Abstract
Soil samples (collected from El-Madina El-Monawara, Kingdom Saudi Arabia) were mixed with human saliva, incubated in media suitable for bacterial and fungal growth and filtered. Eighteen bacterial and five fungal species were isolated and identified. The bacterial and fungal filtrates as well as the isolated species were evaluated for their antimicrobial activities against some pathogenic microbes causing dermatological diseases (Staphylococcus aureus, methicillin resistant S. aureus (MRSA) and Aspergillus niger). The bacterial filtrate showed significant antagonistic effect against S. aureus and methicillin resistant S. aureus (MRSA), whereas showed non inhibitory action on the pathogenic fungus. In contrast, the fungal filtrate antagonized the growth of the pathogenic fungus (A. niger) and did not produce any inhibitory effect on the two tested pathogenic bacteria. The isolated bacterial species showed different levels of antagonistic activities against the three tested microbes. Bacillus subtilis was described as potent isolate against the three pathogens, followed by Esherichia coli. However, Bacillus megaterium strongly inhibited the growth of the pathogenic bacteria only. On the other side, all the fungal filtrates of the isolated species, except Cochliobolus lanatus showed antagonistic activity against the pathogenic fungus (A. niger). The filtrate of Fusarium oxysporum and Emericella nidulans counteracted the growth of S. aureus, whereas, the growth of MRSA was inhibited only by the filtrate of E. nidulans. From the passage way of our respected prophet, how is never tells from him self, if any person complains from awound or ulcer, the messenger of Allah (prayers and peace be upon him) put his forefinger on the ground and lift it then he says: (In the Name of God, soil of our land, with the saliva of some of us, our sick person will get well after the permission of our God) Al-Bukhari. The meaning of this Hadith that the prophet takes his saliva on the forefinger then he put it on the soil and wipe on the wound place while saying the above Hadith that is shows the Prophet’s miracle, which is evidence of healing by using soil and saliva.
Keywords: Antimicrobial activity, Soil microorganisms, Saliva, Pathogenic microbes, Staphylococcus aureus, MRSA, Aspergillus niger
1. Introduction
The dermatological pathogenic microbes could endure in the skin through the cracks, ulcers causing skin infections. The treatment depends on using antibiotics, which have several side effects. The remarkable increase in antibiotics resistant bacterial species (Kina, 2003, Motta et al., 2004) lead to search for new sources of antibiotics through the isolation and identification of new types of microorganisms such as bacteria, fungi and actinomycetes (Alexander, 1982). The antibiotics produced by bacteria have been gaining importance by many investigators. Bacterial species producing antibiotics have been used as biocontrol agents against pathogenic fungi (Yilmaz et al., 2005, Gebree1 et al., 2008).
Soil is considered one of the most suitable environments for microbial growth (Cavalcanti et al., 2006), for that the microorganisms which have been isolated from the soil having leading in this area. The genus Streptomyces which is antibiotics producer has been isolated from the soil of Yemen (Ahmed, 2003). Also, one hundred bacterial isolates were isolated from six different soil samples collected from Egypt, 20 of them could antagonized some selected plants and human pathogenic fungi such as Apergillus sp., Fusarium oxysporum, Penecillium digitatum and Alternaria solani (Gebree1 et al., 2008). Moreover, 20 bacterial strains isolated from soil stressed ecological niches of Eastern Uttar Pradesh, India showed strong antimicrobial activities (Singh et al., 2009).
On the other hand, some studies showed that saliva contains many antimicrobial substances. B-defensins which are cationic peptides with broad-spectrum antimicrobial activity are produced by human salivary glands and oral mucosal surfaces and most abundant in tissues of associated inflammations. Human B-defensins play an important role in the innate defenses against oral microorganisms (Mathews et al., 1999). Murakami et al. (2002) detected cathelicidin antimicrobial peptides in human saliva, contributing to broad-spectrum defense of the oral cavity. Also, cathelicidin are essential for the protection of skin against invasive bacterial infection. In addition, salivary histatins are potent in vitro antifungal agents and have great promise as therapeutic agent in humans with oral candidiasis caused by Candida albicans. Histatins caused loss of fungal cell integrity followed by its death (Edgerton et al., 1998). Further more, Liu et al. (2008) purified and cloned a noval antimicrobial peptide from salivary glands of hard tick named ixoxin-B.
Based on the above mentioned literatures, this study is an attempt to investigate the antimicrobial activity of certain soil microorganisms isolated from the soil of El-Medina El-Monawara mixed with human saliva against some pathogenic microorganisms that cause wounds inflammation and ulcers such as Staphylococcus aureus, methicillin resistant S. aureus (MRSA) and the pathogenic fungus Aspergillus niger isolated from dermatological infections as fungal foot disease (Kown-chung and Bennett, 1992). Moreover, identification of the most active isolates was done.
