the mekk1 mutant. These observations suggest that MEKK1 may play a structural role independent of its protein kinase activity (Suarez-Rodriguez et al., 2007). In addition, avrPto and avrPtoB suppress defense signaling upstream of AtMEKK1 (He et al., 2006), and the MEKK1-MKK1/MKK2-MPK4 signaling module was proposed to serve as a guardee in effector-triggered immunity (Zhang et al., 2012; Zhou et al., 2014).
The MEKK1-MKK1/MKK2-MPK4-MKS1-WRKY33-PAD3 module regulates the accumulation of camalexin, a phytoalexin induced in response to bacterial and fungal pathogens in Arabidopsis (Andreasson et al., 2005; Qiu et al., 2008; Ren et al., 2008; Mao et al., 2011). Interestingly, MEKK1 can bypass the MKK1/MKK2-MPK4 module and directly regulate downstream targets (Miao et al., 2007). Phosphorylation of WRKY53 by MEKK1 increases its DNA-binding activity in vitro and increases the transcription of a reporter gene driven by the WRKY53 promoter in vivo. Thus, MEKK1 can bypass MAPK signaling by directly phosphorylating a transcription factor (Miao et al., 2007).
In soybean (Glycine max), virus-induced gene silencing (VIGS) mediated by Bean pod mottle virus (BPMV) has been used successfully to study MAPK gene function (Liu et al., 2015, 2016). These studies demonstrate that, while soybean GmMPK4 plays a negative role, GmMPK6 plays both positive and negative roles in defense responses (Liu et al., 2011, 2014). In this study, we determined to identify the MAP3Ks and MAP2Ks that function upstream of GmMPK4 or GmMPK6. We found that silencing GmMEKK1, an Arabidopsis MEKK1 homolog, resulted in similar but more severe constitutive defense phenotypes than GmMPK4-silenced plants. These phenotypes included dwarfed stature, induction of PR genes, increased accumulation of SA and H2O2, and enhanced pathogen resistance. Reverse transcription quantitative PCR (RT-qPCR) and RNA sequencing (RNA-seq) analyses of GmMEKK1-silenced plants confirmed that the constitutively activated defense responses occur at the expense of plant growth and development (Liu et al., 2011). The activated defense in GmMEKK1-silenced plants was associated with a gain of resistance to Soybean mosaic virus (SMV) and downy mildew (Peronospora manshurica). In addition, we provide evidence that GmMEKK1 plays a positive role in GmMPK6 activation but a negative role in GmMPK3 activation, and the correlation between the inactivation of GmMPK4 and induced cell death is not observed in soybean. Our data suggest that there are similarities but also important differences in MAPK signaling modules of soybean and Arabidopsis.
RESULTS
Silencing GmMEKK1 Results in Both Local and Systemic Hypersensitive Response
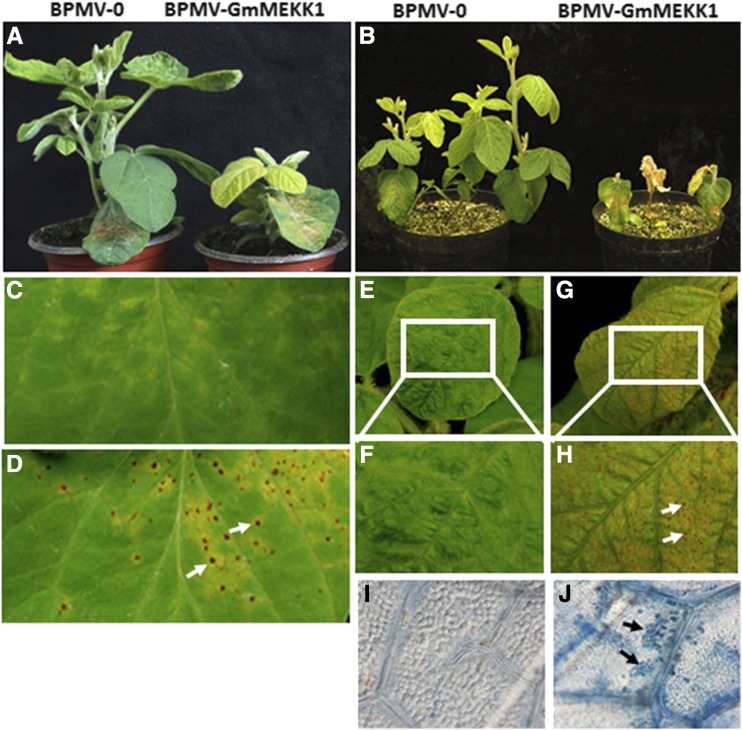
Previously, we demonstrated that, while GmMPK4 negatively regulates defense responses, GmMPK6 plays both positive and negative roles in regulating defense responses (Liu et al., 2011, 2014). To identify the MAP3Ks or MAP2Ks that function upstream of GmMPK4 or GmMPK6, we silenced 20 GmMP3Ks and 12 GmMP2Ks using BPMV-VIGS. When a soybean MAP3K closely related to Arabidopsis MEKK1 was silenced [Glyma.04G253500 (Wm82.a2.v1), hereafter GmMEKK1a], severe stunting was observed relative to the vector control plants (Fig. 1, A and B). While no HR cell death was observed in control plants (Fig. 1C), inoculated leaves of the GmMEKK1-silenced plants displayed a clear HR (Fig. 1D). As silencing progressed, numerous micro-HR lesions appeared on the upper systemic leaves of GmMEKK1-silenced plants (Fig. 1, G and H) relative to controls (Fig. 1, E and F). The HR was further confirmed by Trypan Blue staining (Fig. 1, I and J). These results suggest that, like its counterpart in Arabidopsis, GmMEKK1 plays a negative role in regulating cell death and defense responses (Suarez-Rodriguez et al., 2007; Gao et al., 2008).
Figure 1.
Silencing GmMEKK1 causes both local and systemic hypersensitive response (HR) cell death in soybean. A and B, Control (BPMV-0) and GmMEKK1-silenced (BPMV-GmMEKK1) plants at 15 d post infection (dpi; A) and 25 dpi (B). C and D, HR response on the bombarded local leaves of BPMV-0 (C) and BPMV-GmMEKK1 (D; white arrows indicate examples of necrotic lesions). E and F, HR response on systemic leaves of BPMV-0 plants. G and H, HR observed on systemic leaves of BPMV-GmMEKK1 plants. The images shown in F and H were enlarged from the boxed areas in E and G, respectively. I and J, Trypan Blue-stained systemic leaves of BPMV-0 control (I) and GmMEKK1-silenced (J) plants. Black arrows indicate the blued-stained, dead cells.
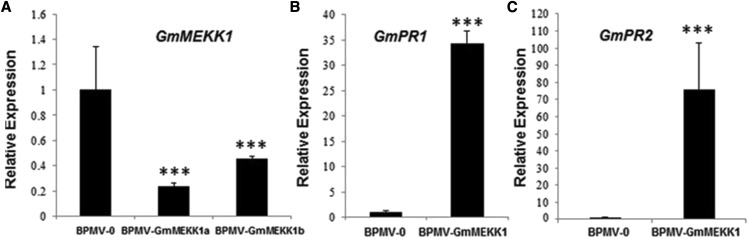
Soybean is a paleopolyploid, and nearly 75% of its genes are present in multiple copies (Schmutz et al., 2010). We have shown previously that GmMPK4 and GmMPK6 each have four homologs that can be divided into two paralogous groups. The amino acid identities within the groups are greater than 96%, and for GmMPK4 we showed that it was necessary to silence all four copies to observe the loss-of-function phenotype (Liu et al., 2011). GmMEKK1 also is duplicated, with GmMEKK1a sharing 94% nucleotide identity with GmMEKK1b [Glyma.06G108900 (Wm82.a2.v1)]. The sequence fragment from GmMEKK1a used for silencing shares 95% nucleotide identity with GmMEKK1b (Supplemental Fig. S1). Because there is sufficient sequence divergence in the 5′ untranslated regions, the expression of GmMEKK1a and GmMEKK1b could be determined by RT-qPCR using gene-specific primers. These analyses verified that GmMEKK1a was silenced, because its transcript level was decreased by 76% in BPMV-GmMEKK1 plants relative to nonsilenced BPMV-0 controls (Fig. 2A). The transcript level of GmMEKK1b also was reduced significantly in the BPMV-GmMEKK1 plants, indicating that the constitutive HR phenotypes resulted from simultaneously silencing GmMEKK1a and GmMEKK1b.
Figure 2.
The transcript levels of GmMEKK1s are reduced and PR genes are induced in GmMEKK1-silenced plants. A, RT-qPCR analysis of GmMEKK1a and GmMEKK1b transcript levels in GmMEKK1-silenced plants compared with BPMV-0 empty vector control plants. B and C, Transcript levels of PR1 and PR2 in GmMEKK1-silenced plants compared with the BPMV-0 empty vector control plants. The β-actin gene (Glyma.15G050200) was used as the endogenous reference gene. Error bars represent sd of three replications. Asterisks indicate significant differences from the control (***, P < 0.001, Student’s t test). The experiment was repeated three times with similar results.
HR usually is associated with increased expression of defense marker genes. To test whether the expression of defense-related genes was activated in the GmMEKK1-silenced plants, the expression of soybean GmPR1 (Glyma.13G251600.1) and GmPR2 (Glyma.03G132700) was examined using RT-qPCR. Consistent with a constitutive defense response phenotype, the levels of both GmPR1 and GmPR2 mRNA transcripts were significantly higher in GmMEKK1-silenced plants compared with BPMV-0 control plants (Fig. 2, B and C).
