Abstract
Occupational and environmental exposures to industrial chemicals are known to cause hepatotoxicity and liver injury, in humans and in animal models. Historically, research has focused on severe acute liver injury (e.g., fulminant liver failure) or endstage diseases (e.g., cirrhosis and HCC). However, it has become recently recognized that toxicants can cause more subtle changes to the liver. For example, toxicant-associated steatohepatitis (TASH), characterized by hepatic steatosis, and inflammation, was recently recognized in an occupational cohort exposed to vinyl chloride. At high occupational levels, toxicants are sufficient to cause liver damage and disease even in healthy subjects with no comorbidities for liver injury. However, it is still largely unknown how exposure to toxicants initiate and possibly more importantly exacerbate liver disease, when combined with other factors, such as underlying non-alcoholic liver disease (NAFLD) caused by poor diet and/or obesity. With better understanding of the mechanism(s) and risk factors that mediate the initiation and progression of toxicant-induced liver disease, rational targeted therapy can be developed to better predict risk, as well as to treat or prevent this disease. The purpose of this review is to summarize established and proposed mechanisms of volatile organic compound-induced liver injury and to highlight key signaling events known or hypothesized to mediate these effects.
Keywords: environmental liver disease, hepatotoxicity, organochlorines, non-alcoholic liver disease (NAFLD), toxicant-associated steatohepatitis (TASH), toxicant
Introduction
The liver has many important metabolic functions. Not only does it serve as a site for conversion, storage and supply of dietary nutrients but also removes toxic compounds. It is the main location of synthesis of multiple key proteins (e.g., albumin and clotting factors), and it synthesizes and excretes bile acids, which is critical for normal uptake of vitamins, lipids and excretion of many xenobiotics. The central location of the liver within the body allows it to act as a biochemical and physical filter to protect other organs from exposure to potentially harmful compounds. The liver has therefore a very high capacity for phase I and II metabolic processes, which is responsible for the liver’s ‘1st pass effect’ in xenobiotic metabolism. By virtue of its function, the liver is often a target of toxicant exposure.
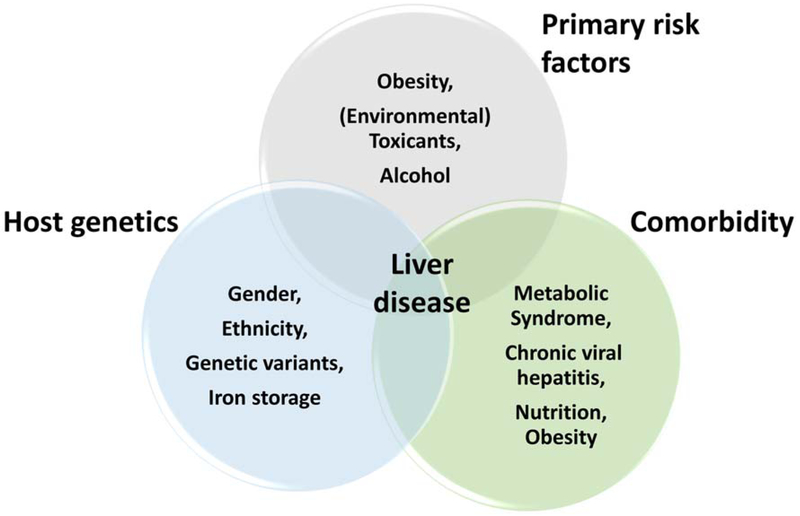
The liver maintains nutrient homeostasis via synthesis and secretion of major carbohydrates and lipids. In that role, hepatocytes, which constitute approximately 80% of the liver by mass (Stanger, 2015), are the major player. Hepatocytes express various enzymes that allow for nutrient metabolism and for detoxification. Among the most diverse and prominent classes of enzymes are the mixed function oxidases, or cytochrome P450 family of enzymes. Such enzymes are capable of metabolizing a variety of drugs, chemicals and toxicants (Raucy et al., 1993). The metabolism of these compounds often results in the formation of active metabolites or reactive intermediates, which may cause more harm than its parent compound, for example via DNA or protein adduct formation, or by inciting an inflammatory response (Liu et al., 2010; Swenberg et al., 1999; Dosanjh et al., 1994; Cai and Guengerich, 2001). Therefore, hepatocytes are often the primary and initial target of damage and injury caused by chemical and/or toxicant exposure. However, hepatocytes are capable of regenerating in order to preserve the function of the entire organ to combat such injuries. Although this mechanism confers protection, the regenerative capacity of the liver is limited. If the liver is continuously damaged or if the damage is too severe, cell death and irreversible injury may occur. It is now a well-established phenomenon that multiple factors can influence and enhance liver injury (Day and James, 1998; Yang et al., 1997). As such, certain factors may sensitize the liver to be more susceptible to damage from other factors, sequentially contributing to the development and progression of liver disease (Figure 1).
Figure 1.
Risk factors of liver injury.
There are well-known primary risk factors causing liver disease, such as obesity, alcohol or toxicants. However, it is less well understood how other mitigating factors such as host genetics or underlying comorbidities will impact or enhance liver injury caused by the primary risk factors. Importantly, although the effects of high-level occupational VOC exposures have been studied, it is becoming clear that low-level occupational and environmental exposure may interact with other risk-modifying factors, such as ‘secondary’ risk factors to cause liver injury at concentrations that are considered ‘safe.’
Liver disease is not a single clinical manifestation; but rather a spectrum of pathologies with various stages of severity (Sayiner et al., 2016). The first stage of liver disease is characterized by lipid accumulation, or steatosis. The influx of lipids within hepatocytes dysregulates both lipid and mitochondrial homeostasis. The resulting disruption of endogenous processes can, when continued chronically, invoke an inflammatory response termed steatohepatitis. Steatohepatitis is identified histologically as lipid accumulation with an infiltration of inflammatory foci. However, if the damage is continued even further, more severe phenotypes develop, such as fibrosis and cirrhosis, which are characterized by an accumulation of extracellular matrix protein resulting in scar formation within the liver tissue. These end-stages of liver disease may ultimately lead to hepatocellular carcinoma (HCC). Although the progressive nature of liver disease is consistent independent of the source of the injury, several unique etiologies have been characterized. These include alcoholic, nonalcoholic, and toxicant-associated fatty liver diseases (Joshi-Barve et al., 2015).
Liver disease development can be influenced by several factors including underlying disease, such as NAFLD and metabolic syndrome caused by poor lifestyle choices, such as diet. But also host genetics, and importantly environmental exposures may play a significant role. Only a portion of patients diagnosed with NAFLD will go on to develop NASH (McPherson et al., 2015; Calzadilla and Adams, 2016). Indeed, it has recently been estimated that approximately 25% of people diagnosed with NAFLD will progress to NASH over a 3 year period (Calzadilla and Adams, 2016). Additionally, several molecular mechanisms are involved in disease progression, such as activation of the inflammatory response and activation of stress responses, such as oxidative and ER stress pathways. Importantly, it is hypothesized that environmental toxicants, such as VOCs, may enhance liver injury and/or accelerate disease progression. While there have been cases demonstrated of augmented end stage liver disease (i.e., fibrosis and cirrhosis; for example there has been shown to be an interaction between alcohol exposure and Hepatitis C (Szabo et al., 2015; Safdar and Schiff, 2004)), currently it is however ‘assumed’ that mechanistically the enhancement of the disease takes place in the early stages of the disease progression, such as during steatosis or steatohepatitis. Furthermore, these earlier disease stages are more readily reversible. It is therefore critical to evaluate risk-modifying factors in the context of environmental exposures that may contribute to enhanced liver disease progression (Figure 1).