Through the guidance of our prophet (peas be upon him) Aisha said: if someone complained to messenger of God (peas be upon him) from ulcer or wound, the prophet used to put his finger on the earth and saying (name of God, our soil, our saliva, to accrue our patient with helping of our lord) Imam Al-Bukhari, 1993, Imam Muslim, 1978 narrated.
2. Materials and methods
Soil samples were collected from different localities at El-Madina El-Monawara city (Qurban El Nazel – Eastern Hora – Ohod mountain) Kingdom Saudi Arabia, and were kept at 25 °C. Human saliva samples were taken from healthy persons. One gram of soil, 1 ml of saliva and 1 ml of mixture (1 g soil + 1 ml of saliva) were inoculated to the nutrient Browth medium for bacterial growth and Sabourand Dextrose liquid medium for fungal growth, incubated for 10 days. Thereafter, the culture media were filtered using sterilized bacterial filters (NALGENE 0.45 Mm) produced by Nalge Nunc International. Isolation of different bacterial and fungal isolates was carried out from the mixture and purified. The bacterial isolates were identified according to Bergey’s manual of systematic bacteriology (Krieg and Holt, 1984, Sneath et al., 1986). The fungal isolates were identified in Botany Department, Faculty of Science, Cairo University, Egypt.
The pathogenic isolates of S. aureus were obtained from King Fahd – Hospital, Jeddah city. The methicillin resistant S. aureus (MRSA) was obtained from King Fahd – Hospital, Gizan. Whereas, the pathogenic fungus A. niger was taken from microbial culture collection (MIRCN), Faculty of Agriculture, Ain Shams University, Egypt.
The nutrient Browth medium was used for quantitative determination of both pathogenic and soil bacterial isolates. The blood agar culture medium recommended by Madigan and Martinko (2005) was used for cultivation of pathogenic bacterial isolates. Whereas, Sabouraud Dextrose agar medium was used for fungal growth (Georg et al., 1954).
The antagonistic activities of soil alone (1 g), saliva alone (1 ml) and the mixture of both (1 g soil and 1 ml saliva) were tested against the pathogenic microbes.
The method described by Umechuruba and Nwachukwa (1997) was used for testing the antimicrobial activities of the cell free culture media of both soil bacteria and fungi on the growth of the tested pathogenic microbes.
The pathogenic fungus and bacteria were treated with different concentrations of fungal and bacterial filtrates of the mixture (soil + saliva).
The dry weight of the pathogenic microbes was determined as mg according to the method of Bouknight and Sadoff (1975).
The antagonistic effects of the bacterial and fungal species isolated from the mixture of soil and saliva were tested according the methods of Weller and Cook, 1983, El-Sohaibani, 1999 for bacteria and Umechuruba and Nwachukwa (1997) for fungi.
3. Results and discussion
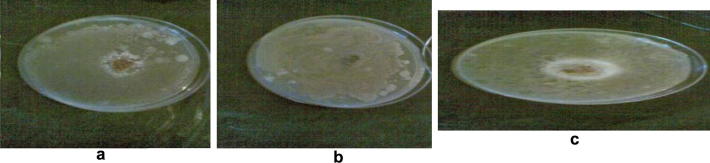
The antimicrobial activities of soil, saliva and mixture of both soil and saliva were tested against the three tested pathogenic organisms. The results revealed that, soil alone showed moderate antimicrobial activity compared to saliva which did not produce any antagonistic effect. On the other hand, mixture of soil and saliva showed the highest antimicrobial activity (Fig. 1). The same trend was observed for the cell free bacterial and fungal culture media. Thus, the pathogenic organisms showed higher sensitivity to the filtrates resulted from mixing soil and saliva. Based on the above mentioned results, all the experiments were carried out using the mixture of soil and saliva. This is may be due to that the alkalinity of saliva may favour the growth of antibiotic producing microorganism. Furthermore, saliva act as oral antiseptic because it contain lysozyme and prevent the growth of dental bacteria. Moreover, when saliva decreased the growth of pathogenic fungi increased leading to different oral diseases.
Figure 1.
The antagonistic activity (expressed by the inhibition zone) of soil alone (a), saliva alone (b), and their mixture (c) on the pathogenic bacteria S. aureus.
Many workers proved that saliva contain antibacterial substances such as lactoferrin (iron binding protein), thiocyanate and lactoperoxidase (Tenovuo, 2002, Komine et al., 2006). In this connection, Dodds et al. (2004) indicated that saliva contain proline-rich proteins such as statherin and histatins that influence Candida infection. Huang (2004) identified and characterized 10 antimicrobial proteins in saliva such as -1-α antrypsin, thioredoxin and peroxiredoxin B. In addition, salivary nitrite enhances its bactericidal effects through the generation of reactive nitrogen intermediates including nitricoxide and nitrosonitrosyl species against Esherichia coli and C. albicans (Bjorne et al., 2006). The presence of horseradish peroxidase–iodide–hydrogen peroxide in saliva increase the salivary bactericidal effect on Fusobacterium nucleatum which associated with the infection of tooth supporting tissues, periodontitis, (Ihalin et al., 2003). Furthermore, Liu et al. (2008) Purified and cloned a noval antimicrobial peptide from salivary glands of hard tick named ixoxin-B.