SA and H2O2 Levels Increase in GmMEKK1-Silenced Plants
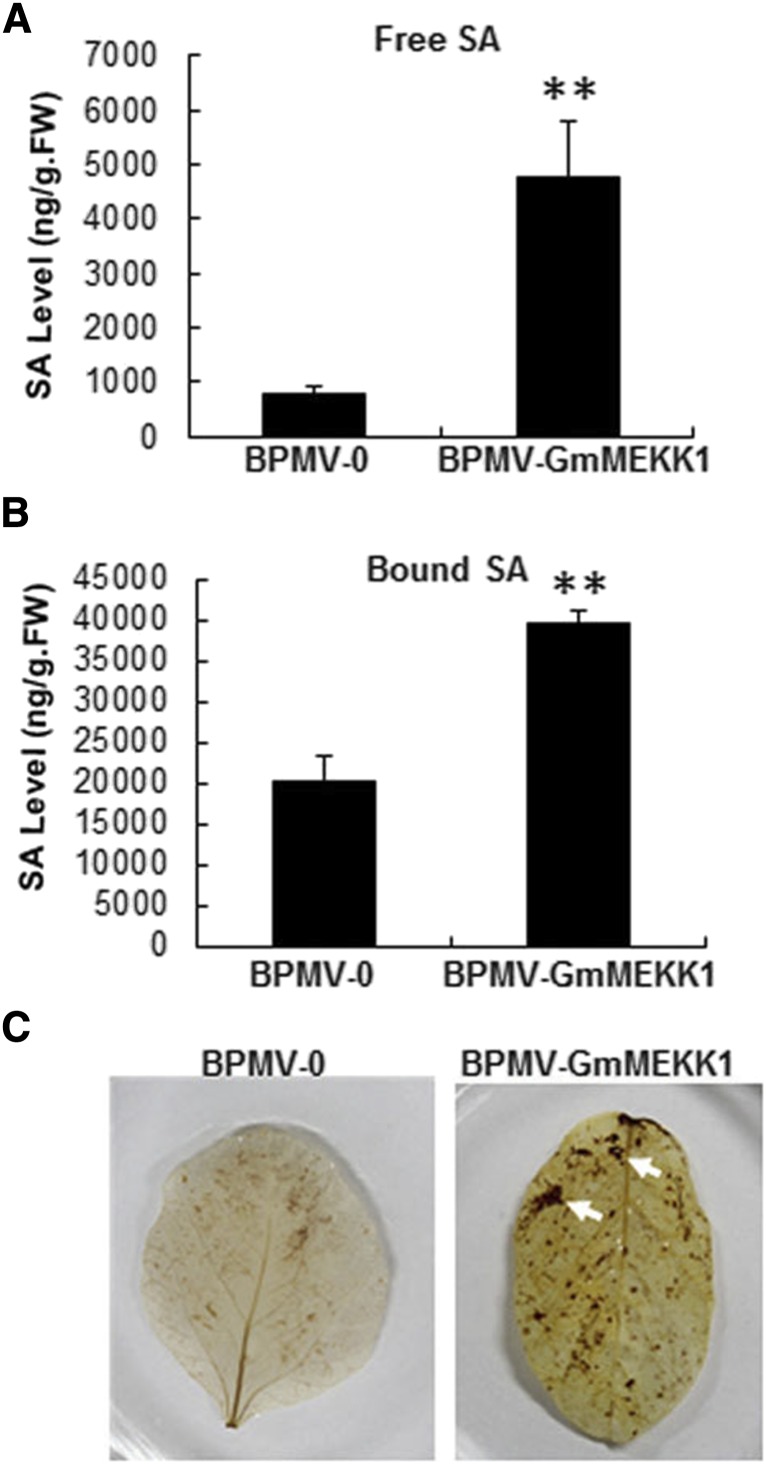
SA is a key hormone that positively regulates defense responses against biotrophic pathogens, and it overaccumulates in Arabidopsis mpk4, mkk1/mkk2, and mekk1 mutants and GmMPK4-silenced soybean plants (Petersen et al., 2000; Suarez-Rodriguez et al., 2007; Gao et al., 2008; Qiu et al., 2008; Liu et al., 2011). To test whether SA overaccumulation occurred in GmMEKK1-silenced plants, both free SA and bound SA were quantified, and the levels increased 5.3- and 1.95-fold, respectively, in GmMEKK1-silenced plants relative to BPMV-0 control plants (Fig. 3, A and B). Increased SA suggested that SA could be involved in the constitutively activated defense responses.
Figure 3.
Elevated accumulation of SA and H2O2 in GmMEKK1-silenced soybean plants. A and B, Free SA (A) and bound SA (SA-O-glycoside; B) levels were quantified in GmMEKK1-silenced and BPMV-0 empty vector control plants at 20 d post BPMV inoculation. Error bars represent sd for three independent samples. Asterisks indicate significant differences from the control (**, P < 0.01, Student’s t test). FW, Fresh weight. C, Presence of H2O2 in soybean leaves visualized by staining with DAB. Oxidized DAB formed a reddish-brown deposit (examples of these deposits are indicated by the white arrows).
H2O2 is a potent trigger of cell death (Lamb and Dixon, 1997; Delledonne et al., 1998). To determine whether H2O2 overaccumulation occurred in GmMEKK1-silenced plants, soybean leaves were stained with 3,3-diaminobenzidine (DAB; Thordal-Christensen et al., 1997; Ren et al., 2002), which forms a brown precipitate when oxidized by H2O2. Brown spots developed in the leaves of GmMEKK1-silenced plants that were more intensely colored compared with BPMV-0 control plants (Fig. 3C), indicating that the cell death observed in GmMEKK1-silenced plants was associated with elevated H2O2 accumulation.
Functional Classification of Differentially Expressed Genes between GmMEKK1-Silenced and BPMV-0 Vector Control Plants
To identify genes differentially expressed between GmMEKK1-silenced and BPMV-0 control samples, RNA-seq analysis was performed (Moran Lauter et al., 2014). We identified 4,007 induced and 4,358 repressed genes (fold change ≥ 2 and false discovery rate [FDR] ≤ 0.001) in GmMEKK-silenced plants relative to BPMV-0 plants (Supplemental Data Sets S1 and S2).
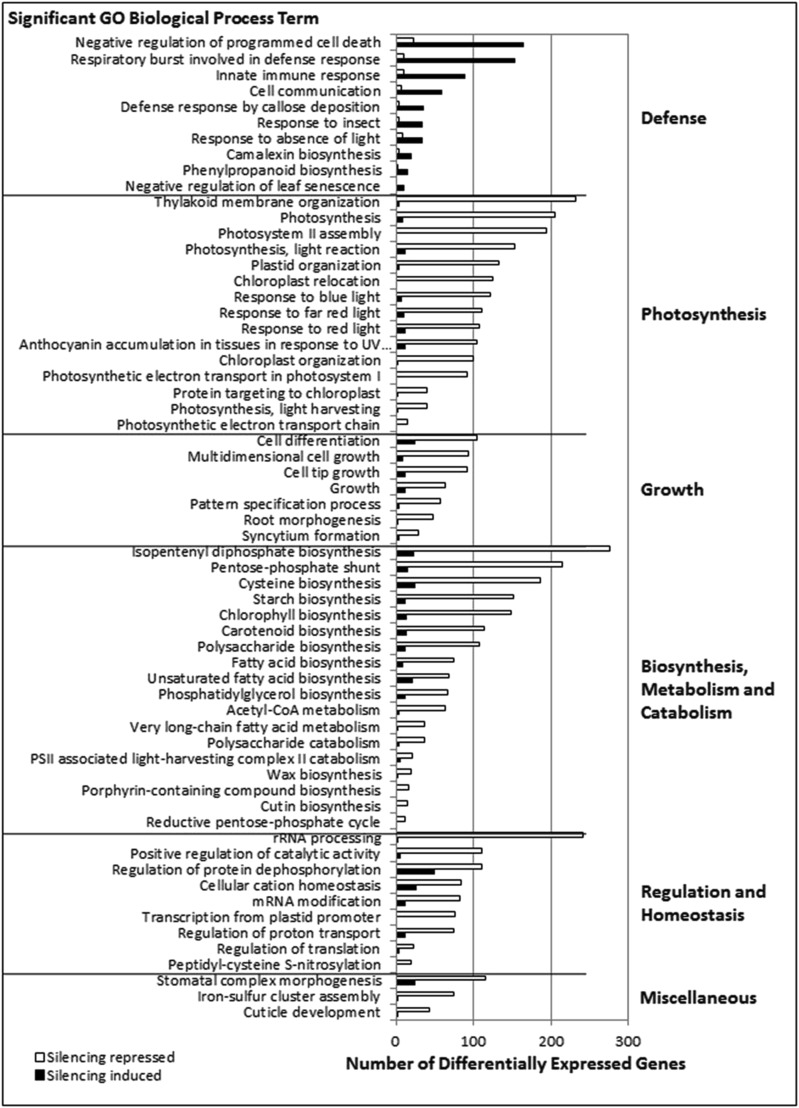
To identify overarching biological processes affected by GmMEKK1 silencing, we used the SoyBase GO (Gene Ontology) Term Enrichment Tool (http://www.soybase.org/goslimgraphic_v2/dashboard.php) to identify GO biological process terms significantly overrepresented among differentially expressed genes relative to all predicted genes in the soybean genome. In total, 71 and 98 GO terms were significantly overrepresented (P < 0.05) among genes induced and repressed, respectively, by GmMEKK1 silencing (Supplemental Data Sets S3 and S4). To visualize these data, we graphed the 62 significant GO terms with genome counts of 20 or more and in which more than 30% of genes were differentially expressed in our analysis (Fig. 4). Of these GO terms, 10 were associated with genes induced by GmMEKK1 silencing and included defense- and stress-related responses such as programmed cell death, innate immunity, cell communication, respiratory burst, and phenylpropanoid and camalexin biosynthesis. In contrast, the remaining 52 GO terms representing genes repressed by GmMEKK1 silencing were associated with photosynthesis, growth, general metabolism, and regulation. Photosynthesis-related GO terms included thylakoid membrane organization, PSII assembly, light reaction, and response to light. Growth responses included multidimensional growth, pattern specification process, and cell differentiation. Metabolism-related GO terms included Cys, starch, chlorophyll, and fatty acid biosynthesis, catabolism of acetyl-CoA, very-long-chain fatty acids, and polysaccharides, and processes related to the pentose-phosphate shunt. Regulation and homeostasis-related GO terms included regulation of translation, protein transport, catalytic activity, and protein phosphorylation, transcription, and mRNA modifications. The down-regulation of genes in growth/development was consistent with the compromised growth and development phenotypes of GmMEKK1-silenced plants (Fig. 1, A and B).
Figure 4.
GO biological process terms significantly (P < 0.05) overrepresented among genes differentially expressed in response to GmMEKK1 silencing. Significantly overrepresented GO terms were identified using the SoyBase GO Term Enrichment Tool (https://www.soybase.org/goslimgraphic_v2/dashboard.php) for genes induced or repressed by GmMEKK1 silencing relative to vector controls. Due to the large number of significant GO terms identified, GO terms were limited to those terms in which 30% of genes in the genome assigned to an individual GO term were differentially expressed. Only GO terms containing 20 or more genes are included. In addition, any GO terms whose genes completely overlapped were mapped to the largest significantly overrepresented GO term. For all significant GO terms, see Supplemental Data Sets S3 and S4.
