Abstract
Piliostigma thonningii (Schumach.) Milne-Redhead. (Leguminosae) is used for various medicinal purposes in African countries. Phytochemical investigation of P. thonningii yielded two compounds newly isolated from natural sources, 2β-methoxyclovan-9α-ol (1), and methyl-ent-3β-hydroxylabd-8(17)-en-15-oate (2), along with 14 known compounds (3–16). Compounds 1 and 4 (alepterolic acid) showed potential selectivity towards Trypanosoma brucei brucei with IC50 7.89 and 3.42 μM, respectively. Compound 2 showed activity towards T. brucei and Leishmania donovani Amastigote with IC50 3.84 and 7.82 μM, respectively. The structure activity relationship (SAR) of the isolated metabolites suggested that hydroxylation at C-2 enhances the antiprotozoal activity towards T. brucei in sesquiterpenes 1 and 3. Similarly hydroxylation at C-3 in labdane diterpenes elevates the antiprotozoal activity towards T. brucei.
Keywords: Piliostigma thonningii, Sesquiterpene, diterpene, Trypanosoma brucei, Leishmania donovani
Introduction
Utilization of plants for different medicinal purposes has been known for thousands of years (Samuelsson 2004). Plants initially used in crude forms such as teas, powders, tinctures, poultices, and other herbal formulations (Samuelsson 2004). In the early 19th century, the use of plants as medicines has involved the isolation of active compounds, beginning with the isolation of morphine from opium (Kinghorn 2001; Samuelsson 2004). Several known active compounds were isolated from African medicinal plants such as Betulinic acid, Combretastatin A4 phosphate, and Harpagoside (Salim et al. 2008). The West African plant Piliostigma thonningii, (Milne-Redhead) belongs to the subfamily Caesalpinioideae in the legume family, Leguminosae/Fabaceae. In African countries P. thonningii is used for various medicinal purposes (Silva et al. 1997). The decoction of the leaves and bark is used for the treatment of ulcers, wounds, heart pain, arthritis, malaria, pyrexia, leprosy, sore throat, diarrhea, toothache, gingivitis, cough, and bronchitis (Ibewuike et al. 1996; Ighodaro and Omole 2012). Its roots and twigs are used in the treatment of dysentery, fever, wound infections, cough, and skin diseases (Asuzu and Onu 1994). The crude extract of P. thonningii was reported to possess antilipidemic (Ighodaro and Omole 2012), antibacterial (Akinpelu and Obuotor 2000), antihelminthic (Asuzu and Onu 1994), and antiinflammatory (Ibewuike et al. 1997) activities.
Previous phytochemical studies on P. thoningii revealed the presence of diverse chemical classes of compounds that possibly accommodate for the various activities of this medicinal plant. Among the identified chemical classes are flavonoids, tannins, kaurane diterpenes, alkaloids, carbohydrates, saponins, terpenes, and volatile oils (Baratta et al. 1999; Egharevba and Folashade 2010; Ibewuike et al. 1997; Ighodaro et al. 2012; Martin et al. 1997). A representative crucial metabolite isolated from P. thonningii is D-3-O-methylchiroinosital, which possesses anthelmintic activity (Asuzu et al. 1999), analgesic, antipyretic, antidiabetic, antioxidant, and antilipidemic activities (Asuzu and Nwaehujor 2013; Nwaehujor et al. 2015); another potential example is C-methyl flavanols, which was identified from the same species and showed antibacterial and antiinflammatory activities (Ibewuike et al. 1997). In continuation to our studies on African medicinal plants (Afolayan et al. 2018; Mohamed et al. 2016a, 2017, 2016b; Mostafa et al. 2016), and based on our in-house battery of screening, we have perused leishmanial and trypnasomal studies on the chemical constituents of P. thonningii.