Arguably, the most prominent health issue affecting the global population is the obesity epidemic. In the US, 62% of adults are overweight (BMI>25 kg/m2) and 26% are obese (BMI>30 kg/m2) (World Health Organization, 2015). Obesity is the most common cause of fatty liver disease in the United States. Non-alcoholic fatty liver disease (NAFLD) is the term for the respective progressions of this disease (Mishra and Younossi, 2012). Canonically, fat accumulation, characterized as simple steatosis, is typically asymptomatic, yet in some cases can be associated with elevated serum transaminase levels (Browning et al., 2004). A major independent risk factor associated with developing NAFLD is metabolic syndrome, which is comprised of several cardiovascular pathologies including high blood pressure, high cholesterol, and high circulating triglycerides (Kaur, 2014). Metabolic syndrome can lead to insulin resistance and type 2 diabetes. The combination of NAFLD and metabolic syndrome can enhance metabolic dysfunction, which in turn, can lead to a progression of further stages of liver disease.
Several genetic variations have recently been recognized as contributing to the risk of developing liver disease (Anstee et al., 2011) (Chalasani et al., 2010; Severson et al., 2016). One of the most studied single nucleotide polymorphisms is in the PNPLA3 gene. The PNPLA3 protein is involved in lipid processing and metabolism in the liver. Indeed, populations expressing a variant of PNPLA3 are more susceptible to develop NAFLD (Romeo et al., 2008; Dongiovanni et al., 2013). In addition to genetic susceptibility, environmental factors are also recognized as risk factors for developing liver disease. Several chemicals and environmental toxicants are also known to directly affect the liver and influence disease progression. In fact, a new term has recently been coined for chemicals that directly induce obesity: obesogens (Grun and Blumberg, 2006; Chamorro-Garcia and Blumberg, 2014). As such, it is critical that multiple factors are evaluated when studying the development of liver disease.
The field of environmental toxicology has started to shift away from analyses of single, high exposures of chemicals and begun to examine lower chronic exposures in conjunction with other potential injurious agents. Such “exposure biology” approaches take into consideration more than one factor when analyzing liver disease susceptibility. For example, the organochlorine compound, vinyl chloride, is a direct hepatotoxicant at high exposures (Cave et al., 2010). More recently, however, studies have described that low-level exposures are sufficient in enhancing underlying liver injury (Anders et al., 2016a,b). Vinyl chloride is only one toxicant of a larger classification known as volatile organic compounds (VOCs). Hepatotoxicity is a common end point of exposure to environmental chemicals (Wahlang et al., 2013). Indeed, 33% of the 677 most common workplace chemicals reported in the National Institute of Occupational Safety and Health Pocket Guide are associated with hepatotoxicity (Tolman and Sirrine, 1998). However, despite its increasing significance, this field of research is still largely understudied and poorly understood. The purpose of this review is therefore to evaluate multiple pathologies and provide mechanistic insight into how different toxicants, specifically VOCs, influence underlying liver disease.
VOCs, an overview
VOCs are organic chemicals that easily vaporize. Ambient levels are often higher indoors than outdoors, and concentrations can be significantly affected by how often windows are opened and the location of the building or residence (i.e., proximity to industrial and traffic pollution) (United States Environmental Protection Agency, 2017a; Cleary et al., 2017). Additionally, VOCs are often ingredients in common household products such as paints, varnishes, cleaning supplies, degreasing agents, gasoline, and dry-cleaned clothing (United States Environmental Protection Agency, 2017b). As such, the typical route for human exposure is via inhalation or dermal absorption (United States Environmental Protection Agency, 2017b). However, VOCs are also common ground water contaminants as they are present at many waste disposal sites and are the most common class of chemical found at National Priority List (NPL) Superfund sites. A substantial number of compounds on the ‘ATSDR hazard substance priority list’ represent VOCs, with many in the top 50 chemicals (Agency for Toxic Substances and Disease Registry, 2017). Although there have been several instances of mass exposure to VOCs, one of the more studied cases is from the Camp Lejeune military base in North Carolina. Up to 1 million people, including military personnel and their families, were exposed to a mixture of VOCs [TCE, PCE, benzene, and vinyl chloride (VC)] via contaminated drinking water in the 1980s (Maslia et al., 2016). A retrospective mortality cohort study demonstrated increased mortality and deaths from cancer, including liver, in personnel exposed at Camp Lejeune compared to a non-contaminated military base (Bove et al., 2014). However, other health effects and long-term outcomes are still being evaluated.
VOCs have long been used as industrial solvents. Therefore, occupational exposure has been the most studied form of VOC exposure and has also been used for risk assessment. Many of these compounds are directly hepatotoxic at high levels. Indeed, studies of high, occupational exposures have been invaluable for elucidating mechanisms of injury and have been instrumental in establishing the current safety regulations for many VOCs (Wahlang et al., 2013). However, with the average global BMI increasing and with nearly 25% of the global population having underlying NAFLD (Younossi et al., 2016), it is necessary to begin to incorporate other contributing factors when developing models of risk assessment and safety guidelines. It is also vital to study lower environmental exposures and/or chronic indoor exposures as probable risk factors for developing liver disease and toxicity. Populations in residential areas surrounding polymer producing facilities or other industrial plants are at risk for chronic exposure. Although many of these chemicals have been known human toxicants for decades, their mechanism of action is still incompletely understood.
As with most disease states, the mechanism of injury is often not solely due to one factor, rather, a combination of events that affect the health of the organ. Here, although several distinct mechanisms are presented separately, it is important to note that injury is a continuous and cyclic process. The reason several of these VOC chemicals are ranked so high on priority lists/IARC categories is due to their properties as direct toxicants with multiple organ targets, very high potential risk for human exposure, and complex mechanisms of action. Some chemicals have been more studied than others and this review will serve to shed light on the need for future mechanistic work on environmental exposure studies with not only VOCs, but environmental contaminants as a whole.
Steatosis
As stated in the Introduction, early pathological changes during NAFLD development include abnormal accumulation of lipids within hepatocytes. There are several mechanisms by which steatosis can occur: increased de novo lipid synthesis, increased free fatty acid (FFA) uptake from circulation, impaired mitochondrial β-oxidation, and impaired VLDL secretion (Cohen et al., 2011). Significant accumulation of not only triglycerides, but also other fatty acid species can lead to lipotoxicity and further tissue injury (Ibrahim et al., 2011). Although obesity can cause all of these processes, several VOCs are known to independently induce hepatic steatosis, or to enhance pre-existing steatosis caused by other factors. For example, vinyl chloride and its metabolite, chloroethanol, have been shown to disrupt hepatic lipid metabolism (Anders et al., 2016a). In this study, mice were exposed to sub-hepatotoxic concentrations of chloroethanol and a secondary inflammatory stimulus of lipopolysaccharide (LPS). Interestingly, even though chloroethanol caused no liver damage per se (normal transaminase levels), steatosis was observed with chloroethanol alone. Moreover, chloroethanol also significantly enhanced macro- and micro-vesicular fat accumulation caused by LPS.
