Abstract
Background.
Following Haemophilus influenzae serotype b (Hib) conjugate vaccine introduction in the 1980s, Hib disease in young children dramatically decreased, and epidemiology of invasive H. influenzae changed.
Methods.
Active surveillance for invasive H. influenzae disease was conducted through Active Bacterial Core surveillance sites. Incidence rates were directly standardized to the age and race distribution of the US population.
Results.
During 2009–2015, the estimated mean annual incidence of invasive H. influenzae disease was 1.70 cases per 100 000 population. Incidence was highest among adults aged ≥65 years (6.30) and children aged <1 year (8.45); many cases in infants aged <1 year occurred during the first month of life in preterm or low-birth-weight infants. Among children aged <5 years (incidence: 2.84), incidence was substantially higher in American Indian and Alaska Natives AI/AN (15.19) than in all other races (2.62). Overall, 14.5% of cases were fatal; case fatality was highest among adults aged ≥65 years (20%). Nontypeable H. influenzae had the highest incidence (1.22) and case fatality (16%), as compared with Hib (0.03; 4%) and non-b encapsulated serotypes (0.45; 11%). Compared with 2002–2008, the estimated incidence of invasive H. influenzae disease increased by 16%, driven by increases in disease caused by serotype a and nontypeable strains.
Conclusions.
Invasive H. influenzae disease has increased, particularly due to nontypeable strains and serotype a. A considerable burden of invasive H. influenzae disease affects the oldest and youngest age groups, particularly AI/AN children. These data can inform prevention strategies, including vaccine development.
Keywords: Haemophilus influenzae, invasive disease, surveillance, epidemiology
Invasive Haemophilus influenzae is an important cause of morbidity and mortality in young children, older adults, and those with certain underlying medical conditions [1–3]. In the United States, after the introduction of H. influenzae serotype b (Hib) polysaccharide vaccine in 1985 and Hib conjugate vaccines during 1987–1990, the incidence of invasive H. influenzae disease among children aged <5 years decreased by >99% [4–7]. Since 2001, the estimated annual incidence of invasive Hib disease has remained below the Healthy People 2020 goal of 0.27 cases per 100 000 children aged <5 years [8, 9].
With the dramatic reduction of Hib disease, the epidemiology of H. influenzae infections changed; recent reports describe an increase in H. influenzae disease caused by non-b serotypes [1, 10–12] and nontypeable strains (nonencapsulated or no serotype identified by available methods) [13]. However, the greatest burden of disease continues to occur in the youngest infants and older adults [1, 2, 14].
Surveillance is essential to monitor the burden and shifts in invasive H. influenzae disease and develop targeted public health prevention strategies. We analyzed data from active population- and laboratory-based surveillance during 2009–2015 to describe the current epidemiology of invasive H. influenzae disease in the United States.
METHODS
Surveillance
Active population- and laboratory-based surveillance for invasive H. influenzae disease was conducted as part of Active Bacterial Core surveillance (ABCs). ABC surveillance is supported by the Centers for Disease Control and Prevention (CDC) as part of the Emerging Infections Program Network [15]. Data from 1 January 2009 through 31 December 2015 were included in this analysis.
The surveillance areas included California (3 San Francisco Bay–area counties, 2009–2015), Colorado (5 Denver-area counties, 2009–2015), Connecticut (statewide, 2009–2015), Georgia (20 Atlanta-area counties, 2009; statewide, 2010–2015), Maryland (statewide, 2009–2015), Minnesota (statewide, 2009–2015), New Mexico (statewide, 2009–2015), New York (15 Rochester- and Albany-area counties, 2009–2015), Oregon (statewide, 2009–2015), and Tennessee (11 counties, 2009; 20 counties, 2010–2015). The population under surveillance ranged in number from 36 748 349 in 2009 to 43 912 997 in 2015, representing 12.0% of the US population in 2009 and 13.7% in 2015 [16].
A case was defined as isolation of H. influenzae from a normally sterile site (eg, blood or cerebrospinal fluid [CSF]) in a surveillance-area resident. Epidemiologic and clinical information was abstracted from medical records. Outcome was based on patient status at hospital discharge. Infants with a gestational age ≤22 weeks were excluded from ABCs.
Cases of invasive H. influenzae disease were categorized as meningitis if a clinical diagnosis of meningitis was recorded in the medical record and H. influenzae was isolated from CSF or other sterile sites, as bacteremic pneumonia if pneumonia was recorded in the patient’s medical record and H. influenzae was isolated from blood or pleural fluid, and as isolated bacteremia if H. influenzae was isolated from blood with no localized clinical syndrome.
