Summary
The plastidic glutamine synthetase isoform (GS2) plays a key role in nitrogen (N) assimilation. We introduced TaGS2‐2Abpro::TaGS2‐2Ab, the favourable allele of TaGS2‐2A in the winter wheat (Triticum aestivum) variety Ji5265. Transgenic expression of TaGS2‐2Ab significantly increased GS2 abundance and GS activity in leaves. Two consecutive field experiments showed the transgenic lines had higher grain yield, spike number, grain number per spike and 1000‐grain weight than did the wild type under both low N and high N conditions. Analysis of N use‐related traits showed that transgenic expression of TaGS2‐2Ab increased root ability to acquire N, N uptake before and after flowering, remobilization of N to grains and N harvest index. Measurement of chlorophyll content and net photosynthesis rate in flag leaves during grain filling stage revealed that the transgenic lines had prolonged leaf functional duration as compared with the wild type. These results suggest that TaGS2 plays important role in N use, and the favourable allele TaGS2‐2Ab is valuable in engineering wheat with improved N use efficiency and grain yield.
Keywords: wheat, glutamine synthetase, nitrogen use efficiency
Introduction
Nitrogen (N) fertilizer is indispensable for growing the world's food. Now the world's food production is facing the challenge of excess N input and low N use efficiency (NUE). An estimation of the amount of excess nutrient application in 17 major crops has revealed that ~60% of N inputs are in excess (West et al., 2014). It is estimated that only 30%–50% of applied N is taken up by crops, and the unused N is lost to the environment which cause soil, water and air pollution (Han et al., 2015; Hirel et al., 2011; Robertson and Vitousek, 2009; West et al., 2014). Therefore, breeding crops with improved NUE represents effective approach in increasing crop productivity with less N Fertilizer input (Han et al., 2015; Hirel et al., 2011).
Conventional breeding and molecular genetics have been employed to improve NUE in crops (Hirel et al., 2011). As NUE is governed by complicated gene networks that mediate the uptake, assimilation, remobilization and storage of N (Good et al., 2004; Masclaux‐Daubresse et al., 2010; McAllister et al., 2012; Xu et al., 2012), much more works of molecular breeding and genetic engineering approaches are required to improve NUE. Currently, many candidate genes have been identified for improving NUE in crop plants, and these candidate genes are existed in pathways relating to uptake, assimilation, remobilization and storage of N (Masclaux‐Daubresse et al., 2010; McAllister et al., 2012; Thomsen et al., 2014). For example, selection of natural variation or overexpression of nitrate transporter has been shown to increase grain yield and NUE in rice (Oryza sativa) (Fan et al., 2016; Hu et al., 2015). Manipulating N assimilation also has been shown great potential in improving NUE. A well‐known example is that transgenic expression of an alanine aminotransferase AlaAT using a root epidermal cell promoter in oilseed rape (Brassica napus) and rice increased yield and biomass under N‐limiting conditions compared with control plants (Good et al., 2007; Shrawat et al., 2008). The plant‐specific DNA BINDING WITH ONE FINGER (Dof) transcription factor Dof1 from maize (Zea mays) has been shown to improve N assimilation and growth of rice under low N conditions by regulating genes involved in the tricarboxylic acid cycle (TCA cycle) and increasing carbon flow towards N assimilation (Kurai et al., 2011).
Once N has entered the plant, it can be assimilated into amino acids and other important nitrogenous compounds. The first step in which inorganic N is assimilated into organic composition is catalysed by glutamine synthetase (GS) (Miflin and Habash, 2002). Based on the subcellular location, GS is classified into cytosolic (GS1) and chloroplastic (GS2) isoforms. GS1 is usually encoded by a multigene family, and GS2 is often encoded by a single gene (Chardon et al., 2012; Miflin and Habash, 2002). We identified three GS1 genes and one GS2 gene in each subgenome of wheat by analysing the reference sequence of the bread wheat variety Chinese Spring (Figure S1). There is accumulating evidence supporting that both GS1 and GS2 play essential roles in efficient N use and high yield potential in major crops including wheat, rice and maize (Chardon et al., 2012; Hirel et al., 2011; Thomsen et al., 2014). And both of these two enzymes have been used to engineering N use efficient crops. For example, overexpressing a GS1 gene from bean with rubisco small subunit (rbcS) promoter from rice exhibited higher root dry weight, grain weight and grain N accumulation than the nontransgenic controls in wheat (Habash et al., 2001). Overexpression of the GS1 gene Gln1 with an ubiquitin promoter led to increased harvest index, N harvest index and spikelet number in rice (Brauer et al., 2011). In maize, overexpressing the GS1 gene Gln1‐3 using a cassava vein mosaic virus (CsVMV) promoter increased grain number and yield (Martin et al., 2006). In sorghum (Sorghum bicolor), enhanced biomass production was observed by the overexpression of the GS1 gene Gln1 under low N conditions (Urriola and Rathore, 2015). However, a recent review revealed that the outcome of overexpressing GS1 has generally been inconsistent, many studies have shown decreases in N use, biomass and yield by overexpressing GS1 (Thomsen et al., 2014). One possible reason underlying the inconsistent outcome is that overexpressing GS1 may cause metabolic imbalances, thus more refined overexpression strategies are needed to overcome metabolic bottlenecks (Thomsen et al., 2014). In contrast to the numerous studies on the transgenic modification of GS1 (Thomsen et al., 2014), only very few studies were carried out on the effects of transgenic modifying GS2. Overexpressing GS2 derived by a leaf‐specific promoter rbcS has been found to increase the growth of tobacco seedlings (Migge et al., 2000), and overexpressing GS2 can enhance photorespiration and tolerance to salt stress in rice (Hoshida et al., 2000). To the best of our knowledge, the influence of overexpressing GS2 on N use and grain productivity has not been reported yet.
In wheat, GS activity is proposed to be one of the best physiological markers that allow the depiction of the plant N status (Kichey et al., 2006, 2007), as leaf GS activity was found to positively correlate with leaf soluble protein and N content (Habash et al., 2007; Kichey et al., 2006, 2007), and grain yield (Kichey et al., 2007), but negatively correlate with leaf senescence (Kichey et al., 2007). As mentioned above, total GS activity is catalysed by GS1 and GS2. GS2 was found to be the major isozyme in the leaf tissues during vegetative growth in wheat (Bernard et al., 2008; Habash et al., 2001). When leaves aged during grain filling, there was a decrease in total GS activity (Habash et al., 2001; Kichey et al., 2007). This was mainly due to loss of GS2 and thus GS1 became a greater contributor to total GS activity (Habash et al., 2001). These dynamic changes in leaf GS1 and GS2 during grain filling made us to think the possibility of engineering GS2 expression in increasing NUE and grain yield in wheat. Our previous study has identified two (a and b), six (a to f) and two (a and b) haplotypes for TaGS2‐2A (former name TaGS2‐A1), TaGS2‐2B (former name TaGS2‐B1) and TaGS2‐2D (former name TaGS2‐D1) in the mini core collection of Chinese wheat varieties, respectively. Association analysis revealed that the TaGS2‐2Ab haplotype showed significant association with higher shoot and root dry weigh at seedling stage, and higher thousand grain weight and higher grain N concentration (Li et al., 2011). Here, we found that TaGS2‐2Ab encoded an enzyme with higher GS activity than other haplotypes did. Field experiments showed that transgenic expression of TaGS2‐2Ab driven by its own promoter increased the growth and nitrate influx rate of the roots, postanthesis N uptake, leaf functional duration, NHI, grain N concentration and grain yield under both low and high N conditions.