Carbon tetrachloride, a now banned chemical solvent, has historically been used as a degreaser, propellant, and as a component in fire extinguishers. However, to this day, carbon tetrachloride is often used as a classic example of occupational hepatotoxicity. A study by Allman et al., described that mice concomitantly exposed to a Western diet and carbon tetrachloride had worse hepatic steatosis and enhanced injury. Additionally, the same study demonstrated that a single dose of carbon tetrachloride was sufficient to induce fat accumulation (Allman et al., 2010).
Another common VOC is perchloroethylene (tetrachloroethylene, PCE). PCE is a class IIa (probable human carcinogen) and is a common contaminant found at many EPA Superfund sites. Philip et al. demonstrated in a repeat dose study that PCE exposure was sufficient to elevate serum transaminase levels and induce hepatic steatosis (Philip et al., 2007). Recently, the Rusyn group has demonstrated that PCE exposure worsens hepatotoxicity upon co-exposure with a high fat diet feeding. Metabolomics analyses from the same studies suggest that PCE toxicity significantly alters lipid homeostasis in vivo, contributing to enhanced steatosis (Cichocki et al., 2017b,a). Additionally, there is evidence that PCE acts via PPARα mediated pathways (Zhou et al., 2017). Importantly, PPARα has been implicated as a key player in NAFLD development as it is a central regulator of hepatic lipid homeostasis and its inhibition has been demonstrated to cause hepatic steatosis (Pawlak et al., 2015). In an occupational cohort study of dry cleaner workers exposed to dimethylformamide (DMF), many of them presented with hepatic fat accumulation (Redlich et al., 1988). It is therefore a reasonable hypothesis that the concomitant exposure to such VOCs with the presence of fatty liver could significantly enhance or predispose individuals to liver injury.
Metabolic disruption and insulin resistance
Both metabolic dyshomeostasis and insulin resistance can be causative factors or a result of developing NAFLD. As mentioned previously, NAFLD induces alterations in pathways regulating lipid metabolism. Similarly, other metabolic pathways such as carbohydrate metabolism can be affected. Furthermore, 50-75% of patients diagnosed with type 2 diabetes also present with hepatic steatosis (Targher et al., 2005) and insulin resistance has been directly correlated with NAFLD development (Seppala-Lindroos et al., 2002; Utzschneider and Kahn, 2006). Several VOCs have been shown to disrupt normal hepatic metabolism, thus contributing to their toxicity.
For example, a sub-lethal dose of carbon tetrachloride significantly increased liver damage and insulin resistance in rats fed a high-fructose diet (Pooranaperundevi et al., 2010). Carbon tetrachloride, at high concentrations, has also been shown to directly impair insulin signaling and sensitivity (Meyer-Alber et al., 1992; Arai et al., 2010).In a recent study with a cohort of highly exposed VC workers, significant liver injury was observed via biopsy, however typical markers of liver injury (i.e. elevated transaminase levels) were not elevated (Cave et al., 2010). In addition to describing toxicant-associated steatohepatitis, Cave et al. showed that occupational exposure to VC was sufficient to increase plasma insulin levels, indicating perturbed insulin and glucose signaling (Cave et al., 2010). A more recent metabolomics analysis on a cohort of occupationally exposed VC workers revealed changes in several lipid metabolites and differential expression of metabolism regulating enzymes, such as AMPK (Guardiola et al., 2016). Moreover, a mouse model of VC metabolite exposure showed disruption of the hepatic metabolism. Both mTOR and AMPK, which are usually activated in opposition, were both activated causing a ‘pseudo-fasted state,’ resulting in paradoxical lipid accumulation and glycogen depletion (Anders et al., 2016a).
A recent analysis of the National Health and Nutrition Examination Survey (NHANES) database looking at urinary concentrations of acrolein metabolites and insulin resistance demonstrated significant, positive associations between acrolein exposure and both diabetes and insulin resistance (Feroe et al., 2016). Another well-studied VOC, benzene is biotransformed via CYP2E1 to its active metabolites. Park and coworkers observed significantly elevated liver transaminase with benzene exposure. Importantly, ‘omics analyses showed that benzene affects lipid and glucose regulators genes in a time dependent manner from the initiation of exposure (Park et al., 2008). Benzene exposure has also recently been shown to cause increased serum cholesterol levels in mouse inhalation study (Abplanalp et al., 2017). Cardiovascular disease risk was the principle parameter of this study. However, as mentioned in the Introduction, metabolic syndrome has a significant cardiovascular component. Importantly, as cardiovascular disease risk is the leading cause of death in NAFLD patients (Ong et al., 2008); the ability of benzene exposure to increase risk for cardiovascular disease provides critical insight to the potential of VOCs’ contribution to the developing of NAFLD.
Oxidative stress
Oxidative stress plays a major role in multiple pathologies and disease states. Oxidative stress results from an imbalance of reactive oxygen and reactive nitrogen species (ROS/RNS) and intracellular antioxidant defenses (Sies, 1985). Mitochondria are a significant source of endogenous ROS as electron leak occurs during normal oxidative respiration (Savini et al., 2013; Kozlov et al., 2017). However, perturbations in cellular homeostasis often disrupt mitochondrial function resulting in irreversible damage. In NAFLD, mitochondrial derived ROS contribute significantly to oxidative damage (Begriche et al., 2013). Specifically, oxidative damage occurs upon covalent adduct formation on major macromolecules in the hepatocyte. Proteins, lipids, and DNA can all be targets of oxidation adducts. Reactive oxygen species are also perpetrators of injury as increased production cause excess damage, deplete intracellular antioxidant pools, and active other stress pathways (Czaja, 2007). Oxidative stress has been examined in depth as a pathogenic modulator of NAFLD (Bellanti et al., 2017). Indeed, patients with NAFLD exhibited higher levels of lipid peroxidation products compared to patients with hepatitis and healthy controls, respectively (Madan et al., 2006).
Active VOC metabolites are oftentimes extremely electrophilic and therefore highly reactive. For example, acrolein is a well-known propagator of oxidative stress by causing lipid peroxidation adducts (Mohammad et al., 2012; Moghe et al., 2015). Similarly, the major metabolites of trichloroethylene (TCE), trichloroacetic acid (TCA) and dichloroacetic acid (DCA), cause oxidative stress through forming lipid peroxidation adducts in vivo. Moreover, exposing AML-12 murine hepatocytes to a combination of both TCA and DCA increased oxidative stress more than either chemical alone (Hassoun and Mettling, 2015; Hassoun et al., 2014).
