Abstract
Although, Patulin and Ochratoxin are produced by the same genera of molds, however, Patulin was the most extensively studied mycotoxins in apple juice and no reports have explored the presence of Ochratoxin A in the apple juice. Therefore, the objective of this study was to explore the presence of Patulin and Ochratoxin A in apple juice in Saudi Arabian market of Jeddah. Potato dextrose agar(PDA) was used to detect fungal contamination. Patulin was determined using HPLC equipped with a UV detector set at 276 nm. Also, HPLC with fluorescence detector was set at 333 and 420 nm as excitation and emission wavelength, respectively,was used for Ochratoxin A separation. All samples of apple juice were free from fungi and yeasts. The Patulin (PAT) was detected in only one type out of 17 types (5.88%) with a concentration of 152.5 ppb, (305%) increased compared with the maximum permitted level (50 ppb). However the occurrence of Ochratoxin A (OTA) in apple juice samples was discovered in 5 types out of 17 types (29.41%). The concentration of OTA ranged from 100 to 200 ppb reaching 5–10-folds compared with the permissible limits (20 ppb).
Keywords: Patulin, Ochratoxin A; Apple juice; HPLC determination
1. Introduction
Mycotoxins are toxic secondary metabolites produced by fungi. Patulin (PAT) as one of these mycotoxins is an unsaturated heterocyclic lactone produced by certain fungal species of Penicillium, Aspergillus and Byssochlamys growing on fruit (Ritieni, 2003). Patulin has been mainly found in apple and apple products and occasionally in pears, grapes, apricots, strawberries, blueberries and peaches (Majerus and Kapp, 2002). As PAT is highly soluble in water and highly stable in aqueous acid media, it reaches apple derivative products, such as juices (Gökmen and Acar, 2000, Armentia et al., 2000). Apple juice contaminated with PAT is hazardous for human health, not only due to the effects of PAT but also due to the toxicity produced when PAT is combined with other mycotoxins. Patulin is toxic for animals; it induces intestinal injuries, including epithelial cell degeneration, inflammation, ulceration, and haemorrhages; it has also been shown to be mutagenic, carcinogenic immunotoxic, neurotoxic, genotoxic and teratogenic (Mahfoud et al., 2002, Iha and Sabino, 2006).
Acute symptoms of Patulin consumption can include agitation, convulsions, edema, ulceration and vomiting (Speijers, 2004). Chronic health effects of Patulin include genotoxicity, immunotoxicity, and neurotoxicity in rodents (Wouters and Speijers, 1996).
The maximum permitted level of Patulin in fruit juices and nectars, in particular apple juices and apple juice ingredients in other beverages marketed in Europe is 50 ppb (European Commission, 2003).
Ochratoxin A (OTA) is the most common naturally occurring mycotoxin produced mainly by Aspergillus ochraceus, A. carbonarius and Penicillium verrucosum, and is receiving increasing attention (FAO/WHO, 2001). While P. verrucosum is the main producer in cereals for OTA in temperate climates (Lund et al., 2003), A. ochraceus is typically associated with coffee, grapes and spices in warm and tropical regions (Codex Alimentarius Commission, 2002). OTA commonly occurs in sub-tropical and temperate climates (European Commission Report, 1999), and can be found in a number of food products, including cereals, beer, coffee beans, cacao, spices, nuts, dried fruit, grape juice, as well as in human blood and animal-derived products (Commission Regulation, 2002). Since OTA is relatively stable like other mycotoxins within the range of conventional food processing temperatures, and partially degraded under fermenting process, it can also be detected in various manufactured food products (Soufleros et al., 2003).
OTA is a well-known nephrotoxic agent and has been associated with fatal human kidney disease, referred to as Balkan Endemic Nephropathy, and with an increased incidence of tumors of the upper urinary effect (FAO/WHO, 2001). The toxin also has a number of toxic effects against various experimental animals: carcinogenic, teratogenic, immunotoxic, genotoxic and possibly neurotoxic (Commission Regulation, 2002).
Although, Patulin was the most extensively studied mycotoxins in apple juice around the world, however, no reports have explored its presence in the apple juice of the Saudi Arabian market. Also, Ochratoxin A was detected in wine and no data were available for its occurrence in the apple juice, although Patulin and Ochratoxin are produced by the same genera of molds. Therefore, the objective of this study was to explore the presence of Patulin and Ochratoxin A in apple juice in Saudi Arabian market of Jeddah.
2. Materials and methods
2.1. Materials
Seventeen different types of apple juices (51 samples) at various retail outlets were collected at Jeddah, Saudi Arabia during 2008. The samples were stored below 4 °C before analyzing.
2.2. Mycological analysis
Fifty milliliters from each of apple juice samples were added to 450 ml sterile peptone-water (0.1%) and mixed thoroughly by shaking. Original serial dilutions and un-diluted juices were cultured (0.1 ml/plate) using potato dextrose agar (PDA), and then the plates were incubated for 5 days at 28 °C and examined for fungal contamination.
