Abstract
Relating biodiversity to ecosystem functioning in natural communities has become a paramount challenge, as links between trophic complexity and multiple ecosystem functions become increasingly apparent. Yet, there is still no generalised approach to address such complexity in biodiversity–ecosystem functioning (BEF) studies. Energy flux dynamics in ecological networks provide the theoretical underpinning of multitrophic BEF relationships. Accordingly, we propose the quantification of energy fluxes in food webs as a powerful, universal tool for understanding ecosystem functioning in multitrophic systems spanning different ecological scales. Although the concept of energy flux in food webs is not novel, its application to BEF research remains virtually untapped, providing a framework to foster new discoveries into the determinants of ecosystem functioning in complex systems.
Keywords: ecosystem multifunctionality, food web, interaction network, metabolic theory, trophic cascade, niche complementarity
Biodiversity and ecosystem functioning in complex systems: challenges and prospects
Anthropogenic effects have put unprecedented pressure on earth’s ecosystems [1], which provide crucial goods and services important for human wellbeing [2]. The erosion of biological diversity has prompted concern regarding the provisioning and stability of ecosystem services (see Glossary) [2,3], substantiated by a large body of empirical research [4,5]. While the foundation of knowledge was built by examining biodiversity effects on ecosystem functioning of single trophic levels or simple food chains [6], emerging evidence suggests that the relationship between biodiversity and the provisioning and stability of multiple services can only be understood if multiple trophic levels (e.g., [7–9]) and the interactions among them are considered [6,10]. Yet, despite growing empirical evidence of diversity effects on function in food webs, we still lack a unified theoretical framework to quantify and explain the underlying mechanisms of these findings in complex systems.
Glossary.
Assimilation efficiency: Proportion of total energy ingested by a consumer that is invested into biomass production, reproduction, and respiration, excluding material excreted as waste.
Ecosystem multifunctionality: A measure of the simultaneous provisioning of multiple ecosystem functions.
Ecosystem service: An ecosystem function that either directly or indirectly benefits humankind.
Food web: An interaction network describing trophic (feeding) interactions among organisms in ecological communities.
Network node: Resource or consumer entity in a food web that stores and/or consumes energy. The level of aggregation of a node (e.g., individual, population, functional feeding guild) is defined by the investigator and should be relevant to the research question.
Network topology: The distribution of interactions among nodes in a network. This can be derived from qualitative links known to exist among particular nodes and can also incorporate weighted links based on interaction strengths.
Trophic complementarity: The degree to which species specialise on different resources in the same location or on the same resources at different times, allowing for greater resource exploitation.
Trophic link: Unidirectional feeding interaction between two food web nodes. In qualitative food webs, links describe the presence of interactions, while in quantitative food webs, the strength of this interaction is described.
One key challenge in this frontier is the identification of a common currency for ecosystem functioning across trophic levels and ecosystem types, allowing for the analysis of multitrophic diversity–function relationships. Such an approach is also the prerequisite to link findings from highly controlled experimental tests of biodiversity–ecosystem function (BEF) theory to more complex natural landscapes [11] that are particularly relevant to humanity [12]. In this paper, we address this challenge by integrating concepts from network theory, metabolic ecology, BEF theory, and ecological stoichiometry. We propose that merging perspectives from community and ecosystem ecology by quantifying energy fluxes in food webs [13,14] can provide a powerful approach for mechanistically understanding the link between the diversity of complex multitrophic systems and both single as well as multiple ecosystem functions.
Food webs link biological diversity, structure, and ecosystem processes
Food-web ecology addresses how biodiversity is organised across trophic levels according to trophic interactions [13], from microscopic autotrophs to carnivorous megafauna. Research in this field has revealed the non-random structure of feeding links among species [15] and how this structure is determined and stabilised by a range of factors, such as the distribution of energy fluxes [16,17] and species traits [18–20] across interaction networks. These fundamental advances have laid the theoretical and methodological groundwork for assessing multiple aspects of ecological stability that can be captured by synthetic indices useful for policy makers and practitioners [21,22], and for investigating how the structure of complex ecological systems modulates ecosystem functioning [23].
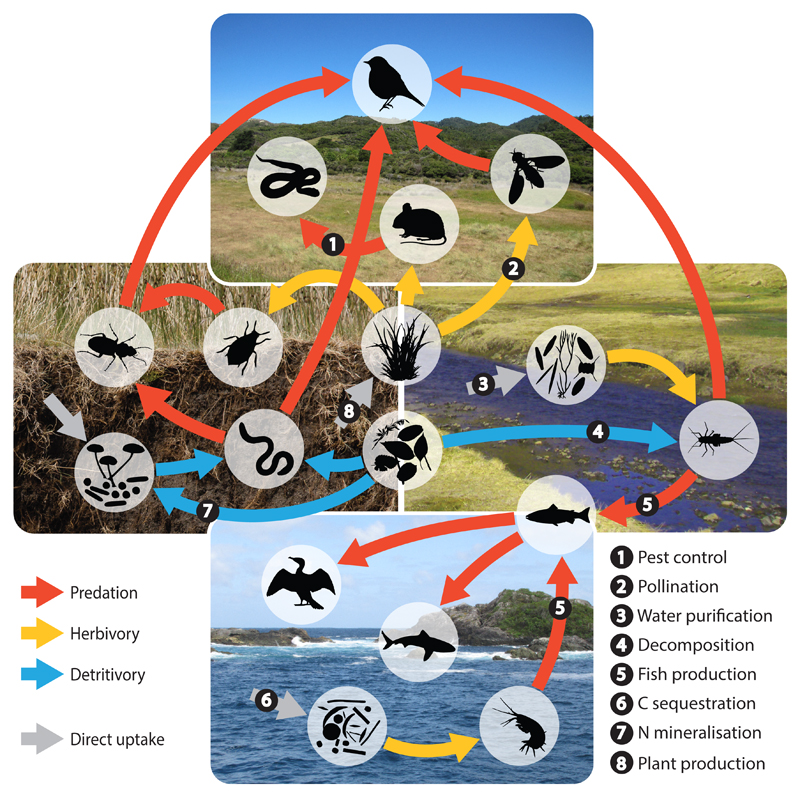
Traditional food-web research has typically described the structure of ecological communities with qualitative feeding relationships. In contrast, quantitative networks [24] that describe fluxes of matter and energy along trophic links [25,26] can be directly associated with multiple functions that are often indicators for ecosystem services (Figure 1), and can allow for comprehensive inference of ecosystem functions that are otherwise difficult to quantify. As one example, herbivory would typically be estimated by assessing leaf damage of host plants, but this excludes any herbivory carried out by sap-feeding herbivores. By quantifying energy flux to all herbivores, however, one can fully assess total herbivory in an ecosystem based on the energetic consumption of the sampled communities. Indeed, a limitation of this approach is in its exclusion of functions driven by non-trophic interactions where energy is often not exchanged among interacting organisms (for example, the mussel-cordgrass mutualism, which has been shown to enhance drought resistance [27]). More recently, though, studies have begun to incorporate non-trophic interactions into food webs (e.g. [28]) and investigate their role in modulating the functioning of ecological networks [29]. Nevertheless, trophic interactions underpin an extensive range of crucial ecosystem functions characterising a large proportion of total ecosystem performance (Figure 1) and have been a central tenet of ecology since Elton’s pioneering work.