The vinyl chloride metabolites chloroethanol and chloroacetaldehyde both have recently been shown to independently cause oxidative damage in vivo and in vitro, respectively (Anders et al., 2016a,b). Indeed, in vivo data from Anders and colleagues demonstrate that chloroethanol exposure significantly increased 4-HNE adduct formation in mice fed a HFD (Anders et al., 2016b). Moreover, in vitro experiments with HepG2 hepatocytes show increased abundance of protein thiol adducts (Anders et al., 2016a). Exposure to dimethylformamide in a human liver cell line exhibited reactive oxygen species production in a dose-dependent manner. This study also highlights that these reactive species inflicted oxidative DNA damage, and double strand DNA breaks, leading to cell death (Wang et al., 2016). Although benzene is a well-known hematoxicant, several mechanisms of action may also be applicable to other organ systems, such as the liver. For example, in a chronic, occupational study, benzene exposure was associated with altered pathway activation of STAT3, a known player in maintaining redox homeostasis (Linher-Melville and Singh, 2017), in peripheral blood monocytes caused via production of reactive oxygen species (Fenga et al., 2016). Reactive oxygen species formation and their interactions with biomolecules results in oxidative stress. The involvement of oxidative stress pathways in both NAFLD pathogenesis and VOC induced hepatotoxicity indicate that understanding the interaction of these two exposures is important for elucidating mechanisms of hepatic injury.
ER stress
The endoplasmic reticulum (ER) is the hub for protein folding and synthesis of the cell. The ER is also tasked with maintaining intracellular calcium and is a part of lipid homeostasis (Fagone and Jackowski, 2009; Pagliassotti et al., 2016). When protein adducts or misfolded proteins are detected, the ER elicits the unfolded protein response (UPR). Chaperone proteins in the ER lumen become phosphorylated and allow activation of three branches of the UPR: RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) (Han and Kaufman, 2016; Wang and Kaufman, 2016). The UPR acts to remove endogenous stress induced by misfolded or adducted proteins. However, if the damage is too great, the system can be overwhelmed and apoptotic cascades become activated. Indeed, ER stress has been implicated as a key step in the transition from NAFLD to NASH by causing inflammation and apoptosis (Sozen and Ozer, 2017; Ozcan et al., 2004; Baiceanu et al., 2016).
It is known that reactive aldehyde species are generated by oxidized lipids and that these aldehydes bind to ER proteins, resulting in ER stress (Vladykovskaya et al., 2012). Acrolein, in addition to being an inducer of oxidative stress, has been shown to be a potent induced of ER stress. Increasing concentrations of acrolein significantly increase expression of several ER stress markers, without protective UPR activation in primary human hepatocytes (Mohammad et al., 2012). Additionally, recent work performed on intestinal epithelial cells show similar results. Significant increases in several ER stress markers were observed, accompanied by an increase in apoptotic cell death (Chen et al., 2017). Although not directly targeting the liver, intestinal barrier function is critical for liver-intestinal homeostasis. Alterations in gut barrier permeability are now known to contribute to, or enhance NAFLD pathogenesis (Cani et al., 2007; Mehal, 2013; Irene et al., 2017; Rahman et al., 2016).
Interestingly, the combined acute exposure of carbon tetrachloride and dimethylformamide in a rat model showed increased ER stress activation and hepatocyte death via apoptosis (Kim et al., 2010). As mentioned previously, Anders et al demonstrated that the reactive aldehyde and VC metabolite, chloroacetaldehyde, is capable of inducing hepatocyte injury. In that study, it was also shown that VC metabolites caused an enhancement of ER stress markers such as ATF3 and CHOP when combined with HFD feeding (Anders et al., 2016a,b). Indeed, ER stress is a known step is NAFLD progression to NASH (Sozen and Ozer, 2017; Puri et al., 2008). Therefore, understanding how VOCs affect this stress pathway will be crucial for understanding how their exposure will affect underlying liver injury.
Inflammation
By definition, NASH is characterized by the presence of chronic inflammation and immune cell infiltration into hepatic tissue. Hepatic inflammation includes altered expression of pro-inflammatory cytokines and chemokines accompanied by innate and adaptive immune cell recruitment and activation at the site of injury (Asrih and Jornayvaz, 2013). The previously discussed mechanisms such as oxidative and ER stress, especially in concert, are capable and sufficient to induce and propagate both local and systemic inflammatory responses (Zhang and Kaufman, 2008). Innate immune cells, specifically hepatic macrophages (Kupffer cells) and neutrophils are key mediators of local inflammation.
Neutrophils are acute responders and important effectors of the innate immune response (Kubes and Mehal, 2012). Neutrophil accumulation within hepatic tissue is a hallmark of NAFLD pathogenesis (Xu et al., 2014). Neutrophils release a cocktail of oxygen radicals and damaging enzymes which cause local tissue damage (Segal, 2005). Hepatic macrophages have also proven to be crucial for the transition to NASH. Macrophages can be activated via sterile or pathogenic stressors and release TNFα and IL-1β, recruiting circulating immune cells to the site of injury (Ma et al., 2002) and have been shown to be necessary for both experimental and human NASH development (Wan et al., 2014; Gadd et al., 2014; Reid et al., 2016). In an acute and chronic exposure study with carbon tetrachloride, it was observed that carbon tetrachloride enhanced monocyte recruitment to the liver and promoted a fibrogenic phenotype (Karlmark et al., 2009). In a mouse model performed by Anders and coworkers, VC metabolites caused significant increases in neutrophil infiltration and pro-inflammatory cytokine expression in mice fed a diet rich in saturated fatty acids. Additionally, inflammasome activation was shown to be a key mechanism of inflammatory injury in this model. Interestingly, mice that were fed a diet rich in unsaturated fatty acids were protected from injury and inflammasome activation in this model (Anders et al., 2016b). More recently, in a chronic low-level VC inhalation study, increased neutrophil accumulation was observed in mice fed a HFD and exposed to VC; however no changes in plasma cytokines or macrophage recruitment were seen. This indicates a specific, localized inflammatory response to tissue injury caused by VC in mice fed a diet rich in saturated fatty acids (Lang et al., 2018).
Another mechanism of inflammation in which neutrophils play a major role is the formation and release of neutrophil extracellular traps (NETs). NETs are an extensive meshwork of decondensed chromatin and hydrolytic enzymes, including myeloperoxidase, which significantly contributes to tissue injury and necrotic cell death (Yang et al., 2016). Interestingly, acrolein has recently been shown to increase hepatic tissue damage after ischemia reperfusion. Increased inflammatory cytokines, such as TNFα and IL-1β, were observed along with enhanced NET formation in isolated neutrophils (Arumugam et al., 2017). The role of the inflammatory response to VOC induced toxicant damage is an area that still remains incompletely understood. However, elucidating the mechanisms of the inflammatory response to VOC toxicity will provide crucial insight into understanding their mechanisms of injury.