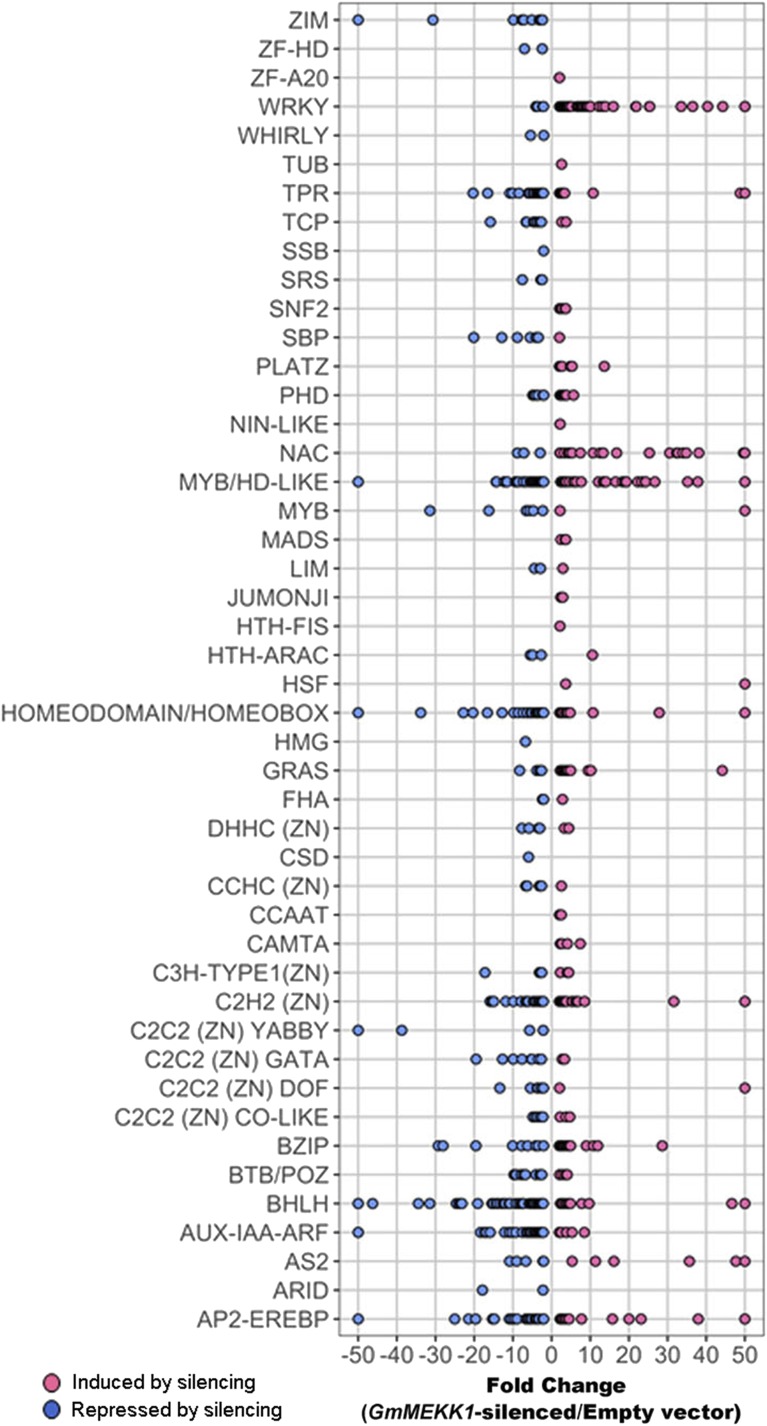
To understand how these complex gene expression networks are regulated downstream of GmMEKK1, we took advantage of the SoyDB transcription factor database (Wang et al., 2010) to find transcription factors responding to GmMEKK1 silencing. Of the 4,946 transcription factors present in the soybean genome (version Wm82.a2.v1), 728 transcription factors, representing 46 families, were differentially expressed in response to GmMEKK1 silencing (Fig. 5; Supplemental Data Set S5). The 389 repressed transcription factors represented 35 families, while the 339 induced transcription factors represented 37 families. Transcription factor families ARID, C2C2(ZN)YABBY, CSD, HMG, SRS, SSB, WHIRLY, ZF-HD, and ZIM were unique to repressed transcription factors. Transcription factor families CAMTA, CCAAT, HSF, HTH-FIS, JUMONJI, MADS, NIN-LIKE, PLATZ, SBP, SNF2, TUB, and ZF-A20 were unique to induced transcription factor families. To identify transcription factor families significantly overrepresented among all transcription factors relative to their genome representation, we used Fisher’s exact test (Fisher, 1966) and Bonferroni correction (Bonferroni, 1935). Three significantly (corrected P < 0.01) overrepresented transcription factor families were identified: WRKY, AUX-IAA-ARF, and ZIM (Fig. 5; Supplemental Data Set S6).
Figure 5.
Expression of transcription factors in response to GmMEKK1 silencing. Significantly differentially expressed transcription factors and families were identified using the SoyDB transcription factor database (Wang et al., 2010). A total of 728 transcription factors representing 46 transcription factor families were identified. Fold change is plotted on the x axis, and transcription factor families are plotted on the y axis. For visualization purposes, transcription factors with a fold change greater than 50 were plotted as 50. Similarly, transcription factors with a fold change less than −50 were plotted as −50. For a full list of transcription factors see Supplemental Data Set S5. Transcription factor families ZIM, WRKY and AUX-IAA-ARF are significantly (FDR < 0.001) overrepresented among differentially expressed transcription factors. For additional details, see Supplemental Data Set S6.
WRKY transcription factors are important regulators of plant defense responses (Cormack et al., 2002; Eulgem and Somssich, 2007; Rushton et al., 2010). We previously showed that, in GmMPK4-silenced plants, 45 and three GmWRKY genes were induced and repressed, respectively (Liu et al., 2011). Similarly, 64 GmWRKY genes were induced and five GmWRKY genes were repressed in GmMEKK1-silenced plants (Fig. 5; Supplemental Data Sets S5 and S6). Among the 64 induced GmWRKYs in GmMEKK1-silenced plants, 29 were in common with GmMPK4-silenced plants (Liu et al., 2011). Auxin is a major hormone that regulates growth and development. Interestingly, AUX-IAA-ARF transcription factors were repressed similarly in both GmMPK4 and GmMEKK1-silenced plants (Fig. 5; Supplemental Data Sets S5 and S6). Our results suggest that both WRKY and AUX-IAA-ARF transcription factors are coregulated in GmMPK4- and GmMEKK1-silenced plants. Furthermore, they support the conclusion that both H2O2 and SA have inhibitory effects on auxin-responsive gene expression (Nakagami et al., 2006; Wang et al., 2007; Cheng et al., 2011).
Silencing GmMEKK1 Enhances Resistance against SMV and Downy Mildew
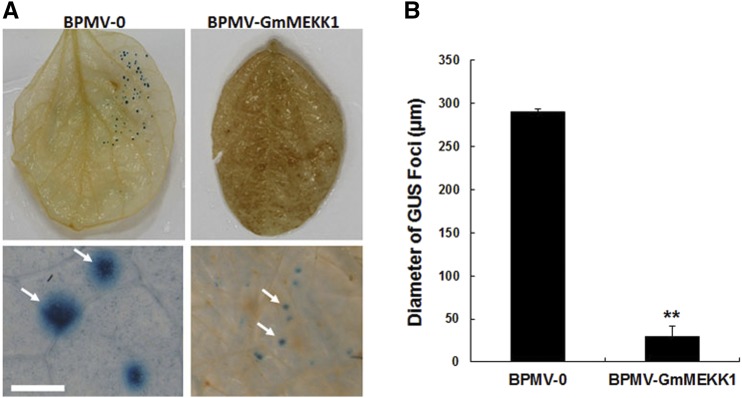
Enhanced disease resistance often is seen in plants in which defense responses are constitutively activated (Liu et al., 2011, 2014), and it has been reported previously that silencing NPK1, which encodes a MAP3K in Nicotiana benthamiana, compromises disease resistance (Jin et al., 2002). To test whether disease resistance is enhanced or compromised in GmMEKK1-silenced plants, SMV strain N tagged with the GUS marker protein (SMV-N-GUS; Wang et al., 2006) was inoculated via biolistic bombardment onto three individual leaves detached from both vector control plants and GmMEKK1-silenced plants. At 3 dpi, the SMV-N-GUS infection was visualized by GUS staining (Fig. 6A). The GUS infection foci were readily seen on the nonsilenced empty vector control leaves (Fig. 6A, left). However, the GUS foci on the GmMEKK1-silenced plants were much smaller and were visible only with a dissecting microscope (Fig. 6, A, right, and B). These SMV-N-GUS foci were approximately 25% of the diameter of those reported previously on the leaves of GmMPK4-silenced plants (Liu et al., 2011), suggesting that GmMEKK1-silenced plants are more resistant to SMV than GmMPK4-silenced plants.
Figure 6.
Silencing GmMEKK1s enhances the resistance of soybean plants to SMV. At 15 dpi with BPMV-0 or BPMV-GmMEKK1, SMV-N-GUS was biolistically delivered into the detached leaves of silenced and nonsilenced plants. At 3 dpi with SMV-N-GUS, the replication and movement of SMV-N-GUS in the biolistically inoculated leaves was detected by GUS staining. The GUS foci were counted and measured. A, Infection foci of SMV-N-GUS on the leaves of BPMV-0 and BPMV-GmMEKK1 plants (top), and closeup images from leaves at top taken with a dissecting microscope (arrows at bottom). Bar = 2 mm. B, Comparison of the diameters of SMV-N-GUS foci on the leaves of BPMV-0 and BPMV-GmMEKK1 plants. Error bars represent sd of the diameters of at least 30 GUS foci measured on each of four independent leaves (at least 120 foci). Asterisks indicate a significant difference from the control (**, P < 0.01, Student’s t test).