Results
In vitro assay of TaGS2 activity
Our previous study has shown that there were three TaGS2 genes located on the homologous group 2 chromosomes and have identified two (a and b), six (a to f) and two (a and b) haplotypes for TaGS2‐2A, TaGS2‐2B and TaGS2‐2D, respectively (Li et al., 2011). The ten haplotypes of TaGS2 genes encode three kinds of putative GS2 proteins (Figure S1), TaGS2‐2A representing the sequences of TaGS2‐2Aa, 2Ab and 2Da, TaGS2‐2B representing the sequences of all TaGS2‐2B haplotypes, and TaGS2‐2Db representing the sequence of TaGS2‐2Db. Prokaryotic expression experiment in vitro showed that TaGS2‐2A had 40% higher GS activity than did TaGS2‐2B and TaGS2‐2Db (Figure 1).
Figure 1.

In vitro assay of TaGS2 activity. GST and N‐terminal GST‐tagged TaGS2‐2A, ‐2B and ‐2Db were overexpressed in Escherichia coli. The expressed proteins were purified to homogeneity. In vitro GS assays were performed as described under ‘Materials and methods'. Data represent means ± SE of four replicates. Asterisks indicate that the difference between the means of the TaGS2 activities was significant at the P < 0.01 (**) level.
Selection of TaGS2‐2Ab transgenic lines
We totally obtained 85 transgenic lines by introducing TaGS2‐2Abpro::TaGS2‐2Ab into the wheat variety Ji2565. Thirty‐seven lines of T3 generation were further selected to evaluate the yield performance under high N conditions in 2011–2012 growing season. The results showed that the grain yield increased first with the GS activity of the flag leaves at 14 DPA, and then declined when the GS activity increased further (Figure S2). Based on the grain yield and GS activity, the three transgenic lines OE53, OE57 and OE94 were selected for further study. In field experiment 2 in 2013–2014 growing season, the transgenic lines had greener canopy than the wild type under both low N and high N conditions (Figure S3). The total TaGS2 mRNA levels and GS activities in the flag leave at 14 DPA were investigated. All the transgenic lines had significantly higher expression TaGS2 (Figures 2a, S4), GS activity (Figure 2b) and GS2 protein abundance (Figure 2c,d) than did the wild type under both low N and high N conditions.
Figure 2.

Molecular characterization of the TaGS2‐2Ab transgenic lines and wild control Ji5265. The transgenic lines (OE53, 57 and 94) and wild type (WT) were grown under low N and high N conditions in field experiment 2. The flag leaves were collected at 14 DPA for molecular characterization of GS2. (a) Relative expression level of total TaGS2. The relative expression levels were normalized to the expression of TaACTIN. (b) Total GS activity. (c) Protein abundance of TaGS2. Ten micrograms total chloroplastid protein was loaded in each lane. Upper panel shows the GS2 immunoblot and lower panel the Ponceau‐stained blot. (d) Quantified intensity of the TaGS2 Western bands present in (c). Data represent means ± SE of at least three replicates. Asterisks indicate that the difference between the means of the transgenic lines and WT was significant at the P < 0.05 (*) and P < 0.01 (**) level.
Grain yield and yield components
The agronomic traits of the transgenic lines and wild type were evaluated under low N and high N conditions in two consecutive field experiments. Both experiments observed significant higher grain yield in the transgenic lines than in the wild type under both N conditions (Figure 3a,e). In experiment 1 in 2012–2013 growing season, the transgenic lines had 5.4%–11.1% and 8.4%–13.5% higher grain yield than did the wild type under low N and high N conditions, respectively (Figure 3a). In experiment 2 in 2013–2014 growing season, the transgenic lines had 8.0%–13.5% and 3.6%–4.9% higher grain yield than did the wild type under low N and high N conditions, respectively (Figure 3e). We further analysed the yield components, the result showed the three transgenic lines had higher spike number, grain number per spike and 1000‐grain weight (TGW) under both low N and high N conditions, but the increasing levels depended on the transgenic line, N treatment and growing season (Figure 3b–d,f–h). We also generated transgenic lines with TaGS2‐2Abpro::TaGS2‐2Ab in another wheat variety Kenong199 and the results showed that the grain yield is higher in transgenic lines than that in the wild type and azygous control line under both high N and low N conditions (Figure S5).
Figure 3.
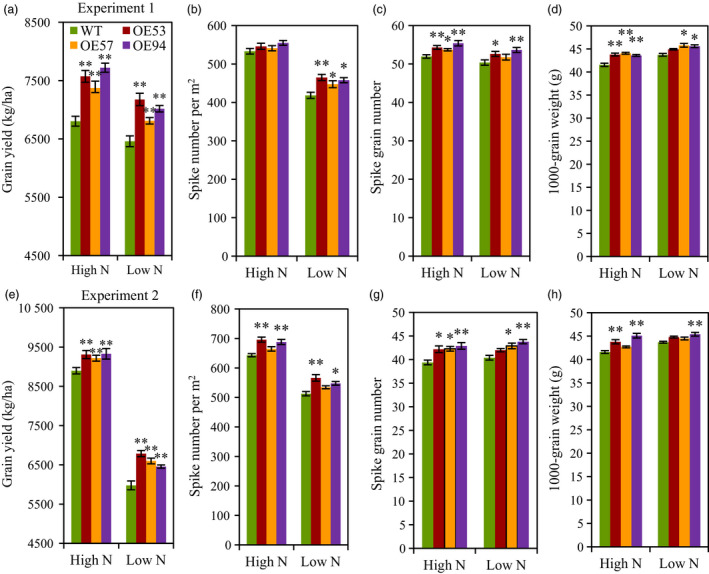
Agronomic traits of the wild type and TaGS2‐2Ab transgenic in the field experiments. (a–d) Grain yield per ha (a), spike number per m2 (b), grain number per spike (c) and 1000‐grain weight (d) in experiment 1 in 2012–2013 growing season. (e–h) Grain yield per ha (e), spike number per m2 (f), grain number per spike (g) and 1000‐grain weight (h) in experiment 2 in 2013–2014 growing season. Data represent means ± SE of four replicates. Asterisks indicate that the difference between the means of the transgenic lines and wild type was significant at the P < 0.05 (*) and P < 0.01 (**) level.