Figure 1. Energy fluxes and their relation to ecosystem services across ecosystems.
Arrows denote the directional flux of energy among functional feeding guilds, indicating how these fluxes permeate ecosystem boundaries (such as terrestrial above and below ground, freshwater, and marine systems as shown here). All energy fluxes in ecosystems are analogous to respective ecosystem functions, many of which can be directly translated to services that are beneficial to human wellbeing, as indicated by numbered examples. Soil cross-section image courtesy of Julia Siebert.
In addition to energy flux expressed in carbon (C) per unit area over time, one can also quantify other nutrient fluxes through networks [30,31] to assess whole-ecosystem processes such as the mineralisation of nitrogen (N) and phosphorus (P) [26,31] (Figure 1). Hence, when integrated with metabolic theory and ecological stoichiometry, food-web theory might provide mechanistic predictions of the distribution and stability of biomass, nutrients and their fluxes across populations in natural communities [26]. Accordingly, an energetic network approach provides a unique conceptual framework that mechanistically links community patterns (i.e., species richness and composition) with ecosystem ecology (the flux of energy and matter) [32] (Box 1).
Box 1. Quantifying energy flux in ecological networks.
Various methods exist to quantify energy fluxes in networks. These methods mainly differ in: i) their approach (theoretical vs. empirical), ii) the level of organization from which parameters are obtained and applied (individuals, species, or aggregated feeding guilds such as herbivores or predators), and iii) the assumptions on which they are based (e.g., steady-state systems, closed systems). Examples include a ‘production-consumption’ approach by Reuman & Cohen [25], and Ecological Network Analysis (ENA), which can be performed in the Ecopath and Ecosim software [34] and more recently has been built into the R package ‘enaR’[35].
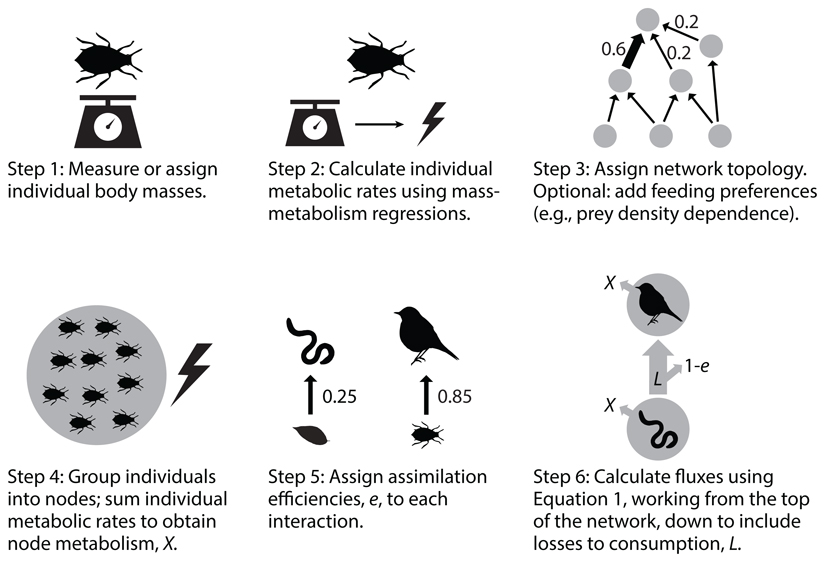
A third method, namely the ‘food web energetics’ approach, uses measured biomass stocks, energetic expenditure, and ecological efficiencies to calculate energy flux between network nodes [30,31,36]. Assuming system equilibrium, energy fluxes among nodes are calculated by balancing energetic demands of biomass stocks with energy outflow. Food-web nodes can be aggregated at different organisational levels (i.e., individuals, species, or general feeding guilds), and strengths of links assigned according to consumer feeding preferences (i.e., the proportional likelihood of feeding on different resources) [26,36]. To test the accuracy of this approach, a previous study compared observed versus inferred fluxes in empirical networks and demonstrated that inferred and observed values closely resemble each other [37]. More recently, this approach has been adapted to incorporate individual-level metabolic demands into network nodes [38] (Figure I), thus better accounting for community composition (i.e., taxonomy, body size structure, and trophic topology) and temperature effects [39] (due to the temperature dependence of metabolism [40,41] and assimilation efficiency [42]). In the adapted ‘food web energetics’ approach, energy flux F to each consumer node is calculated using Equation 1:
| (Equation 1) |
where e is the diet-specific assimilation efficiency, X is the summed metabolic demands of individuals in a consumer node, and L is the loss of energy to higher trophic levels via consumption (e.g., predation or herbivory). The process by which energy flux is calculated using the ‘food web energetics’ approach is practically very straightforward (Figure I), and is therefore an ideal tool for both empirical and theoretical ecologists to robustly quantify energy fluxes in multitrophic systems using readily obtainable data (see Online Supplementary Material S1 and Supplementary Data S1 for a worked example).