Laboratory Methods
State public health laboratories serotyped H. influenzae and sent isolates to the CDC’s Bacterial Meningitis Laboratory, where species was confirmed by Haemophilus quad identification plates, API Neisseria-Haemophilus strips, and real-time polymerase chain reaction (PCR), and serotype was confirmed by slide agglutination and PCR [17, 18]. If the serotype results were discordant, the CDC PCR result was used. If an isolate was nonviable upon arrival at CDC after being sent twice by the state, the serotype result from the state laboratory was used. Haemophilus influenzae isolates were classified by capsular serotype: serotypes a, b, c, d, e, and f and nontypeable.
Statistical Analysis
Race was categorized as white, black, American Indian and Alaska Native (AI/AN), or Asian/Pacific Islander. Patients with unknown race were distributed based upon the known racial distribution in each ABCs site and age group. Case-fatality ratios were calculated using the proportion of cases with known outcomes as the denominator. Wilcoxon rank-sum tests were used to compare continuous variables, and Pearson’s χ2 test was used for categorical variables.
Incidence rates were reported as cases per 100 000 population and calculated using National Center for Health Statistics’ bridged-race postcensal population estimates [16] for the ABCs sites; nationwide estimates were calculated by directly standardizing to the age and race distribution of the US population. Incidence in infants aged <1 month was calculated using live birth estimates. The 95% confidence intervals (CIs) around the directly standardized rates were calculated using a method derived from the relationship between the Poisson distribution and the gamma distribution, whereas estimated age, race, and serotype-specific 95% CIs were calculated using exact CI for a Poisson random variable [19]. The time periods of 2002–2008 (a subset of data previously published by MacNeil et al [1]) versus 2009–2015 were compared to assess recent changes in incidence. Incidence trends over time were assessed using Cochrane-Armitage tests for trend. A negative binomial model with 95% CIs was used to estimate annual percentage changes in incidence from 2002–2015.
The CDC determined this surveillance to be public health nonresearch. At each ABCs site, it was deemed either a public health assessment or human subjects research, for which approval was granted by local institutional review boards.
RESULTS
General Haemophilus influenzae Epidemiology: 2009–2015
During 2009–2015, 4924 cases of invasive H. influenzae disease were reported from ABCs sites; 715 (14.5%) were fatal. The median annual incidence among ABCs sites was 1.69 cases per 100 000, with site-specific incidences ranging from 1.42 (California) to 2.31 (New Mexico). Nationwide, the estimated annual number of cases ranged from 5009 in 2009 to 6054 in 2015 (estimated annual mean, 5327), with an annual estimated national incidence of 1.70 cases per 100 000.
Estimated national invasive H. influenzae incidence was highest among children aged <1 year (8.45) and adults aged ≥65 years (6.30); case-fatality ratio was highest among adults aged ≥65 years (19.9%) (Table 1). Overall, 45.6% of patients were male, 68.6% were white, 16.8% were black, 2.4% were AI/AN, 2.7% were Asian/Pacific Islander, and 9.6% were of unknown race (Table 2). Median patient age was 64 years (range, 0–103 years). Haemophilus influenzae was isolated from blood in 93.1% of cases, CSF in 4.4%, pleural fluid in 1.4%, joint fluid in 1.3%, and peritoneal fluid in 1.0%. In 2.1% of cases, H. influenzae was isolated from both blood and CSF. Information on clinical syndrome was available for 91.4% of cases: 61.9% were categorized as bacteremic pneumonia, 26.3% as bacteremia, and 7.0% as meningitis. The median age of patients with bacteremic pneumonia was 70 years (interquartile range [IQR], 57–82 y), whereas the median age was 53 years (IQR, 15–73 y) among patients with bacteremia and 31 years (IQR, 1–58 y) among those with meningitis (P < .0001).
Table 1. Annual Estimated Incidence and Case-Fatality Ratios Associated With Invasive Haemophilus influenzae Disease, by Age Group and Serotype—United States, 2009–2015.
| Serotype b | Non-b Serotypes | Nontypeable | Totala | |||||
|---|---|---|---|---|---|---|---|---|
| Age, y | Incidenceb (95% CI) | CFR, % | Incidenceb (95% CI) | CFR, % | Incidenceb (95% CI) | CFR, % | Incidenceb (95% CI) | CFR, % |
| <1 | 0.30 (.13–.50) | 10.0 | 2.53 (2.05–3.11) | 3.4 | 5.63 (4.92–6.49) | 9.7 | 8.45 (7.55–9.46) | 8.2 |
| 1–4 | 0.08 (.04–.14) | 0 | 0.71 (.57–.85) | 2.0 | 0.68 (.55–.82) | 4.1 | 1.47 (1.27–1.66) | 3.5 |
| 5–17 | 0.02 (.01–.03) | 0 | 0.09 (.07–.13) | 4.7 | 0.24 (.20–.29) | 3.8 | 0.35 (.30–.41) | 3.4 |
| 18–34 | 0.01 (.00–.02) | 0 | 0.08 (.06–.10) | 12.0 | 0.35 (.31–.40) | 9.1 | 0.44 (.39–.49) | 8.8 |
| 35–49 | 0.00 (.00–.01) | 0 | 0.23 (.19–.27) | 10.0 | 0.45 (.40–.51) | 7.0 | 0.68 (.62–.75) | 7.8 |
| 50–64 | 0.04 (.02–.06) | 0 | 0.67 (.59–.73) | 12.6 | 1.08 (1.00–1.18) | 13.7 | 1.78 (1.68–1.90) | 12.9 |
| >65 | 0.04 (.03–.08) | 11.8 | 1.27 (1.14–1.37) | 13.7 | 4.99 (4.78–5.23) | 21.3 | 6.30 (6.05–6.55) | 19.9 |
| Total | 0.03 (.02–.03) | 3.9 | 0.45 (.42–.47) | 10.8 | 1.22 (1.18–1.26) | 16.1 | 1.70 (1.65–1.74) | 14.5 |
Abbreviations: CFR, case-fatality ratio; CI, confidence interval.