Cell death
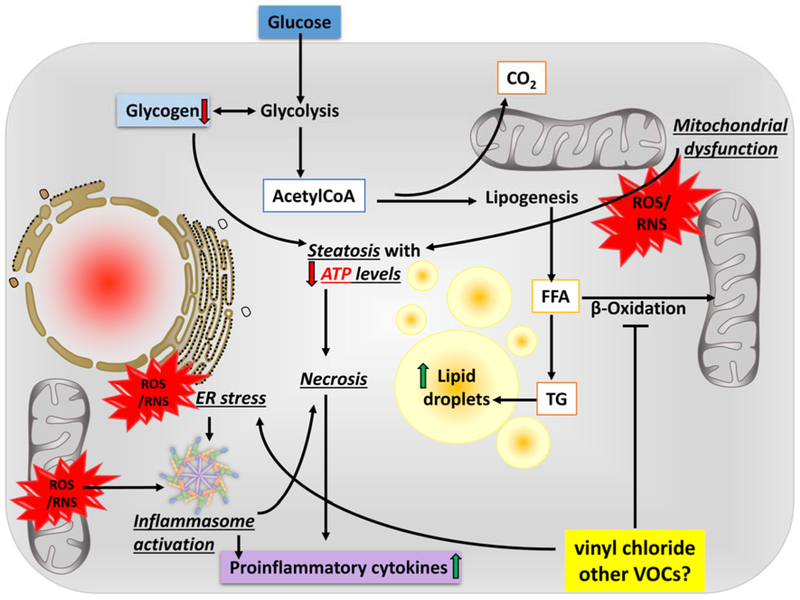
The combined and prolonged effect of mechanisms of injury culminate with activation of cellular death processes. Apoptotic hepatocyte cell death is a hallmark of NAFLD (Feldstein et al., 2003). However, upon extreme damage or loss of intracellular energy stores, necrotic death occurs, resulting in increased local inflammation and tissue damage. Indeed, all of the VOCs discussed in this review are inducers of cell death via various mechanisms (Figure 2). For example, the mechanism of vinyl chloride-induced hepatocyte death is primarily necrosis. Cave et al demonstrated serum CK-18 levels in exposed workers were consistent with a necrotic phenotype (Cave et al., 2011). Supporting this, in mice, VC metabolite exposure causes an overall increase in cell death, while hepatocyte apoptosis is decreased (Anders et al., 2016a), suggesting that there is enhanced necrosis rather than apoptotic cell death caused by VC metabolites.
Figure 2.
Potential mechanism(s) of liver injury by VOC exposure.
Upon exposure to VC, reactive intermediates form though bio activation processes and diet-induced obesity decreases their elimination. Through carbonyl stress and the generation of reactive oxygen and nitrogen species (ROS/RNS), VC metabolites cause ER stress leading to mitochondrial damage, which impairs oxidative phosphorylation; the cell increases flux through glycolysis to compensate for this loss of ATP yield. The increased demand for glucose depletes glycogen stores. AcetylCoA is being shunted to lipid synthesis (causing steatosis) rather than β-oxidation, even under conditions of ATP depletion, resulting in an increase in lactate production. The combined metabolic stress of VC exposure and ATP depletion likely causes ‘liponecrosis’ associated with increased oxidative stress, ER stress, and inefficient mitochondrial respiration and energy production.
Conversely, carbon tetrachloride toxicity leads to hepatocyte death predominantly via apoptosis. Several studies have examined the role of caspase activation in the apoptotic pathway and hepatotoxicity of carbon tetrachloride. Kim et al showed enhanced apoptotic activation via caspase-3 activity with the combination of dimethlformamide and carbon tetrachloride exposure (Kim et al., 2010). Carbon tetrachloride biotransformation via CYP2E1 is critical in its cytotoxicity. In vivo rat data showed increased activity of caspase 3, 8, and 9 following carbon tetrachloride exposure (Ijiri et al., 2017). In another study it was demonstrated that cell death caused by carbon tetrachloride exposure was alleviated in caspase 12 knockout mice, indicating a critical role of caspase 12 in carbon tetrachloride-induced cytotoxicity (Liu et al., 2014).
Xu and coworkers have also examined the caspase cascade activation with trichloroethylene exposure in L02 human hepatocytes. Indeed, they observed both a time and dose dependent increases in pro-apoptotic proteins, BAD and BAX (Xu et al., 2012). Identifying mechanisms of VOC inflicted cell death are crucial for understanding their toxicities. Taken together, as apoptotic cell death is a key characteristic mechanism of NAFLD pathogenesis, by understanding the mechanisms by which these chemicals cause cell death, it will fill knowledge gaps regarding VOC and NAFLD co-exposure and risk for potentially enhanced hepatic damage.
Diagnostic relevance of VOCs for human disease
Independent of the industrial VOCs used commercially; there are also several VOCs that are produced endogenously through metabolism and exhaled through the breath which can be used for diagnoses of human disease. Common VOCs in exhaled breath include ethane, pentane, aldehydes, and isoprene (Boots et al., 2012). In the past few decades, the diagnostic potential for breath and urine analyses regarding VOCs has gained traction. For example, exhaled VOC profiles can be used for the diagnoses of COPD and asthma (Boots et al., 2012)and diabetes (Das et al., 2016). Additionally, VOC analysis has been used in the diagnoses of several forms of cancer (Peng et al., 2010). More recently, VOC analyses have begun to be applied to both gastrointestinal and liver diseases. Indeed, patients with irritable bowel syndrome have been shown to have unique VOC breath fingerprints compared to healthy controls (Arasaradnam et al., 2016).
In regards to aiding in liver disease diagnosis, Arasaradnam et al., recently showed that urinary VOC analysis can be used to distinguish between healthy controls and patients presenting with both NAFLD and NASH, and also was able to differentiate between NASH and NAFLD (Arasaradnam et al., 2015). Moreover, it has also been demonstrated that obese individuals have a unique VOC profile extracted from the fecal microbiome compared to healthy controls (Raman et al., 2013). The diagnostic potential of VOCs in both exhaled breath and urine are beginning to be examined in more detail. Using VOC analytes for diagnosis is less invasive and faster than traditional diagnostic measures, such as biopsies. Indeed, this area has made significant progress over the last ten years; however, more research is needed to broaden the application of such methods. To that extent, using VOCs as diagnostic tools may also aid in emphasizing the importance of understanding the mechanisms of their hepatotoxicity and how their exposure impacts human health.
Summary and Conclusions
Although industrial chemical exposures have long been known to cause fatty liver both in humans and animal models, toxicant-induced steatosis was typically not emphasized as its clinical significance was considered doubtful. Only recently, it has become clear that these toxicants can induce steatohepatitis (TASH) and that steatohepatitis may lead to meaningful clinical outcomes including decompensated cirrhosis, HCC, and cardiometabolic disease. However, the interaction of environmental chemicals, such as VOCs with other factors, such as genetics, diet or underlying diseases, such as NAFLD, is still largely understudied.
Animal research to predict human disease serves several critical roles. Not only does this research inform on new potential mechanism(s) by which injury occurs, but may also lead to applied knowledge that can be directly translated into the clinics. For example, if there are already therapies available that target those mechanisms, then these can be ‘repurposed’ for the toxicity. Furthermore, specific mechanisms of injury may have specific ‘footprints’ in biological tissues, such as altered protein, expression or metabolite profiles. Therefore, better mechanistic insight into toxicity could identify biomarkers (or surrogate biomarkers) to determine inter-individual risk. However, such advances only are possible when the mechanisms are validated in human cohorts. For this reason, it is absolutely critical that prospectively designed human studies incorporates (or at least allows in future work) the investigation of variables that may validate the mechanisms identified in animal models. Although some studies in humans have done exactly that for environmental exposures, such bench-to-bedside translation is needed more universally. Therefore, more research is needed to understand the mechanisms of toxicant-induced liver injury, so that more effective diagnostics and therapeutics may be developed.