Figure I.
Using the ‘food web energetics’ approach, energy flux is quantified following six general steps: For a given community, individual body masses are assigned (Step 1) and used to calculate individual metabolic rates. This can be done using body mass-metabolic rate regressions available from the literature for many taxonomic groups. Alternatively, metabolic rates can be directly measured for study organisms. (Step 2). Network topology is then constructed and feeding preferences defined, either through experimentation or via literature review (Step 3), followed by the calculation of metabolic demands of each node (Step 4) by summing all individual metabolic rates of the respective group. Assimilation efficiencies are assigned (Step 5) based on the resource of a consumer, which can be measured, derived from existing literature for specific consumer types or temperatures (e.g., [31,42]), or scaled depending on resource stoichiometry if e.g. C/N content is measured for organisms in the food web (e.g., [45]). Single fluxes throughout the network are then calculated, starting from the highest trophic level and working to the bottom (Step 6). See Online Supplementary Material S1 for a worked example.
Because energy flux reflects aggregate processes (e.g., primary production, herbivory), it could also be used to provide a standardised index of ecosystem ‘multifunctionality.’ An emerging concept in biodiversity research, multifunctionality reflects the provisioning of multiple ecosystem functions (see Glossary), although capturing this phenomenon statistically has proved historically challenging[33]. Fluxes reflect most of the functions commonly measured in biodiversity experiments, and could replace or complement these functions in future investigations. Using multitrophic flux to quantify ecosystem multifunctionality, however, ignores functions arising from non-trophic processes, such as those related to cultural ecosystem services. In such cases, aggregate indices incorporating energy flux (including multiple fluxes from different resources) and uncorrelated additional functions may prove particularly insightful [33].
Ecological stoichiometry and energy flux
Energy flux in ecological networks characterises the rate of energy flow among nodes, expressing energy consumption by different trophic groups and describing the energetic structure of communities (Figure 1). While this is particularly intriguing for investigating ecological dynamics and processes, researchers might also be interested in how these flows relate to the flux of specific chemical elements. Therefore, while energy flux is often directly related to C flow (as metabolic rates are typically based on organismal respiration and are thus directly related to C uptake [40]), there are techniques that allow for the conversion of energy fluxes to various other elemental fluxes, providing aa range of ecosystem processes. Furthermore, the relative availability of different elements to consumers can have important consequences for the structure of ecosystems [43], the distribution of biomass across populations [44] and the rate of energy flux to consumers [45]. Ecological stoichiometry studies the balance between nutrient requirements of consumers and nutrient availability in their food, and the consequences for the life-histories and dynamics of populations [43]. While many studies in ecological stoichiometry focus on single interactions between a consumer and its resource [46], there is growing interest in the role of stoichiometric effects in food webs, as stoichiometry might influence the structure, function, and stability of food webs in various ways [47].
Inspired by early research in marine systems, it has been shown that, across different ecosystem types, important elemental ratios (e.g., C to N ratio) in primary producer tissue are highly conserved [48]. Because elemental ratios change along a food chain [49,50], by quantifying the C consumption of organisms (via conversion of energy flux to C consumption per unit time), it is possible to calculate rates of other elemental fluxes such as N and P among trophic nodes in networks [31]. As global element cycles are tightly linked through biotic interactions [51], they are consequently affected by associated changes in biodiversity and energy flux in ecological networks. Thus, quantifying network energy fluxes allows for the exploration of individual relationships of multiple currencies (e.g., C, N, P) of ecosystem functioning with biodiversity (Box 2).
Box 2. Unifying classical BEF theory with the energetic network concept.
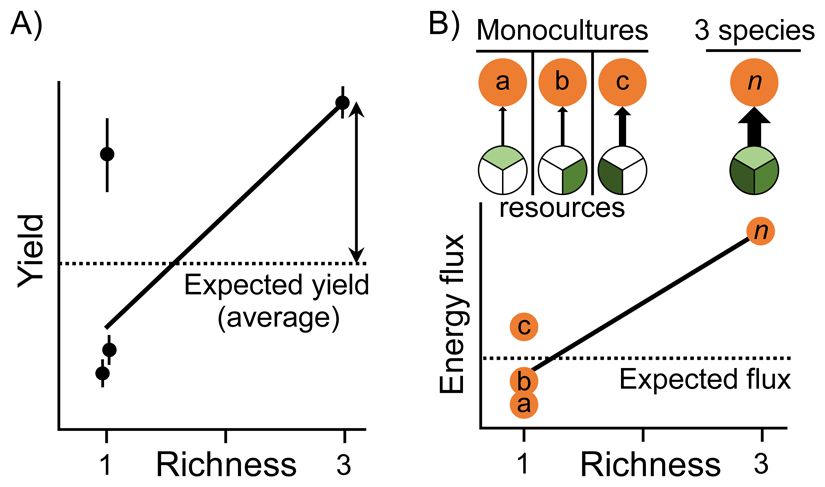
Over the last two decades, >600 experiments have attempted to isolate the independent effects of changing biodiversity on ecosystem processes. Most of these experiments were designed to test the central theoretical hypothesis that a diverse mixture would perform differently than predicted based on the mean or maximum monoculture [63]. The typical design contained at least: (i) a diverse mixture comprising species from a local species pool, and, (ii) each constituent species in monoculture. Starting biomass (or density) is equalised among treatments, such that the most diverse mixture has the same total biomass (or density) as corresponding single-species treatments [63]. Under this design, a diversity effect is observed if ecosystem functioning (such as biomass yield) in the mixture deviates positively from the null expectation; i.e., the averaged function of each monoculture (Figure II A).