Includes cases with an unknown serotype.
Cases per 100 000 persons per year.
Table 2. Epidemiologic and Clinical Characteristics of Patients With Invasive Haemophilus influenzae Disease, by Serotype—Active Bacterial Core Surveillance, 2009–2015.
| Characteristic | Serotype b | Non-b Serotypes | Nontypeable | Totala |
|---|---|---|---|---|
| Age, median (range) | 49 y (0–88) | 58 y (0–99) | 67 y (0–103) | 64 y (0–103) |
| Sex | ||||
| Male | 55.8% | 42.6% | 47.0% | 45.6% |
| Race | ||||
| White | 61.0% | 65.2% | 70.2% | 68.6% |
| Black | 13.0% | 19.7% | 15.7% | 16.8% |
| AI/AN | 15.6% | 4.6% | 1.4% | 2.4% |
| Asian/PI | 2.6% | 1.2% | 3.1% | 2.7% |
| Unknown | 7.8% | 9.4% | 9.6% | 9.6% |
| Ethnicity | ||||
| Hispanic/Latino | 6.5% | 7.9% | 67.1% | 7.2% |
| Non-Hispanic/Latino | 76.6% | 65.1% | 66.3% | 66.3% |
| Unknown | 16.9% | 27.0% | 26.6% | 26.5% |
| Clinical syndromeb | ||||
| Bacteremic pneumonia | 64.2% | 63.6% | 62.4% | 61.9% |
| Bacteremia | 10.4% | 17.6% | 29.5% | 26.3% |
| Meningitis | 16.4% | 12.0% | 5.4% | 7.0% |
| Other | 9.0% | 6.7% | 2.7% | 4.8% |
| Hospitalized | 97.4% | 93.8% | 93.3% | 92.8% |
| Duration of hospitalization, median (range) | 7 d (0–107) | 6 d (0–112) | 6 d (0–170) | 6 d (0–170) |
Abbreviations: AI/AN, American Indian and Alaska Natives; PI, Pacific Islander.
Includes cases with an unknown serotype.
Information on clinical syndrome was available for 4499 (91.4%) cases.
The majority (92.8%) of patients were hospitalized; median duration of hospitalization was 6 days (range, 0–170 d). Hospitalization varied by age; only 88.2% of children aged 1–4 years and 72.6% of children aged 5–17 years were hospitalized. The proportion of patients hospitalized also varied by syndrome—87.0% for bacteremia, 96.4% for bacteremic pneumonia, and 99.4% for meningitis (P < .0001)—as did duration of hospitalization—median of 6 days (range, 0–170 d) for bacteremia, 7 days (range, 0–156 d) for bacteremic pneumonia, and 9 days (range, 0–112 d) for meningitis (P < .0001).
Isolates were available for serotyping at the CDC or state health departments for 4368 (88.7%) cases. Of these, 3126 (71.6%) were nontypeable, 710 (16.3%) serotype f, 253 (5.8%) serotype a, 196 (4.5%) serotype e, 77 (1.8%) serotype b, and 6 (0.1%) serotype d (Figures 1 and 2). Nontypeable H. influenzae had the highest incidence (1.22) and case-fatality ratio (16.1%), as compared with Hib and non-b encapsulated serotypes. Among the non-b encapsulated serotypes, serotype a primarily affected children aged <5 years, serotype c was not detected, serotype d rarely caused disease but had the highest case-fatality ratio (50.0%), serotype e also had a high case-fatality ratio (18.4%), and serotype f had the highest overall incidence (0.27) (Table 3).
Figure 1.