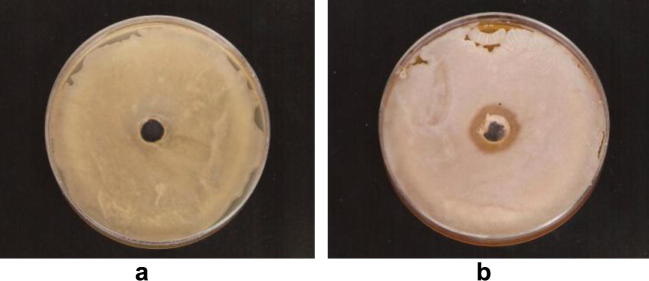
The results revealed that the bacterial cell free culture medium showed highly effective antibacterial action against the growth of S. aureus (Fig. 2) and methicillin resistant S. aureus (MRSA) (Fig. 3) as appeared from the inhibition zones around the holes filled with the bacterial filtrates.
Figure 2.
The antagonistic effect of bacterial cell free culture medium on the growth of Staphylococcus aureus. (a) Control culture of S. aureus (b) S. aureus cultures with hole filled with bacterial filtrate.
Figure 3.
The antagonistic effect of bacterial cell free culture medium on the growth of methicillin resistant S. aureus (a) control culture of MRSA (b) MRSA culture with holes filled with bacterial filtrate.
The effects of different concentrations of bacterial and fungal cell free culture media on the dry weight of the two tested pathogenic bacterial species were presented in (Table 1). As appeared from the data, the dry weight of S. aureus and MRSA decreased with increasing the concentration of the bacterial filtrate and consequently the percentage of inhibition increased. Thus, 8% of the bacterial filtrate inhibited the growth of S. aureus and MRSA by 85.7% and 50%, respectively below the control level. Although the bacterial filtrate exerted strong antagonistic effect, MRSA showed more resistance than S. aureus to the inhibitory action of the bacterial filtrate. In contrast, the fungal cell free culture medium did not show any antagonistic action against the two pathogenic bacteria. Thus, the dry weights of the treated cultures were nearyl the same as the control weight at all concentrations of the fungal filtrate.
Table 1.
Effect of different concentrations of bacterial and fungal filtrates (cell free culture media) on the dry weight (mg) of S. aureus and methicillin resistant S. aureus after four days of incubation (±standard deviation).
| Treatment | (%) Conc. |
S. aureus |
MRSA |
||
|---|---|---|---|---|---|
| Dry weight | % Inhibition | Dry weight | % Inhibition | ||
| Bacterial filtrate | Control | 140.0 ± 33.0 | 0.0 | 40.00 ± 0.58 | 0.0 |
| 2 | 100.0 ± 0.58⁎⁎ | 28.57 | 35.0 ± 0.33⁎⁎ | 12.5 | |
| 4 | 82.0 ± 0.58⁎⁎ | 41.43 | 33.0 ± 1.00⁎⁎ | 17.5 | |
| 6 | 60.0 ± 0.33 | 57.14 | 25.0 ± 0.58⁎⁎ | 37.5 | |
| 8 | 20.00 ± 1.00⁎⁎ | 85.71 | 20.0 ± 1.53⁎⁎ | 50.0 | |
| Fungal filtrate | Control | 140.33 ± 0.58 | 0.0 | 40.67 ± 1.15 | 0.0 |
| 2 | 139.33 ± 0.58⁎⁎ | 0.71 | 38.33 ± 0.58⁎⁎ | 5.75 | |
| 4 | 140.00 ± 1.53⁎⁎ | 0.23 | 40.0 ± 1.00⁎⁎ | 1.65 | |
| 6 | 138.00 ± 1.00⁎⁎ | 1.66 | 39.33 ± 0.58⁎⁎ | 3.29 | |
| 8 | 140.33 ± 0.58⁎⁎ | 0.00 | 40.67 ± 1.15⁎⁎ | 0.00 | |
∗Significant at 5%.
Significant at 1%.
All the concentrations used from the bacterial filtrates did not show any antifungal effect against the growth of the tested pathogenic fungus (A. niger) at all incubation periods (Table 2). However, the fungal filtrate exerted strong antagonistic action against the growth of A. niger. Thus, concentration of 8% inhibited the fungal growth by 64.7% at the end of the incubation period. This results could be confirmed by the appearance of the inhibition zone of the fungal growth in response to treatment with fungal filtrate not with bacterial one (Fig. 4). These results are in confirmity with those of Hentschel et al. (2006) who indicated that most of bacterial isolates showed antagonistic effects against bacteria more than fungi specially the strains isolated from hospitals as Staplylococcus epidermis and S. aureus. The ability of bacterial filtrate to antagonize the pathogenic bacteria whereas the fungal filtrate counteract the pathogenic fungus may be referred to that some microbial species produce antimicrobial substances which affect only the systematically related species (Abu Arkoub, 2002).