2.3. Extraction of Patulin
The extraction of Patulin was performed according to AOAC (2000). In this method, 10 ml of ethyl acetate was added to 5 ml of juice and vigorously shaken for 1 min using vortex mixer; the upper layer was transferred into culture tube. This step was repeated 3× and then 2 ml of 1.5%Na2CO3 was added to the ethyl acetate layer and vigorously mixed. The upper ethyl acetate layer was dried by adding 1 g of Na2SO4 and then evaporated under nitrogen stream at 40 °C. The residue was dissolved in 0.5 ml acetic acid, and retained to HPLC determination.
2.4. HPLC conditions for Patulin
Patulin was determined using a HPLC Agilent 1100 system equipped with a quaternary pump model G1311A, a detector DAD G13158 UV set at 276 nm, an autosampler model G1329a and a C18 (Phenomenex) column (150 × 4.6 mm); 5 μm was used for Patulin separation. The mobile phase tetrahydrofuran 5% was used at 0.5 ml/min flow rate.
2.5. Extraction of Ochratoxin A
Ochratoxin A was determined according to AOAC (2000). Fifty millilitres of sample, 25 ml 0.1 M phosphoric acid and 250 ml chloroform were shaken on a vortex mixer for 3 min at medium speed. Near end of blending 10 g of diatomaceous earth was added. The extract was filtered through filter paper covered with 10 g diatomaceous earth. Ten millilitres of 3% sodium bicarbonate were added to 50 ml filtrate and shacked followed by centrifugation for 2 min at 2000 rpm; 5 ml of biocarbonate phase was loaded on SPE C18 (3 ml, 100 mg) column and eluted by 8 ml ethyl acetate–methanol–acetic acid (95 + 5 + 0.5). The resulting elution was evaporated under gentle stream of nitrogen and the residue was reconstituted in 0.5 ml of mobile phase for HPLC determination.
2.6. HPLC conditions for Ochratoxin A
HPLC Agilent 1100 system was equipped with a quaternary pump model G1311A, a fluorescence detector sitting on 333 and 420 nm as excitation and emission wavelength, respectively, an autosampler model G1329a and a C18 phenomenex column, (150 × 4.6 mm); 5 μm was used for Ochratoxin A separation. The mobile phase consists of water: acetonitrile: acetic (99:99:2) and was used at 1 ml/min flow rate.
3. Results and discussion
An attempt was made to detect fungal contamination in the apple juice, however, all samples of apple juice explored in this study were free from fungi and yeasts. Similar manifestations in apple juices were reported by Tournas et al. (2006). However, the same investigators isolated small numbers of Penicillium and Fusarium spp. in 20% whereas Geotrichum spp. was present in 40% of the grapefruit juice. Mendoza et al. (1982) reported the isolation of Penicillium and Cladosporium from pasteurized fruit juices. Heat treatments along with preservatives may be the reason that no fungal count was detected in the samples under study.
Patulin was detected in 3 samples that belong to only one type out of the 17 types (5.88%) collected in this study (Table 1). The maximum concentration of the positive samples was 152.5 ppb which is more than 3-folds of the maximum permitted limit (50 ppb) with a mean concentration of 140 ppb. No data were available for the presence of Patulin in apple juice of the Saudi Arabian market in Jeddah. However, worldwide interest in Patulin contamination dramatically increased over the last few years following a series of articles that published the results of several surveys conducted in different countries.
Table 1.
Level of Patulin in local and imported apple juice from food stores located at Jeddah, Saudi Arabia.
| Samples | Local | Imported | Total |
|---|---|---|---|
| No. of samples | 36 | 15 | 51 |
| No. of apple juice types | 12 | 5 | 17 |
| No. of positive samples | 3 | 0 | 3 |
| % of positive samples | 8.3 | – | 5.88 |
| Range of Patulin (ppb) | ND – 152.5 | ND | ND – 152.5 |
| Over all mean of positive samples (ppb) ± SE | 140 ± 7.85 | – | 140 ± 7.85 |
A German survey reported that six out of 28 apple juice samples tested contained Patulin (21%) at trace levels (Aping, 1982). A survey conducted in UK during 1980 showed that Patulin was detected in 70% of the tested samples at concentrations ranging from 1 to 38 ppb (Ministry of Agriculture, 1980). Patulin levels ranging from 106 to 216 ppb were reported in 15% of the apple juices samples collected from New Zealand (Wilson, 1981). All the 215 samples of apple juice concentrates analyzed in Turkey for Patulin in 1994 were contaminated ranging from 7 to 376 ppb and 98 of these samples contained Patulin level above 50 ppb (Gökmen and Acar, 1998). In Brazil 111 samples of processed fruit juices and 38 samples of sound fruits were analyzed for Patulin. Only one out of 30 samples of apple juice was found to be positive at 17 ppb. However, 150–267 ppb of Patulin was found in 14 spoiled apple samples (De Sylos and Rodriguez-Amaya, 1999). Forty-one percent of the 68 apple juices (32 clear and 36 cloudy) marketed in Portugal were contaminated with Patulin with a maximum level of 42 ppb. A higher incidence of positive samples was detected in cloudy juices (67%) when compared with clear ones (13%) (Barreira et al., 2010).