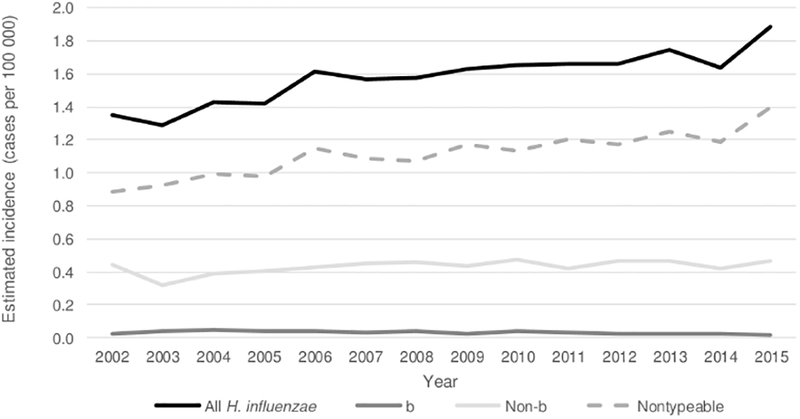
Trends in estimated incidence of invasive Haemophilus influenzae disease, by serotype—United States, 2002–2015. The 2002–2008 cases are a subset of data previously published by MacNeil et al [1]. Abbreviation: H. influenzae, Haemophilus influenzae.
Figure 2.
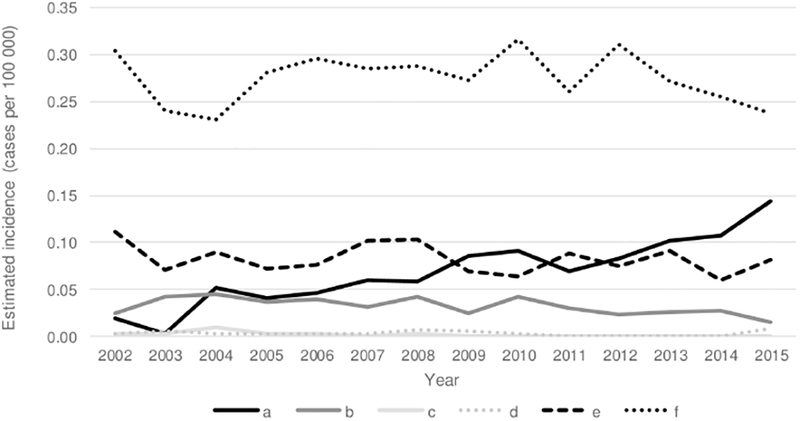
Trends in estimated incidence of invasive Haemophilus influenzae disease caused by non-b encapsulated serotypes—United States, 2002–2015. The 2002–2008 cases are a subset of data previously published by MacNeil et al [1].
Table 3. Annual Estimated Incidence and Case-Fatality Ratios Associated With Invasive Non-b Haemophilus influenzae Disease, by Age Group and Serotype—United States, 2009–2015.
| Serotype a | Serotype d | Serotype e | Serotype f | |||||
|---|---|---|---|---|---|---|---|---|
| Age, y | Incidencea (95% CI) | CFR, % | Incidencea (95% CI) | CFR, % | Incidencea (95% CI) | CFR, % | Incidencea (95% CI) | CFR, % |
| <1 | 1.52 (1.16–1.99) | 5.7 | 0.00 (.00–.11) | N/A | 0.17 (.07–.37) | 0 | 0.83 (.57–1.19) | 0 |
| 1–4 | 0.40 (.31–.51) | 0 | 0.00 (.00–.03) | N/A | 0.03 (.01–.07) | 0 | 0.28 (.20–.37) | 5.1 |
| 5–17 | 0.02 (.01–.04) | 0 | 0.00 (.00–.01) | N/A | 0.01 (.00–.02) | 0 | 0.06 (.05–.09) | 6.7 |
| 18–34 | 0.02 (.01–.03) | 8.3 | 0.00 (.00–.01) | N/A | 0.01 (.01–.03) | 44.4 | 0.05 (.03–.06) | 3.4 |
| 35–49 | 0.05 (.03–.07) | 16.7 | 0.00 (.00–.01) | 0 | 0.03 (.01–.04) | 7.1 | 0.16 (.12–.19) | 8.6 |
| 50–64 | 0.09 (.07–.12) | 10.4 | 0.00 (.00–.01) | 100.0 | 0.13 (.10–.16) | 20.0 | 0.45 (.39–.50) | 10.6 |
| >65 | 0.15 (.11–.19) | 16.0 | 0.01 (.00–.03) | 50.0 | 0.28 (.23–.34) | 19.1 | 0.83 (.73–.91) | 10.9 |
| Total | 0.10 (.08–.11) | 8.3 | 0.00 (.00–.00) | 50.0 | 0.08 (.07–.09) | 18.4 | 0.27 (.26–.29) | 9.3 |
No serotype c cases were reported in 2009–2015.
Abbreviations: CFR, case-fatality ratio; CI, confidence interval; N/A, not applicable.