Table 2.
Effect of different concentrations of bacterial and fungal filtrates (cell free culture media) on the dry weight (mg) of Aspergillus niger at different incubation periods (±standard deviation).
| Treatment | (%) Conc. | 4 days of incubation | 8 days of incubation | ||
|---|---|---|---|---|---|
| Dry weight | % Inhibition | Dry weight | % Inhibition | ||
| Bacterial filtrate | Control | 280 ± 2.00 | 0.0 | 550 ± 0.58 | 0.0 |
| 2 | 280 ± 0.33⁎⁎ | 0.0 | 540 ± 1.67⁎⁎ | 1.82 | |
| 4 | 280 ± 0.67⁎ | 0.0 | 550 ± 0.33 | 0.0 | |
| 6 | 279.33 ± 0.58⁎ | 2.3 | 550 ± 0.58⁎ | 0.0 | |
| 8 | 279 ± 0.67⁎ | 0.35 | 550 ± 1.45⁎ | 0.0 | |
| Fungal filtrate | Control | 103.33 ± 15.28 | 0.0 | 134.0 ± 16.37 | 0.0 |
| 2 | 60.33 ± 0.58⁎⁎ | 41.61 | 67.0 ± 12.12⁎⁎ | 50.0 | |
| 4 | 50.33 ± 1.53⁎⁎ | 51.29 | 70.33 ± 3.51⁎⁎ | 47.51 | |
| 6 | 40.33 ± 0.58⁎⁎ | 60.97 | 70.33 ± 2.52⁎⁎ | 47.51 | |
| 8 | 35.0 ± 1.00⁎⁎ | 66.13 | 64.67 ± 1.15⁎⁎ | 51.74 | |
Significant at 5%.
Significant at 1%.
Figure 4.
The antagonistic effect of fungal cell free culture medium on the growth of Aspergillus niger. (a) Control culture of A. niger (b) A. niger culture with holes filled with fungal filtrate.
On the other side, some soil bacteria showed antifungal properties because of the production of chitinase which may be a part of a lytic system that enables bacteria for living on hyphae as actual growth substrate (De Boer et al., 1998). At the same time, Barakate et al. (2002) indicated that some soil Streptomycetes were active against yeasts and some filamentous fungi. Also, the antifungal effect of some microbes may be due to the production of extracellular antifungal secondary metabolites such as iron chelators produced by some bacterial species such as pyocheline, pyoverdine and pseudopactin which inhibit or prevent the growth of pathogenic organisms (Osman, 2004). De Boer et al. (2007) indicated in vitro suppression of some plant pathogenic fungi by non-antagonistic soil bacteria. They referred the antifungal effect to the sensitivity of fungi to bacterial secondary metabolites, and to the competitive interactions between bacterial strains. At the same time, Park et al. (2008) isolated extracellular 17 KDa antimicrobial protein from A. oryza and active against pathogenic fungi and bacteria.
Data presents in (Table 3) show that 18 bacterial species were isolated from the soil samples collected from different localities from El-Madina Al-Monwara city mixed with human saliva. The bacterial isolates were identified according to Bergeys manual (Holt et al., 1994). As appeared from the data, Bacillus was the most dominant genus constituting about 50% of the isolated genera. This could explain the strong antimicrobial activity of El-Madina soil. These results are in conformity with others who confirmed the effective antimicrobial activity of this genus (Bacillus) specially against the pathogenic microbes (Aunpad and Na-Bangchang, 2007, Dhanapathi et al., 2008).
Table 3.
The bacterial species isolated from the soil of El-Madina mixed with saliva.
| The species | No. of species | (%) |
|---|---|---|
| Bacillus sp. | 9 | 50.00 |
| Lactobacillus sp. | 2 | 11.11 |
| Arthrobacter sp. | 1 | 5.56 |
| Micrococcus sp. | 3 | 16.67 |
| Sporosarcia sp. | 1 | 5.56 |
| Sporocytophage sp. | 1 | 5.56 |
| Esherichia coli | 1 | 5.56 |
The isolated bacterial genera were tested for their antimicrobial activities against the three tested pathogenic microbes (Table 4). As appeared from the table, Bacillus megaterium (D3) and Bacillus subtilis (D10) showed the most inhibitory effects against the two pathogenic bacteria. Thus, the inhibition zone reached (17 mm) by B. megaterium (D3) and 11 and 12 mm by B. subtilis D10 against S. aureus and MRSA, respectively (Figure 5, Figure 6, 6a and b). This followed by Bacillus sp. and Sporosacia sp. which produced inhibition zone reached (10 and 11 m) against S. aureus and MRSA, respectively. The bacterial genera D1, D2, D4, D13, D14 produced 9 mm inhibition zone against S. aureus whereas, the other genera could not produce any antimicrobial activity. However, only seven genera showed antimicrobial activity against the pathogenic fungus A. niger.