Table 1 showed that 8.33% of the local samples were contaminated with Patulin with a maximum level of 152.5 ppb and a mean value of 140 ppb. However, no Patulin was detected in imported samples.
Apple juice in Saudi Arabia market is either imported as a final apple juice product or imported as apple juice concentrate and locally mixed and packed to produce the final local product. So the local apple juice samples in this study means an imported apple juice concentrate with minimal local processing steps to produce the final product. The presence of Patulin only in local apple juice samples may be due to the poor quality of the imported concentrate rather than contamination during processing.
The presence of Patulin in apple-producing countries is well documented and the high possibility of Patulin contamination of the apple juice concentrate and apple juice final product in the international market is shown. Patulin concentrations up to 497 ppb were detected in some retailed apple juices sold in UK (MAFF, 1993). Overall, about 50% of apple juice samples analyzed worldwide have been shown to contain detectable levels of Patulin (Kubacki, 1986, Prieta et al., 1994). The contamination of apple juice products may sometimes be as high as 8000 ppb in apple juices made from partly rotten apples (Brackett and Marth, 1979), and even higher in apple cider obtained from industrial premises where decayed apples were not sorted out prior to processing (Wilson and Nuovo, 1973). The results from the South African survey of 60 commercial apple products for Patulin contamination showed that none of the samples had Patulin higher than 50 ppb and the maximum level was 45 ppb (Leggott and Shephard, 2001).
Published surveys in 1994 reported the incidence of Patulin in fruit juices sampled in Spain and Austria between 43% and 85% with Patulin levels of 10–185 ppb (Pittet, 1998). In Australia, 328 apple, pear and mixed fruit products were collected from various retail outlets and from producers in Sydney and rural New South Wales between August 1989 and May 1990. Twenty-three percent of the samples were contaminated with Patulin and 22% of these samples contained Patulin levels between 51 and 1130 ppb (Burda, 1992). In Spain, of the 100 apple juice samples, between April and December 1992, 82 samples were contaminated with Patulin, 7 of these being above 50 ppb, with a mean of 13.8 ppb and the highest Patulin concentration being 170 ppb. (Prieta et al., 1994).
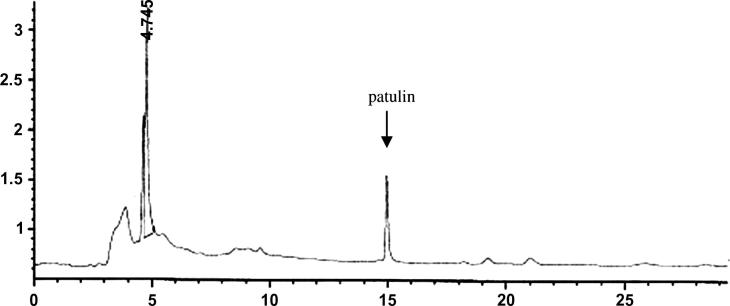
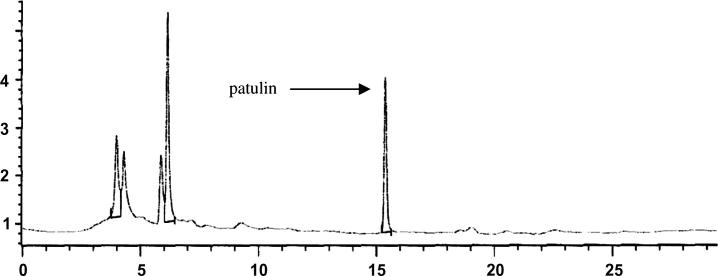
Figure 1, Figure 2 showed the Patulin HPLC chromatogram of the standard and the positive sample of the maximum concentration of 152.5 ppb. The chromatogram showed no interference peaks due to the different types of apple juice matrices used in this study. This means that the method used in the detection and determination of Patulin was adequate in respect to the matrix type of the apple juice.
Figure 1.
HPLC chromatogram of patulin standard.
Figure 2.
HPLC chromatogram of apple juice sample with 152.5 ppb patulin/ml.
Also, Ochratoxin A was detected in wine and no data were available for its occurrence in the apple juice, although Patulin and Ochratoxin are produced by the same genus of fungi. Therefore, the objective of this study was to explore the presence of Patulin and Ochratoxin A in apple juice in Saudi Arabian market of Jeddah.
Table 2 recorded the occurrence of Ochratoxin A in apple juice samples, which was detected in 5 types out of 17 types (29.41%). The concentrations ranged between 100 and 200 ppb (5–10-folds) when compared with the permissible limit (20 ppb).
Table 2.