Material and methods
General experimental
A Bruker model AMX 500 NMR and 400 NMR spectrometer operating on a standard pulse system collected 1H and 13C NMR spectra. The instrument ran at 500 and 400 MHz for 1H and 125–100 MHz for 13C. CDCl3, CD3OD, DMSO-d6, and C5D5N were used as solvents, and TMS was used as an internal standard. HR-MS was performed on Agilent 1100 HPLC coupled to a JOEL AccuTOF (JMS-T100LC) (Peabody, MA). FT-IR spectrum 100 was used to record neat IR spectra for the isolated compounds. ESI-MS was analyzed in Orbitrap (Mass error on the instrument <2 ppm). TLC was performed on precoated silica gel GF254 plates and Column Chromatography was performed on silica gel (200–300 mesh) (Sorbent Technologies, Atlanta, GA, USA).
Plant material
P. thoningii leaves were collected during the rainy season (June 2016) from the medicinal plant garden at the Sheda Science and Technology Complex (SHESTCO), Abuja, Nigeria. The leaves were identified and authenticated at the Forestry Research Institute of Nigeria (FRIN), Ibadan, Oyo state, Nigeria, by Mr. A. Adeyemo, where a voucher specimen was deposited with the assigned number FHI 110688.
Extraction and isolation
P. thoningii leaves were dried and grounded and the ground leaves (1.5 kg) were extracted using MeOH (7 L). The extract was filtered and concentrated at 40 °C yielding 235.6 g of crude methanolic extract. The crude methanolic extract (80 g) was triturated with water: MeOH (50:50, 500 mL) and partitioned successively using CH2Cl2 (500 mL), EtOAc (500 mL), and n-butanol (500 mL). Each fraction was evaporated to yield 8.7 g CH2Cl2 fraction (A), 8.5 g EtOAc fraction (B), and 32.4 g n-butanol fraction (C).
Fraction A (8 g) was loaded onto a silica gel column and eluted using n-hexanes-acetone gradient to yield 19 fractions (A1–A19). Fraction A1 (240 mg) was purified over silica gel column using n-hexanes— EtOAc gradient yielded 5.5 mg of α-tocopherol (vitamin E, 8) and 2.6 mg of β-amyrin (7). Fraction A2 (900 mg) was loaded on silica gel column and eluted with n-hexanes—EtOAc gradient yielded stigmasterol (15, 150 mg) and 5.6 mg of 2β-methoxyclovan-9α-ol (1, about 90% purity based on its NMR spectral data). Fractions A3 and A4 were pooled together (150 mg) and purified over silica gel column using EtOAc—n-hexanes to yield 11.3 mg of methyl ent-3β-hydroxylabd-8(17)-en-15-oate (2). Fraction A12 was identified to be piliostigmin (9, 3.0 mg). Fraction A13 (150 mg) yielded two compounds while purifying it over silica gel column using n-hexanes—EtOAc gradient with increasing polarity, which were identified as alepterolic acid (4, 19.5 mg) and chlorae-2β, 9α-diol (3, 2.4 mg).
Fraction B (8 g) was further fractionated on normal phase VLC using a mixture of EtOAc, CH2Cl2, MeOH, and H2O in three ratios (15:8:4:1; 10:6:4:1; 6:4:4:1) to give three fractions (B1–B3). The first fraction B1 (2 g) was loaded on a normal phase column and eluted with CH2Cl2:MeOH gradient with increasing polarity to yield six fractions (D1–D6). Fraction D1 was identified as anticopalic acid (5, 14.2 mg), Fraction D2 (500 mg) was subjected to column chromatography over silica gel using CH2Cl2 and MeOH gradient with increasing polarity yielded 3.5 mg of (3R,5R,6R)-trihydroxy-7E-megastigmen-9-one (6), 21.9 mg of (+)-epicatechin (10), and 2.4 mg of quercetin (11). Fraction D3 (200 mg) was loaded on silica gel column and eluted with CH2Cl2 and MeOH gradient with increasing polarity yielded 12.2 mg of β-sitosterol glucoside (16), 3.5 mg of kampferol-3-O-rhamnoside (afzelin, 13), and 31.4 mg of quercetin-3-O-rhamnoside (quercitrin, 12). Fraction B2 (2.5 g) was loaded on silica gel column and eluted with CH2Cl2 and MeOH gradient with increasing polarity to yield eight fractions (E1–E8). Fraction E2 was identified as 3-hexenyl-1-O-β-D-glucopyranoside (14, 3.5 mg).