N use‐related traits
We investigated N use‐related traits in experiment 2. Under high N conditions, the N concentration in whole aerial parts of the three transgenic lines was 5.9%–15.4% higher at stem elongation stage (Figure 4a), 6.9%–7.1% higher at anthesis stage (Figure 4b) and 6.6%–9.2% higher at 14 days postanthesis (DPA, Figure 4c) than did the wild type. Under low N conditions, the N concentration in whole aerial parts of the three transgenic lines was 5.7%–11.8% higher at stem elongation stage (Figure 4a), 10.5%–16.3% higher at anthesis stage (Figure 4b) and 14.8%–23.2% higher at 14 DPA (Figure 4c) than did the wild type. At maturity stage, the transgenic lines had 4.8%–14.6% higher and 6.9%–16.2% higher aerial N accumulation than did the wild type under high N and low N conditions, respectively (Figure 4d). A pot experiment by 15N labelling fertilizer at stem elongation and flowering stage showed that the transgenic lines, OE53 and OE94, absorbed more N than did the wild type before and after flowering (Table S1). The field experiment also found that transgenic expression of TaGS2‐2Ab altered N distribution in aerial parts by allocating more N in grains, as the transgenic lines displayed lower leaf and stem N concentration (Figure 4e,f), higher grain N concentration (Figure 4g), N harvest index (NHI, Figure 4h) and harvest index (HI, Figure 4i) than did the wild type under both N conditions. In line with the results of grain N concentration, the transgenic lines had significantly higher concentrations of the total amino acids in grains than did the wild type (Figure S6, Table S2).
Figure 4.
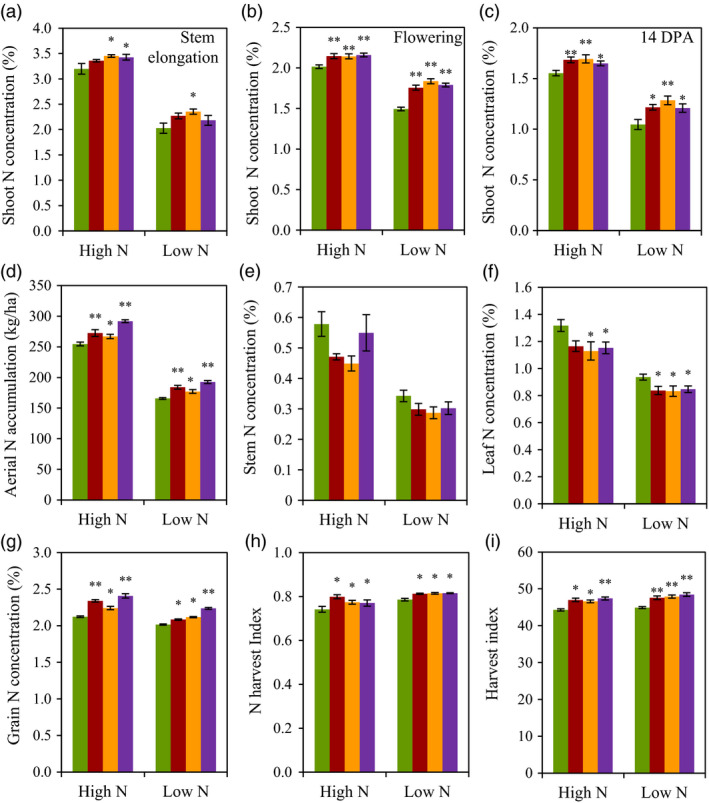
N use‐related traits of the wild type and the transgenic lines in field experiment 2 in 2013–2014 growing season. (a–c) Shoot N concentration at stem elongation (a), flowering (b) and 14 DPA (c). (d–i) Aerial N accumulation (d), stem N concentration (e), leaf N concentration (f), grain N concentration (g), nitrogen harvest index (h) and Harvest index (i) at maturity. Data represent means ± SE of four replicates. Asterisks indicate that the difference between the means of the transgenic lines and wild type was significant at the P < 0.05 (*) and P < 0.01 (**) level.
Wheat root morphology, nitrate flux rate and expression of nitrate transporters
To understand the mechanisms by which TaGS2‐2Abpro::TaGS2‐2Ab increased N uptake, we first investigated how TaGS2‐2Ab affected root growth. In a hydroponic culture system with plants at the seedling stage, we observed that the three transgenic lines had longer primary root length and more lateral root number than did the wild type (Figure 5). We next measured nitrate flux rates of roots. The transgenic lines displayed significantly higher nitrate influx rates at root surfaces 5, 10 and 20 mm from the root tip than that did the wild type when the nitrate flux rate was recorded in a measuring solution containing 0.2 mm nitrate (Figure 6). We also analysed the expression of nitrate transporter genes. In roots, the three transgenic lines had higher TaNRT2.1 and TaNPF6.3 expression than did the wild type (Figure S7). These results suggest that the overexpression of TaGS2‐2Abpro::TaGS2‐2Ab up‐regulated the expression of genes involved in nitrate transport. These results also indicate that overexpression of TaGS2‐2Abpro::TaGS2‐2Ab in wheat enhanced nitrate uptake rate, possibly by up‐regulating the expression of nitrate transporters.
Figure 5.

Root morphological parameters of the wild type wheat (J5265) and the transgenic lines. Wheat seedlings (7 days after germination) were grown for 12 days in a nutrient solution that contained 2 mm nitrate (high N) or 0.2 mm nitrate (low N). (a) Primary root length. (b) Lateral root number. Data represent means ± SE of at least five replicates. Asterisks indicate that the difference between the means of the transgenic lines and wild type was significant at the P < 0.05 (*) and P < 0.01 (**) level.
Figure 6.

Nitrate flux rate. NO3 − flux rate was measured by SIET with 0.2 mm NO3 − in the measuring solution. Seedlings were pretreated with 0.2 mm nitrate and were then used to measure nitrate influx rate at the root tip surface in measuring solutions that contained 0.2 mm nitrate. Positive and negative flux values indicate efflux and influx, respectively. Data represent means ± SE of at least three replicates. Asterisks indicate that difference between the wild type and the transgenic lines was significant at the P < 0.05 level.
N concentration, chlorophyll concentration and net photosynthesis rate in flag leaves
During grain filling, the flag leaves of the transgenic lines had higher N concentration before 21 DPA under high N condition (Figure 7a) and before 14 DPA under low N conditions (Figure 7b), higher chlorophyll concentration after 21 DPA under high N conditions (Figure 7c) and at all the measuring dates under low N conditions (Figure 7d), and higher Pn at all the measuring dates under both N conditions (Figure 7e,f) than those of wild type. We also found that the transgenic lines had larger flag leaves (Figure S8), higher soluble proteins at 14 DPA (Figure S9), and higher superoxide dismutase (SOD) activity (Figure S10a,b), lower malondialdehyde (MDA) concentration (Figure S10c,d) in the flag leaves at 7 DPA–35 DPA than did the wild type under both N conditions. These results indicated that the transgenic lines had increased flag leaf size and prolonged flag leaf functional duration.
Figure 7.