Cases per 100 000 persons per year.
Haemophilus influenzae Serotype b Epidemiology
From 2009–2015, 77 Hib cases were reported to ABCs, with a median patient age of 49 years (range, 0–88 y; IQR, 4–62 y). Among the 23 (29.9%) Hib patients aged <5 years, 22 (95.7%) had available information on clinical syndrome: 9 (40.9%) had meningitis, 6 (27.3%) had bacteremic pneumonia, 3 (13.6%) had bacteremia, and 4 (18.2%) had other presentations. Two (8.7%) were too young to have received Hib vaccine, 6 (26.1%) were unvaccinated, and 10 (43.5%) were undervaccinated (n = 8/10 had received the 3-dose primary series but were missing a booster dose at 12–15 months). Five (21.7%) were age-appropriately vaccinated and had no reported underlying conditions; 2 were 3-month-old infants who had been age-eligible for only the first dose of Hib vaccine.
Of the 54 (70.1%) Hib patients aged ≥5 years, 9 (16.7%) were aged 5–17 years, 28 (51.9%) were aged 18–64 years, and 17 (31.5%) were aged ≥65 years. Information on clinical syndrome was available for 45 (83.3%): 37 (82.2%) had bacteremic pneumonia, 4 (8.9%) had bacteremia, and 2 (4.4%) each had meningitis or other presentations. Thirty-four (62.9%) had reported underlying conditions, including smoking, chronic obstructive pulmonary disease, diabetes, obesity, and athero-sclerotic cardiovascular disease.
Haemophilus influenzae Epidemiology in Children Aged <5 Years
During 2009–2015, 545 (11.1%) cases of invasive H. influenzae were reported in children aged <5 years, corresponding to an estimated national incidence of 2.84 cases per 100 000: 8.45 among infants aged <1 year and 1.47 among children aged 1–4 years (Table 1, Figure 3). The estimated incidence was 0.13 for Hib disease, 1.65 for nontypeable H. influenzae, and 1.06 for non-b serotypes (Figure 4). Compared with children with Hib disease or non-b serotypes, children with nontypeable H. influenzae were more likely to present with bacteremia than bacteremic pneumonia or meningitis (P < .0001).
Figure 3.
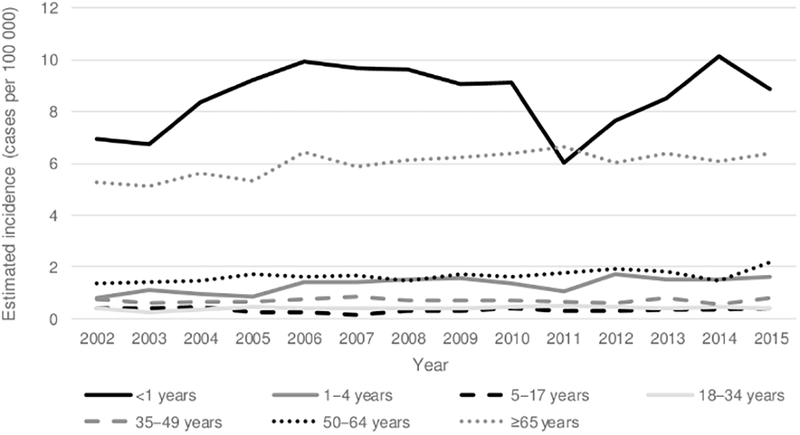
Trends in estimated incidence of invasive Haemophilus influenzae disease, by age group—United States, 2002–2015. The 2002–2008 cases are a subset of data previously published by MacNeil et al [1].
Figure 4.
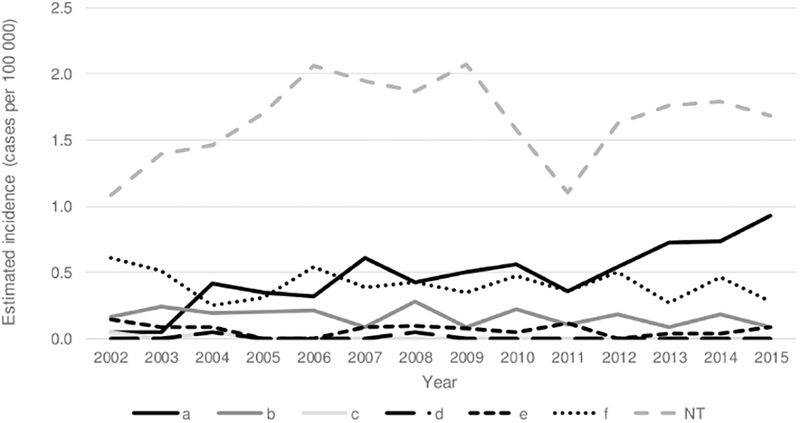
Trends in estimated incidence of invasive Haemophilus influenzae disease among children aged <5 years, by serotype—United States, 2002–2015. The 2002–2008 cases are a subset of data previously published by MacNeil et al [1]. Abbreviation: NT, nontypeable.