2β-methoxyclovan-9α-ol (1)
Yellow oil; [α]D25 = +52.8 (c 0.009, MeOH); IR (neat): νmax 3416, 2928, 1453 cm−1; for 1H and 13C NMR data, see Table 1; HR-MS [M+Na]+ m/z 275.1869 (calc. for C16H28NaO2 275.1987).
Table 1.
13C and 1H NMR data for compounds 1 and 2 in CDCl3 (δC and δH in ppm; J in Hz)
| Position | 1a |
2a |
||
|---|---|---|---|---|
| 13C NMR | 1H NMR | 13C NMR | 1H NMR | |
| 1 | 44.3 | – | 37.2 | 1.77 m, 1.17 m |
| 2 | 90.3 | 3.32 m | 28.1 | 1.70 m, 1.60 m |
| 3 | 44.2 | 1.70 m, 1.45 m | 79.0 | 3.25 dd (4.5, 12.0) |
| 4 | 37.1 | – | 39.3 | – |
| 5 | 50.7 | 1.40 m | 54.7 | 1.08 dd (2.5, 12.5) |
| 6 | 20.7 | 1.40 m | 24.1 | 1.73 m, 1.34 m |
| 7 | 33.2 | 2.33 m | 38.3 | 2.38 m, 1.96 m |
| 8 | 34.9 | – | 148.2 | – |
| 9 | 75.4 | 3.30 m | 56.8 | 1.53 m |
| 10 | 26.1 | 1.61 m | 39.5 | – |
| 11 | 26.7 | 1.98 m, 1.66 m | 21.1 | 1.40 m |
| 12 | 36.7 | 1.61 m, 1.25 m | 35.8 | 1.32 m, 1.11 m |
| 13 | 25.5 | 0.85 s | 31.0 | 1.91 m |
| 14 | 31.4 | 1.02 s | 42.0 | 2.27 dd (6, 15), 2.12 dd (8, 15) |
| 15 | 28.5 | 0.96 s | 173.9 | – |
| 16 | – | – | 19.8 | 0.94 d (6.5) |
| 17 | – | – | 106.8 | 4.82 d (1.5), 4.48 d (1.5) |
| 18 | – | – | 28.4 | 0.99 s |
| 19 | – | – | 15.5 | 0.77 s |
| 20 | – | – | 14.6 | 0.68 s |
| 2-OCH3 | 58.4 | 3.35 s | – | – |
| 15-OCH3 | – | – | 51.5 | 3.66 s |
1H NMR carried out at 500 MHz, 13C NMR carried out at 125 MHz
The assignments were based on 1H–1H COSY, HSQC, and HMBC experiments.
Methyl ent-3β-hydroxylabd-8(17)-en-15-oate (2)
Yellow oil; IR (neat): νmax3419, 2924, 1733 cm−1; 1H and 13C NMR data, Table 1; HR-MS [M+Na]+ m/z 359.2524 (calc. for C21H36NaO3 359.2562).
Biological evaluation
In vitro antitrypanosomal assays
Blood stage forms of Trypanosoma brucei brucei was grown in IMDM medium supplemented with 10% fetal bovine serum. The culture was maintained at 37 °C in 5% CO2 incubator. Two-day-old culture of T. brucei was diluted to 5000 parasites/ml. Diluted T. brucei parasite culture was dispensed in clear flat-bottom culture well plates and treated with test compounds. The antitrypanosomal screening assay was based on Alamar blue-based fluorometric growth analysis at a concentration range of 10–0.4 μg/ml. Active compounds were further screened at a concentration range of 10–0.0032 μg/ml. Difluoromethy lornithine was used as positive drug controls. IC50 values were computed from the dose response growth inhibition curve by XLfit version 5.2.2 (Mohamed et al. 2016a; Tarawneh et al. 2018).