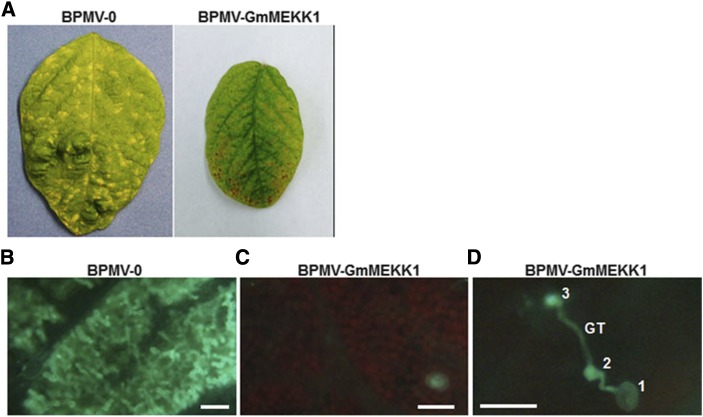
We have reported that silencing GmMPK4 and GmMPK6 also enhances soybean resistance against downy mildew (Liu et al., 2011, 2014). To test whether silencing GmMEKK1 has the same effect, we inoculated BPMV-0 control plants and GmMEKK1-silenced plants (12 dpi) with P. manshurica. Symptomatic chlorotic lesions of P. manshurica infection were observed on BPMV-0 control leaves at 7 dpi (Fig. 7A, left). However, similar lesions were not observed on GmMEKK1-silenced leaves (Fig. 7A, right). To further validate these results, P. manshurica mycelia were stained within inoculated leaves using Aniline Blue. Abundant hyphae were readily observed in mesophyll cells of vector control plant leaves with chlorotic lesions (Fig. 7B), while no hyphae were observed within the mesophyll of the GmMEKK1-silenced plants (Fig. 7C). As in GmMPK4-silenced plants (Liu et al., 2011), sporangia that germinated on the leaves of GmMEKK1-silenced plants (Fig. 7D, 1) often produced germ tubes (Fig. 7D, GT) with multiple appressoria (Fig. 7D, 2 and 3), indicating that multiple unsuccessful penetrations were sometimes attempted (Fig. 7D). This behavior was not observed on the leaves of the BPMV-0 control plants, suggesting that the resistance of GmMEKK1-silenced plants to P. manshurica may have occurred at the penetration stage. Together, these results clearly show that silencing GmMEKK1 enhanced resistance against SMV and P. manshurica.
Figure 7.
Silencing GmMEKK1 enhances the resistance of soybean to downy mildew. A, Typical chlorotic lesion symptoms of soybean downy mildew detected on the leaves of BPMV-0 empty vector control plants (left) but not on the leaves of GmMEKK1-silenced plants (right) 1 week after inoculation with P. manshurica. B, P. manshurica hyphae observed in the mesophyll of BPMV-0 control plants at 1 week after inoculation. C, Hyphae absent from the mesophyll of GmMEKK1-silenced plants 1 week after inoculation with P. manshurica. D, Germ tubes (GT) that formed multiple appressoria unable to penetrate the epidermal surface observed on GmMEKK1-silenced plants. 1, Sporangium; 2 and 3, appressoria. These experiments were repeated three times (at least three plants each time) with similar results. Bars = 100 μm in B and C and 50 μm in D.
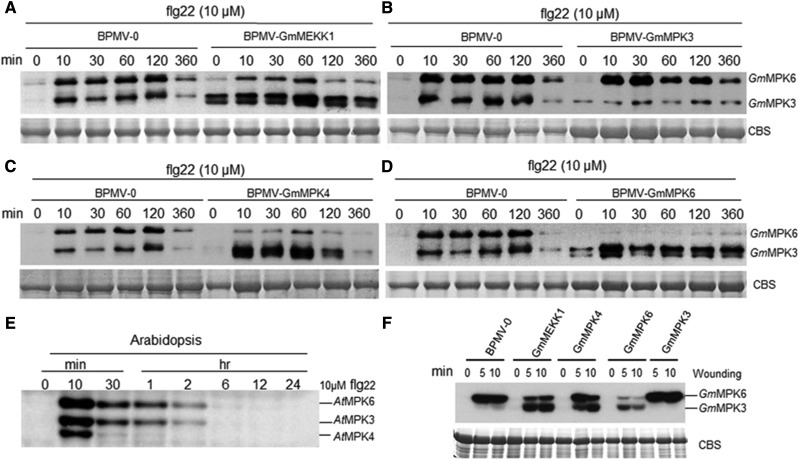
Activation of GmMPK6 Is Repressed and Activation of GmMPK3 Is Enhanced in GmMEKK1-Silenced Plants in Response to flg22
Activation of MPK4 but not MPK3/6 in response to the flg22 peptide is abolished in the Arabidopsis mekk1 mutant (Suarez-Rodriguez et al., 2007; Gao et al., 2008). To examine whether GmMPK4 or GmMPK3/6 functions downstream of GmMEKK1, we tested for GmMPK4 or GmMPK3/6 phosphorylation in GmMEKK1-silenced plants following flg22 treatment. To detect the phosphorylated forms of these three MPKs, we used the phospho-p44/42 MAP Erk1/2 antibody that recognizes the phosphorylation of Arabidopsis MPK3/4/6 at the sites corresponding to Thr-202/Tyr-204 of human (Homo sapiens) p44 MAPK (Zhao et al., 2014). This phosphorylation site is highly conserved between the human p44 MAPK epitope and Arabidopsis or soybean MPK3/4/6 (Supplemental Fig. S2). GmMPK3-, GmMPK4-, and GmMPK6-silenced plants were used as controls. Compared with BPMV-0 control plants, activation of GmMPK6 by flg22 was partially dependent on GmMEKK1, because less GmMPK6 phosphorylation was observed in GmMEKK1-silenced plants at all time points after flg22 treatment (Fig. 8A). In contrast, GmMPK3 activation was strongly enhanced in GmMEKK1-silenced plants even in the absence of flg22 treatment (Fig. 8A). Interestingly, silencing GmMPK4 and GmMPK6 also enhanced the activation of GmMPK3 by flg22 (Fig. 8, C and D). As expected, activation of GmMPK3 or GmMPK6 by flg22 was reduced significantly in the corresponding GmMPK3- or GmMPK6-silenced control plants (Fig. 8, B and D), respectively.
Figure 8.
The activation of GmMPK3 and GmMPK6 in response to flg22 is enhanced and reduced, respectively, in GmMEKK1-silenced plants. A to D, Leaf discs from the indicated soybean plants were incubated on moist filter paper for 24 h to allow recovery from wounding before treatment with 10 µm flg22 or diluted DMSO for the indicated times. The kinase activities were detected by immunoblotting using phospho-p44/42 MAP Erk1/2 antibody. E, Five-day-old whole Arabidopsis seedlings (Columbia-0 ecotype) were treated with 10 µm flg22 or diluted DMSO over the indicated time course. The kinase activities were detected by immunoblotting using phospho-p44/42 MAP Erk1/2 antibody. F, Leaf discs from the indicated soybean plants silenced for various GmMPKs were wounded for 0, 5, and 10 min. Kinase activities were detected subsequently by in gel kinase assay. Myelin basic protein was embedded in the gel to serve as a substrate for MPKs. CBS, Coomassie Blue staining for a loading control.
GmMPK4 Activity Is Not Activated by Either flg22 or SA
To our surprise, GmMPK4 was not activated in either BPMV-0 control plants or in GmMEKK1- or GmMPK3-, GmMPK4-, and GmMPK6-silenced plants (Fig. 8), even though MPK3, MPK4, and MPK6 are activated in Arabidopsis (Fig. 8E). This result implied that the antibody does not recognize the phosphorylated GmMPK4. However, the amino acid sequences of the Arabidopsis and soybean MPK3/4/6 peptides, which correspond to the human p44 MAPK epitope used to raise the antibody, are identical (Supplemental Fig. S2). To resolve this issue, an in gel kinase activity assay was performed. Similar to the antibody-based assay, the in gel kinase activity assay also did not detect the activation of GmMPK4 either in the vector control plants or in the plants silenced for GmMEKK1 or GmMPK3, GmMPK4, and GmMPK6 (Fig. 8F). Activation of GmMPK6 was reduced but activation of GmMPK3 was enhanced in GmMEKK1-silenced plants, which also was consistent with the antibody-based assay (Fig. 8A). As expected, activation of GmMPK6 and GmMPK3 was reduced in the corresponding GmMPK6- and GmMPK3-silenced control plants (Fig. 8F).
We noticed that activation of GmMPK6 kinase activity was reduced in GmMPK4-silenced plants compared with nonsilenced BPMV-0 control plants (Fig. 8C). To investigate the possibility of cross silencing between GmMPK4 and GmMPK6, we performed immunoblot analyses using Arabidopsis MPK3, MPK4, and MPK6 antibodies. The signals detected by these antibodies in soybean were highly related to the amino acid sequence identities between the Arabidopsis epitopes used for raising the antibodies and the corresponding soybean peptides (Supplemental Fig. S3, A and B). The protein levels of GmMPK3, GmMPK4, and GmMPK6 were barely detectable in the respective silenced plants compared with nonsilenced control plants (Supplemental Fig. S3A), indicating that silencing of each gene occurred. Consistent with the kinase activation assay (Fig. 8C), the level of GmMPK6 protein indeed decreased in GmMPK4-silenced plants relative to vector control plants (Supplemental Fig. S3A). However, the level of GmMPK4 protein was not reduced in GmMPK6-silenced plants (Supplemental Fig. S3A), excluding the possibility of cross silencing between these two genes. Taken together, these results indicated that the silencing mediated by the BPMV-VIGS was specific to the targeted GmMPK.