Of the 317 (6.4%) cases of invasive H. influenzae reported in children aged <1 year, 26 (8.2%) were fatal; clinical syndrome was categorized as bacteremia in 53.3%, meningitis in 25.1%, and bacteremic pneumonia in 15.9%. Most patients (92.7%) were hospitalized, for a median duration of 14 days (range, 0–170 d). Among the 294 (92.7%) cases in children aged <1 year with a known serotype, 196 (66.7%) were caused by nontypeable H. influenzae, 53 (18.0%) by serotype a, 29 (9.9%) by sero-type f, 10 (3.4%) by serotype b, and 6 (2.0%) by serotype e.
Of the 140 cases in infants aged <1 month (estimated national incidence, 45 per 100 000 live births), 18 (12.9%) died. Gestational age and birth weight were known for 133 (95.0%) infants: 32 (24.1%) were full-term, 22 (16.5%) were 34–36 weeks, 9 (6.8%) were 32–33 weeks, 38 (28.6%) were 28–31 weeks, and 32 (24.1%) were 22–28 weeks. The majority (n = 86; 63.7%) of infants aged <1 month were low birth weight (<2500g); 52 (38.5%) were very low birth weight (<1500g), and 26 (19.3%) were extremely low birth weight (<1000g). In 127 (90.7%) patients aged <1 month, disease onset occurred during the first week of life (<7 days), with the majority (n = 109; 77.9%) occurring at birth. Most patients aged <1 month (80.7%) had bacteremia, and 98.5% had nontypeable H. influenzae.
The burden of H. influenzae disease among AI/AN children is much greater than the among the general US population, with an estimated incidence of 15.19 per 100 000 among AI/AN children aged <5 years (5.8 times the incidence among all other races combined) and 34.36 among AI/AN infants aged <1 year (4.3 times the incidence among all other races combined). Non-b serotypes, mainly serotype a, caused the highest incidence of invasive disease in AI/AN children, whereas nontypeable H. influenzae was the primary cause of disease among other races (Table 4).
Table 4. Annual Estimated Incidence of Haemophilus influezae Disease, by Serotype and Race, Among Children Aged <5 years—United States, 2009–2015.
| Serotype b | Non-ba | Nontypeable | Totalb | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | White | Black | AI/AN | Asian/PI | White | Black | AI/AN | Asian/PI | White | Black | AI/AN | Asian/PI | White | Black | AI/AN | Asian/PI |
| Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
Incidencec (95% Cl) |
|
| <1 y | 0.23 (.06–.45) |
0.25 (.03–.90) |
3.88 (.82–11.61) |
0.00 (.00–1.55) |
1.98 (1.51–2.65) |
2.36 (1.42–3.68) |
19.76 (10.13–31.09) |
1.27 (.26–3.69) |
4.82 (4.08–5.83) |
6.70 (4.92–8.59) |
9.24 (2.91–17.29) |
4.60 (2.31–8.28) |
7.54 (6.70–8.89) |
10.42 (7.86–12.34) |
34.36 (21.42–48.85) |
5.87 (2.91–9.36) |
| 1 y | 0.04 (.00–.22) |
0.12 (.00–.69) |
1.30 (.03–7.27) |
0.00 (.00–1.49) |
0.78 (.50–1.25) |
2.45 (1.22–3.35) |
15.65 (9.98–30.63) |
0.00 (.00–1.49) |
0.58 (.36–1.01) |
2.21 (1.13–3.20) |
2.61 (.32–9.42) |
2.01 (.89–5.27) |
1.48 (1.08–2.07) |
4.68 (3.00–5.99) |
22.16 (13.91–37.10) |
2.01 (.89–5.27) |
| 2–4 y | 0.06 (.02–.15) |
0.04 (.00–.23) |
1.69 (.46–4.33) |
0.00 (.00–.48) |
0.27 (.17–41) |
0.77 (.47–1.21) |
3.37 (1.19–6.10) |
0.39 (.03–95) |
0.60 (.41–75) |
0.41 (.22–80) |
0.44 (.01–2.36) |
0.26 (.03–.95) |
1.02 (.78–1.24) |
1.35 (1.00–1.99) |
6.37 (2.93–9.41) |
0.65 (.21–1.53) |
| <5 y | 0.09 (.04–.15) |
0.10 (.03–.25) |
2.05 (.89–4.06) |
0.00 (.00–.30) |
0.71 (.58–88) |
1.43 (1.02–1.76) |
9.14 (6.28–12.53) |
0.49 (.13–94) |
1.43 (1.23–1.65) |
2.03 (1.56–2.45) |
2.65 (1.06–4.40) |
1.46 (.92–2.38) |
2.40 (2.17–2.71) |
3.83 (3.12–4.33) |
15.19 (10.89–18.72) |
1.94 (1.23–2.86) |
Abbreviations: AI/AN, American Indian and Alaska Natives; CI, confidence interval; PI, Pacific Islander.