Early multitrophic BEF experiments tested hypotheses about whether the core BEF relationship within a trophic level varied with food chain length (e.g., [64]), or how diversity at one trophic level affected performance at an adjacent level (e.g., [65,66]). These pioneering studies revealed that diversity effects differed from the null expectation because of, for example, variation in foraging behaviour or intra-guild predation [67]. Such experiments continued to test and expand the basic BEF theoretical framework, grounded in mechanisms of selection and complementarity. However, these mechanisms have not been extended to questions about network complexity and ecosystem function, likely due to logistical constraints of quantifying ecosystem functioning for complex food webs.
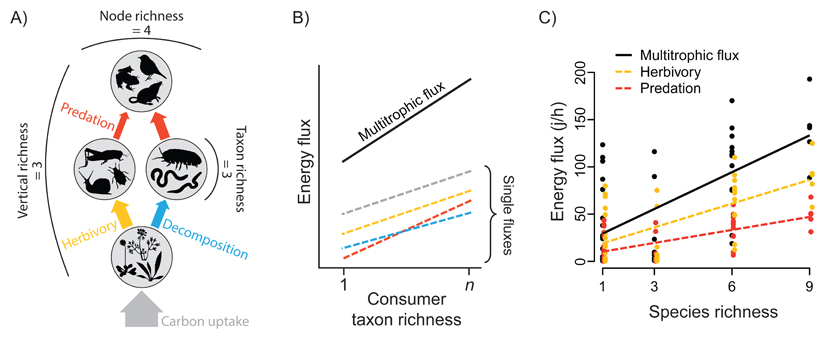
Under a network approach, classical BEF hypotheses could be tested by comparing energy fluxes to species in monoculture with multi-species mixtures (Figure II B). This allows for the quantification of diversity effects via manipulation of diversity at one trophic level to create a diversity gradient across networks (e.g., manipulating producer diversity within the same node across multiple identical networks). One can then extract information on node diversity from each replicated network and regress corresponding fluxes on selected diversity variables (Figure II B) to test the hypothesis that node diversity, as a measure of network diversity, affects net ecosystem fluxes. This framework can be further extended to multiple nodes of differing diversity across structurally identical networks, or multiple networks of increasing trophic complexity [10] (Figure III).
To demonstrate the applicability of these methods, we reanalyse a multitrophic BEF experiment that manipulated nine species of estuarine consumers, including fishes, crabs, shrimps and other epifaunal invertebrates [53]. Using reproducible R code (provided in the Online Supplementary Material), we computed energy flux in each replicate of varying diversity (1, 3, 6, or 9 species) and regressed these values against diversity. As in the previous analysis of single functions [53], increasing diversity enhanced multitrophic flux, both within and across trophic levels (Figure II C). This example should convey that quantifying energy flux in multitrophic systems does not require substantially different experimental designs; indeed, they can be applied without much additional effort to existing BEF experiments.
Figure II.
Definition of a ‘true’ diversity effect in classical BEF experiments (A), with the translation of how one would conceptualise such an effect in ecological networks using energy flux (B). The double-headed arrow in (A) indicates the magnitude of the diversity effect on yield in a hypothetical three-species mixture. In (B), orange nodes denote three hypothetical species consuming a heterogeneous resource (consumption is indicated by coloured segments), with a complementarity effect on total energy flux depicted for the three-species mixture (all resources consumed simultaneously).
Figure III.
Different measures of diversity obtained from the network (A) can be directly related to individual energy fluxes for specific functions, or to summed network fluxes to infer ecosystem multifunctionality (B). Line colours in (B) correspond to individual functions depicted in (A), with network multifunctionality indicated by these summed fluxes as ‘multitrophic flux’ in (B).
A stoichiometric approach to quantifying energy flux is not only useful for calculating multiple currencies of elemental fluxes, but also allows for more precise quantification of energy flux rates in food webs. This is because consumers might respond differently to arising stoichiometric constraints; e.g., by lowering their production efficiency [52] or by increasing feeding rates [45]. These two responses yield different effects on rates of fluxes, interaction strengths, and, hence, stability [16]. Thus, understanding the stoichiometric composition of food webs allows for the conversion of energy fluxes in ecological networks to a range of specific elemental fluxes, as well as providing crucial information for understanding interaction strengths and rates of energy flux in ecosystems.
Integrating food webs, fluxes, and ecosystem functioning
Despite an increasing focus of BEF research on multitrophic systems (primarily on marine food chains [6,53,54]), there has been very little concentration on systems with high species richness and trophic complexity (Box 2). Because of this, BEF research has not yet been able to directly investigate and define the bioenergetic mechanisms underlying the relationship between biodiversity and ecosystem functioning in complex, multitrophic systems [32]. Yet, studies that establish whether and how complex network structure determines ecosystem functioning have been repeatedly called for in recent years [6,10,23,32]. While not necessarily touted in the context of BEF research, food-web studies quantifying energy flux among trophic nodes have already begun to answer this call (e.g., [16,17]). To demonstrate the ease with which existing experimental designs can incorporate energy fluxes, we provide a worked example (Box 2; data and code available in the Online Supplementary Material).
BEF experiments have identified general mechanisms underlying the relationship between biodiversity and ecosystem functioning in simplified systems (e.g., selection and complementarity) [12] that are directly transposable to energy fluxes in a multitrophic context (Box 2). For example, niche complementarity (or trophic complementarity [55]) can be an important mechanism driving ecosystem functioning in food webs, in which greater consumer diversity reduces competition over resources, thus increasing total energy flux to consumers [55,56]. Alternatively, selection effects, whereby species of unique functional importance come to dominate species mixtures, might also modulate the diversity-function relationship in food webs [57]. Extending beyond the framework of BEF, total energy flux can also depend on network complexity (e.g., species diversity or links per species [58]) due to increased exploitation of additional energy stocks [59,60]; an emergent property of networks that can be independent of total system biomass [61]. In a similar vein, one aspect of network theory that has received considerable attention is that of ‘keystone’ effects, caused by species that have disproportionate effects on the persistence of other species [13]. This ‘keystone’ concept can be extended to understand the relationship between diversity and keystone effects on energy fluxes in networks.