Table 4.
The antagonistic effect of bacterial suspensions of species isolated from mixture of soil and saliva against S. aureus, MRSA and Aspergillus niger expressed by inhibition zones (mm).
| Sym. | Bacterial species | Inhibition zone (mm) |
||
|---|---|---|---|---|
| S. aureus | MRSA | A. niger | ||
| D1 | Bacillus sp. | 9 | 0.0 | 0.0 |
| D2 | Escherichia coli | 9 | 7 | 30 |
| D3 | Bacillus megaterium | 17 | 17 | 0.0 |
| D4 | Micrococcus sp. | 9 | 0.0 | 0.0 |
| D5 | Bacillus sp. | 0.0 | 0.0 | 13 |
| D6 | Micrococcus sp. | 0.0 | 0.0 | 4 |
| D7 | Sporosarcia sp. | 10 | 10 | 0.0 |
| D8 | Bacillus sp. | 10 | 11 | 0.0 |
| D9 | Bacillus sp. | 0.0 | 2 | 5 |
| D10 | Bacillus subtilis | 11 | 12 | 40 |
| D11 | Micrococcus sp. | 0.0 | 0.0 | 9 |
| D12 | Bacillus sp. | 0.0 | 0.0 | 0.0 |
| D13 | Bacillus sp. | 9 | 0.0 | 0.0 |
| D14 | Arthrobacter sp. | 9 | 0.0 | 0.0 |
| D15 | Bacillus sp. | 0.0 | 0.0 | 17 |
| D16 | Lactobacillus sp. | 0.0 | 3 | 0.0 |
| D17 | Lactobacillus sp. | 0.0 | 0.0 | 0.0 |
| D18 | Sporocytophage sp. | 0.0 | 0.0 | 0.0 |
Figure 5.
Antagonistic effect of bacterial suspension of (a) Bacillus megaterium (D3) (b) Bacillus subtilis (D10) on the growth of S. aureus compared to the control culture.
Figure 6.
Antagonistic effect of bacterial suspension of (a) Bacillus megaterium (D3) (b) Bacillus subtilis (D10) on the growth of MRSA compared to the control culture.
B. subtilis D10 showed the strongest antifungal activity followed by E. coli D2 producing 40 and 30 mm inhibition zone, respectively against A. niger (Fig. 7). B. subtilis (D10) and E. coli (D2) showed inhibitory actions against the three tested microbes. These results were supported by several studies which indicated the efficient antimicrobial activity of soil bacteria specially the Bacilli. In this connection, Aunpad and Na-Bangchang (2007) concluded that Bacillus pumilus isolated from the soil of Thailand produced new antimicrobial peptide with broad spectrum antibacterial activity including MRSA. The compound has potential for use as an alternative antibacterial agent for the treatment of infection with MRSA. Furthermore, the antimicrobial compound subtilin produced by B. subtilis was found to be effective against the commonly occurring gram positive S. aureus and Gram negative E. coli (Beima et al., 2002, Dhanapathi et al., 2008).
Figure 7.
Antagonistic effect of bacterial suspension of (a) Bacillus subtilis D10 (b) Bacillus megaterium D3 on the growth of Aspergillus niger expressed by the inhibition zone.
Bacillus sp. isolated from soil showed antimicrobial activity against some pathogenic fungi (Sclerotium rolfsii, F. oxysporum and Rhizoctonia solani) and the pathogenic bacteria S. aureus (Ghai et al., 2007). In addition, B. subtilis produce antifungal peptides (Iturine) which have been used as biocontrol agents (Levenfors et al., 2004, Chung et al., 2005).
Also, some lactic acid bacteria (Magnusson et al., 2003) and other Bacillus sp. (Yilmaz et al., 2005) produced antifungal substances. Whereas, E. coli produced chitinase enzyme which dissolve the wall of the pathogenic fungus F. oxysporum which cause wilt disease in Cucumber (Soohee and Dal, 2007).