Level of Ochratoxin A in local and imported apple juice from food stores located at Jeddah, Saudi Arabia.
| Samples | Local | Imported | Total |
|---|---|---|---|
| No. of samples | 36 | 15 | 51 |
| No. of apple juice types | 12 | 5 | 17 |
| No. of positive samples | 12 | 3 | 15 |
| % of Positive samples | 33.33 | 20 | 29.41 |
| Range of Ochratoxin A (ppb) | ND – 200 | ND – 110 | ND – 200 |
| Over all mean of positive samples (ppb) ± SE | 127.5 ± 12.66 | 105 ± 2.89 | 123 ± 10.33 |
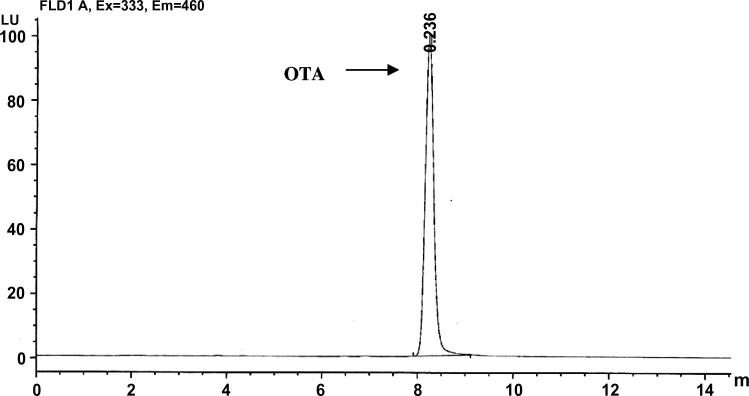
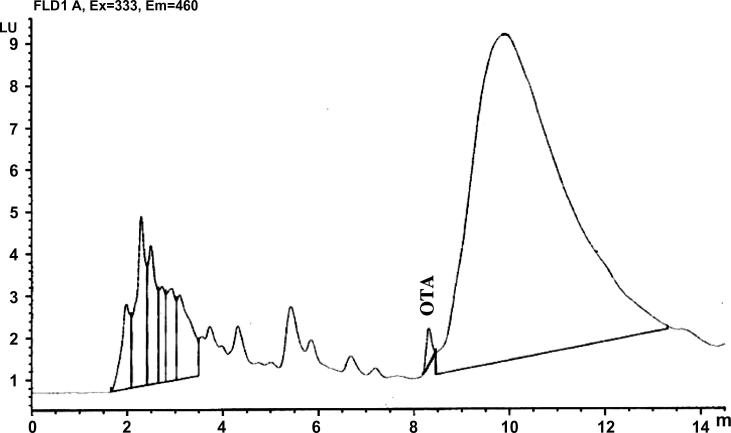
The Ochratoxin A HPLC chromatogram of the standard (Fig. 3) and the positive sample of the maximum concentration of 200 ppb (Fig. 4) showed that the method used for the extraction of Ochratoxin A was not highly selective that is because of the huge peak adjacent to the OTA peak (Fig. 4) and more study should be focused in this area.
Figure 3.
HPLC chromatogram of Ochratoxin A standard.
Figure 4.
HPLC chromatogram of apple juice sample with 200 ppb Ochratoxin A/ml.
According to the available literatures, this may be the first report of Ochratoxin A detection in apple juices, although several literatures reported the presence of OTA producing fungi in apple. OTA is produced mainly by A. ochraceus, and P. verrucosum, as well as by Aspergillus belonging to the section Nigri (black Aspergilli), and in particular by A. carbonarius (FAO/WHO, 2001, Battilani and Pietri, 2002). Joosten et al. (2001) reported that A. carbonarius M325 isolated from apples was not able to produce OTA where it was capable to produce OTA from other sources. Also, Asan (2004) reported the presence of A. niger on apple.
Also, no data were accessible to show that Ochratoxin A was detected in apple juice, although it was frequently detected in wine. The first data on OTA occurrence in wine were reported by Zimmerli and Dick (1996). Also, Burdaspal and Legarda (1999) reported the contamination of wine by OTA in Spain. Most of their samples were domestic, but some of them were imported. Further surveys have been reported that the overall% of contaminated samples was 51.5% and the highest OTA concentration was 15.25 ppb in wine (Medina et al., 2005, Medina et al., 2006, Mateo et al., 2006, Hernández et al., 2006).
In Italy, wines have been extensively surveyed for this toxin. OTA incidence was higher in red wines (78.4%), followed by rosé, and dessert and white wines. The highest level (7.63 ppb) was found in red wine (Brera et al., 2005, Bacaloni et al., 2005, Spadaro et al., 2010). In Germany, a value of 7.0 ppb was found in Italian red wine exported to Germany (Majerus and Otteneder, 1996, Majerus et al., 2000).