Another advantage of integrating network energy fluxes into BEF research is the knowledge that can be gained from patterns in food web stability and resilience and, thus, the stability of ecosystem functions. Network topology, for example, can provide an indication of functional redundancy, whereby food webs with more generalists (consuming energy from multiple stocks) might have a lower likelihood of complete functional losses as other species can replace their functional counterparts [56]. The distribution of energy fluxes (which also infer interaction strengths) is also important for determining stability of networks; trophic loops that contain weak links [17], as well as energetically bottom-heavy food-web structure where energy fluxes are larger at lower compared to higher trophic levels [62], can confer greater stability to food webs. These aforementioned mechanisms that modulate ecosystem functioning and stability depend, at least in part, on biodiversity in ecological networks, supporting our proposal that an understanding of these mechanisms can be advanced by focusing on complex multitrophic systems through the lens of energy fluxes in food webs.
Promising directions for integrating network energy fluxes into BEF research
Linking food web stability and ecosystem function
Food-web research has a long-standing history in tackling the problem of stability [68,69], initiated and sustained by the question of how diversity and complexity of interaction networks affect their stability [70]. While random interaction networks are intrinsically destabilised by the diversity and complexity of a community, natural food webs are stabilised by non-random patterns in their (i) network structure [71], (ii) distribution of interaction strengths across links [16,17,39,72] and body-mass structure [18,73], as well as (iii) adaptive feeding processes [20]. A range of metrics have been developed and employed to assess the stability of ecological networks [e.g., 74–76] Discrepancy between these metrics has been treated by comparing different metrics within studies [18] or developing multi-stability indices [22]. Thus, several decades of research on ecological stability have provided mechanistic insights into the structure and dynamics of communities, as well as a set of well-developed methods to investigate ecological stability.
Applying a multitrophic energy flux approach to BEF research opens the possibility to measure the stability of multiple functions in complex systems. Similar to the aforementioned food-web approaches, estimation of certain aspects of stability such as temporal variability of fluxes between pairs of populations, between functional groups (e.g., herbivory as the flux from plants to herbivores), or throughout entire communities is possible within this framework. Empirically, the calculation of energy fluxes has unravelled the stability constraints imposed by network structure [17] and community assembly [78]. More recently, models of multitrophic communities have been used to predict changes in energy fluxes following changes in consumer species diversity [59]. While such studies have quantified time-averaged fluxes as measurements of ecosystem functioning, analyses of temporal variation in fluxes have not yet been investigated. Further exploration in this direction could stimulate entirely new research questions targeting the relationships between the stability of biomass stocks (at the population, feeding guild, or community level) and the temporal stability of the fluxes between these stocks.
Understanding species identity effects on ecosystem performance
Human-driven biodiversity change is the combined net effect of local or global species extinctions, changes in species composition and realised interactions, as well as introductions of exotic species. Pursuant to its original theoretical motivations, early BEF research concentrated on the consequences of random species loss [2,79]. While changes in species diversity, itself, can influence ecosystem functioning, the directional or non-random loss (or gain) of particular species can have disproportionate effects on ecosystem properties [57,80]. Such effects often occur as a result of species’ interactions that initiate cascading effects through multitrophic systems following the loss or gain of ‘keystone species’ [13].
One potentially exciting avenue of research would be to extend this concept to understand how strongly interacting species affect energy flux to other populations in complex food webs. For example, the loss of a predator could result in increased herbivory and reduced producer biomass (due to predatory release), destabilisation of herbivore populations (due to increased top-down pressure on producers), and thus destabilisation of remaining predator species (due to increased bottom-up forces on predators). In modelled food webs in which interaction strengths are defined by energy fluxes, keystone species could be identified based on their aggregate effects on energy fluxes across the network. While this application of the keystone species concept contradicts the classic demonstration that demographic and biomass responses best capture keystone effects [13], adapting this concept to per capita (or mass-specific) energy fluxes might provide new insights into diversity-function relationships and how these might depart from diversity-community structure relationships characterized by abundance. Further tests could involve shifting population structure, such as population density or body size distributions; both of which can have important impacts on ecosystem functioning [69].
Following the functional trait approach to BEF work, similar empirical approaches could experimentally remove or introduce species based specifically on functional traits related to energy flux that are predicted to play a central role for ecosystem functioning. Total and per capita energy fluxes can be calculated for these empirical food webs, before and after experimental manipulation, and compared to food webs where species are lost or gained at random. Such experiments could disentangle the importance of extinction and invasion of keystone species for ecosystem functioning, relative to changes in species diversity per se. Beyond identifying keystone effects on ecosystem functioning, model simulations based on results from experimental manipulations of food webs could also focus on identifying species combinations that maximise whole-network energy fluxes to ascertain at which point multitrophic systems will reach peak performance.
Cross-ecosystem processes and the spatial scaling of multitrophic ecosystem functioning
Perhaps one of the greatest challenges for operationalising BEF theory in “real world” ecosystems, beyond the carefully controlled experimental conditions used to test hypotheses, is to scale up from plot-level manipulations of communities with relatively low diversity and trophic complexity to highly complex, landscape-scale systems that span multiple habitats [12]. Unlike many other often-measured functions (such as standing-stock biomass), energy flux is not spatially restricted but, instead, mechanistically characterises functions that link populations and communities in space (e.g., via movement or cross-ecosystem interactions and subsidies) [81,82] (Figure 1), allowing for cross-ecosystem predictions of ecosystem functioning [83].
In multitrophic systems, organism mobility (or habitat use) typically increases with body size and trophic level [84], leading to energy export across ecosystem boundaries that is dependent on both body size and trophic structure of communities. In addition, multitrophic systems are open to neighbouring systems via trophic interactions [81], further contributing to outflow of energy (or spillover). Global change drivers, such as habitat fragmentation and climate change, can further influence home range size [85] and dispersal [86], leading to important, scale-dependent impacts on energy flux in ecosystems subjected to various drivers of environmental change.