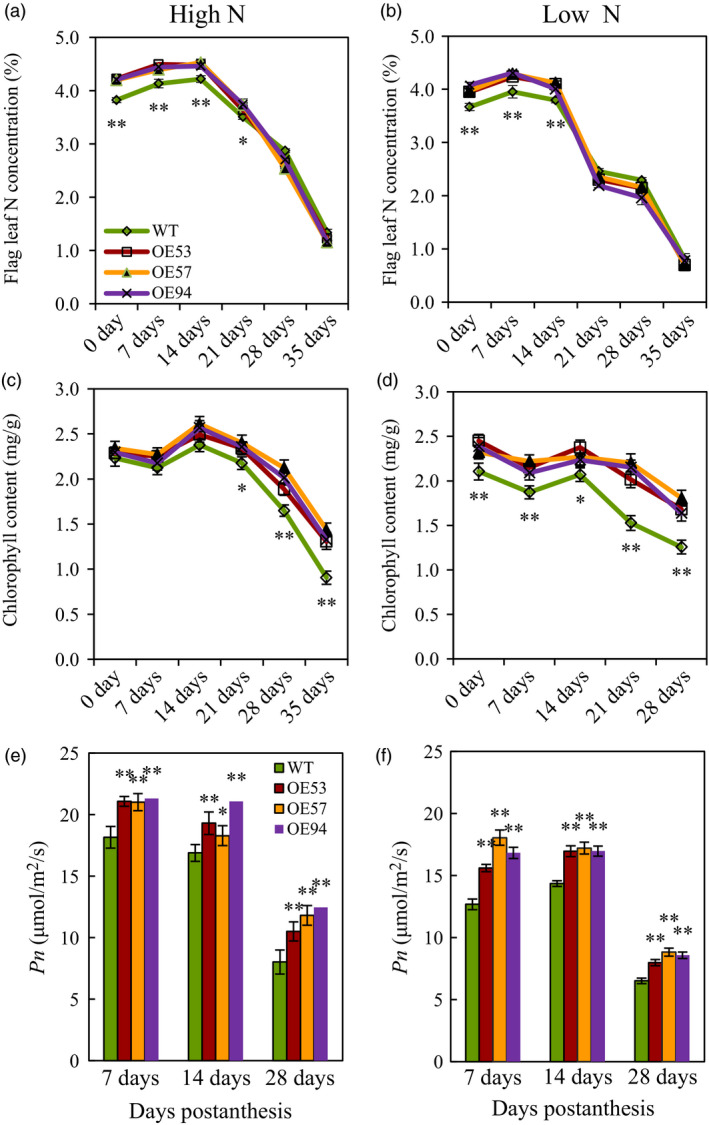
N content, chlorophyll content and net photosynthesis rate in flag leaves of the transgenic lines and wild type in experiment 2 in 2013–2014 growing season. (a and b) Flag leaf N content under high N (a) and low N (b) conditions. (c and d) Chlorophyll content in flag leaves under high N (c) and low N (d) conditions. (e and f) Net photosynthesis rate (Pn) under high N (e) and low N (f) conditions. Data represent the mean ± SE of 4 replications. Asterisks indicate that the difference between the means of the transgenic lines and wild type was significant at the P < 0.05 (*) and P < 0.01 (**) level.
Discussion
Transgenic expression of TaGS2‐2Ab leads to an N efficient wheat ideotype with high grain mass, large harvest index and N harvest index, and high grain N concentration
Manipulating N assimilation has been proposed an important strategy to improve NUE. GS, which catalyses the first step in assimilating inorganic N into organic composition (Miflin and Habash, 2002), has been used to improve NUE in many crops including wheat (Chardon et al., 2012; Hirel et al., 2011; Thomsen et al., 2014). However, the majority of the studies were conducted in greenhouse to evaluate the phenotypes of the transgenic lines. And the effects of overexpressing GS on N use‐related physiological traits, biomass and yield have generally been inconsistent (Thomsen et al., 2014). The two consecutive field experiments in present study showed that transgenic expression of TaGS2‐2Ab increased flag leaf GS activity, leaf functional duration, N uptake, grain N concentration, NHI, HI, grain yield and yield components under both low N and high N conditions. These merits of the transgenic lines meet the requirement for an N efficient wheat ideotype with high grain mass, large HI and NHI, and high GPC (Chardon et al., 2012). Two strategies might be important for the successful use of TaGS2‐2Ab in engineering N efficient wheat. The first one was to optimize leaf GS activity. The correlation between flag leaf GS activity and grain yield of T3 transgenic lines showed that extremely high leaf GS activity had negative effect on grain yield (Figure S2). We observed that the transgenic lines with very high leaf GS activity exhibited excessive stay‐green and had reduced TGW. The second one was to drive the expression of TaGS2‐2Ab by its own promoter. Overexpression of GS may cause metabolic imbalances and inhibit N use and plant growth, thus more refined overexpression strategies combined with tissue and cell specific targeting are needed to overcome metabolic bottlenecks in engineering N efficient crops (Thomsen et al., 2014). Optimizing GS activity and driving TaGS2‐2Ab by its own promoter may avoid the possible metabolic imbalances.
Transgenic expression of TaGS2‐2Ab increases N uptake and remobilization
The size and architecture of root system is an important variable for ensuring adequate access to N in plant (Miller and Cramer, 2005). The increased primary root length and lateral root number (Figure 5) combined with the increased nitrate influx rates (Figure 6) provided a strong basis for the TaGS2‐2Ab‐transgenic wheat lines to efficiently acquire soil N resources, as has been shown in the field experiment 2 (Figure 4d) and in the pot experiment (Table S1). We found that transgenic expressing TaGS2‐2Ab increased expression of TaNRT2.1 and TaNPF6.3 (Figure S7). NRT2 function as high‐affinity nitrate transporter. Increasing expression of OsNRT2.1 through transgenic approach enchanted N uptake before and after heading in rice (Chen et al., 2016). In wheat, it has been reported that root expression of transporter TaNRT2.1 is related with postanthesis N uptake (Taulemesse et al., 2015). TaNPF6.3 is orthologous to Arabidopsis AtNPF6.3/NRT1.1 which functions as dual‐affinity nitrate transporter (Wang et al., 1998). Overexpression of OsNPF6.3/NRT1.1b increased N uptake and grain yield in rice (Hu et al., 2015). As such, the increased expression of TaNRT2.1 and TaNPF6.3 may partially explain the enhanced nitrate influx (Figure 6), and N uptake before and after flowering (Table S1) by transgenic expressing TaGS2‐2Ab.