In vitro antileishmanial assays
Promastigote culture of Leishmanial donovani was grown in RPMI medium with 10% fetal bovine serum (FBS) with pH 7.4 at 26 °C. Axenic amastigote culture of Leishmanial donovani was grown in RPMI medium with 10% FBS with pH 5.5 at 37 °C in 5% CO2 incubator. The antileishmanial activity of the compounds was tested in vitro against promastigotes, axenic amastigotes, and macrophage internalized amastigote form of Leishmania donovani parasite. Promastigotes and axenic amastigotes assays were based on Alamar blue fluorometric growth analysis. Differentiated THP1 cells were been used in the macrophage internalized amastigote assay. The macrophage internalized amastigote method was based on parasite rescued and transformation assay described earlier; pentamidine was used as positive standards (Jain et al. 2012; Tarawneh et al. 2018).
Results and discussion
Compound 1 was obtained as yellow oil. The HR-MS data indicated a molecular formula C16H28O2, based on the [M+Na]+ ion signal at m/z 275.1869 (calc. 275.1987). The 1H NMR data (Table 1), showed three singlets at δH 0.85, 0.96, and 1.02 attributed to three methyls CH3-13, 15, and 14, respectively. Based on HSQC and HMBC correlations, the multiplets at δH 3.27–3.34 [2H] were assigned to CH-2 and 9, the methoxy group appears as singlet at δH 3.35 is assigned to [2-OMe]. The 13C NMR data (Table 1) of 1 showed resonances of 16 carbon atoms, which were classified by DEPT 135 and HSQC experiments as three methyls, one methoxy, six methylenes, three methines, and three quaternary carbons. The HMBC spectrum of compound 1 showed the following key correlations: methoxy protons singlet at δH 3.35 showed 3J correlation with δC 90.3 (C-2), indicated the methoxylation at C-2. The methyl singlet at δH 0.96 exhibited 2J and 3J correlation with carbons at δC 33.2 (C-7), 75.4 (C-9), and 36.7 (C-12) indicated the attachment of this methyl group at C-8. The orientations of the two stereo centers at C-2 and 9 were assigned to be β and α respectively, by comparison with the previously reported data (Collado et al. 1996, 1998). The overall NMR data were in full agreement with the data of 2β-methoxyclovan-9α-ol (Collado et al. 1996), which were obtained from the biotransformation of (−)- caryophyllene oxide. However, this is the first time to be isolated from natural source.
Compound 2 was also isolated as yellow oil. Its HR-MS data showed a molecular formula C21H36O3, based on the [M+Na]+ ion signal at m/z 359.2524 (calc. 359.2562). The 1H NMR data (Table 1), showed three singlets at δH 0.67, 0.76, and 0.98 for three methyls CH3-20, 19, and 18, respectively. The doublet at δH 0.93[J = 6.6 Hz] to be assigned to the methyl CH3-16. The singlet at δH 3.65 was attributed to the methoxy group at C-15. A doublet of doublet of the appeared at δH 3.25 [J = 4.4, 11.8 Hz], was attributed to oxymethine CH-3. Two singlets observed at δH 4.82 and 4.48 were assigned to exomethylene CH2-17.
The 13C NMR data of 2 (Table 1) exhibited the resonances of 21 carbons, which were classified as four methyls, one methoxy, eight methylenes, four methines, and four quaternary carbons via DEPT 135 and HSQC experiments. The exocyclic methylene protons doublets at δH 4.82 and 4.48 showed 3J HMBC correlations with δC 38.3 (C-7), and 56.8 (C-9) indicated the presence of double bond between C-8 and C-17. The methoxy protons at δH 3.66 showed 3J correlation to δC 173.9 (C-15), indicated the presence of methyl ester at C-15. The methyl singlet δH at 0.99 exhibited 2J and 3J HMBC correlations with carbons at δC 79.0 (C-3), 39.3 (C-4), 54.7 (C-5), and 15.5 (C-19), the methyl singlet at δH 0.77 exhibited 2J and 3J HMBC correlations with carbons at δC 79.0 (C-3), 39.3 (C-4), 54.7 (C-5), and 28.4 (C-18) indicated that these two geminal methyl groups are directly attached to C-4. The doublet at δH 0.94 showed 2J and 3J HMBC correlations to carbons at δC 31.0 (C-13), 35.8 (C-12), and 42.0 (C-14) confirmed the presence of the methyl group at C-13. The structure proposed for the major component is consistent with previously synthesized compound methyl-ent-3β-hydroxylabd-8(17)-en-15-oate (2, Fig. 1). This compound has been synthesized as part of confirming the carboxyl functional group in ent-3β-hydroxylabd-8(17)-en-15-oic acid by reaction with diazomethane (Branco et al. 2004). However, this is the first time that this is being reported from a natural source.