For a given ecosystem (e.g., a grassland patch of known size), Equation 1 (Box 1) could be modified to account for home range size and across-system energy loss by: (i) including an additional loss term that parameterises the outflow of energy due to, e.g., the body size-dependent emigration from a consumer node, and (ii) reducing energy loss to consumers, L, from Equation 1 (assuming increasing home-range size—scaled by body size—reduces the impact of a consumer on a given resource node). Within this framework, future models can be developed to integrate organism mobility and home range size into energy flux calculations and incorporate permeability properties of system boundaries in several dimensions.
Concluding remarks
For the last two decades, intensive efforts have cemented the central role that biodiversity plays in determining ecosystem functioning. Though the central motivation of BEF experiments was to establish the importance of biodiversity for the integrated performance of ecosystems, trophic complexity has been frequently excluded from many of these studies. Furthermore, the majority of BEF studies (both experimental and observational) have focused on standing-stock biomass, rather than measuring fluxes of material and energy [87]. These limitations have been pointed out repeatedly, with calls for the incorporation of multitrophic complexity, as well as the quantification of rates and fluxes to increase realism and, thus, the applicability of BEF theory to natural systems [11,88].
In this review, we have outlined the importance of integrating a network perspective into BEF research. Unlike previous such syntheses, however, we provide an applied framework based on ecosystem energetics within which ecologists can approach the question of how biodiversity determines ecosystem functioning in complex, multitrophic systems. By synthesising past work that has contributed toward this research frontier, we provide examples of how the quantification of energy fluxes in ecological networks opens new avenues of BEF research, expanding previous boundaries of ecological scale, complexity, and context. The application of energy fluxes in ecological networks to BEF research is, however, only a small part of the utility of this framework. In a broader context, energy fluxes can be used to understand the impacts of a vast range of drivers of environmental change (e.g., climate change, species invasions, N deposition, etc.) in highly diverse systems spanning many trophic levels. Thus, by embracing ecosystem complexity through the expression of ecosystem functioning as energy flux in ecological networks, we improve our ability to understand whether and how natural systems will persist and continue to provide multiple ecosystem services in a changing world.
Acknowledgements
A.D.B, U.B., and N.E. acknowledge support by the German Research Foundation (FZT 118); A.D.B., M.J., C.S., U.B., and N.E. were also supported by the German Research Foundation within the framework of the Jena Experiment (FOR 1451). Further, M.J. was supported by the Swiss National Science Foundation and N.E. acknowledges funding by the European Research Council (ERC Starting Grant 677232, ECOWORM).
References
- 1.Butchart SHM, et al. Global biodiversity: indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 2.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 3.Mulder C, et al. 10 years later: revisiting priorities for science and society a decade after the millennium ecosystem assessment. Adv Ecol Res. 2015;53:1–53. [Google Scholar]
- 4.Lefcheck JS, et al. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat Commun. 2015;6 doi: 10.1038/ncomms7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isbell F, et al. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477:199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 6.Duffy JE, et al. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol Lett. 2007;10:522–538. doi: 10.1111/j.1461-0248.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 7.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 8.Soliveres S, et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature. 2016;536:456–459. doi: 10.1038/nature19092. [DOI] [PubMed] [Google Scholar]
- 9.Seabloom EW, et al. Food webs obscure the strength of plant diversity effects on primary productivity. Ecol Lett. 2017;20:505–512. doi: 10.1111/ele.12754. [DOI] [PubMed] [Google Scholar]
- 10.Hines J, et al. Towards an integration of biodiversity-ecosystem functioning and food web theory to evaluate relationships between multiple ecosystem services. Adv Ecol Res. 2015;53:161–199. [Google Scholar]
- 11.Brose U, Hillebrand H. Biodiversity and ecosystem functioning in dynamic landscapes. Philos Trans R Soc B Biol Sci. 2016 doi: 10.1098/rstb.2015.0267. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer N, et al. Biodiversity-ecosystem function experiments reveal the mechanisms underlying the consequences of biodiversity change in real world ecosystems. J Veg Sci. 2016;27:1061–1070. [Google Scholar]
- 13.Paine RT. Food webs: linkage, interaction strength and community infrastructure. J Anim Ecol. 1980;49:667–685. [Google Scholar]
- 14.Loreau M. From populations to ecosystems: Theoretical foundations for a new ecological synthesis. Princeton University Press; 2010. [Google Scholar]
- 15.Williams RJ, Martinez ND. Simple rules yield complex food webs. Nature. 2000;404:180–3. doi: 10.1038/35004572. [DOI] [PubMed] [Google Scholar]
- 16.de Ruiter PC, et al. Energetics, patterns of interaction strengths, and stability in real ecosystems. Science. 1995;269:1257–1260. doi: 10.1126/science.269.5228.1257. [DOI] [PubMed] [Google Scholar]
- 17.Neutel A, et al. Stability in real food webs : weak links in long loops. Science. 2002;296:1120–1123. doi: 10.1126/science.1068326. [DOI] [PubMed] [Google Scholar]