Grain yield and GPC are two major targets of wheat breeding. However, the strong genetic negative correlation between grain yield and GPC in wheat makes it difficult to improve these two traits simultaneously (Bogard et al., 2010). Our current study showed transgenic expression of TaGS2‐2Ab increased not only grain yield but also grain N concentration under both low N and high N condition, enabling TaGS2‐2Ab valuable in shifting the negative correlation between grain yield and GPC. Usually, increased grain N concentration can be accomplished through two ways, one is to increase N uptake, and another one is to improve partitioning of N to the grain through remobilization. Transgenic expressing TaGS2‐2Ab increased N uptake before anthesis and more apparently after anthesis (Table S1). It has been proposed that increasing postanthesis N uptake is important to increase GPC while maintaining high grain productivity (Bogard et al., 2010; Monaghan et al., 2001). Several lines of evidence supported that transgenic expressing TaGS2‐2Ab increased N remobilization. Firstly, the transgenic lines had higher shoot N concentrations at flowering (Figure 4b), but lower leaf and stem N concentrations than did the wild type at maturity (Figure 4e,f). Secondly, the N concentrations in flag leaves of the transgenic lines were higher than those of wild type at early stage of grain filling, but were similar with those of wild type at later stage of grain filling (Figure 7a,b). Finally, the transgenic lines had higher NHI than did the wild type (Figure 4h). GS1 and GS2 isoforms play nonoverlapping roles in plant N assimilation. During leaf senescence, GS1 was the predominant isoform of GS enzymes and was thought to play major roles in assimilating ammonia during the critical phases of remobilization of N to the grain in wheat (Bernard et al., 2008) while GS2 was proposed a major role in ammonium assimilation within photorespiratory N cycling (Bernard and Habash, 2009). Our data clearly showed that GS2 was also important in remobilization of N to the grain. However, the underlying mechanisms are needed to explore in future research.
Transgenic expression of TaGS2‐2Ab prolongs leaf functional duration
Leaf senescence is the final stage of leaf development in nature which is an important process for crop yield. Delaying leaf senescence and extending the duration of leaf photosynthesis during grain filling is a possible route for increasing grain yields (Gaju et al., 2011; Hebbar et al., 2014; Kichey et al., 2007; Richards, 2000). Our current study found that transgenic expressing TaGS2‐2Ab prolonged leaf functional duration (Figure 7c–f), providing strong leaf metabolic basis for the increased grain yield and TGW. Although the transgenic lines had higher chlorophyll content after 21 DPA (Figure 7c,d) and higher Pn at 28 DPA (Figure 7e,f) than did the wild type, they had similar leaf N concentrations with the wild type after 21 DPA (Figure 7a,b). This phenomenon implied that the transgenic lines had higher photosynthetic capacity using similar amount of N. Although the underlying mechanisms are worthy to be elucidated, the increased SOD activity and reduced MDA content (Figure S10) by expressing TaGS2‐2Ab might gave partial explanation. In north China (where our field experiments located), during the late grain filling stage, wheat often experiences hot weather combined with high sun light, which may cause photo‐oxidative damage to photosynthetic apparatus (Yang et al., 2006). The increased SOD activity and reduced MDA content suggested that the transgenic lines had a more efficient antioxidant system and higher capacity for the scavenging of reactive oxygen species favourable for resistance to photo‐oxidative stress. It has been shown that during leaf senescence, GS2 experienced rapid loss and GS1 became the major GS isoform (Habash et al., 2001). The prolonged leaf functional duration by transgenic expressing TaGS2‐2Ab suggested that a low GS2 activity may be a limiting factor for leaf function during the late grain filling stage.
In summary, TaGS2‐2Ab is a valuable gene resource for breeding wheat with grain yield and GPC under a range of soil N conditions. Besides transgenic modification, marker‐assisted selection of different TaGS2 haplotypes may be effective in improving NUE and yield, as our previous study has already shown that the favourable haplotypes of TaGS2 were associated with N use‐ and yield‐related traits (Li et al., 2011). Although we observed that transgenic expression of TaGS2‐2Ab increased root ability to acquire N and N remobilization, further studies are needed to dissect the underlying mechanisms.
Materials and methods
Plant materials
The wheat (Triticum aestivum) varieties Xiaoyan 54, Zhengmai 9023 and Ji5265 were used in this study. Xiaoyan 54 was used to isolate the sequences of the TaGS2‐2Ab gene, and Zhengmai 9023 was used to isolate the cDNA sequences of TaGS2‐2A, TaGS2‐2B and TaGS2‐2Db. Ji5265 was used to generate the transgenic lines. Ji5265 is a winter wheat variety which was commercially released in 2007.
In vitro assay of TaGS2 activity
We used the total cDNA from wheat variety Zhengmai 9023 as the template and amplified the ORF region for TaGS2‐2A, TaGS2‐2B and TaGS2‐2Db cDNA by PCR with the universal primers of TaGS2. The primers used were forward (5′‐CGGGATCCATGGCGCAGGCGGTGGT‐3′) and reverse (5′‐AAGGAAAAAAGCGGCCGCTCATACCTTCAGCGCCAGCTTC‐3′). These amplified ORFs were inserted into pGEX4T‐1 plasmid at BamHI and NotI sites. All the constructs of three TaGS2 cDNA were confirmed by sequencing the ligation products of pGEX4T‐1 plasmids. The constructs, pGEX4T‐1‐TaGS2‐2A, pGEX4T‐1‐TaGS2‐2B, pGEX4T‐1‐TaGS2‐2Db and empty pGEX4T‐1 vector, were transformed into BL21 (DE3) plysS cells (TransGen Biotech Inc., Beijing, China). Protein productions were induced by the addition of isopropyl‐β‐d‐thiogalactoside to a final concentration of 0.05 mm and incubated in shaker with 150 rpm overnight at 20 °C. After induction, cells were harvested by centrifugation at 6000 g for 30 min at 4 °C. After decanting the supernatant, the resulting pellet was suspended in phosphate buffer solution (PBS, pH 7.4) and sonicated with three 5‐s bursts using an ultrasonic homogenizer (Scientz‐IID; Ningbo Scientz Biotechnology Co. Ltd., Ningbo, China). The lysate was centrifuged at 1 4000 g for 30 min at 4 °C, and the supernatants were transferred to an equilibrated for purification. GST‐TaGS2‐2A, GST‐TaGS2‐2B and GST‐TaGS2‐2Db fusion proteins were produced and purified to homogeneity as described previously (Huang et al. 2007). In brief, crude protein extracts were loaded onto a glutathione sepharose 4B column (GE Healthcare, Waukesha, WI), and then, after washing seven times with PBS buffer, GST‐TaGS2‐2A, GST‐TaGS2‐2B and GST‐TaGS2‐2Db fusion proteins were eluted using 5 mm reduced glutathione in 50 mm Tris–HCl, pH 8.0 and then concentrated by Amicon Ultra‐15 Centrifugal Filter Units (Millipore, Darmstadt, Germany). The GS activities of the purified proteins were determined using Glutamine Synthetase Detection Kit A047 (Nanjing Jiancheng Biotechnology, Nanjing, China).