In Greece, more than 66% of samples showed detectable OTA levels and both red and sweet wines showed the highest levels (Soufleros et al., 2003, Stefanaki et al., 2003). More than 50% and 100% of samples analyzed in Cyprus and Turkey, respectively, had detectable levels of the toxin (Ioannou-Kakouri et al., 2004, Anli et al., 2005). Eder et al. (2002) detected OTA in only 1/116 Austrian wines. Research performed by the German Federal Ministry of Health between 1995 and 1998 showed that total OTA incidence was 40% but contamination was considerably higher in red wines and wines from southern Europe regions.
In France, Ospital et al. (1998) found OTA in 29 wines (0.01–0.27 ppb) but a value of 0.78 ppb was found in a French red wine exported to Germany. In Portugal, Festas et al. (2000) did not find OTA in 64 domestic wines but Soleas et al. (2001) detected it in 5 out of 37 samples of Portuguese wine. A survey of 340 Portuguese wine revealed that OTA was detectable in 20.3% of the samples and the highest level was 2.1 ppb (Ratola et al., 2004).
According to the SCOOP task 3.2.7 report, Soleas et al. (2001) analyzed 71 samples of US red wine and 40 samples of US white wine for OTA and they found that only 8 red wine samples exceeded 0.05 ppb. They also found OTA in 16.6% of 580 red wine samples and 3.9% of 362 white wine samples marketed in Canada but the toxin was not detected in their US samples. Canadian wines, when compared with imported products, showed both lower OTA occurrence and lower contamination level.
Rosa et al. (2004) detected OTA in 24% of 42 wine samples from Brazil, Argentina and Chile. The problem of OTA contamination in wines is not as concerning as in Europe (Chulze et al., 2006). However, Soleas et al. (2001) found OTA in Argentinean wines; most samples contained OTA below 0.05 ppb and the highest level was 0.62 ppb (Leong et al., 2006). Sugita-Konishi et al. (2006) studied wines commercialized in Japan and found that 6 out of 10 wines had detectable OTA levels (0.07–0.72 ppb). In South Africa, Shephard et al. (2003) detected this toxin in 24 local samples. The highest level of OTA (2.67 ppb) was found in noble wine by Stander and Steyn (2002). Filali et al. (2001) found OTA in 30 wine samples from Morocco.
A total of 106 samples, including grape juices, pulp of frozen grapes, red, rose and white wines from Brazil, Chile and Argentina were analyzed for OTA contamination by HPLC using immunoaffinity columns, where the limit of detection was 0.021 ppb and the recovery of the method used was 80–90% and Ochratoxin A was detected in 29% and 12.5% of the grape juice and pulp of frozen grape samples, respectively, at levels ranging from 0.021 to 0.1 ppb (Rosa et al., 2004). Also, Tessini et al. (2010) concluded that the combination of C-18 cartridges with conventional particle packed columns and HPLC–florescence detector is the most appropriate alternative for Ochratoxin A analysis in wine. This could also be an alternative to solve the problem of the huge adjacent peak in the OTA chromatogram.
It can be concluded from the results of this study that the incidence of Patulin concentrates in apple juice in Saudi Arabia is low, with a significant proportion of the products exceeding the 50 ppb limit for apple juice set by the WHO and certain European countries. The incidence of Ochratoxin A is high with the proportion exceeding 20 ppb (the maximum permitted level) and this confirms the importance of establishing the level of OTA in apple and its products in local and international standards. It should also be pointed out that the method used for determining the concentrations of Patulin in apple juice concentrates was found to be very useful in terms of ease of operation, speed, economy and sensitivity, however, the method used for determining Ochratoxin A needs more effort to get rid of that huge peak in the HPLC chromatogram which may affect the sensitivity of the method.
Acknowledgements
The author is thankful to the Ministry of Higher Education, King Abdulaziz University, Deanship of Scientific Research, Jeddah, Saudi Arabia for the financial support to carry out this research work.
References
- Anli E., Çabuk B., Vural N., Başpinar E. Ochratoxin A in Turkish wines. J. Food Biochem. 2005;29:611–623. [Google Scholar]
- AOAC (Association of Official Analytical Chemists), 2000. Official Methods of Analysis. Neutral Toxins. pp. 1–64 (Chapter 49).
- Aping, R., 1982. Die Patulin bildung durch Penicillium expansum in Äpfeln und Äpfelsäften in Abhängigkeit von der Sorte, dem Standort und der Düngung im Vergleich zu relevanten Fruchtmerkmalen (Dissertation), Giessen, Germany.