- 18.Brose U, et al. Allometric scaling enhances stability in complex food webs. Ecol Lett. 2006;9:1228–1236. doi: 10.1111/j.1461-0248.2006.00978.x. [DOI] [PubMed] [Google Scholar]
- 19.Coux C, et al. Linking species functional roles to their network roles. Ecology Letters. 2016;19:762–770. doi: 10.1111/ele.12612. [DOI] [PubMed] [Google Scholar]
- 20.Heckmann L, et al. Interactive effects of body-size structure and adaptive foraging on food-web stability. Ecol Lett. 2012;15:243–250. doi: 10.1111/j.1461-0248.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- 21.Donohue I, et al. Navigating the complexity of ecological stability. Ecol Lett. 2016;19:1172–1185. doi: 10.1111/ele.12648. [DOI] [PubMed] [Google Scholar]
- 22.Donohue I, et al. On the dimensionality of ecological stability. Ecol Lett. 2013;16:421–429. doi: 10.1111/ele.12086. [DOI] [PubMed] [Google Scholar]
- 23.Gravel D, et al. The meaning of functional trait composition of food webs for ecosystem functioning. Philos Trans R Soc B Biol Sci. 2016;371 doi: 10.1098/rstb.2015.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banašek-Richter C, et al. Complexity in quantitative food webs. Ecology. 2009;90:1470–1477. doi: 10.1890/08-2207.1. [DOI] [PubMed] [Google Scholar]
- 25.Reuman DC, Cohen JE. Estimating relative energy fluxes using the food web, species abundance, and body size. Adv Ecol Res. 2005;36:137–182. [Google Scholar]
- 26.Moore JC, de Ruiter PC. Energetic food webs: An analysis of real and model ecosystems. Oxford University Press; 2012. [Google Scholar]
- 27.Angelini C, et al. A keystone mutualism underpins resilience of a coastal ecosystem to drought. Nat Commun. 2016;7 doi: 10.1038/ncomms12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kéfi S, et al. Network structure beyond food webs: Mapping non-trophic and trophic interactions on Chilean rocky shores. Ecology. 2015;96:291–303. doi: 10.1890/13-1424.1. [DOI] [PubMed] [Google Scholar]
- 29.Terry JCD, et al. Trophic interaction modifications: an empirical and theoretical framework. Ecol Lett. 2017;20:1219–1230. doi: 10.1111/ele.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt HW, et al. The detrital food web in a shortgrass prairie. Biol Fertil Soils. 1987;3:57–68. [Google Scholar]
- 31.de Ruiter PC, et al. Calculation of nitrogen mineralization in soil food webs. Plant Soil. 1993;157:263–273. [Google Scholar]
- 32.Thompson RM, et al. Food webs: reconciling the structure and function of biodiversity. Trends Ecol Evol. 2012;27:689–697. doi: 10.1016/j.tree.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Byrnes JEK, et al. Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions. Methods Ecol Evol. 2014;5:111–124. [Google Scholar]
- 34.Pauly D, et al. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J Mar Sci. 2000;57:697–706. [Google Scholar]
- 35.Borrett SR, Lau MK. enaR: An R package for Ecosystem Network Analysis. Methods Ecol Evol. 2014;5:1206–1213. [Google Scholar]
- 36.O’Neill RV. Indirect estimation of energy fluxes in animal food webs. J Theor Biol. 1969;22:284–290. doi: 10.1016/0022-5193(69)90006-x. [DOI] [PubMed] [Google Scholar]
- 37.Neutel AM, Thorne MAS. Interaction strengths in balanced carbon cycles and the absence of a relation between ecosystem complexity and stability. Ecol Lett. 2014;17:651–661. doi: 10.1111/ele.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes AD, et al. Consequences of tropical land use for multitrophic biodiversity and ecosystem functioning. Nat Commun. 2014;5 doi: 10.1038/ncomms6351. 5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz B, et al. Warming alters the energetic structure and function but not resilience of soil food webs. Nat Clim Chang. 2017 doi: 10.1038/s41558-017-0002-z. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehnes RB, et al. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol Lett. 2011;14:993–1000. doi: 10.1111/j.1461-0248.2011.01660.x. [DOI] [PubMed] [Google Scholar]
- 41.Andrews RM, Pough FH. Metabolism of Squamate Reptiles : Allometric and Ecological Relationships. Physiol Zool. 1985;58:214–231. [Google Scholar]
- 42.Lang B, et al. Temperature and consumer type dependencies of energy flows in natural communities. Oikos. 2017;4:1–9. [Google Scholar]
- 43.Hillebrand H, et al. Think ratio! A stoichiometric view on biodiversity–ecosystem functioning research. Basic Appl Ecol. 2014;15:465–474. [Google Scholar]
- 44.Ott D, et al. Unifying elemental stoichiometry and metabolic theory in predicting species abundances. Ecol Lett. 2014;17:1247–1256. doi: 10.1111/ele.12330. [DOI] [PubMed] [Google Scholar]
- 45.Jochum M, et al. Decreasing stoichiometric resource quality drives compensatory feeding across trophic levels in tropical litter invertebrate communities. Am Nat. 2017;190:131–143. doi: 10.1086/691790. [DOI] [PubMed] [Google Scholar]
- 46.Sterner RW, Elser JJ. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press; 2002. [Google Scholar]
- 47.Hessen DO, et al. Carbon sequestration in Ecosystems : The role of stoichiometry. Ecology. 2004;85:1179–1192. [Google Scholar]
- 48.McGroddy ME, et al. Scaling of C:N:P stoichiometry in forests worldwide: implications of terrestrial Redfield-type ratios. Ecology. 2004;85:2390–2401. [Google Scholar]
- 49.Fanin N, et al. An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter-microbe system. Ecol Lett. 2013;16:764–772. doi: 10.1111/ele.12108. [DOI] [PubMed] [Google Scholar]
- 50.Fagan WF, et al. Nitrogen in insects: implications for trophic complexity and species diversification. Am Nat. 2002;160:784–802. doi: 10.1086/343879. [DOI] [PubMed] [Google Scholar]
- 51.Hessen DO, et al. Ecological stoichiometry: An elementary approach using basic principles. Limnol Oceanogr. 2013;58:2219–2236. [Google Scholar]
- 52.Anderson TR, et al. Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am Nat. 2005;165:1–15. doi: 10.1086/426598. [DOI] [PubMed] [Google Scholar]