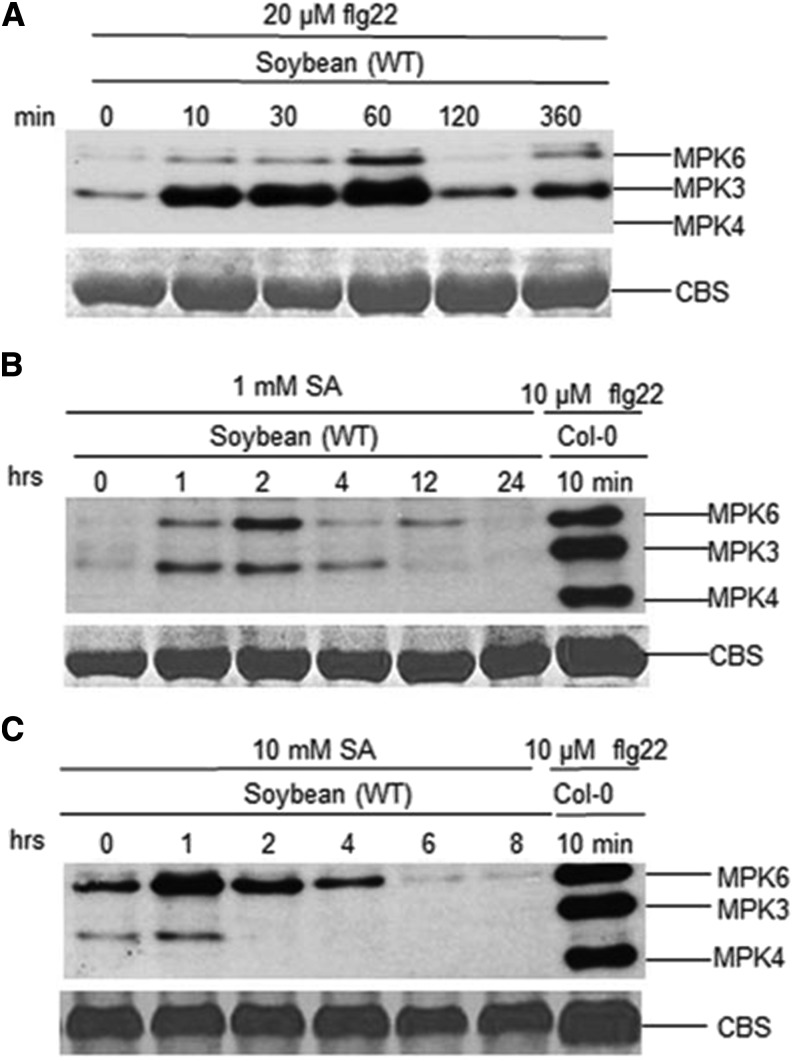
To test the possibility that GmMPK4 activation requires higher concentrations of flg22 and SA and/or has different kinetics from Arabidopsis MPK4, we treated soybean leaf tissues with 20 µm flg22 and 1 or 10 mm SA for different periods of time (Fig. 9). Regardless of the concentrations of flg22 and SA or the time points, the activation of GmMPK4 was not observed. Together, these results indicated distinct differences between soybean and Arabidopsis in terms of MPK4 activation, raising the possibility that GmMEKK1 negatively regulates cell death independent of GmMPK4 phosphorylation status.
Figure 9.
GmMPK4 kinase activation was not detected in the presence of flg22 or SA regardless of concentrations and time points. Leaf discs from wild-type (WT) soybean were incubated on wet filter paper for 24 h to allow recovery from wounding before being treated with 20 µm flg22 (A), 1 mm SA (B), or 10 mm SA (C) for the indicated time periods. Kinase activities were detected by immunoblotting using phospho-p44/42 MAP Erk1/2 antibody, which recognizes phosphorylated MPK3, MPK4, and MPK6 across kingdoms. The activation of Arabidopsis MPK3/4/6 by 10 µm flg22 was used as a control. CBS, Coomassie Blue staining for a loading control.
GmMEKK1 Interacts with Multiple Downstream GmMKKs
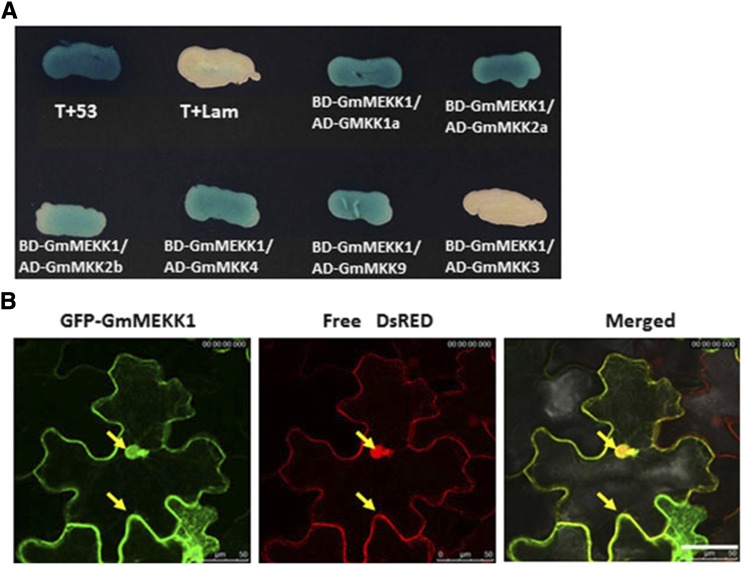
The MEKK1-MKK1/2-MPK4 module negatively regulates Arabidopsis defense responses (Gao et al., 2008), and MEKK1 interacts directly with MKK1 and MKK2 in yeast two-hybrid (Y2H) and bimolecular fluorescence complementation assays (Ichimura et al., 1998; Mizoguchi et al., 1998; Gao et al., 2008). MEKK1-MKK4/5 and MKK9 are involved in the activation of MPK3/MPK6 and camalexin production, but their direct interaction has not been reported previously (Ren et al., 2008; Xu et al., 2008). To test whether GmMEKK1 interacts with GmMKK1/GmMKK2, GmMKK4/GmMKK5, or GmMKK9, Y2H assays were performed. Consistent with Arabidopsis, GmMEKK1 interacted with GmMKK1a, GmMKK2a, and GmMKK2b, but it also interacted with GmMKK4 and GmMKK9 (Fig. 10A), suggesting that GmMEKK1 might exert its function through multiple downstream GmMKKs. However, interaction was not detected between GmMEKK1 and GmMKK3 (Fig. 10A).
Figure 10.
Identification of GmMEKK1-interacting partners and subcellular localization of GmMEKK1. A, A Y2H assay was performed between GmMEKK1 (Glyma.04G253500) and several GmMKKs, including GmMKK1a (Glyma.15G172600), GmMKK2a (Glyma.17G052400), GmMKK2b (Glyma.13G106900), GmMKK4 (Glyma.08G223400), and GmMKK9 (Glyma.09G172500). T-antigen and p53 were included as a positive interaction control, and Lam and T-antigen were included as a negative interaction control. B, GmMEKK1a localization in both the cytoplasm and nucleus of N. benthamiana epidermal cells. Agrobacterium tumefaciens carrying 35S::GFP-GmMEKK1a and 35S::DsRed constructs was coinfiltrated into N. benthamiana leaves. At 2 dpi, the infiltrated area of the leaves was excised and viewed by confocal microscopy. The left image shows the transient expression of GFP-GmMEKK1a in N. benthamiana cells; the middle image shows the transient expression of free DsRed in N. benthamiana cells; and the right image shows the merged image. Bar = 100 μm.
GmMEKK1 Localizes to the Cytosol and Nucleus
To investigate the subcellular location of GmMEKK1, the full-length GmMEKK1 was fused to the C terminus of GFP and transiently coexpressed in N. benthamiana leaves with a free DsRed-expressing construct via agroinfiltration. 35S::GFP-GmMEKK1 colocalized with free 35S::DsRed, indicating that GmMEKK1 was present in both the cytosol and the nucleus (Fig. 10B). Because the strong constitutive 35S promoter could potentially bias the results, we transiently expressed a GmMEKK1-GFP fusion protein driven by the native GmMEKK1 promoter (∼1.2 kb upstream of the start codon). Although the fluorescence intensity was reduced significantly compared with the expression driven by the 35S promoter, GmMEKK1-GFP was observed similarly in the cytoplasm and nucleus (Supplemental Fig. S4). The subcellular localization of MEKK1 in other plant species has not been reported previously, even though bimolecular fluorescence complementation assays have shown MEKK1 interacting with MKK1 or MKK2 only at the plasma membrane in Arabidopsis protoplasts (Gao et al., 2008). GmMEKK1 shares similar subcellular localization with its potential downstream target GmMPK4 (Liu et al., 2011).
Transient Overexpression of GmMEKK1a in N. benthamiana Induces HR
We have shown previously that GmMPK6 plays both positive and negative roles in cell death and defense pathways (Liu et al., 2014). To test whether GmMEKK1a also plays a positive role in inducing HR, we transiently expressed 35S-GmMEKK1a using agroinfiltration (Liu et al., 2005). Overexpression of GmMEKK1a induced HR cell death in N. benthamiana leaves (Supplemental Fig. S5), indicating that it also may promote defense responses. Interestingly, transient overexpression of a kinase-dead version of GmMEKK1 (K321M, equivalent to K361M of Arabidopsis MEKK1 as described by Suarez-Rodriguez et al. [2007]) did not induce cell death (Supplemental Fig. S5), indicating that the kinase activity of GmMEKK1 is required to induce cell death. As expected, transient overexpression of 35S::PtoY207D (active Pto) triggered HR-like cell death (Rathjen et al., 1999). This result indicates that GmMEKK1a can induce cell death when expressed ectopically in the leaves of N. benthamiana, further confirming that the kinase activity in MAPK signaling pathways must be tightly controlled.
DISCUSSION
Does GmMEKK1 Function Upstream of GmMPK4?
Compared with GmMPK4-silenced plants, silencing GmMEKK1 resulted in a more severe constitutively activated defense response, which was manifested by the more severe HR observed on both the inoculated local and systemic leaves (Fig. 1; Liu et al., 2011). For example, GmMEKK1-silenced plants typically died by 25 dpi, while GmMPK4-silenced plants remained alive (Liu et al., 2011). These constitutive defense phenotypes are consistent with the idea that GmMEKK1 might be the MAP3K that functions upstream of GmMPK4. In Arabidopsis, the mekk1, mkk1/mkk2, and mpk4 mutants exhibit a gradient of phenotypic severities, and the activation of MPK4 activity in response to flg22 in mekk1 and mkk1/mkk2 mutants is abolished, supporting their proposed functional hierarchy in a signaling cascade (Ichimura et al., 2006; Nakagami et al., 2006; Suarez-Rodriguez et al., 2007; Gao et al., 2008; Qiu et al., 2008).
We have shown previously that silencing GmMPK4 induced HR and defense responses, and both GmMKK1 and GmMKK2 interacted with GmMPK4 in vivo and phosphorylated GmMPK4 in vitro (Liu et al., 2011). Here, we showed that GmMEKK1 interacted with GmMKK1/2 in Y2H assays (Fig. 10A). In addition, there was a significant overlap in the genes induced in GmMEKK1- and GmMPK4-silenced plants (Supplemental Data Set S7). It seems that a similar GmMEKK1-GmMKK1/GmMKK2-GmMPK4 module also might be operating in soybean. However, this signaling module cannot be firmly established because the activation of GmMPK4 by flg22 or SA was not observed in the BPMV-0 control plants (Figs. 8 and 9). Therefore, we could not determine whether GmMEKK1 kinase activity is required for GmMPK4 activation. These results indicate that a distinctive difference exists in MPK4 activation between soybean and Arabidopsis (Supplemental Fig. S6). In addition, unlike Arabidopsis, in which both MPK3 and MPK6 are activated to much higher levels in mekk1 plants than in wild-type plants in response to flg22 (Ichimura et al., 2006), silencing GmMEKK1 enhanced the activation of GmMPK3 but reduced the activation of GmMPK6 in response to flg22 (Fig. 8, B and D), further supporting that distinctive differences exist between soybean and Arabidopsis in the activation of MAPKs.