Acknowledgements
Funding: Supported by awards from the National Institutes of Health (K01 DK096042, R03 DK107912, T32ES011564, and P42 ES023716). Research was also supported by two Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P20GM113226 and P20GM113226 as well as the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number P50AA024337. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abplanalp W, DeJarnett N, Riggs DW, Conklin DJ, McCracken JP, Srivastava S, Xie Z, Rai S, Bhatnagar A, and O'Toole TE (2017) Benzene exposure is associated with cardiovascular disease risk. PLoS One 12, e0183602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. ATSDR's Substance Priority List. 2017. Ref Type: Online Source [Google Scholar]
- Allman M, Gaskin L, and Rivera CA (2010) CCl4-induced hepatic injury in mice fed a Western diet is associated with blunted healing. J Gastroenterol Hepatol. 25, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders LC, Lang AL, Anwar-Mohamed A, Douglas AN, Bushau AM, Falkner KC, Hill BG, Warner NL, Arteel GE, Cave M, McClain CJ, and Beier JI (2016a) Vinyl chloride metabolites potentiate inflammatory liver injury caused by LPS in mice. Toxicol. Sci 151, 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders LC, Yeo H, Kaelin BR, Lang AL, Bushau AM, Douglas AN, Cave M, Arteel GE, McClain CJ, and Beier JI (2016b) Role of dietary fatty acids in liver injury caused by vinyl chloride metabolites in mice. Toxicol. Appl. Pharmacol 311, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee QM, Daly AK, and Day CP (2011) Genetic modifiers of non-alcoholic fatty liver disease progression. Biochim. Biophys. Acta 1812, 1557–1566. [DOI] [PubMed] [Google Scholar]
- Arai H, Awane N, Mizuno A, Fukaya M, Sakuma M, Harada N, Kawaura A, Yamamoto H, Okumura H, Taketani Y, Doi T, and Takeda E (2010) Increasing early insulin secretion compensate adequately for hepatic insulin resistance in CCl4- induced cirrhosis rats. J Med Invest 57, 54–61. [DOI] [PubMed] [Google Scholar]
- Arasaradnam RP, McFarlane M, Daulton E, Skinner J, O'Connell N, Wurie S, Chambers S, Nwokolo C, Bardhan K, Savage R, and Covington J (2016) Non-invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease (IBD). Dig. Liver Dis 48, 148–153. [DOI] [PubMed] [Google Scholar]
- Arasaradnam RP, McFarlane M, Daulton E, Westenbrink E, O'Connell N, Wurie S, Nwokolo CU, Bardhan KD, Savage RS, and Covington JA (2015) Non-invasive distinction of non-alcoholic fatty liver disease using urinary volatile organic compound analysis: early results. J Gastrointestin. Liver Dis 24, 197–201. [DOI] [PubMed] [Google Scholar]
- Arumugam S, Girish SK, Kemparaju K, and Thirunavukkarasu C (2017) Neutrophil extracellular traps in acrolein promoted hepatic ischemia reperfusion injury: therapeutic potential of NOX2 and p38MAPK inhibitors. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Asrih M, and Jornayvaz FR (2013) Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 218, R25–R36. [DOI] [PubMed] [Google Scholar]
- Baiceanu A, Mesdom P, Lagouge M, and Foufelle F (2016) Endoplasmic reticulum proteostasis in hepatic steatosis. Nat. Rev. Endocrinol 12, 710–722. [DOI] [PubMed] [Google Scholar]
- Begriche K, Massart J, Robin MA, Bonnet F, and Fromenty B (2013) Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 58, 1497–1507. [DOI] [PubMed] [Google Scholar]
- Bellanti F, Villani R, Facciorusso A, Vendemiale G, and Serviddio G (2017) Lipid oxidation products in the pathogenesis of non-alcoholic steatohepatitis. Free Radic. Biol. Med 111, 173–185. [DOI] [PubMed] [Google Scholar]
- Boots AW, van Berkel JJ, Dallinga JW, Smolinska A, Wouters EF, and van Schooten FJ (2012) The versatile use of exhaled volatile organic compounds in human health and disease. J Breath. Res 6, 027108. [DOI] [PubMed] [Google Scholar]
- Bove FJ, Ruckart PZ, Maslia M, and Larson TC (2014) Evaluation of mortality among marines and navy personnel exposed to contaminated drinking water at USMC base Camp Lejeune: a retrospective cohort study. Environ. Health 13, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, and Hobbs HH (2004) Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40, 1387–1395. [DOI] [PubMed] [Google Scholar]
- Cai H, and Guengerich FP (2001) Reaction of trichloroethylene oxide with proteins and dna: instability of adducts and modulation of functions. Chem. Res. Toxicol 14, 54–61. [DOI] [PubMed] [Google Scholar]
- Calzadilla BL, and Adams LA (2016) The natural course of non-alcoholic fatty liver disease. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, and Burcelin R (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772. [DOI] [PubMed] [Google Scholar]
- Cave M, Falkner KC, Henry L, Costello B, Gregory B, and McClain CJ (2011) Serum cytokeratin 18 and cytokine elevations suggest a high prevalence of occupational liver disease in highly exposed elastomer/polymer workers. J. Occup. Environ. Med 53, 1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M, Falkner KC, Ray M, Joshi-Barve S, Brock G, Khan R, Bon Homme M, and McClain CJ (2010) Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology 51, 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Guo X, Loomba R, Goodarzi MO, Haritunians T, Kwon S, Cui J, Taylor KD, Wilson L, Cummings OW, Chen YD, and Rotter JI (2010) Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology 139, 1567–76, 1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-Garcia R, and Blumberg B (2014) Transgenerational effects of obesogens and the obesity epidemic. Curr. Opin. Pharmacol 19, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Wang M, Zhang J, Barve SS, McClain CJ, and Joshi-Barve S (2017) Acrolein disrupts tight junction proteins and causes ER stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am J Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki JA, Furuya S, Konganti K, Luo YS, McDonald TJ, Iwata Y, Chiu WA, Threadgill DW, Pogribny IP, and Rusyn I (2017a) Impact of nonalcoholic fatty liver disease on toxicokinetics of tetrachloroethylene in mice. J Pharmacol Exp. Ther 361, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki JA, Furuya S, Luo YS, Iwata Y, Konganti K, Chiu WA, Threadgill DW, Pogribny IP, and Rusyn I (2017b) Nonalcoholic fatty liver disease is a susceptibility factor for perchloroethylene-induced liver effects in mice. Toxicol Sci 159, 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary E, Asher M, Olawoyin R, and Zhang K (2017) Assessment of indoor air quality exposures and impacts on respiratory outcomes in River Rouge and Dearborn, Michigan. Chemosphere 187, 320–329. [DOI] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, and Hobbs HH (2011) Human fatty liver disease: old questions and new insights. Science 332, 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja MJ (2007) Cell signaling in oxidative stress-induced liver injury. Semin. Liver Dis 27, 378–389. [DOI] [PubMed] [Google Scholar]