Plasmid construction and wheat transformation
The complete open reading fragment (ORF) of TaGS2‐2Ab (GQ169685.1) was amplified from the total cDNA prepared from wheat variety Xiaoyan 54 using an reverse transcription (RT)‐PCR system (TaKaRa, Dalian, China) in accordance with the manufacturer's instructions. The primers used were forward (5′‐ATGGCGCAGGCGGTGGTGCCGGCGATG‐3′) and reverse (5′‐TCATACCTTCAGCGCCAGCTTCTTG‐3′). The promoter of TaGS2‐2Ab (TaGS2‐2Abpro) was amplified from the genomic DNA of Xiaoyan 54. The primers used were forward (5′‐TGGAGGGTGACTGCTCCAGAGTTC‐3′) and reverse (5′‐CTTCCCCTTCAGCGCTAGCTGATC‐3′). The amplified TaGS2‐2Ab ORF was inserted in frame between Ubiquitin promoter and 3′NOS at BamHI and KpnI sites of the modified pACH25 vector (Christensen et al., 1992). Then, the Ubiquitin promoter was replaced with TaGS2‐2Abpro at PstI and BamHI sites, resulting in the TaGS2‐2Abpro::TaGS2‐2Ab‐pACH25 construct. The construct was transformed into immature embryos of wheat variety Ji5265 using the method described by Wang et al. (2013).
Field experiments
The field experiments were conducted in the Dishang Experimental Station which belongs to the Institute of Grain and Oil Crops, Hebei Academy of Agriculture and Forestry Sciences in Shijiazhuang, Hebei Province. Two consecutive field experiments were conducted in 2012–2013 (transgenic T4 generation, experiment 1) and 2013–2014 (transgenic T5 generation, experiment 2) growing seasons. Both experiments had two treatments each with four replications. The high N treatment had 22.5 g N/m2 in the form of urea with 13.5 g N/m2 applied prior to sowing and 9.0 g N/m2 applied at the stem elongation stage. The low N treatment had no N application in experiment 1 and 6.75 g N/m2 applied prior to sowing in experiment 2. Both treatments were applied 8 g/m2 phosphorus (P) as calcium superphosphate and 10 g/m2 potassium (K) as potassium sulphate. For each genotype in each replicate, the plot was 1.7 m × 8.4 m which includes eight rows and the rows were spaced 20 cm apart. The sowing density was set at 270 germinating seeds per m2. Ten plants in each plot were randomly collected to measure N concentration in aerial parts at stem elongation, anthesis and 14 DPA. At maturity, twenty plants in each plot were randomly collected to measure N concentration and dry weight of leaves, stems and grains, and grain number per spike, and 1000‐grain weight (TGW). Plant density and spike density were recorded on two rows each with 1 m long in each plot. Grain yield was recorded in the whole plot. Aerial N accumulation at maturity was estimated as (grain yield × grain N concentration)/NHI. Total N concentration in plant samples was measured using a semi‐automated Kjeldahl method (Tecator Kjeltec Auto 1030 Analyzer; Tecator, Hoganas, Sweden).
Quantitative real‐time PCR
Total RNA from plant tissues was extracted with TRIzol reagent (Thermo Fisher Scientific, Waltham, MA). The first‐strand cDNA was synthesized from 2 μg of DNase I‐treated total RNA using murine leukaemia virus reverse transcriptase (Promega, Madison, WI). Quantitative real‐time RT–PCR analysis was performed with a LightCycler 480 engine (Roche, Mannheim, Germany) using the LightCycler480 SYBR Green I Master Mix (Roche). ACTIN2 mRNA was used as an internal control. The primers used for TaGS2‐2A were TaGS2F (5′‐CCGAGACCACGATCCTGT‐3′) and TaGS2R (5‐GGTCCTTCATACCTTCAGCG‐3′). The primers used for ACTIN2 were TaActinF (5′‐ACCTTCAGTTGCCCAGCAAT‐3′) and TaActinR (5′‐CAGAGTCGAGCACAATACCAGTTG‐3′).
Western blot analysis
The abundant of TaGS2 protein was detected the flag leaves at 14 DPA. Twenty flag leaves were randomly collected in each plot in experiment 2. The chloroplast protein was extracted using a commercial Chloroplast Protein Isolation Kit (BestBio, Shanghai, China). Protein concentrations were determined using the BCA protein assay kit (Thermo Fisher Scientific). The extracted proteins were separated on 10% acrylamide gels (Invitrogen Life Technologies, Carlsbad, CA), and Western blot analyses were performed using a GS specific antibody raised against GS2 in rabbits (Abmart, Shanghai, China). Broad range prestained standards (GenStar Biosolutions Co. Ltd., Beijing, China) were used as markers.
Measurement of GS activity
Twenty flag leaves were randomly collected in each plot 14DPA in experiment 2. The samples were grounded to a fine powder under liquid N and then homogenized in an extraction buffer containing 50 mm Tris‐HCl (pH 7.6), 1.0 mm EDTA, 1.0 mm MgC12, 10 mm 2‐ME, 1.0 mm DTT and 0.5% (w/v) insoluble PVP. The homogenate was centrifuged at 10 000 g for 20 min for two times at 4 °C. The supernatant fraction was used for the assay of GS activity. The GS activity was determined using Glutamine Synthetase Detection Kit A047 (Nanjing Jiancheng Biotechnology).
Measurement of chlorophyll concentration and net photosynthesis rate
Net photosynthesis rates of ten randomly selected flag leaves in each plot were measured at 0, 7, 14, 21, 28 and 35 DPA with a LI‐COR 6400 portable photosynthesis system (LiCor Inc., Lincoln, NE). All measurements were conducted in the morning from 9:00 to 11:00 am on a clear day. For measuring chlorophyll concentration, 20 flag leaves were randomly collected. Chlorophyll concentration was measured as described by Arnon (1949).
Root morphological analysis
The root morphological parameters of the transgenic lines (T5 generation) and wild type Ji5265 were measured. The nutrient solution and plant growth conditions were described previously (Wang et al., 2013). Briefly, wheat seedlings (7 days after germination) were grown for 12 days in a nutrient solution that contained 0.2 mm nitrate (low N) or 2.0 mm nitrate (high N). The length of longest primary root was measured by a ruler, and the lateral root number was accounted manually.
Measurements of root nitrate flux rate
Wheat seedlings (7 days after germination) were grown for 7 days in a nutrient solution that contained 0.2 mm nitrate. The roots of these plants were then transferred to a measuring solution containing 0.2 mm KNO3, 0.1 mm CaCl2 and 0.3 mm MES (pH6.0), and allowed to balance for 10 min. The net nitrate fluxes were measured as described previously (He et al., 2015). The maximum nitrate flux rates along the root were recorded.
Statistical analysis of data
One‐way analysis of variance was performed using SAS statistical software (SAS Institute Inc., Cary, NC).
Author contributions
M.H. and X.Z. performed most of the experiments; Q.L. generated transgenic lines; Y.Z. participated in field experiments; L.S. identified transgenic lines; X.H. and W.Z. performed Western bolt experiment and measured enzyme activity; Y.T. and H.L. designed the experiments and analysed the data; Y.T. and X.Z. supervised and complemented the writing.