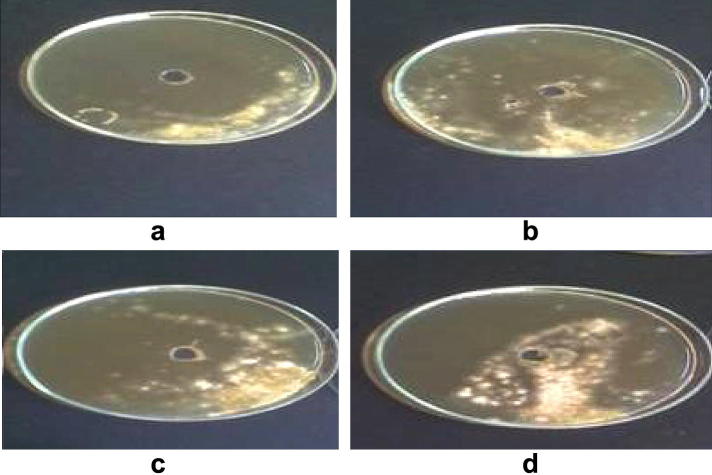
Five fungal species were isolated from the mixture of soil and saliva which were Chaetomium globosum (F1), Fusarium oxysorum (F2), A. biplane (F3), Cochliobolus lanatus (F4) and Emericella nidulans (F5). The fungal suspensions of the isolates showed efficient antagonistic activities against the two tested pathogenic bacteria and completely prevent their growth on solid media (Fig. 8). At the same time, the fungal suspensions present in the agar well have been grown vigorously indicating their ability to prevent the pathogenic bacteria from nutrients and consequently prevent their growth and reproduction (Berg et al., 1994). In contrast, the fungal isolates could not counteract the pathogenic tested fungus A. niger, although, its growth was inhibited by the filtrate of fungi isolated from the mixture of soil and saliva as mentioned previously.
Figure 8.

The growth of the isolated fungi (used as fungal suspensions in the mid-hole) completely prevent the growth of the pathogenic bacteria mixed with the media.
The cell free filtrates of the fungal cultures were tested for their antimicrobial activities against the tested pathogenic microbes (Table 5). As appeared from the table, the filtrate of Emericella indulans (F5) showed strong antagonistic activities against the three tested pathogenic microbes. Whereas, the filtrate of F. oxysporum (F2) counteracted the growth of S. aureus and A. niger efficiently (Figure 9, Figure 10). At the same time, the pathogenic fungus A. niger, showed high sensitivity to the filtrate of Chaetomium globosum (F1), E. nidulans (F5), Fusarium oxysorum (F2) and A. biplane (F3) (Fig. 11).
Table 5.
Effect of cell free culture filtrates of isolated fungi on the growth of Staphylococcus aureus, methicillin resistant MRSA and Aspergillus niger (+ low growth, ++ medium growth, +++ high growth).
| Sym. | Isolated fungi | Staphylococcus aureus | MRSA | A. niger |
|---|---|---|---|---|
| F1 | Chaetomium globosum | ++ | ++ | + |
| F2 | Fusarium oxysporum | + | ++ | + |
| F3 | Aspergillus biplane | +++ | ++ | + |
| F4 | Cochliobolus lanatus | ++ | ++ | ++ |
| F5 | Emericella nidulans | + | + | + |
Figure 9.
Antagonistic effect of the filtrate of (a) Fusarium oxysporum F2 (b) Emericella nidulans F5 on the growth of S. aureus.
Figure 10.

The antagonistic effect of the filtrate of Emericella nidulans F5 on the growth of MRSA.
Figure 11.
The antagonistic effect of the filtrate of (a) Haetomium globosum (F1) (b) Emericella nidulans (F5) (c) Fusarium oxysorum (F2) and (d) Aspergillus biplane (F3) on the growth of Aspergillus niger.
These results could be explained supported by those of Wen et al. (2005) who concluded that some microbes secrete some extracellular enzymes such as chitinase, cellulase, glucanase and protease which digest the fungal mycelia. E. nidulans NK-62 produce cellulase and xylanase which hydrolyse the fungal wall (Kango et al., 2003). Also, E. nidulans antagonized the growth of the pathogenic fungus F. oxysporum which causes wilt disease in tomato (Sibounnavong et al., 2008).
References
- Abu Arkoub M.M. The Academic Library; Cairo, Egypt: 2002. Antibiotics and Three Resistors. [Google Scholar]
- Ahmed, A.A., 2003. Biological Study on Some Actinomycetes Isolates. PhD. Thesis. Faculty of Science, AL Azhar University, Cairo, Egypt.
- Alexander, M., 1982. Soil Microbiology, second ed. John Wiley and Sons, Inc., New York.
- Aunpad, R., Na-Bangchang, K., 2007. A novel antimicrobial peptide with anti-MRSA activity. 17th European Congress of Clinical Microbiology and Infectious Disease ICC, Munich, Germany.
- Barakate M., Ouhdouch Y., Oufdou K.H., Beaulieu C. Characterization of rhizospheric soil streptomycetes from Moroccan habitats and their antimicrobial activities. World J. Microbiol. Biotechnol. 2002;18(1):49–54. [Google Scholar]
- Beima, A., Neodet, S., Yavuz, B., 2002. Determination of some properties of Bacillus isolated from soil. Turkish J. Biol. 41–48.