- Das S, Pal S, and Mitra M (2016) Significance of exhaled breath test in clinical diagnosis: a special focus on the detection of diabetes mellitus. J Med Biol. Eng 36, 605–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP, and James OF (1998) Steatohepatitis: a tale of two "hits"? Gastroenterology. 114, 842–845. [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Anstee QM, and Valenti L (2013) Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment. Curr. Pharm. Des 19, 5219–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosanjh MK, Chenna A, Kim E, Fraenkel-Conrat H, Samson L, and Singer B (1994) All four known cyclic adducts formed in DNA by the vinyl chloride metabolite chloroacetaldehyde are released by a human DNA glycosylase. Proc. Natl. Acad. Sci USA 91, 1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagone P, and Jackowski S (2009) Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 50 (Suppl.), S311–S316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, and Gores GJ (2003) Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125, 437–443. [DOI] [PubMed] [Google Scholar]
- Fenga C, Gangemi S, Giambo F, Tsitsimpikou C, Golokhvast K, Tsatsakis A, and Costa C (2016) Low-dose occupational exposure to benzene and signal transduction pathways involved in the regulation of cellular response to oxidative stress. Life Sci 147, 67–70. [DOI] [PubMed] [Google Scholar]
- Feroe AG, Attanasio R, and Scinicariello F (2016) Acrolein metabolites, diabetes and insulin resistance. Environ. Res 148, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, and Clouston AD (2014) The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 59, 1393–1405. [DOI] [PubMed] [Google Scholar]
- Grun F, and Blumberg B (2006) Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinol. 147, S50–S55. [DOI] [PubMed] [Google Scholar]
- Guardiola JJ, Beier JI, Falkner KC, Wheeler B, McClain CJ, and Cave M (2016) Occupational exposures at a polyvinyl chloride production facility are associated with significant changes to the plasma metabolome. Toxicol. Appl. Pharmacol 313, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, and Kaufman RJ (2016) The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 57, 1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun E, Cearfoss J, Mamada S, Al-Hassan N, Brown M, Heimberger K, and Liu MC (2014) The effects of mixtures of dichloroacetate and trichloroacetate on induction of oxidative stress in livers of mice after subchronic exposure. J Toxicol Environ. Health A 77, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun E, and Mettling C (2015) Dichloroacetate and Trichloroacetate Toxicity in AML12 Cells: Role of Oxidative Stress. J Biochem. Mol Toxicol 29, 508–512. [DOI] [PubMed] [Google Scholar]
- Ibrahim SH, Kohli R, and Gores GJ (2011) Mechanisms of lipotoxicity in NAFLD and clinical implications. J Pediatr. Gastroenterol Nutr 53, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri Y, Kato R, Sadamatsu M, Takano M, Yasuda Y, Tanaka F, Oishi C, Imano H, Okada Y, Tanaka K, and Hayashi T (2017) Contributions of caspase-8 and -9 to liver injury from CYP2E1-produced metabolites of halogenated hydrocarbons. Xenobiotica 1–13. [DOI] [PubMed] [Google Scholar]
- Irene P, Chiara R, Laura A, Maria GD, Melania G, Cristina F, Chiara S, Valeria M, Loris S, Eleonora M, Claudio P, Emma B, Luciano T, Antonio G, Marco M, Samuele M, Sergio U, Saverio C, Gastalderi A, and Gianluca SB (2017) Lack of NLRP3-inflammasome leads to gut-liver axis derangement, gut dysbiosis and a worsened phenotype in a mouse model of NAFLD. Sci Rep. 7, 12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi-Barve S, Kirpich I, Cave MC, Marsano LS, and McClain CJ (2015) Alcoholic, nonalcoholic, and toxicant-associated steatohepatitis: mechanistic similarities and differences. Cell Mol Gastroenterol Hepatol. 1, 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, and Tacke F (2009) Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274. [DOI] [PubMed] [Google Scholar]
- Kaur J (2014) A comprehensive review on metabolic syndrome. Cardiol Res. Pract 2014, 943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim TH, Kim YW, Shin SM, Kim CW, Yu IJ, and Kim SG (2010) Synergistic hepatotoxicity of N,N-dimethylformamide with carbon tetrachloride in association with endoplasmic reticulum stress. Chem. Biol. Interact 184, 492–501. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Lancaster JR Jr., Meszaros AT, and Weidinger A (2017) Mitochondria-meditated pathways of organ failure upon inflammation. Redox. Biol 13, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, and Mehal WZ (2012) Sterile inflammation in the liver. Gastroenterology 143, 1158–1172. [DOI] [PubMed] [Google Scholar]
- Lang AL, Chen L, Poff GD, Ding WX, Barnett RA, Arteel GE, and Beier JI (2018) Vinyl chloride dysregulates metabolic homeostasis and enhances diet-induced liver injury in mice. Hepatol. Commun 2, 270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linher-Melville K, and Singh G (2017) The complex roles of STAT3 and STAT5 in maintaining redox balance: Lessons from STAT-mediated xCT expression in cancer cells. Mol Cell Endocrinol. 451, 40–52. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang Z, and Nowicki MJ (2014) Caspase-12 mediates carbon tetrachloride-induced hepatocyte apoptosis in mice. World J Gastroenterol 20, 18189–18198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Zhu MX, and Xie JP (2010) Mutagenicity of acrolein and acrolein-induced DNA adducts. Toxicol Mech. Methods 20, 36–44. [DOI] [PubMed] [Google Scholar]
- Ma XD, Ma X, Sui YF, and Wang WL (2002) Expression of gap junction genes connexin32 and connexin43 mRNAs and proteins, and their role in hepatocarcinogenesis. World J Gastroenterol. 8, 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan K, Bhardwaj P, Thareja S, Gupta SD, and Saraya A (2006) Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J Clin. Gastroenterol 40, 930–935. [DOI] [PubMed] [Google Scholar]
- Maslia ML, Aral MM, Ruckart PZ, and Bove FJ (2016) Reconstructing historical VOC concentrations in drinking water for epidemiological studies at a U. S. military base: summary of results. Water (Basel) 8, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson S, Hardy T, Henderson E, Burt AD, Day CP, and Anstee QM (2015) Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 62, 1148–1155. [DOI] [PubMed] [Google Scholar]
- Mehal WZ (2013) The Gordian Knot of dysbiosis, obesity and NAFLD. Nat. Rev. Gastroenterol Hepatol 10, 637–644. [DOI] [PubMed] [Google Scholar]
- Meyer-Alber A, Hartmann H, Stumpel F, and Creutzfeldt W (1992) Mechanism of insulin resistance in CCl4-induced cirrhosis of rats. Gastroenterology 102, 223–229. [DOI] [PubMed] [Google Scholar]
- Mishra A, and Younossi ZM (2012) Epidemiology and natural history of non-alcoholic fatty liver disease. J Clin. Exp. Hepatol 2, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, and Joshi-Barve S (2015) Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci 143, 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C, and Joshi-Barve S (2012) Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol. Appl. Pharmacol 265, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JP, Pitts A, and Younossi ZM (2008) Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 49, 608–612. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, and Hotamisligil GS (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461. [DOI] [PubMed] [Google Scholar]
- Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, and Gentile CL (2016) Endoplasmic reticulum stress in obesity and obesity-related disorders: an expanded view. Metabolism 65, 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Oh JH, Yoon S, and Rana SV (2008) Time dependent gene expression changes in the liver of mice treated with benzene. Biomark. Insights 3, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M, Lefebvre P, and Staels B (2015) Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 62, 720–733. [DOI] [PubMed] [Google Scholar]
- Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Tisch U, and Haick H (2010) Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J Cancer 103, 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip BK, Mumtaz MM, Latendresse JR, and Mehendale HM (2007) Impact of repeated exposure on toxicity of perchloroethylene in Swiss Webster mice. Toxicol. 232, 1–14. [DOI] [PubMed] [Google Scholar]
- Pooranaperundevi M, Sumiyabanu MS, Viswanathan P, Sundarapandiyan R, and Anuradha CV (2010) Insulin resistance induced by high-fructose diet potentiates carbon tetrachloride hepatotoxicity. Toxicol Ind. Health 26, 89–104. [DOI] [PubMed] [Google Scholar]
- Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, and Sanyal AJ(2008) Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134, 568–576. [DOI] [PubMed] [Google Scholar]
- Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, Wu P, Liu X, Yu Y, Farris AB, Nusrat A, Parkos CA, and Anania FA (2016) Loss of junctional adhesion molecule A promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology 151, 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, Bailey J, Myers RP, and Rioux KP (2013) Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol Hepatol 11, 868–875. [DOI] [PubMed] [Google Scholar]
- Raucy JL, Kraner JC, and Lasker JM (1993) Bioactivation of halogenated hydrocarbons by cytochrome P4502E1. Crit Rev. Toxicol 23, 1–20. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Beckett WS, Sparer J, Barwick KW, Riely CA, Miller H, Sigal SL, Shalat SL, and Cullen MR (1988) Liver disease associated with occupational exposure to the solvent dimethylformamide. Ann. Intern. Med 108, 680–686. [DOI] [PubMed] [Google Scholar]
- Reid DT, Reyes JL, McDonald BA, Vo T, Reimer RA, and Eksteen B (2016) Kupffer cells undergo fundamental changes during the development of experimental NASH and are critical in initiating liver damage and inflammation. PLoS One 11, e0159524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, and Hobbs HH (2008) Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet 40, 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar K, and Schiff ER (2004) Alcohol and hepatitis C. Semin. Liver Dis 24, 305–315. [DOI] [PubMed] [Google Scholar]
- Savini I, Catani MV, Evangelista D, Gasperi V, and Avigliano L (2013) Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci 14, 10497–10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayiner M, Koenig A, Henry L, and Younossi ZM (2016) Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin. Liver Dis 20, 205–214. [DOI] [PubMed] [Google Scholar]
- Segal AW (2005) How neutrophils kill microbes. Annu. Rev. Immunol 23, 197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, and Yki-Jarvinen H (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin. Endocrinol. Metab 87, 3023–3028. [DOI] [PubMed] [Google Scholar]
- Severson TJ, Besur S, and Bonkovsky HL (2016) Genetic factors that affect nonalcoholic fatty liver disease: A systematic clinical review. World J Gastroenterol 22, 6742–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H (1985). Oxidative stress: introductory remarks In: Oxidative Stress Sies H, ed. (Academic Press: London: ), pp. 1–8. [Google Scholar]
- Sozen E, and Ozer NK (2017) Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated mini-review. Redox. Biol 12, 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ (2015) Cellular homeostasis and repair in the mammalian liver. Annu. Rev. Physiol 77, 179–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenberg JA, Bogdanffy MS, Ham A, Holt S, Kim A, Morinello EJ, Ranasinghe A, Scheller N, and Upton PB (1999) Formation and repair of DNA adducts in vinyl chloride- and vinyl fluoride-induced carcinogenesis. IARC Sci Publ. 29–43. [PubMed] [Google Scholar]
- Szabo G, Saha B, and Bukong TN (2015) Alcohol and HCV: implications for liver cancer. Adv. Exp. Med Biol 815, 197–216. [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, and Falezza G (2005) Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54, 3541–3546. [DOI] [PubMed] [Google Scholar]
- Tolman KG, and Sirrine R (1998) Occupational hepatotoxicity. Clin Liver Dis 563–589. [Google Scholar]
- United States Environmental Protection Agency. Technical Overview of Volatile Organic Compounds. 2017a. Ref Type: Online Source. [Google Scholar]
- United States Environmental Protection Agency. Volatile organic compounds' impact on indoor air quality. 2017b. Ref Type: Online Source. [Google Scholar]
- Utzschneider KM, and Kahn SE (2006) Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin. Endocrinol. Metab 91, 4753–4761. [DOI] [PubMed] [Google Scholar]
- Vladykovskaya E, Sithu SD, Haberzettl P, Wickramasinghe NS, Merchant ML, Hill BG, McCracken J, Agarwal A, Dougherty S, Gordon SA, Schuschke DA, Barski OA, O'Toole T, D'Souza SE, Bhatnagar A, and Srivastava S (2012) Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J. Biol. Chem 287, 11398–11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Beier JI, Clair HB, Bellis-Jones HJ, Falkner KC, McClain CJ, and Cave MC (2013) Toxicant-associated steatohepatitis. Toxicol Pathol 41, 343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, Lotersztajn S, and Pavoine C (2014) M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 59, 130–142. [DOI] [PubMed] [Google Scholar]
- Wang C, Yang J, Lu D, Fan Y, Zhao M, and Li Z (2016) Oxidative stress-related DNA damage and homologous recombination repairing induced by N,N-dimethylformamide. J Appl Toxicol 36, 936–945. [DOI] [PubMed] [Google Scholar]
- Wang M, and Kaufman RJ (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity and overweight. 2015. 2-19-2016. Ref Type: Online Source. [Google Scholar]
- Xu R, Huang H, Zhang Z, and Wang FS (2014) The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 11, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XY, Liu YF, Lu LW, Ke YB, Mao JY, and Mao KL (2012) Altered expression of hepatic metabolic enzyme and apoptosis-related gene transcripts in human hepatocytes treated with trichloroethylene. Hum. Exp. Toxicol 31, 861–867. [DOI] [PubMed] [Google Scholar]
- Yang H, Biermann MH, Brauner JM, Liu Y, Zhao Y, and Herrmann M (2016) New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front Immunol. 7, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Lane MD, Clemens M, and Diehl AM (1997) Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA 94, 2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, and Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84. [DOI] [PubMed] [Google Scholar]
- Zhang K, and Kaufman RJ (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YH, Cichocki JA, Soldatow VY, Scholl E, Gallins P, Jima D, Yoo HS, Chiu WA, Wright FA, and Rusyn I (2017) Comparative dose-response analysis of liver and kidney transcriptomic effects of trichloroethylene and tetrachloroethylene in B6C3F1 mouse. Toxicol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]