Supporting information
Figure S1 (a) Alignment of TaGS2 protein sequences. (b) Phylogenetic tree of glutamine synthetase and its homologues in plants.
Figure S2 Correlation of GS activity and grain yield.
Figure S3 Growth performance of the TaGS2‐2Ab transgenic lines (a) and wild control Ji5265 under low N conditions (b).
Figure S4 Expression levels of TaGS2 in shoots (a) and roots (b) of the transgenic lines and wild type Ji5265 grown in high N and low N.
Figure S5 Grain yield of the wild type Kenong199 and TaGS2‐2Ab transgenic in the field experiments.
Figure S6 The amino acids content in grains of the transgenic lines and wild type Ji5265.
Figure S7 Expression levels of TaNRT2.1 and TaNPF6.3 in shoots and roots of of the transgenic lines and wild type Ji5265 grown in high N and low N.
Figure S8 Flag leaf senescence in transgenic lines is delayed as compared with wild type Ji5265.
Figure S9 Soluble protein concentrations in the flag leaves of the transgenic lines and wild type Ji5265 at 14 days postanthesis.
Figure S10 The SOD activity and MDA concentration of the flag leaves in the wild type Ji5265 and transgenic lines during grain filling in field experiment 2.
Table S1 Aerial N accumulation in the pot experiment.
Table S2 The amino acids content in grains of the transgenic lines and wild type Ji5265.
Acknowledgements
This research was supported by the Keystone Project of Transfergene in China (2016ZX08002‐005), the National Natural Science Foundation of China (31272222), and the Special Fund for Strategic Pilot Technology Chinese Academy of Sciences A (XDA0801040401).
Accession numbers: TaGS2‐2Aa, GQ169684; TaGS2‐2Ab, GQ169685; TaGS2‐2Bb, GQ169686; TaGS2‐2Db, GQ169689; ACTIN2, AB181991.1.
Contributor Information
Hui Li, Email: zwslihui@163.com.
Yiping Tong, Email: yptong@genetics.ac.cn.
References
- Arnon, D.I. (1949) Copper enzymes in isolated chloroplasts – polyphenoloxidase in beta‐vulgaris. Plant Physiol. 24, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, S.M. and Habash, D.Z. (2009) The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 182, 608–620. [DOI] [PubMed] [Google Scholar]
- Bernard, S.M. , Moller, A.L. , Dionisio, G. , Kichey, T. , Jahn, T.P. , Dubois, F. , Baudo, M. et al. (2008) Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Mol. Biol. 67, 89–105. [DOI] [PubMed] [Google Scholar]
- Bogard, M. , Allard, V. , Brancourt‐Hulmel, M. , Heumez, E. , Machet, J.M. , Jeuffroy, M.H. , Gate, P. et al. (2010) Deviation from the grain protein concentration‐grain yield negative relationship is highly correlated to post‐anthesis N uptake in winter wheat. J. Exp. Bot. 61, 4303–4312. [DOI] [PubMed] [Google Scholar]
- Brauer, E.K. , Rochon, A. , Bi, Y.M. , Bozzo, G.G. , Rothstein, S.J. and Shelp, B.J. (2011) Reappraisal of nitrogen use efficiency in rice overexpressing glutamine synthetase1. Physiol. Plant. 141, 361–372. [DOI] [PubMed] [Google Scholar]
- Chardon, F. , Noel, V. and Masclaux‐Daubresse, C. (2012) Exploring NUE in crops and in Arabidopsis ideotypes to improve yield and seed quality. J. Exp. Bot. 63, 3401–3412. [DOI] [PubMed] [Google Scholar]
- Chen, J.G. , Zhang, Y. , Tan, Y.W. , Zhang, M. , Zhu, L.L. , Xu, G.H. and Fan, X.R. (2016) Agronomic nitrogen‐use efficiency of rice can be increased by driving OsNRT2.1 expression with the OsNAR2.1 promoter. Plant Biotechnol. J. 14, 1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, A.H. , Sharrock, R.A. and Quail, P.H. (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 18, 675–689. [DOI] [PubMed] [Google Scholar]
- Fan, X. , Tang, Z. , Tan, Y. , Zhang, Y. , Luo, B. , Yang, M. , Lian, X. et al. (2016) Overexpression of a pH‐sensitive nitrate transporter in rice increases crop yields. Proc. Natl Acad. Sci. USA, 113, 7118–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaju, O. , Allard, V. , Martre, P. , Snape, J.W. , Heumez, E. , Le Gouis, J. , Moreau, D. et al. (2011) Identification of traits to improve the nitrogen‐use efficiency of wheat genotypes. Field. Crop. Res. 123, 139–152. [Google Scholar]
- Good, A.G. , Shrawat, A.K. and Muench, D.G. (2004) Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 9, 597–605. [DOI] [PubMed] [Google Scholar]
- Good, A.G. , Johnson, S.J. , De Pauw, M. , Carroll, R.T. and Savidov, N. (2007) Engineering nitrogen use efficiency with alanine aminotransferase. Can. J. Bot. 85, 252–262. [Google Scholar]
- Habash, D.Z. , Massiah, A.J. , Rong, H.L. , Wallsgrove, R.M. and Leigh, R.A. (2001) The role of cytosolic glutamine synthetase in wheat. Ann. Appl. Biol. 138, 83–89. [Google Scholar]
- Habash, D.Z. , Bernard, S. , Schondelmaier, J. , Weyen, J. and Quarrie, S.A. (2007) The genetics of nitrogen use in hexaploid wheat: N utilisation, development and yield. Theor. Appl. Genet. 114, 403–419. [DOI] [PubMed] [Google Scholar]
- Han, M. , Okamoto, M. , Beatty, P.H. , Rothstein, S.J. and Good, A.G. (2015) The genetics of nitrogen use efficiency in crop plants. Annu. Rev. Genet. 49(49), 269–289. [DOI] [PubMed] [Google Scholar]
- He, X. , Qu, B. , Li, W. , Zhao, X. , Teng, W. , Ma, W. , Ren, Y. et al. (2015) The nitrate‐inducible NAC transcription factor TaNAC2‐5A controls nitrate response and increases wheat yield. Plant Physiol. 169, 1991–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbar, K.B. , Rane, J. , Ramana, S. , Panwar, N.R. , Ajay, S. , Rao, A.S. and Prasad, P.V.V. (2014) Natural variation in the regulation of leaf senescence and relation to N and root traits in wheat. Plant Soil, 378, 99–112. [Google Scholar]
- Hirel, B. , Tetu, T. , Lea, P.J. and Dubois, F. (2011) Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability, 3, 1452–1485. [Google Scholar]
- Hoshida, H. , Tanaka, Y. , Hibino, T. , Hayashi, Y. , Tanaka, A. , Takabe, T. and Takabe, T. (2000) Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol. Biol. 43, 103–111. [DOI] [PubMed] [Google Scholar]
- Hu, B. , Wang, W. , Ou, S. , Tang, J. , Li, H. , Che, R. , Zhang, Z. et al. (2015) Variation in NRT1.1B contributes to nitrate‐use divergence between rice subspecies. Nat. Genet. 47, 834–838. [DOI] [PubMed] [Google Scholar]
- Huang, Y.F. , Wang, Y. and Watford, M. (2007) Glutamine directly downregulates glutamine synthetase protein levels in mouse C2C12 skeletal muscle myotubes. J. Nutr. 137, 1357–1362. [DOI] [PubMed] [Google Scholar]