- Berg, G., Knaape, C., Balline, G., Seidel, D., 1994. Biological control Verticillium dahliae Kelp. by natural occurring rhizosphere bacteria. Arch. Phytopathol. Plant Protc. 29 (30), 249–262.
- Bjorne H., Weitzberg E., Lundberg J.O. Intragastric generation antimicrobial nitrogen oxides from saliva – physiological and therapeutic consideration. Biol. Med. 2006;41(9):1404–1412. doi: 10.1016/j.freeradbiomed.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Bouknight, R.R., Sadoff, H.L., 1975. Tryptophan catabolism in Bacillus megaterium. J. Bacteriol. 121 (1), 70–76. [DOI] [PMC free article] [PubMed]
- Cavalcanti, M.A., Oliveira, L.G., Fernandes, M.J., Lima, D.M., 2006. Filamentous fungi isolated from soil in districts of the Xingó region, Braz. Acta Bot. Bras. 20 (4), 831–837.
- Chung W.C., Huang J.W., Huang H.C. Formulation of soil biofungicidefor control of Chinese cabbage (Barssica chinensis) caused by Rhizoctonia solani. Biol. Control. 2005;32(2):287–294. [Google Scholar]
- De Boer, W., Paulien, J.A., Gunnewiek, K., Lafeber, P., Janse, J.D., Spit, B.E., Woldendorp, J.W., 1998. Anti-fungal properties of chitinolytic dune soil bacteria. Soil Biol. Biochem. 30 (2), 193–203.
- De Boer, W., Wagennaar, AM., Klein Gunnewiek, PJ., Van Veen, JA., 2007. In vitro suppression of fungi caused by combinations of apparently non-antagonistic soil bacteria. FEMS Microbiol. Ecol. Engl. 59 (1), 177–185. [DOI] [PubMed]
- Dhanapathi, Prabhakar, T.G., Prabhakar, P., 2008. Antibacterial activity of Bacillus subtilis is extraction pathogenic organisms. Tamil Nadu J. Vet. Anim. Sci. 4 (4), 150–153.
- Dodds M.W.J., Johnson D.A., Yeh C.-K. Health benefits of saliva: a review. J. Dent. 2004;33(3):223–233. doi: 10.1016/j.jdent.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Edgerton M., Koshlukova S.E., Lo T.E., Chrzan B.G., Straubinger R.M., Raj P.A. Candidacidal activity of salivary histatins. J. Biol. Chem. 1998;273(32):2038–2044. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- El-Sohaibani, M., 1999. Studies on the biological control and some biochemical effects of true heavy metals on the growth and some metabolic activities of the pathogenic fungus Fusarium oxysporum. PhD – College of Education for Girls, Jeddah.
- Gebree1 H.M., E1-Mehalawy A.A., E1-Kholy I.M., Rifaat H.M., Humid A.A. Antimicrobial activities of certain bacteria isolated from Egyptian soil against pathogenic fungi. J. Agric. Biol. Sci. 2008;4(4):331–339. [Google Scholar]
- Georg L.K., Ajello L., Papageorge C. Use of cycloheximide in the selective isolation of fungi pathogenic to man. J. Lab. Clin. Med. 1954;44:422–428. [PubMed] [Google Scholar]
- Ghai S., Sood S.S., Jain R.K. Antagonistic and antimicrobial activities of sorr bacterial isolates collected from soil samples. Ind. J. Microbiol. 2007;47:77–80. doi: 10.1007/s12088-007-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel, U., Schmid, M., Wagner, M., Fieseler, L., Gernert, C., Hacker, J., 2006. Isolation and phylogenetic analysis of bacteria with antimicrobial activities from the Med sponges Aplysina aerophoba and Aplysina cavernicola. FEMS Microbiol. Ecol. 35 (3), 305–312. [DOI] [PubMed]
- Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T., Williams, S.T., 1994. Bergey’s Manual of Determinative Bacteriology, ninth ed. Williams and Wilkins, Baltimore, London.
- Huang, C.M., 2004. Comparative proteomic analysis of human whole saliva. Oral Biol. 49 (12), 951–962. [DOI] [PubMed]
- Ihalin R., Nuutila J., loimaranta V., Lenander M., Tenovuo J., Lilius E.M. Susceptibility of Fusobacterium nucleatum to killing by peroxidase- iodide- hydrogen peroxide combination in buffer solution and in human whole saliva. Anaerobe. 2003;9(1):23–30. doi: 10.1016/S1075-9964(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Imam Al-Bukhari, 1993. Sahih Bukhari. Dar Ibn Katheir, Beirut.
- Imam Muslim, 1978. Al-Msnd Al-Saheh. Dar al-Fikr, Beirut.
- Kango, N., Agrawal, S.C., Jain, P.C., 2003. Production of xylanase by Emericella nidulans NK-62 on low-value lignocellulosic substrates. World J. Microbiol. Biotechnol. 19 (7), 691–694.