Fig. 1.
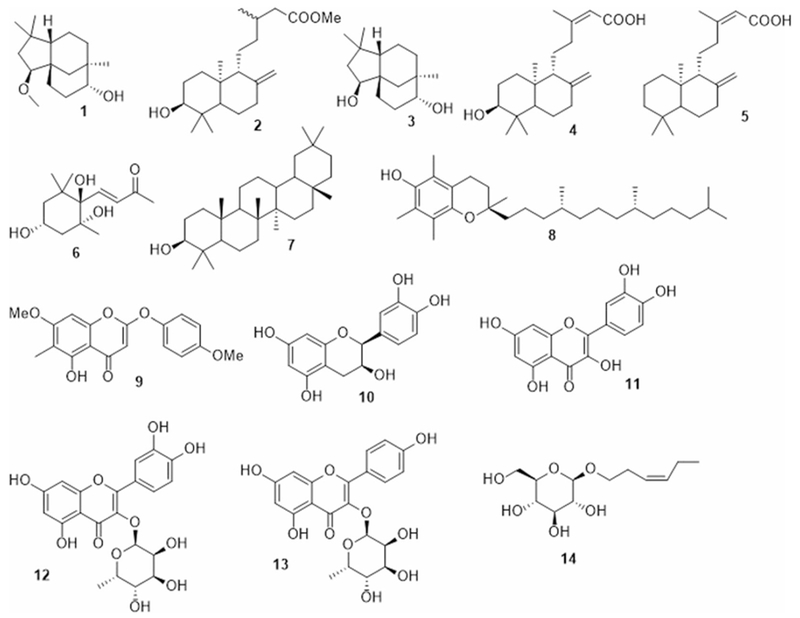
Isolated compounds from P. thonningii
The known isolated compounds 3–16 (Fig. 1) were identified by comparing their spectral data to those in the literature and were identified as clovane-2β,9α-diol (3) (Collado et al. 1998), alepterolic acid (4) (Braun and Breitenbach 1977), anticopalic acid (5) (Villegas Gómez et al. 2009), (3S,5R,6S)-trihydroxy-7E-megastigmen-9-one (6) (Park et al. 2011), β-amyrin (7) (Okoye et al. 2014), Vitamin E (8) (Matsuo and Urano 1976), piliostigmin (9) (Ibewuike et al. 1996), (+)-epicatechin (10) (Foo et al. 1996), quercetin (11), quercitrin (12) (Aderogba et al. 2013), Afzelin (13) (Aderogba et al. 2013), 3-hexenyl-1-O-β-D-glucopyranoside (14) (Lee et al. 2005), stigmasterol (15), and β-sitosterol glucoside (16) (Fig. 2).
Fig. 2.