Estimated incidence of serotype a cases in AI/AN children: aged <1 year (16.94 per 100000); aged 1 year (14.44 per 100000); aged 2–4 years (3.37 per 100000);
Includes cases with unknown serotype.
Cases per 100000 persons per year.
Haemophilus influenzae Epidemiology in Adults Aged ≥65 Years
Among adults aged ≥65 years, the risk of disease increased with increasing age: incidence was 3.48 per 100 000 adults aged 65–69 years; 4.65 among persons aged 70–74 years, 6.48 among persons aged 75–79 years, 8.56 among persons aged 80–84 years, and 13.56 among persons aged ≥85 years. Most strains in persons aged ≥65 years were nontypeable (79.3%) or non-b serotypes (19.9%; serotype f most common); only 0.8% were Hib. Of the 2416 (49.1%) cases of invasive H. influenzae reported in adults aged ≥65 years, 19.9% were fatal; 73.4% were categorized as bacteremic pneumonia, 22.4% as bacteremia, and 2.1% as meningitis; and 95.5% were hospitalized. At least 1 underlying condition was present in 74% of patients aged ≥65 years, with ≥2 conditions present in 47%. The most frequently reported underlying conditions were chronic obstructive pulmonary disease, atherosclerotic cardiovascular disease, diabetes, and chronic heart failure.
Temporal Changes in Haemophilus influenzae Incidence: 2002–2008 Versus 2009–2015
From 2002–2015, 8482 cases of H. influenzae disease were reported from ABCs sites; during both 2002–2008 and 2009–2015, 88%–89% had isolates available for serotyping. Between these 2 time periods, the estimated incidence of H. influenzae disease increased by 16%, from 1.47 to 1.70 cases per 100 000 (Table 5). The increase was primarily due to increases in disease caused by serotype a (148% increase) and nontypeable strains (21% increase). From 2002 to 2015, overall incidence of invasive H. influenzae increased by 2% annually. Incidence of nontypeable disease increased by 3% annually, incidence of serotype a disease increased by 13% annually, and incidence of all other non-b serotypes remained stable or decreased.
Table 5. Change in Annual Estimated Incidence of Haemophilus influenzae Disease Serotypes—United States, 2002–2015.
| Serotype | 2002–2008 average annual incidencea,b | 2009–2015 average annual incidencea | Percent change in incidence (2002–2008b vs. 2009–2015) | 2002–2015 annual percent change in incidence (95% CI) |
|---|---|---|---|---|
| Hib | 0.04 | 0.03 | −25% | −4% (−5% to −3%) |
| Non-b | 0.41 | 0.45 | 10% | 1% (1%–2%) |
| a | 0.04 | 0.10 | 148% | 13% (12%–15%) |
| c | 0.004 | 0 | −100% | −27% (−33% to −21%) |
| d | 0.004 | 0.002 | −45% | −5% (−9% to 0%) |
| e | 0.09 | 0.08 | −15% | −1% (−2% to −1%) |
| f | 0.27 | 0.27 | 0% | 0% (−1% to 0%) |
| Nontypeable | 1.01 | 1.22 | 21% | 3% (3%–3%) |
| Totalc | 1.47 | 1.70 | 16% | 2% (2%–2%) |
Abbreviations: CI, confidence interval
Cases per 100 000 persons per year.
The 2002–2008 cases are a subset of data previously published by MacNeil et al [1].
Includes cases with unknown serotype.
Among children aged <1 year, H. influenzae incidence increased from 6.92 per 100 000 in 2002 to 8.87 in 2015 (22% increase), mainly due to increases in disease caused by sero-type a and nontypeable strains. Among adults aged ≥65 years, H. influenzae incidence increased by 18%, mostly due to an increase in disease due to nontypeable strains. Between 2002–2008 and 2009–2015, the median age of patients increased slightly from 61 years to 64 years (P < .05).
DISCUSSION
Although some aspects of invasive H. influenzae disease epidemiology have remained consistent throughout the post-vaccine era, some recent changes have been observed. A considerable burden of H. influenzae continues to affect the oldest and youngest age groups, and AI/AN populations are disproportionately affected. Many cases in children aged <1 year occur during the first few days of life, suggesting peri-natal transmission. These cases often occur in preterm or low-birth-weight infants and are predominately due to nontypeable strains [1, 20, 21]; although the majority of these cases were due to H. influenzae as confirmed by PCR targeting the hpd gene, some may be due to Haemophilus quentini [22]. Consistent with other reports of H. influenzae epidemiology post-Hib implementation [13, 21, 23], disease due to nontypeable H. influenzae currently has the highest incidence (1.22 cases per 100 000) and case-fatality ratio (16.1%) compared with disease caused by Hib and non-b serotypes. Haemophilus influenzae serotype b disease incidence remains low, and most Hib disease occurs in adults. Most Hib cases in children aged <5 years are in unvaccinated or undervaccinated children and are therefore preventable. Of the non-b serotypes, serotype f remains the most common cause of invasive H. influenzae disease.