- Kina, J., 2003. Alternatives to antibiotics. Translation Yasser Al-Otaibi. To Jean de Vries. First Printing. ALObeikan Library.
- Komine K.I., Kuroishi T., Ozawa A., Komine Y., Minami T., Shimauchi H., Sugawara S. Cleaved inflammatory lactoferrin peptides in parotid saliva of periodontitis patients. Mol. Immunol. 2006;44(7):1498–1508. doi: 10.1016/j.molimm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Kown-chung, K., Bennett, J., 1992. Medical Mycology. Lea and Febiger, Philadelphia, p. 114.
- Krieg N.R., Holt J.G. Williams and Wilkins; Baltimore, London: 1984. Bergey’s Manual of Systematic Bacteriology. (V1) [Google Scholar]
- Levenfors J.J., Hedman R., Thaning C., Gerhardson B., Welch C.J. Broad-spectrum antifungal metabolites produced by the soil bacterium Serratia plymuthica A153. Soil Biol. Biochem. 2004;36:677–685. [Google Scholar]
- Liu, Z., Liu, X., Liu, H., Wu, X., 2008. Purification and cloning of a novel antimicrobial peptide from salivary glands of the hard tick, Ixodes sinensis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. Engl. 149 (4), 557–561. [DOI] [PubMed]
- Madigan, M., Martinko, J., 2005. Brock Biology of Microorganisms, 11th ed. Prentice Hall. ISBN 0131443291.
- Magnusson, J., Stron, K., Rooa, R., Sjogren, J., Schniirer, J., 2003. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol. 219, 129–135. [DOI] [PubMed]
- Mathews, M., Jia, H.P., Guthmiller, J.M., Losh, G., Graham, S., Johnson, G.K., Tack, B.F., McCray Jr., P.B., 1999. Production of β-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect. Immun. 67 (6), 2740–2745. [DOI] [PMC free article] [PubMed]
- Motta A.S., Olivera F.C., Brandelli A. Screening antimicrobial activity among bacteria isolated from the Amazon Basin. Braz. J. Microbiol. 2004;35:307–310. [Google Scholar]
- Murakami, M., Ohtake, T., Dorschner, R.A., Gallo, R.L., 2002. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J. Dent. Res. 81 (12), 845–850. [DOI] [PubMed]
- Osman M.M. Factors affecting the antifungal properties of Brevibacterium linens. Int. Dairy J. 2004;14(8):713–722. [Google Scholar]
- Park S.C., Yoo N.C., Kim J.Y., Park B.J., Shin S.Y., Cheong H., Park Y., Hahm K.S. Isolation and characterization of an extracellular antimicrobial protein from Aspergillus oryzae. J. Agric. Food Chem. 2008;56(20):9647–9652. doi: 10.1021/jf802373h. [DOI] [PubMed] [Google Scholar]
- Sibounnavong P., Cynthia D.C., Kanokmedhakul S., Soytong K. The new antagonistic fungus, Emericella nidulans strain EN against fusarium wilt of tomato. J. Agric. Technol. 2008;4(1):89–99. [Google Scholar]
- Singh, V., Praveen, V., Banga, J., Tripathi, C.K., 2009. Antimicrobial activities of microbial strains isolated from soil of stressed ecological niches of Eastern Uttar Pradesh. Ind. J. Exp. Biol. India 47 (4), 298–303. [PubMed]
- Sneath P.H., Mair N.S., Sharp E. Williams and Wilkins; Baltimore and London: 1986. Bergey’s Manual of Systematic Bacteriology. [Google Scholar]
- Soohee, C., Dal, K.S., 2007. Escherichia coli can produce recombinant chitinase in the soil to control the pathogenesis by Fusarium oxysporum without colonization. J. Microbiol. Biotechnol. 17(3), 474–480. [PubMed]
- Tenovuo J. Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme and lactoferrin in xerostomia: efficacy and safety. Oral Dis. 2002;8:23–29. doi: 10.1034/j.1601-0825.2002.1o781.x. [DOI] [PubMed] [Google Scholar]
- Umechuruba C.I., Nwachukwa E.O. The effect of filtrates of seed borne fungi of African yam bean on seed germination and seedling development. Global J. Pure Appl. Sci. 1997;3(2):165–176. [Google Scholar]
- Weller D.M., Cook R.J. Suppression of take-all wheat by seed treatment with fluorescent pseudomonals. Phytopathology. 1983;37:463–469. [Google Scholar]
- Wen, Z., Liao, W., Chen, S., 2005. Production of cellulase by Trichoderma reesei from dairy manure. Bioresource Technol. 96 (4), 491–499. [DOI] [PubMed]
- Yilmaz M., Soran H., Beyatli Y. Antimicrobial activities of some Bacillus spp. Strain isolated from the soil. Microbiology. 2005;161:127–131. doi: 10.1016/j.micres.2005.07.001. [DOI] [PubMed] [Google Scholar]