- 53.Lefcheck JS, Duffy JE. Multitrophic functional diversity predicts ecosystem functioning in experimental assemblages of estuarine consumers. Ecology. 2015;96:2973–2983. doi: 10.1890/14-1977.1. [DOI] [PubMed] [Google Scholar]
- 54.O’Connor MI, et al. A general biodiversity-function relationship is mediated by trophic level. Oikos. 2016;126:18–31. [Google Scholar]
- 55.Poisot T, et al. Trophic complementarity drives the biodiversity-ecosystem functioning relationship in food webs. Ecol Lett. 2013;16:853–861. doi: 10.1111/ele.12118. [DOI] [PubMed] [Google Scholar]
- 56.Peralta G, et al. Complementarity and redundancy of interactions enhance attack rates and spatial stability in host-parasitoid food webs. Ecology. 2014;95:1888–1896. doi: 10.1890/13-1569.1. [DOI] [PubMed] [Google Scholar]
- 57.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 58.Riede JO, et al. Scaling of Food-Web Properties with Diversity and Complexity Across Ecosystems. Adv Ecol Res. 2010;42:139–170. [Google Scholar]
- 59.Schneider FD, et al. Animal diversity and ecosystem functioning in dynamic food webs. Nat Commun. 2016;7 doi: 10.1038/ncomms12718. 12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morriën E, et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat Commun. 2017;8 doi: 10.1038/ncomms14349. 14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnes AD, et al. Species richness and biomass explain spatial turnover in ecosystem functioning across tropical and temperate ecosystems. Philos Trans R Soc B. 2016;371 doi: 10.1098/rstb.2015.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rip JMK, McCann KS. Cross-ecosystem differences in stability and the principle of energy flux. Ecol Lett. 2011;14:733–740. doi: 10.1111/j.1461-0248.2011.01636.x. [DOI] [PubMed] [Google Scholar]
- 63.Schmid B, et al. A guide to analyzing biodiversity experiments. J Plant Ecol. 2017;10:91–110. [Google Scholar]
- 64.Duffy JE, et al. Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecol Lett. 2005;8:301–309. [Google Scholar]
- 65.Gamfeldt L, et al. Species richness changes across two trophic levels simultaneously affect prey and consumer biomass. Ecol Lett. 2005;8:696–703. [Google Scholar]
- 66.Bruno JF, O’Connor MI. Cascading effects of predator diversity and omnivory in a marine food web. Ecol Lett. 2005;8:1048–1056. [Google Scholar]
- 67.Finke DL, Denno RF. Predator diversity and the functioning of ecosystems: The role of intraguild predation in dampening trophic cascades. Ecol Lett. 2005;8:1299–1306. [Google Scholar]
- 68.May RM. Will a Large Complex System be Stable? Nature. 1972;238:413–414. doi: 10.1038/238413a0. [DOI] [PubMed] [Google Scholar]
- 69.Brose U, et al. Predicting the consequences of species loss using size-structured biodiversity approaches. Biol Rev. 2016;49 doi: 10.1111/brv.12250. 0–0. [DOI] [PubMed] [Google Scholar]
- 70.Montoya JM, et al. Ecological networks and their fragility. Nature. 2006;442:259–264. doi: 10.1038/nature04927. [DOI] [PubMed] [Google Scholar]
- 71.Jacquet C, et al. No complexity-stability relationship in empirical ecosystems. Nat Commun. 2016;7 doi: 10.1038/ncomms12573. 12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gross T, et al. Generalized Models Reveal Stabilizing Factors in Food Webs. Science. 2009;325:747–750. doi: 10.1126/science.1173536. [DOI] [PubMed] [Google Scholar]
- 73.Otto SB, et al. Allometric degree distributions facilitate food-web stability. Nature. 2007;450:1226–1229. doi: 10.1038/nature06359. [DOI] [PubMed] [Google Scholar]
- 74.Allesina S, Tang S. Stability criteria for complex ecosystems. Nature. 2012;483:205–208. doi: 10.1038/nature10832. [DOI] [PubMed] [Google Scholar]
- 75.Fussmann KE, et al. Ecological stability in response to warming. Nat Clim Chang. 2014;4:206–210. [Google Scholar]
- 76.Curtsdotter A, et al. Robustness to secondary extinctions: Comparing trait-based sequential deletions in static and dynamic food webs. Basic Appl Ecol. 2011;12:571–580. [Google Scholar]
- 77.McCann K, et al. Weak trophic interactions and the balance of nature. Nature. 1998;395:794–798. [Google Scholar]
- 78.Neutel AM, et al. Reconciling complexity with stability in naturally assembling food webs. Nature. 2007;449:599–U11. doi: 10.1038/nature06154. [DOI] [PubMed] [Google Scholar]
- 79.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486:105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 80.Ehrenfeld JG. Ecosystem Consequences of Biological Invasions. Annu Rev Ecol Evol Syst. 2010;41:59–80. [Google Scholar]
- 81.Knight TM, et al. Trophic cascades across ecosystems. Nature. 2005;437:880–883. doi: 10.1038/nature03962. [DOI] [PubMed] [Google Scholar]
- 82.Gravel D, et al. Source and sink dynamics in meta-ecosystems. Ecology. 2010;91:2172–2184. doi: 10.1890/09-0843.1. [DOI] [PubMed] [Google Scholar]
- 83.Loreau M, et al. Meta-ecosystems: A theoretical framework for a spatial ecosystem ecology. Ecol Lett. 2003;6:673–679. [Google Scholar]
- 84.Tucker MA, et al. Evolutionary predictors of mammalian home range size: Body mass, diet and the environment. Glob Ecol Biogeogr. 2014;23:1105–1114. [Google Scholar]
- 85.Haskell JP, et al. Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature. 2002;418:527–530. doi: 10.1038/nature00840. [DOI] [PubMed] [Google Scholar]
- 86.Barnes AD, et al. Individual behaviour mediates effects of warming on movement across a fragmented landscape. Funct Ecol. 2015;29:1543–1552. [Google Scholar]
- 87.Duffy JE, et al. Biodiversity mediates top-down control in eelgrass ecosystems: a global comparative-experimental approach. Ecol Lett. 2015 doi: 10.1111/ele.12448. [DOI] [PubMed] [Google Scholar]
- 88.Gamfeldt L, et al. Marine biodiversity and ecosystem functioning: What’s known and what’s next? Oikos. 2015;124:252–265. [Google Scholar]