- Kichey, T. , Heumez, E. , Pocholle, D. , Pageau, K. , Vanacker, H. , Dubois, F. , Le Gouis, J. et al. (2006) Combined agronomic and physiological aspects of nitrogen management in wheat highlight a central role for glutamine synthetase. New Phytol. 169, 265–278. [DOI] [PubMed] [Google Scholar]
- Kichey, T. , Hirel, B. , Heumez, E. , Dubois, F. and Le Gouis, J. (2007) In winter wheat (Triticum aestivum L.), post‐anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field. Crop. Res. 102, 22–32. [Google Scholar]
- Kurai, T. , Wakayama, M. , Abiko, T. , Yanagisawa, S. , Aoki, N. and Ohsugi, R. (2011) Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low‐nitrogen conditions. Plant Biotechnol. J. 9, 826–837. [DOI] [PubMed] [Google Scholar]
- Li, X.P. , Zhao, X.Q. , He, X. , Zhao, G.Y. , Li, B. , Liu, D.C. , Zhang, A.M. et al. (2011) Haplotype analysis of the genes encoding glutamine synthetase plastic isoforms and their association with nitrogen‐use‐ and yield‐related traits in bread wheat. New Phytol. 189, 449–458. [DOI] [PubMed] [Google Scholar]
- Martin, A. , Lee, J. , Kichey, T. , Gerentes, D. , Zivy, M. , Tatout, C. , Dubois, F. et al. (2006) Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell, 18, 3252–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux‐Daubresse, C. , Daniel‐Vedele, F. , Dechorgnat, J. , Chardon, F. , Gaufichon, L. and Suzuki, A. (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann. Bot. 105, 1141–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister, C.H. , Beatty, P.H. and Good, A.G. (2012) Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnol. J. 10, 1011–1025. [DOI] [PubMed] [Google Scholar]
- Miflin, B.J. and Habash, D.Z. (2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 53, 979–987. [DOI] [PubMed] [Google Scholar]
- Migge, A. , Carrayol, E. , Hirel, B. and Becker, T.W. (2000) Leaf‐specific overexpression of plastidic glutamine synthetase stimulates the growth of transgenic tobacco seedlings. Planta, 210, 252–260. [DOI] [PubMed] [Google Scholar]
- Miller, A.J. and Cramer, M.D. (2005) Root nitrogen acquisition and assimilation. Plant Soil, 274, 1–36. [Google Scholar]
- Monaghan, J.M. , Snape, J.W. , Chojecki, A.J.S. and Kettlewell, P.S. (2001) The use of grain protein deviation for identifying wheat cultivars with high grain protein concentration and yield. Euphytica, 122, 309–317. [Google Scholar]
- Richards, R.A. (2000) Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 51, 447–458. [DOI] [PubMed] [Google Scholar]
- Robertson, G.P. and Vitousek, P.M. (2009) Nitrogen in agriculture: balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 34, 97–125. [Google Scholar]
- Shrawat, A.K. , Carroll, R.T. , DePauw, M. , Taylor, G.J. and Good, A.G. (2008) Genetic engineering of improved nitrogen use efficiency in rice by the tissue‐specific expression of alanine aminotransferase. Plant Biotechnol. J. 6, 722–732. [DOI] [PubMed] [Google Scholar]
- Taulemesse, F. , Le Gouis, J. , Gouache, D. , Gibon, Y. and Allard, V. (2015) Post‐flowering nitrate uptake in wheat is controlled by N status at flowering, with a putative major role of root nitrate transporter NRT2.1. PLoS ONE, 10, e0120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen, H.C. , Eriksson, D. , Moller, I.S. and Schjoerring, J.K. (2014) Cytosolic glutamine synthetase: a target for improvement of crop nitrogen use efficiency? Trends Plant Sci. 19, 656–663. [DOI] [PubMed] [Google Scholar]
- Urriola, J. and Rathore, K.S. (2015) Overexpression of a glutamine synthetase gene affects growth and development in sorghum. Transgenic Res. 24, 397–407. [DOI] [PubMed] [Google Scholar]
- Wang, R. , Liu, D. and Crawford, N.M. (1998) The Arabidopsis CHL1 protein plays a major role in high‐affinity nitrate uptake. Proc. Natl Acad. Sci. USA, 95, 15134–15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Sun, J. , Miao, J. , Guo, J. , Shi, Z. , He, M. , Chen, Y. et al. (2013) A phosphate starvation response regulator Ta‐PHR1 is involved in phosphate signalling and increases grain yield in wheat. Ann. Bot. 111, 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, P.C. , Gerber, J.S. , Engstrom, P.M. , Mueller, N.D. , Brauman, K.A. , Carlson, K.M. , Cassidy, E.S. et al. (2014) Leverage points for improving global food security and the environment. Science, 345, 325–328. [DOI] [PubMed] [Google Scholar]
- Xu, G.H. , Fan, X.R. and Miller, A.J. (2012) Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63, 153–182. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Chen, X. , Ge, Q. , Li, B. , Tong, Y. , Zhang, A. , Li, Z. et al. (2006) Tolerance of photosynthesis to photoinhibition, high temperature and drought stress in flag leaves of wheat: a comparison between a hybridization line and its parents grown under field conditions. Plant Sci. 171, 389–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (a) Alignment of TaGS2 protein sequences. (b) Phylogenetic tree of glutamine synthetase and its homologues in plants.
Figure S2 Correlation of GS activity and grain yield.
Figure S3 Growth performance of the TaGS2‐2Ab transgenic lines (a) and wild control Ji5265 under low N conditions (b).
Figure S4 Expression levels of TaGS2 in shoots (a) and roots (b) of the transgenic lines and wild type Ji5265 grown in high N and low N.
Figure S5 Grain yield of the wild type Kenong199 and TaGS2‐2Ab transgenic in the field experiments.
Figure S6 The amino acids content in grains of the transgenic lines and wild type Ji5265.
Figure S7 Expression levels of TaNRT2.1 and TaNPF6.3 in shoots and roots of of the transgenic lines and wild type Ji5265 grown in high N and low N.
Figure S8 Flag leaf senescence in transgenic lines is delayed as compared with wild type Ji5265.
Figure S9 Soluble protein concentrations in the flag leaves of the transgenic lines and wild type Ji5265 at 14 days postanthesis.
Figure S10 The SOD activity and MDA concentration of the flag leaves in the wild type Ji5265 and transgenic lines during grain filling in field experiment 2.
Table S1 Aerial N accumulation in the pot experiment.
Table S2 The amino acids content in grains of the transgenic lines and wild type Ji5265.