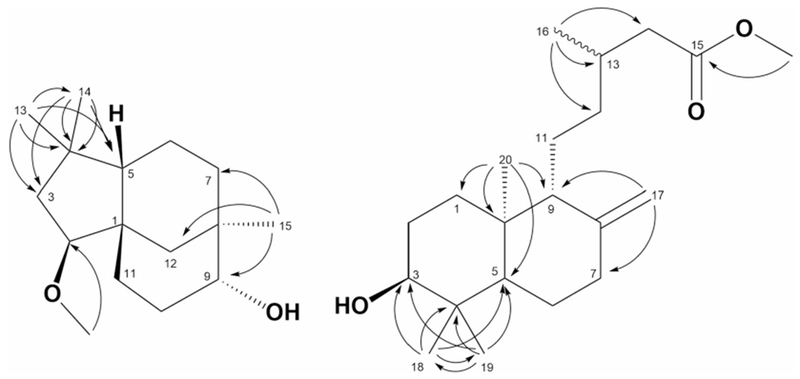
Key HMBC correlations of compounds 1 and 2
The total extract and isolated compounds were tested for their antiprotozoal activity (Table 2). Only the fractions containing major compounds 1, 2, and pure compound 4 showed activity against Trypanosoma brucei with IC50 values of 7.89, 3.84, and 3.42 μM, respectively (used standard for T. brucei, difluoromethylornithine IC50 3.593 μM). In addition, the fraction contains major constituent as compound 2 showed activity towards Leishmania donovani with IC50 7.82 μM (used standard for L. donovani Amastigote, Pentamidine IC50 1.666 μM). The structure activity relationship (SAR) of sesquiterpenoids 1 and 3 suggested that the introduction of the hydroxyl group at C-2 enhanced the activity. Similarly, comparing the activities of 2, 4, and 5 towards T. brucei indicated the importance of the hydroxyl group at C-3 for the activity. There were few reports for the antiprotozoal activity of the labdane diterpenes (Fokialakis et al. 2006; Jassbi et al. 2016; Richomme et al.1991; Siheri et al 2014) and these compounds possess structural similarities to the active andrographolides (Sinha et al. 2000).
Table 2.
Bioassay results of active compounds
| Sample code | L. donovani Amastigote/THP IC50 | T. brucei IC50 |
|---|---|---|
| Pentamidine | 1.666 | |
| Difluoromethylornithine | 3.593 | |
| 1 | >10 | 7.89 |
| 2 | 7.82 | 3.84 |
| 4 | >10 | 3.42 |
Conclusions
Phytochemical evaluation of P. thonningii yielded two new compounds 1–2, and fourteen known compounds (3–16). Compounds 1 and 2 were isolated for the first time from the nature. Compounds 3–8, 10, 13, and 14 were reported from this plant for the first time. Compounds 1 and 4 showed selectivity towards T. brucei with IC50 7.89 and 3.42 μM, respectively. Compound 2 showed moderate activity towards T. brucei and L. donovani Amastigote with IC50 3.84 and 7.82 μM, respectively. The structure activity relationship (SAR) suggested that hydroxylation at C-2 enhances the antileishmanial activity in sesquiterpenes 1 and 3. Similarly the hydroxylation at C-3 in labdane diterpenes (2, 4, and 5) elevates the activity towards T. brucei.
Supplementary Material
Acknowledgements
The project was supported by Sheda Science and Technology Complex, Nigeria and National Center for Natural Product Research, University of Mississippi, USA. We acknowledge Award Number P20GM104932 from the National Institute of General Medical Sciences for bioassay results. The authors wish to thank Dr. Charles L. Cantrell and Amber C. Reichley, USDA-ARS-NPURU for HR MS results.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00044-018-2238-1) contains supplementary material, which is available to authorized users.