- Armentia A., Jalon M., Urieta I., Macho M.L. Vigilancia de la presencia de Patulin a en zumos de manzana y sidras comercializados en la comunidad auto´noma del Paı´s Vasco. Alimentaria. 2000;310:65–70. [Google Scholar]
- Asan A. Aspergillus, Penicillium, and related species reported from Turkey. Mycotaxon. 2004;89(1):155–157. [Google Scholar]
- Bacaloni A., Cavaliere C., Faberi A., Pastorini E., Samperi R., Laganà A. Automated on-line solid-phase extraction-liquid chromatography–electrospray tandem mass spectrometry method for the determination of Ochratoxin A in wine and beer. J. Agric. Food Chem. 2005;53:5518–5525. doi: 10.1021/jf050254+. [DOI] [PubMed] [Google Scholar]
- Barreira M.J., Alvito P.C., Almeida C.M.M. Occurrence of Patulin in apple-based-foods in Portugal. Food Chem. 2010;121:653–658. [Google Scholar]
- Battilani P., Pietri A. Ochratoxin A in grapes and wine. Eur. J. Plant Pathol. 2002;108P:639–643. [Google Scholar]
- Brackett R.E., Marth E.H. Ascorbic acid and ascorbate cause disappearance of Patulin from buffer solutions and apple juice. J. Food Protec. 1979;42:864–866. doi: 10.4315/0362-028X-42.11.864. [DOI] [PubMed] [Google Scholar]
- Brera C., Soriano J.M., Debegnach F., Miraglia M. Exposure assessment to Ochratoxin A from the consumption of Italian and Hungarian wines. Microchem. J. 2005;79:109–113. [Google Scholar]
- Burda K. Incidence of Patulin in apple, pear and mixed fruit products marketed in New South Wales. J. Food Protec. 1992;55:796–798. doi: 10.4315/0362-028X-55.10.796. [DOI] [PubMed] [Google Scholar]
- Burdaspal P.A., Legarda T.M. Ocratoxina A en vinos, mostos y zumos de uva elaborados en España y otros países europeos. Alimentaria. 1999;299:107–113. [Google Scholar]
- Chulze S.N., Magnoli C.E., Dalcero A.M. Occurrence of Ochratoxin A in wine and ochratoxigenic mycoflora in grapes and dried vine fruits in South America. Int. J. Food Microbiol. 2006;111(Suppl. 1):S5–S9. doi: 10.1016/j.ijfoodmicro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius Commission, 2002. Proposed draft code of Practice for the prevention (reduction) of mycotoxin contamination in cereals, including annexes on Ochratoxin A, Zearalenone, Fumonisins and Trichothecenes, CX/FAC 02/21. Joint FAO/WHO Food Standards Programme, Rotterdam, The Netherlands.
- Commission Regulation EC No. 472/2002 of 12 March, 2002. Amending Regulation (EC) No 466/2001 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union L75, pp. 18–20 (16.03).
- De Sylos C.M., Rodriguez-Amaya D.B. Incidence of Patulin in fruits and fruit juices marketed in Campinas, Brazil. Food Addit. Contam. 1999;16(2):71–74. doi: 10.1080/026520399284226. [DOI] [PubMed] [Google Scholar]
- Eder R., Paar E., Edinger W., Lew H. Untersuchungen über den Gehalt an Ochratoxin A (OTA) in Weinen, insbesondere Prädikatsweinen aus Österreich. Mitteilungen Klosterneuburg, Rebe und Wein, Obstbau und Früchteverwertung Austria. 2002;52:125–131. [Google Scholar]
- European Commission, 2003. Commission Regulation No. 1425/2003 of 11 August 2003 amending Regulation (EC) No. 466/2001 as regards Patulin. Official Journal of the European Union L203, pp. 1–3.
- European Commission Report, 1999. Opinion on the relationship between the use of plant protection products on food plants and the occurrence of mycotoxins in foods. European Commission SCP/RESI063, Belgium.
- FAO/WHO (Food and Agricultural Organization/World Health Organization), 2001. Ochratoxin A. In: Safety Evaluations of Specific Mycotoxins. Prepared by the fifty-sixth meeting of the Joint FAO/WHO Expert Committee on Food Additives, 6–15 February, Geneva.