As the cell-death phenotype develops in Arabidopsis mekk1, mkk1/2, and mpk4 mutant plants under normal growth conditions, the activation of MPK4 is barely detectable even in wild-type Columbia-0 plants (Ichimura et al., 2006; Nakagami et al., 2006; Gao et al., 2008). Therefore, the statement that the inactivation of MPK4’s kinase activity is necessary for the activated defense responses observed in Arabidopsis mekk1, mkk1/2, and mpk4 mutant plants may not be established unequivocally. Thus, it is possible that a function of MPK4 other than its kinase activity may be required to suppress cell death under normal growth conditions. It will be interesting to test whether a kinase-dead version of MPK4 can rescue the cell-death phenotypes of an mpk4 mutant, which would parallel the rescue of some mekk1 phenotypes by a kinase-dead version of MEKK1 (Suarez-Rodriguez et al., 2007). Because GmMPK4 cannot be activated by either flg22 or SA, it may not be involved in the processes triggered by these stimuli. However, we cannot eliminate the possibility that a slight attenuation of MPK4 activation, which is undetectable by the methods we used, is sufficient to trigger the cell death in Arabidopsis mekk1 and mkk1/mkk2 mutants and in GmMEKK1-silenced plants.
A recent study showed that constitutively activated MPK3 triggers defense responses in Arabidopsis, including dwarfed stature, massive induction of defense-related gene expression, and spontaneous cell death (Genot et al., 2017). Silencing GmMEKK1, GmMPK4, or GmMPK6 increased the activation of GmMPK3 (Fig. 8, A, C, D, and F), which coincides with the spontaneous cell death and constitutive activation of defenses (Liu et al., 2011, 2014). Therefore, the activated defense responses and cell death observed in GmMEKK1-, GmMPK4-, or GmMPK6-silenced plants could be the result of GmMPK3 activation. In contrast to GmMPK3, the activation of GmMPK6 by flg22 was compromised in GmMEKK1-silenced plants (Fig. 8A). Therefore, the activation of GmMPK3 and GmMPK6 is differentially regulated in GmMEKK1-silenced plants. Because GmMEKK1 also interacts with multiple GmMKKs, our results raise the question of whether GmMEKK1 exerts its function through a linear GmMEKK1-GmMKK1/2-GmMPK4 pathway or through a branched network (Supplemental Fig. S6).
An additional band was detected below GmMPK3 in the GmMEKK1- and GmMPK6-silenced plants in our kinase activation assays (see band below GmMPK3 in Fig. 8, A and D), and a band corresponding to Arabidopsis MPK4 was not detected in either vector control or any of the silenced soybean plants (Fig. 8). One may argue that the band below GmMPK3 could represent GmMPK4, but the following three lines of evidence may exclude this possibility. First, the band sizes between Arabidopsis MPK3 and GmMPK3, MPK4 and GmMPK4, and MPK6 and GmMPK6 were almost identical in our immunoblot analysis using the respective Arabidopsis antibodies (Supplemental Fig. S3A). Second, the size of the band below GmMPK3 was significantly larger than Arabidopsis MPK4 in our kinase activation assays (Supplemental Fig. S3C). Third, MEKK1 functions upstream of MPK4 in Arabidopsis, and the activation of MPK4 is abolished in mekk1 (Suarez-Rodriguez et al., 2007; Gao et al., 2008). If the function of MEKK1 is conserved between Arabidopsis and soybean, the band below GmMPK3 is unlikely to be GmMPK4.
Overlapping But Distinct Transcriptome Patterns between GmMEKK1- and GmMPK4-Silenced Plants
Kovtun et al. (2000) showed that the H2O2-MAPK cascade could repress auxin responses in protoplasts. The mekk1 and mpk4 mutants are compromised in the expression of auxin-induced genes (Nakagami et al., 2006; Qiu et al., 2008; Pitzschke et al., 2009a). Genome-wide microarray analysis indicated that even though a large cohort of genes is coregulated in mpk4, mkk1/2, and mekk1 mutants, distinct sets of genes are expressed specifically in each mutant, suggesting both conserved and divergent roles of these kinases in regulating gene expression (Pitzschke et al., 2009a).
Analyses of GmMPK4-silenced plants were conducted previously using the soybean Affymetrix GeneChip Soybean Genome Array (Liu et al., 2011). Of the 56,044 genes present in the current genome assembly, 18,619 (33%) were represented on the array. To compare gene expression patterns between GmMEKK1- and GmMPK4-silenced plants, we converted the GmMPK4-silencing data to current gene calls. Of the 2,413 genes induced by GmMPK4 silencing, 1,243 (52%) also were induced by GmMEKK1 silencing. Not surprisingly, GO analyses revealed that the commonly induced genes were significantly (corrected P < 0.05) overrepresented with GO terms related to defense, immunity, and stress (Supplemental Data Set S7). Similarly, of the 3,471 genes repressed by GmMPK4 silencing, 1,412 (41%) also were repressed by GmMEKK1 silencing. These commonly repressed genes were significantly overrepresented with GO terms related to growth, photosynthesis, and metabolism (Supplemental Data Set S7). Since only a subset of genes present in the genome were present on the array used to characterize GmMPK4-silenced plants, we cannot absolutely analyze genes unique to GmMEKK1 silencing, but we can examine genes unique to GmMPK4 silencing. The 1,170 uniquely induced genes also were significantly overrepresented with GO terms associated with defense and stress responses (Supplemental Data Set S7). The 2,059 uniquely repressed genes were overrepresented with GO terms related to the cell cycle, cell division, methylation, and gene silencing (Supplemental Data Set S7). These results indicate strong overlapping but distinct transcriptome patterns between GmMEKK1- and GmMPK4-silenced plants. Similar transcriptome patterns also are shared by mekk1, mkk1/mkk2, and mpk4 mutants in Arabidopsis (Qiu et al., 2008; Pitzschke et al., 2009a). The distinct expression patterns between GmMEKK1- and GmMPK4-silenced plants could be explained by the high-level complexity of the MAPK pathway. It is highly possible that GmMEKK1 can regulate gene expression independent of GmMPK4 and vice versa.
Is GmMEKK1 a Double-Edged Sword in Regulating Cell Death?
Transient overexpression of GmMEKK1 possessing kinase activity was required for HR on N. benthamiana leaves (Supplemental Fig. S5). Our numerous efforts to generate transgenic Arabidopsis plants that overexpress wild-type GmMEKK1 under the control of the constitutive cauliflower mosaic virus 35S promoter or an inducible promoter failed, implying that it also might play a positive role in defense. Similarly, overexpression of MEKK1 in Arabidopsis is lethal (Suarez-Rodriguez et al., 2007), suggesting that, regardless of plant species, the expression of MEKK1 must be tightly regulated to properly control growth/development and defense. Defense responses will be activated if MEKK1 activity is either too low or too high, which will lead to defense-oriented transcriptomic reprogramming. This seemingly dual role is not unexpected for genes in soybean MAPK pathways based on our previous observation that either silencing or overexpressing GmMPK6 triggers defense (Liu et al., 2014).
CONCLUSION
Our results revealed similarities and distinct differences between soybean and Arabidopsis MEKK1 (Supplemental Fig. S6). Like its Arabidopsis counterpart, GmMEKK1 is both a positive and negative regulator of soybean cell death and defense responses. Unlike in Arabidopsis, where MPK4 functions downstream of MEKK1, we were unable to demonstrate conclusively that GmMPK4 functions downstream of GmMEKK1. In addition, we found that GmMEKK1 positively regulates GmMPK6 but negatively regulates GmMPK3 activity in response to flg22. These interesting differences in the functional relationships between GmMEKK1 and GmMPK3/4/6 will need to be resolved in the future. Furthermore, significantly increased activation of GmMPK3 in response to flg22 was observed in GmMEKK1-, GmMPK4-, and GmMPK6-silenced plants (Fig. 8, A, C, and D), which is associated with cell death and the constitutively activated defense observed in these plants (Fig. 1; Liu et al., 2011, 2014). Thus, the relevance of the GmMPK3 activation in cell death and defense will be a focus in future studies. Together, our results indicate that the MAPK signaling pathway in soybean is more complex than expected. It appears that the soybean MAPK components we have studied cooperate in ways not predicted from previous work in Arabidopsis to form an interconnected, multidirectional network rather than unidirectional linear pathways.
MATERIALS AND METHODS
Plant Materials
Seeds of soybean (Glycine max ‘Williams 82’) and Nicotiana benthamiana used in this study were harvested from greenhouse-grown plants. Soybean plants were maintained in the greenhouse or growth chamber at 22°C with a photoperiod of 16 h, unless indicated otherwise.
BPMV-Mediated VIGS
BPMV strains, BPMV-VIGS constructs, and inoculation of soybean seedlings with DNA-based BPMV constructs via biolistic particle bombardment using a Biolistic PDS-1000/He system (Bio-Rad Laboratories) have been described previously (Zhang et al., 2010, 2013). The orthologs of MEKK1 were identified by reciprocal best BLASTn searches (cutoff value < 0.001) between Arabidopsis (Arabidopsis thaliana) MEKK1 and soybean gene models available in the Phytozome database (www.phytozome.org). A 360-bp fragment of GmMEKK1 was amplified by PCR using the following primers: GmMEKK1a-F (Glyma.04G253500), 5′-aagGGATCCATGCATTACCTATCTCGGATT-3′, and GmMEKK1-R, 5′-ttgGGTACCCCTCGGGAATATCGAGGTTATCG-3′. The underlined sequences are BamHI and KpnI restriction sites, respectively, that were used to clone the PCR fragment into the BPMV-VIGS (IA-D35). The boldface letter indicates an extra nucleotide in reverse primers needed to maintain the reading frame.
RNA Isolation and RT-qPCR
RNA isolation and RT-qPCR were performed as described elsewhere (Liu et al., 2014). The RT-qPCR tests were performed using an iCycler (Bio-Rad Laboratories) and the Platinum SYBRGreen qPCR SuperMix UDG (Invitrogen). Total RNA samples were treated with DNaseI according to the manufacturer’s instructions (Invitrogen). The primers used for qPCR are listed in Supplemental Table S1.