References
- Aderogba MA, Ndhlala AR, Rengasamy KRR, Staden JV (2013) Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules 18:12633–12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afolayan M, Srivedavyasasri R, Asekun OT, Familoni OB, Ross SA (2018) Chemical and biological studies on Bridelia ferruginea grown in Nigeria. Nat Prod Res. in Press 2018, 10.1080/14786419.2018.1440225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinpelu DA, Obuotor EM (2000) Antibacterial activity of Piliostigma thonningii stem bark. Fitoterapia 71:442–443 [DOI] [PubMed] [Google Scholar]
- Asuzu IU, Gray AI, Waterman PG (1999) The anthelmintic activity of D-3-O-methylchiroinositol isolated from Piliostigma thonningii stem bark. Fitoterapia 70:77–79 [Google Scholar]
- Asuzu IU, Nwaehujor CO (2013) The Anti-diabetic, hypolipidemic and anti-oxidant activities of D-3-O-methylchiroinositol in alloxan-induced diabetic rats. Hygeia J Drug Med 5:27–33 [Google Scholar]
- Asuzu IU, Onu OU (1994) Antihelmintic activity of the ethanolic extract of Piliostigma thinningii bark in Ascaridia galli infected chickens. Fitoterapia 65:291–294 [Google Scholar]
- Baratta MT, Ruberto G, Tringali C (1999) Constituents of the pods of Piliostigma thonningii. Fitoterapia 70:205–208 [Google Scholar]
- Branco A, Pinto AC, Braz Filho R (2004) Chemical constituents from Vellozia graminifolia. Acad Bras Cienc 76:505–518 [DOI] [PubMed] [Google Scholar]
- Braun S, Breitenbach H (1977) Strukturaufklärung einer neuen diterpensäure aus metasequoia glyptostroboides mit hilfe der 13CNMR-spektroskopie. Tetrahedron 33:145–150 [Google Scholar]
- Collado IG, Hanson JR, Macias-Sanchez AJ (1996) The cleavage of caryophyllene oxide catalysed by tetracyanoethylene. Tetrahedron 52:7961–7972 [Google Scholar]
- Collado IG, Hanson JR, Macias-Sanchez AJ, Mobbs D (1998) The biotransformation of some clovanes by Botrytis cinerea. J Nat Prod 61:1348–1351 [DOI] [PubMed] [Google Scholar]
- Egharevba HO, Folashade KO (2010) Preliminary phytochemical and proximate analysis of the leaves of Piliostigma thonningii (Schumach.) Milne-Redhead. Ethnobot Leafl 14:570–577 [Google Scholar]
- Fokialakis N, Kalpoutzakis E, Tekwani BL, Skaltsounis AL, Duke SO (2006) Antileishmanial activity of natural diterpenes from Cistus sp. and semisynthetic derivatives thereof. Biol Pharm Bull 29:1775–1778 [DOI] [PubMed] [Google Scholar]
- Foo LY, Newman R, Waghorn G, McNabb WC, Ulyatt MJ (1996) Proanthocyanidins from Lotus corniculatus. Phytochemistry 41:617–624 [Google Scholar]
- Ibewuike JC, Ogundaini AO, Ogungbamila FO, Martin M-T, Gallard J-F, Bohlin L, Paies M (1996) Piliostigmin, a 2-phenoxychromone, and C-methylflavonols from Piliostigma thonningii. Phytochemistry 43:687–690 [Google Scholar]
- Ibewuike JC, Ogundaini AO, Ogungbamila FO, Ogundaini AO, Okeke IN, Bohlin L (1997) Antiinflammatory and antibacterial activities of C-methylflavonols from Piliostigma thonningii. Phytother Res 11:281–284 [Google Scholar]
- Ighodaro OM, Agunbiade SO, Omole JO, Kuti OA (2012) Evaluation of the chemical, nutritional, antimicrobial and antioxidantvitamin profiles of Piliostigma thonningii leaves (Nigerian species). J Med Plants Res 6:537–543 [Google Scholar]
- Ighodaro OM, Omole JO (2012) Effects of Nigerian Piliostigma thonningii species leaf extract on lipid profile in Wistar rats. ISRN Pharmacol, Article ID 387942, 10.5402/2012/387942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Sahu R, Walker LA, Tekwani BL (2012) A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J Vis Exp 70:4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassbi AR, Zare S, Firuzi O, Xiao J (2016) Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem Rev 15:829–867 [Google Scholar]