- Festas L., Herbert P., Santos L., Cabral M., Barros P., Alves A. Ochratoxin A in some Portuguese wines: method validation and screening in Port wine and Vinho Verde. Am. J. Enol. Vitic. 2000;51(2):150–154. [Google Scholar]
- Filali A., Ouammi L., Betbeder A.M., Baudrimont I., Soulaymani R., Benayada A., Creppy E.E. Ochratoxin A in beverages from Morocco: a preliminary survey. Food Addit. Contam. 2001;18:565–568. doi: 10.1080/02652030117365. [DOI] [PubMed] [Google Scholar]
- Gökmen V., Acar J. Incidence of Patulin in apple juice concentrates produced in Turkey. J. Chromatogr. A. 1998;815:99–102. doi: 10.1016/s0021-9673(97)01280-6. [DOI] [PubMed] [Google Scholar]
- Gökmen V., Acar J. Long-term survey of Patulin in apple juice concentrates produced in Turkey. Food Addit. Contam. 2000;17(11):933–936. doi: 10.1080/026520300750038117. [DOI] [PubMed] [Google Scholar]
- Hernández M.J., Valme García-Moreno M., Durán E., Guillén D., Barroso C.G. Validation of two analytical methods for the determination of Ochratoxin A by reversed-phase high-performance liquid chromatography coupled to fluorescence detection in musts and sweet wines from Andalusia. Anal. Chim. Acta. 2006;566:117–121. [Google Scholar]
- Iha M.H., Sabino M. Determination of Patulin in apple juice by liquid chromatography. Food Chem. Cont. 2006;89:139–143. [PubMed] [Google Scholar]
- Ioannou-Kakouri E., Aletrari M., Christou E., Ralli A., Koliu A., Christofidou M. Occurrence and control of mycotoxins in foodstuffs in Cyprus. In: Logrieco A., Visconti A., editors. An Overview on Toxigenic Fungi and Mycotoxins in Europe. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2004. pp. 51–65. [Google Scholar]
- Joosten H.M.L.J., Goetz J., Pittet A., Schellenberg M., Bucheli P. Production of Ochratoxin A by Aspergilluscarbonarius on coffee cherries. Int. J. Food Microbiol. 2001;65:39–44. doi: 10.1016/s0168-1605(00)00506-7. [DOI] [PubMed] [Google Scholar]
- Kubacki S.J. The analysis and occurrence of Patulin in apple juice. In: Steyn P.S., Vleggaar R., editors. Mycotoxins and Phycotoxins – A Collection of Invited Papers Presented at the Sixth International Iupac Symposium on Mycotoxins and Phycotoxins, Pretoria, South Africa, 1985. Elsevier; Amsterdam: 1986. pp. 293–304. [Google Scholar]
- Leggott N.L., Shephard G.S. Patulin in South Africa commercial apple products. Food Control. 2001;12:73–76. [Google Scholar]
- Leong S.L., Hocking A.D., Pitt J.I., Kazi B.A., Emmett R.W., Scott E.S. Australian research on ochratoxigenic fungi and Ochratoxin A. Int. J. Food Microbiol. 2006;111(1):10–17. doi: 10.1016/j.ijfoodmicro.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Lund F., Frisvad J.C. Penicillium verrucosum in wheat and barley indicates presence of Ochratoxin A. J. Appl. Microbiol. 2003;95(5):1117–1123. doi: 10.1046/j.1365-2672.2003.02076.x. [DOI] [PubMed] [Google Scholar]
- Mahfoud R., Maresca M., Garmy N., Fantini J. The mycotoxin Patulin alters the barrier function of the intestinal epithelium: mechanism of action of the toxin and protective effects of glutathione. Toxicol. Appl. Pharmacol. 2002;181:209–218. doi: 10.1006/taap.2002.9417. [DOI] [PubMed] [Google Scholar]
- Majerus P., Bresch H., Otteneder H. Ochratoxin A in wines, fruit juices and seasonings. Archiv für Lebensmittelhygiene. 2000;51:95–97. [Google Scholar]
- Majerus, P., Kapp, K., 2002. Reports on tasks for scientific cooperation, task 3.2.8. Assessment of dietary intake of Patulin by the population of EU Member States. Brussels: SCOOP Report 2002. <http://europa.eu.int/comm/food/fs/scoop/3.2.8_en.pdf>.
- Majerus P., Otteneder H. Nachweis und Vorkommen von Ochratoxin A in Wein und Traubensaft. Deutsche Lebesnmittel-Rundschau. 1996;92:338–390. [Google Scholar]
- Mateo, R., Medina, A., Mateo, F., Valle-Algarra, F.M., Gimeno-Adelantado, J.V., Jiménez, M., 2006. Optimization of the methodology for the determination of Ochratoxin A in wine and study of its occurrence in wines consumed in Spain. In: Proceedings of the 29th World Congress of the Vine and Wine, CD ed., Logroño, Spain.
- Medina A., Jiménez M., Gimeno-Adelantado J.V., Valle-Algarra F.M., Mateo R. Determination of Ochratoxin A in beer marketed in Spain by liquid chromatography with fluorescence detection using lead hydroxyacetate as a clean-up agent. J. Chromatogr. 2005;1083:7–13. doi: 10.1016/j.chroma.2005.05.089. [DOI] [PubMed] [Google Scholar]
- Medina A., Valle-Algarra F.M., Gimeno-Adelantado J.V., Mateo R., Mateo F., Jiménez M. New method for determination of Ochratoxin A in beer using zinc acetate and solid-phase extraction silica cartridges. J. Chromatogr. 2006;1121:178–183. doi: 10.1016/j.chroma.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Mendoza S., Montemayor L., Boscan L.A., Barreiro J.A. Microflora in pasteurized fruit juices in Venezuela. Arch. Latinoam. Nutr. 1982;32(3):617–629. [PubMed] [Google Scholar]
- Ministry of Agriculture, 1980. Fisheries and Food, Survey of Mycotoxins in the United Kingdom, HMSO, London. pp. 23–24.
- Ministry of Agriculture, Fisheries and Food (MAFF), 1993. Mycotoxins: third report. In: Food Surveillance Paper No. 36. Ministry of Agriculture, Fisheries and Food. HMSO, London. pp. 46–50.