RNA-Seq Analysis
Sequencing was performed at the National Center for Genome Resources on an Illumina Genome Analyzer II as described by Moran Lauter et al. (2014). In brief, eight multiplex libraries were prepared from four biological replicates of BPMV empty vector control and GmMEKK1-silenced plants. Sequencing produced a total of 177,027,059 single-end reads. TopHat (version 2.0.3; Trapnell et al., 2009) was used to align reads to the cv Williams 82 reference genome using default settings (version G. max 2.1; Schmutz et al., 2010). SAMtools (Li et al., 2009; Schmutz et al., 2010) was used to remove unreliably mapped reads. The resulting mapping files (bam) were imported into the statistical program R (R Development Core Team, 2006). The Bioconductor package rtracklayer (Lawrence et al., 2009) was used to import the gene feature file corresponding to G. max version 2.1. The package GenomicRanges was used to count reads and output a matrix containing gene counts for each sample. Prior to normalization and statistical analysis, counts assigned to GmMEKK1a and GmMEKK1b were removed, as they could be of viral origin. Genes with counts per million < 1 in at least three of the four biological replicates were eliminated from the analyses. The Bioconductor package edgeR (Robinson et al., 2010) was used for single-factor, pairwise comparisons to calculate normalization factors and determine differential expression. Differentially expressed genes were considered significant if their fold change was greater than 2 with an FDR < 0.001. Significantly differentially expressed genes were annotated using the SoyBase Genome Annotation Report page (www.soybase.org/genomeannotation), which provided best Arabidopsis homologs, and GO information inferred from Arabidopsis (TAIR version 10; www.arabidopsis.org). Significantly (P < 0.05) overrepresented biological process GO terms were identified using the SoyBase GO Term Enrichment Tool (https://www.soybase.org/goslimgraphic_v2/dashboard.php), which uses Fisher’s exact test (Fisher, 1966) and Bonferroni correction (Bonferroni, 1935). The SoyDB transcription factor database was used to identify transcription factors among differentially expressed genes (Wang et al., 2010). To facilitate comparisons between GmMEKK1- and GmMPK4-silenced plants, we used the SoyBase Gene Model Correspondence Lookup (https://www.soybase.org/correspondence/).
SA Quantification
SA was quantified using an Agilent 1100 high-performance liquid chromatograph with fluorometric detection (Agilent Technologies) as described previously (Liu et al., 2011). The column was a 4.6-by-75-mm Agilent RR XDB C18 with an isocratic mobile phase comprising 75% (v/v) 20 mm formate (pH 3.8), 20% methanol (v/v), and 5% acetonitrile (v/v) at a flow rate of 0.75 mL min−1 at 35°C. SA-O-glucoside was measured after converting to free SA by acid hydrolysis. Recovery rates were determined using o-anisic acid as an internal standard and were typically greater than 60% (v/v).
H2O2 Detection by DAB Staining
H2O2 was detected by an endogenous peroxidase-dependent in situ histochemical staining procedure using DAB (Sigma-Aldrich; Ren et al., 2002). Leaves were detached and placed in a solution containing 1 mg mL−1 DAB (pH 5.5) for 2 h. The leaves were cleared by boiling in 96% ethanol for 10 min and then stored in 96% ethanol (v/v). H2O2 production was visualized as a reddish-brown precipitate in cleared leaves (Ren et al., 2002).
SMV-N-GUS Inoculation, GUS Staining, and GUS Foci Measurements
SMV-N-GUS inoculation, GUS staining, and GUS foci measurements were described previously (Liu et al., 2011). Briefly, at 15 dpi with BPMV empty vector (BPMV-0) or BPMV-GmMEKK1 constructs by rub inoculation, the second fully expanded soybean trifoliate leaves counting from the top were detached and biolistically inoculated with SMV-N-GUS (Wang et al., 2006; Zhang et al., 2009). After SMV-N-GUS inoculation, the detached leaves were incubated for 3 d on moist filter paper in petri dishes maintained on a lit growth shelf. GUS staining was then performed as described (Jefferson et al., 1987). The leaves were viewed and photographed using a stereomicroscope (Olympus SZX16). The GUS foci were counted, and their diameters were measured using Image-Pro Plus 6.0 (Media Cybernetics).
Downy Mildew Infection
The isolate of Peronospora manshurica used in these studies was described previously (Liu et al., 2011). Vector control and GmMEKK1-silenced soybean plants were inoculated by spraying with a suspension of P. manshurica sporangia in deionized water. Plants were kept in the dark at high humidity overnight and then moved to the greenhouse for 7 d. The photographs for the symptoms were taken, and samples for microscopy were collected 1 week after inoculation. Pathogen structures in the infected foci were visualized using a KOH-Aniline Blue staining procedure (Hood and Shew, 1996). Tissue samples were placed in 1 m KOH for 24 h and then heated in 1 m KOH for 30 min at 80°C. Samples were rinsed three times with distilled water and soaked in 0.05% Aniline Blue in 0.7 m K2HPO4, pH 9, for 15 min. Specimens were mounted in the same staining solution and observed with a Leitz Fluovert epifluorescence microscope with UV illumination (exciter filter, BP 340–380; dichroic mirror, RKP 400; barrier filter, LP 430). Autofluorescence was observed in leaf specimens that were fixed in boiling 95% ethanol and cleared for several days in saturated chloral hydrate (Heath, 1984). The cleared specimens were mounted in 50% glycerol and observed with blue illumination (exciter filter, BP 420–490; dichroic mirror, RKP 510; barrier filter, LP 520).
Subcellular Localization of GmMEKK1
The GmMEKK1 (Glyma.04G253500) open reading frame was amplified by RT-PCR from total RNA extracted from cv Williams 82 soybean plants. The PCR product was initially cloned into the pENTR/D TOPO vector (Invitrogen) and then recombined into the binary destination vector pB7WGF2,0 (Karimi et al., 2002) to generate the GFP-GmMEKK1 fusion construct. This fusion construct and the free DsRed construct were coinfiltrated into N. benthamiana leaves as described (Liu et al., 2005). Images were captured with a confocal laser-scanning microscope (Leica TCS SP5 AOBS).
Immunoblot Analysis for Detecting Phosphorylated MPKs
Protein was extracted from soybean leaf tissues using native extraction buffer (50 mm Tris-MES, pH 8, 0.5 m Suc, 1 mm MgCl2, 10 mm EDTA, 5 mm DTT, and protease inhibitor cocktail S8830 [Sigma-Aldrich]). The extract was centrifuged at 12,000 rpm at 4°C for 30 min. For immunoblotting, proteins were separated by SDS-PAGE (10% acrylamide gel) and transferred to PVDF membranes (Millipore) by semidry electrotransfer (Bio-Rad). The membrane was blocked in 1× TBS buffer containing 5% skim milk powder and incubated further with anti-phospho-p44/p42 MAPK (anti-pTEpY) diluted at 1:2,000 (Cell Signaling Technology) and subsequently with secondary antibody diluted at 1:6,000. Finally, the bands were detected using chemiluminescent horseradish peroxidase (HRP) substrate (Millipore).
In Gel Kinase Activity Assay
Protein was extracted from soybean leaf tissues as described (Liu and Zhang, 2004), and the concentration was determined using the Bio-Rad protein assay kit with BSA as the standard. In gel kinase assay was performed essentially as described previously using myelin basic protein as the substrate (Zhang and Klessig, 1997).
Y2H Assay for MEKK1-Interacting Partners
A Y2H screen was performed as described in the BD Matchmaker Library Construction and Screening Kits (Clontech). The complete coding sequences of GmMEKK1a (Glyma.04G253500), GmMKK1a (Glyma.15G172600), GmMKK2a (Glyma.17G052400), GmMKK2b (Glyma.13G106900), GmMKK4 (Glyma.07G0465000 or Glyma.08G223400), and GmMKK9 (Glyma.09G172500) were fused to either the GAL4 DNA-binding domain (pGBKT7) or activation domain (pGADT7) to generate pGBKT7- or pGADT7-MAP3K or -MAP2K and then cointroduced into yeast (Saccharomyces cerevisiae) strain Y187. Detection of the interactions between the MAP2Ks and GmMEKK1 was performed according to the manufacturer’s manual (Clontech). The primers used for making the constructs are listed in Supplemental Table S2.
Accession Numbers
The full RNA-seq data sets generated in this study were deposited in the National Center for Biotechnology Small Reads Archive under BioProject identifier PRJNA374025 (accession no. SRP099206).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Nucleotide sequence alignment of the GmMEKK1a fragment used for silencing and the corresponding sequence of GmMEKK1b.
Supplemental Figure S2. Amino acid sequence alignment of the peptide used to raise the phospho-p44/42 MAP Erk1/2 antibody and the corresponding peptide sequences of Arabidopsis and soybean MPK3, MPK4, and MPK6.
Supplemental Figure S3. Confirmation of the specificity and effectiveness of GmMPK silencing using the BPMV-VIGS system.
Supplemental Figure S4. The GFP-GmMEKK1a fusion protein is localized in the cytoplasm and the nucleus when its expression is driven by a ∼1.2-kb native promoter of GmMEKK1.
Supplemental Figure S5. Transient overexpression of GmMEKK1a in N. benthamiana leaves results in HR-like cell death.
Supplemental Figure S6. Comparison of MEKK1-regulated cell death pathways in Arabidopsis and soybean.
Supplemental Table S1. Primers used for RT-PCR or RT-qPCR.
Supplemental Table S2. Primers used for making constructs.
Supplemental Data Set S1. Genes induced by GmMEKK1 silencing relative to vector control plants.
Supplemental Data Set S2. Genes repressed by GmMEKK1 silencing relative to vector control plants.
Supplemental Data Set S3. GO terms significantly overrepresented within genes induced by GmMEKK1 silencing.
Supplemental Data Set S4. GO terms significantly overrepresented within genes repressed by GmMEKK1 silencing.
Supplemental Data Set S5. Transcription factors differentially expressed in response to GmMEKK1 silencing.
Supplemental Data Set S6. Significantly overrepresented (P < 0.05) transcription factor families in GmMEKK1-silenced plants.
Supplemental Data Set S7. Comparisons of GO terms significantly overrepresented among genes common to GmMEKK1- and GmMPK4-silenced plants and genes unique to GmMPK4-silenced plants.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Drs. John Hill and Alan Eggenberger for providing the SMV-N-GUS construct and technical advice, Dr. Shuqun Zhang for the Arabidopsis MPK antibodies, and Dr. Jianwei Pan for providing the pBI101 vector. We also thank Jaime Dittman for technical support.