- Kinghorn AD (2001) Pharmacognosy in the 21st century. J Pharm Pharmacol 53:135–148 [DOI] [PubMed] [Google Scholar]
- Lee KH, Choi SU, Lee KR (2005) Sesquiterpenes from Syneilesis palmata and their cytotoxicity against human cancer cell lines In vitro. Arch Pharm Res 28:280–284 [DOI] [PubMed] [Google Scholar]
- Martin M-T, Paies M, Ogundaini AO, Ibewuike JC, Ogungbamila FO (1997) Complete 1H and 13C NMR assignment of a kaurane diterpene from Piliostigma thonningii. Magn Reson Chem 35:896–898 [Google Scholar]
- Matsuo M, Urano S (1976) 13C nmr spectra of tocopherols and 2,2-dimethylchromanols. Tetrahedron 32:229–231 [Google Scholar]
- Mohamed NM, Makboul MA, Farag SF, Jain SK, Jacob MR, Tekwani BL, Ross SA (2016a) Triterpenes from the roots of Lantana montevidensis with antiprotozoal activity. Phytochem Let 15:30–36 [Google Scholar]
- Mohamed NM, Makboul MA, Farag SF, Tarawneh AH, Khan S, Brooks TA, Wang Y, Ross SA (2017) Iridoid and phenylpropanoid glycosides from the roots of Lantana montevidensis. Med Chem Res 26:1117–1126 [Google Scholar]
- Mohamed SM, Bachkeet EY, Bayoumi SA, Ross SA (2016b) New cycloartane saponin and monoterpenoid glucoindole alkaloids from Mussaenda luteola. Fitoterapia 110:129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa AE, Elhela AA, Mohammed EI, Cutler SJ, Ross SA (2016) New triterpenoidal saponins from Koelreuteria paniculata. Phytochem Lett 17:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaehujor CO, Udegbunam R, Asuzu IU (2015) Analgesic, anti-inflammatory and anti-pyretic activities of D-3-O-methylchiroinositol isolated from stem bark of Piliostigma thonningii. Med Chem Res 24:4139–4145 [Google Scholar]
- Okoye NN, Ajaghaku DL, Okeke HN, Ilodigwe EE, Nworu CS, Okoye FB (2014) beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm Biol 52:1478–1486 [DOI] [PubMed] [Google Scholar]
- Park JH, Lee DG, Yeon SW, Kwon HS, Ko JH, Shin DJ, Park HS, Kim YS, Bang MH, Baek NI (2011) Isolation of Megastigmane sesquiterpenes from the silkworm (Bombyx mori L.) droppings and their promotion activity on HO-1 and SIRT1. Arch Pharm Res 34:533–542 [DOI] [PubMed] [Google Scholar]
- Richomme P, Godet MC, Foussard F, Toupet L, Sevenet T, Bruneton J (1991) A novel leishmanicidal labdane from Polyalthia macropoda. Planta Med 57:552–554 [DOI] [PubMed] [Google Scholar]
- Salim A, Chin YW, Kinghorn A (2008) Drug Discovery from Plants In: Ramawat K, Merillon J (eds) Bioactive Molecules and Medicinal Plants. Springer, Berlin, Heidelberg, p 1–24 [Google Scholar]
- Samuelsson G (2004) Drugs of Natural Origin: a Textbook of Pharmacognosy. 5th Swedish Pharmaceutical Press, Stockholm [Google Scholar]
- Siheri W, Igoli JO, Gray AI, Nasciemento TG, Zhang T, Fearnley J, Clements CJ, Carter KC, Carruthers J, Edrada-Ebel R, David GW (2014) The solation of Antiprotozoal Compounds from Libyan Propolis. Phytother Res 28:1756–1760 [DOI] [PubMed] [Google Scholar]
- Silva O, Barbosa S, Diniz A, Valdeira ML, Gomes E (1997) Plant extracts antiviral activity against Herpes simplex virus type 1 and African swine fever virus. Int J Pharmacogn 35:12–16 [Google Scholar]
- Sinha J, Mukhopadhyay S, Das N, Basu MK (2000) Targeting of liposomal andrographolide to L. donovani-infected macrophages in vivo. Drug Deliv 7:209–213 [DOI] [PubMed] [Google Scholar]
- Tarawneh AH, Al-Momani LA, León F, Jain SK, Gadetskaya AV, Abu-Orabi ST, Tekwani BL, Cutler SJ (2018) Evaluation of triazole and isoxazole derivatives as potential anti-infective agents. Med Chem Res 27:1269–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas Gómez C, Martínez-Vázquez M, Esquivel B (2009) Antifeedant activity of anticopalic acid isolated from Vitex hemsleyi. Z Naturforsch C 64:502–508 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