- Ospital, M., Cazabeil, J.M., Betbeder, A.M., Tricard, C., Creppy, E., Medina, B., 1998. L’Ochratoxine A dans les vins. Revue Françoise D’énologie, vol. 169, pp. 16–19.
- Pittet A. Natural occurrence of mycotoxins in foods and feeds – an updated review. Revue de Medecine Veterinaire. 1998;149:479–492. [Google Scholar]
- Prieta J., Moreno M.A., Diaz S., Suarez G., Dominguez L. Survey of Patulin in apple juice and children’s apple food by the biphasic dialysis membrane procedure. J. Agric. Food Chem. 1994;42:1701–1703. [Google Scholar]
- Ratola N., Martins L., Alves A. Ochratoxin A in wines – assessing global uncertainty associated with the results. Anal. Chim. Acta. 2004;513:319–324. [Google Scholar]
- Ritieni A. Patulin in Italian commercial apple products. J. Agric. Food Chem. 2003;51:6086–6090. doi: 10.1021/jf034523c. [DOI] [PubMed] [Google Scholar]
- Rosa C.A.R., Magnoli C.E., Fraga M.E., Dalcero A.M., Santana D.M. Occurrence of Ochratoxin A in wine and grape juice marketed in Rio de Janeiro, Brazil. Food Addit. Contam. 2004;21:358–364. doi: 10.1080/02652030310001639549. [DOI] [PubMed] [Google Scholar]
- Shephard G.S., Fabiani A., Stockenstrom S., Mshicileli N., Sewram V. Quantitation of Ochratoxin A in South African wines. J. Agric. Food Chem. 2003;51:1102–1106. doi: 10.1021/jf0259866. [DOI] [PubMed] [Google Scholar]
- Soleas G.J., Yan J., Goldberg D.M. Assay of Ochratoxin A in wine and beer by high-pressure liquid chromatography photodiode array and gas chromatography mass selective detection. J. Agric. Food Chem. 2001;49:2733–2740. doi: 10.1021/jf0100651. [DOI] [PubMed] [Google Scholar]
- Soufleros E.H., Tricard C., Boloumpasi E.C. Occurrence of Ochratoxin A in Greek wines. J. Sci. Food Agric. 2003;83:173–179. [Google Scholar]
- Spadaro D., Lorè A., Garibaldi A., Gullino M.L. Occurrence of Ochratoxin A before bottling in DOC and DOCG wines produced in Piedmont (Northern Italy) Food Control. 2010;21:1294–1297. [Google Scholar]
- Speijers G.J.A. Patulin. In: Magan N., Olsen M., editors. Mycotoxins in Food: Detection and Control. CRC Press; Boca Raton, Fla: 2004. pp. 339–352. [Google Scholar]
- Stander A., Steyn P.S. Survey of Ochratoxin A in South African wines. South African J. Enol. Viticul. 2002;23:9–13. [Google Scholar]
- Stefanaki I., Foufa E., Tsatsou-Dritsa A., Dais P. Ochratoxin A concentrations in Greek domestic wines and dried vine fruits. Food Addit. Contam. 2003;20:74–83. doi: 10.1080/0265203021000031537. [DOI] [PubMed] [Google Scholar]
- Sugita-Konishi Y., Nakajima M., Tabata S., Ishikuro E., Tanaka T., Norizuki H., Itoh Y., Aoyama K., Fujita K., Kai S., Kumagai S. Occurrence of aflatoxins, Ochratoxin A, and fumonisins in retail foods in Japan. J. Food Protec. 2006;69:1365–1370. doi: 10.4315/0362-028x-69.6.1365. [DOI] [PubMed] [Google Scholar]
- Tessini C., Mardones C., von Baer D., Vega M., Herlitz E., Saelzer R., Silva J., Torres O. Alternatives for sample pre-treatment and HPLC determination of Ochratoxin A in red wine using fluorescence detection. Anal. Chim. Acta. 2010;660:119–126. doi: 10.1016/j.aca.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Tournas V.H., Heeres J., Burgess L. Moulds and yeasts in fruit salads and fruit juices. Food Microbiol. 2006;23(7):684–688. doi: 10.1016/j.fm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Wilson R.D. Surveying some apple juices for Patulin. Food Technol. New Zealand. 1981;16:27–31. [Google Scholar]
- Wilson D.M., Nuovo G.J. Patulin production in apples decayed by Penicillium expansum. Appl. Microbiol. 1973;26:124–125. doi: 10.1128/am.26.1.124-125.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters M.F.A., Speijers G.J.A. Food Additives Series 35. Toxicological Evaluation of Certain Food Additives and Contaminants. World Health Organisation; Geneva, Switzerland: 1996. Patulin; pp. 337–402. [Google Scholar]
- Zimmerli B., Dick R. Ochratoxin A in table wine and grape juice: occurrence and risk assessment. Food Addit. Contam. 1996;13:655–668. doi: 10.1080/02652039609374451. [DOI] [PubMed] [Google Scholar]