Footnotes
This work was supported by the Natural Science Foundation of China (31571423 and 31371401 to J.-Z.L.), by the Qianjiang Talent Program of Zhejiang Province (2013R10074 to J.-Z.L.), by the Xin Miao Program from Zhejiang Normal University (2013R404005 to J.-Z.L.), by the National Science Foundation Plant Genome Research Program (0820642 to S.A.W.), and by the U.S. Department of Agriculture-Agricultural Research Service projects (5030-21220-005-00D to M.A.G; 58-3092-5-001 to P.A.N).
Articles can be viewed without a subscription.
References
- Andreasson E, Jenkins T, Brodersen P, Thorgrimsen S, Petersen NH, Zhu S, Qiu JL, Micheelsen P, Rocher A, Petersen M, et al. (2005) The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J 24: 2579–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Bonferroni CE. (1935) Il calcolo delle assicurazioni su gruppi di teste. Studi in Onore del Professore Salvatore Ortu Carbon 13–60 [Google Scholar]
- Cheng NH, Liu JZ, Liu X, Wu Q, Thompson SM, Lin J, Chang J, Whitham SA, Park S, Cohen JD, et al. (2011) Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J Biol Chem 286: 20398–20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack RS, Eulgem T, Rushton PJ, Köchner P, Hahlbrock K, Somssich IE (2002) Leucine zipper-containing WRKY proteins widen the spectrum of immediate early elicitor-induced WRKY transcription factors in parsley. Biochim Biophys Acta 1576: 92–100 [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394: 585–588 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10: 366–371 [DOI] [PubMed] [Google Scholar]
- Fisher RA. (1966) The Design of Experiments. Oliver & Boyd, Oxford [Google Scholar]
- Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, Zhang Y (2008) MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res 18: 1190–1198 [DOI] [PubMed] [Google Scholar]
- Genot B, Lang J, Berriri S, Garmier M, Gilard F, Pateyron S, Haustraete K, Van Der Straeten D, Hirt H, Colcombet J (2017) Constitutively active Arabidopsis MAP Kinase 3 triggers defense responses involving salicylic acid and SUMM2 resistance protein. Plant Physiol 174: 1238–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadiarto T, Nanmori T, Matsuoka D, Iwasaki T, Sato K, Fukami Y, Azuma T, Yasuda T (2006) Activation of Arabidopsis MAPK kinase kinase (AtMEKK1) and induction of AtMEKK1-AtMEK1 pathway by wounding. Planta 223: 708–713 [DOI] [PubMed] [Google Scholar]
- He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nürnberger T, Sheen J (2006) Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell 125: 563–575 [DOI] [PubMed] [Google Scholar]
- Heath MC. (1984) Relationship between heat-induced fungal death and plant necrosis in compatible and incompatible interactions involving the bean and cowpea rust fungi. Phytopathology 74: 1370–1376 [Google Scholar]
- Hood ME, Shew HD (1996) Applications of KOH-aniline blue fluorescence in the study of plant-fungal interactions. Phytopathology 86: 704–708 [Google Scholar]
- Ichimura K, Mizoguchi T, Irie K, Morris P, Giraudat J, Matsumoto K, Shinozaki K (1998) Isolation of ATMEKK1 (a MAP kinase kinase kinase)-interacting proteins and analysis of a MAP kinase cascade in Arabidopsis. Biochem Biophys Res Commun 253: 532–543 [DOI] [PubMed] [Google Scholar]
- Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang SQ, Hirt H, Wilson C, et al. (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7: 301–308 [DOI] [PubMed] [Google Scholar]
- Ichimura K, Casais C, Peck SC, Shinozaki K, Shirasu K (2006) MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J Biol Chem 281: 36969–36976 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Axtell MJ, Dahlbeck D, Ekwenna O, Zhang S, Staskawicz B, Baker B (2002) NPK1, an MEKK1-like mitogen-activated protein kinase kinase kinase, regulates innate immunity and development in plants. Dev Cell 3: 291–297 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Lawrence M, Gentleman R, Carey V (2009) rtracklayer: an R package for interfacing with genome browsers. Bioinformatics 25: 1841–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HHB, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang S (2004) Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16: 3386–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Blancaflor EB, Nelson RS (2005) The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol 138: 1853–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Horstman HD, Braun E, Graham MA, Zhang C, Navarre D, Qiu WL, Lee Y, Nettleton D, Hill JH, et al. (2011) Soybean homologs of MPK4 negatively regulate defense responses and positively regulate growth and development. Plant Physiol 157: 1363–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Braun E, Qiu WL, Shi YF, Marcelino-Guimarães FC, Navarre D, Hill JH, Whitham SA (2014) Positive and negative roles for soybean MPK6 in regulating defense responses. Mol Plant Microbe Interact 27: 824–834 [DOI] [PubMed] [Google Scholar]
- Liu JZ, Graham MA, Pedley KF, Whitham SA (2015) Gaining insight into soybean defense responses using functional genomics approaches. Brief Funct Genomics 14: 283–290 [DOI] [PubMed] [Google Scholar]
- Liu JZ, Fang Y, Pang H (2016) The current status of the soybean-soybean mosaic virus (SMV) pathosystem. Front Microbiol 7: 1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S (2011) Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhang S (2013) MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 51: 245–266 [DOI] [PubMed] [Google Scholar]
- Mészáros T, Helfer A, Hatzimasoura E, Magyar Z, Serazetdinova L, Rios G, Bardóczy V, Teige M, Koncz C, Peck S, et al. (2006) The Arabidopsis MAP kinase kinase MKK1 participates in defence responses to the bacterial elicitor flagellin. Plant J 48: 485–498 [DOI] [PubMed] [Google Scholar]
- Miao Y, Laun TM, Smykowski A, Zentgraf U (2007) Arabidopsis MEKK1 can take a short cut: it can directly interact with senescence-related WRKY53 transcription factor on the protein level and can bind to its promoter. Plant Mol Biol 65: 63–76 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Ichimura K, Irie K, Morris P, Giraudat J, Matsumoto K, Shinozaki K (1998) Identification of a possible MAP kinase cascade in Arabidopsis thaliana based on pairwise yeast two-hybrid analysis and functional complementation tests of yeast mutants. FEBS Lett 437: 56–60 [DOI] [PubMed] [Google Scholar]
- Moran Lauter AN, Peiffer GA, Yin T, Whitham SA, Cook D, Shoemaker RC, Graham MA (2014) Identification of candidate genes involved in early iron deficiency chlorosis signaling in soybean (Glycine max) roots and leaves. BMC Genomics 15: 702–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Soukupová H, Schikora A, Zárský V, Hirt H (2006) A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem 281: 38697–38704 [DOI] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, et al. (2000) Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Pitzschke A. (2015) Modes of MAPK substrate recognition and control. Trends Plant Sci 20: 49–55 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Djamei A, Bitton F, Hirt H (2009a) A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol Plant 2: 120–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzschke A, Schikora A, Hirt H (2009b) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12: 421–426 [DOI] [PubMed] [Google Scholar]
- Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, Petersen K, Mackinlay J, Loake GJ, Mundy J, Morris PC (2008) Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol 148: 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MW, Roux M, Petersen M, Mundy J (2012) MAP kinase cascades in Arabidopsis innate immunity. Front Plant Sci 3: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen JP, Chang JH, Staskawicz BJ, Michelmore RW (1999) Constitutively active Pto induces a Prf-dependent hypersensitive response in the absence of avrPto. EMBO J 18: 3232–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2006) R A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Ren D, Yang H, Zhang S (2002) Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem 277: 559–565 [DOI] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S (2008) A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 105: 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15: 247–258 [DOI] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183 [DOI] [PubMed] [Google Scholar]
- Smeets K, Opdenakker K, Remans T, Forzani C, Hirt H, Vangronsveld J, Cuypers A (2013) The role of the kinase OXI1 in cadmium- and copper-induced molecular responses in Arabidopsis thaliana. Plant Cell Environ 36: 1228–1238 [DOI] [PubMed] [Google Scholar]
- Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, Zhang S, Bent AF, Krysan PJ (2007) MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol 143: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15: 141–152 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X (2007) Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol 17: 1784–1790 [DOI] [PubMed] [Google Scholar]
- Wang L, Eggenberger A, Hill J, Bogdanove AJ (2006) Pseudomonas syringae effector avrB confers soybean cultivar-specific avirulence on Soybean mosaic virus adapted for transgene expression but effector avrPto does not. Mol Plant Microbe Interact 19: 304–312 [DOI] [PubMed] [Google Scholar]
- Wang Z, Libault M, Joshi T, Valliyodan B, Nguyen HT, Xu D, Stacey G, Cheng J (2010) SoyDB: a knowledge database of soybean transcription factors. BMC Plant Biol 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chua NH (2012) Dehydration stress activates Arabidopsis MPK6 to signal DCP1 phosphorylation. EMBO J 31: 1975–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang S (2015) Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci 20: 56–64 [DOI] [PubMed] [Google Scholar]
- Xu J, Li Y, Wang Y, Liu H, Lei L, Yang H, Liu G, Ren D (2008) Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J Biol Chem 283: 26996–27006 [DOI] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (1997) Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9: 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yang C, Whitham SA, Hill JH (2009) Development and use of an efficient DNA-based viral gene silencing vector for soybean. Mol Plant Microbe Interact 22: 123–131 [DOI] [PubMed] [Google Scholar]
- Zhang C, Bradshaw JD, Whitham SA, Hill JH (2010) The development of an efficient multipurpose bean pod mottle virus viral vector set for foreign gene expression and RNA silencing. Plant Physiol 153: 52–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Whitham SA, Hill JH (2013) Virus-induced gene silencing in soybean and common bean. Methods Mol Biol 975: 149–156 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wu Y, Gao M, Zhang J, Kong Q, Liu Y, Ba H, Zhou J, Zhang Y (2012) Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 11: 253–263 [DOI] [PubMed] [Google Scholar]
- Zhao C, Nie H, Shen Q, Zhang S, Lukowitz W, Tang D (2014) EDR1 physically interacts with MKK4/MKK5 and negatively regulates a MAP kinase cascade to modulate plant innate immunity. PLoS Genet 10: e1004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wu S, Chen X, Liu C, Sheen J, Shan L, He P (2014) The Pseudomonas syringae effector HopF2 suppresses Arabidopsis immunity by targeting BAK1. Plant J 77: 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]