The AI/AN populations have the highest burden of invasive H. influenzae disease, especially among children. An estimated 10% of disease among children aged <5 years occurred in AI/AN children, who accounted for only 1.9% of children aged <5 years in the United States in 2015. Historically, AI/AN children have been at increased risk for Hib disease, a disparity that may be attributable to adverse living conditions (eg, household crowding, poverty, and poor indoor air quality) disproportionally experienced by many AI/AN children [11, 24]. Despite the decline in overall Hib incidence, AI/AN children continue to be at higher risk, with 23 times the incidence of Hib compared with all other races. American Indian/Alaska Native children are also disproportionately affected by non-b disease, with 11 times the incidence among all other races, primarily due to serotype a. These data are consistent with other reports of increases in serotype a disease in the post-Hib era, particularly among North American indigenous populations [10, 25–28]. This increase is concerning because serotype a disease has been reported to be similar to Hib disease in clinical presentation and severity [27–29], possibly due to capsule similarities [29, 30].
This description of current invasive H. influenzae disease burden may help guide prevention measures, such as new vaccine development and policy. Given the increases in nontypeable H. influenzae in the post-Hib era, a vaccine targeting nontypeable H. influenzae could have substantial impact on invasive disease burden. Exploration of potential nontypeable vaccine candidates is ongoing, although antigenic heterogeneity complicates vaccine development [31, 32]. Nontypeable H. influenzae protein D is a carrier protein in a 10-valent pneumococcal conjugate vaccine (Synflorix, GlaxoSmithKline Biologicals) used in some countries, although vaccine impact on nasopharyngeal carriage of nontypeable H. influenzae is unclear [33, 34], and impact on invasive H. influenzae disease has not been evaluated [32]. Monovalent serotype a vaccines are also in early development [35]; similarities between serotype a and Hib capsules, clinical presentation, and infection immunology suggest that a bivalent serotype a–Hib conjugate vaccine could offer protection against carriage and disease [29]. Because serotype f causes the highest incidence of disease among the non-b serotypes, a vaccine targeting serotype f could be considered as well.
Following the introduction of Hib vaccines, invasive H. influenzae disease incidence dramatically decreased from an estimated 100 cases per 100 000 children aged <5 years in the prevaccine era to 2.84 cases per 100 000 in the present report. However, a substantial burden of non-b and nontypeable disease in the youngest and oldest age groups remains and is increasing, with mortality similar to or higher than that historically seen with Hib disease. In particular, serotype a incidence more than doubled from 2002–2008 to 2009–2015, with the highest burden among young children and AI/AN populations. Further investigation into the serotype a disease increase and a better understanding of perinatal transmission of nontypeable disease to neonates [20] in the United States are needed. Focusing interventions in populations at increased risk (ie, AI/AN children, neonates) should be a key consideration for H. influenzae disease prevention strategies such as vaccines or chemoprophylaxis. These data indicate a need for new vaccines to continue the momentum toward decreasing invasive H. influenzae disease burden.
Acknowledgments.
The authors are grateful to the following individuals for their contributions to the establishment and maintenance of the ABCs system. California Emerging Infections Program: Susan Brooks and Hallie Randel; Colorado Emerging Infections Program: Benjamin White, Deborah Aragon, Meghan Barnes, and Jennifer Sadlowski; Connecticut Emerging Infections Program: Matt Cartter, Carmen Marquez, and Michelle Wilson; Georgia Emerging Infections Program: Stephanie Thomas, Amy Tunali, and Wendy Baughman; Maryland Emerging Infections Program: Joanne Benton, Terresa Carter, Rosemary Hollick, Kim Holmes, and Andrea Riner; Minnesota Emerging Infections Program: Kathryn Como-Sabetti, Lori Triden, Corinne Holtzman, Richard Danila, and Kerry MacInnes; New Mexico Emerging Infections Program: Kathy Angeles, Joseph Bareta, Lisa Butler, Sarah Khanlian, Robert Mansmann, and Megin Nichols; New York Emerging Infections Program: Kari Burlaff, Suzanne McGuire, Glenda Smith, and Nancy Spina; Oregon Emerging Infections Program: Mark Schmidt, Jamie Thompson, and Tasha Poissant; Tennessee Emerging Infections Program: Brenda Barnes, Karen Leib, Katie Dyer, and Lura McKnight; Centers for Disease Control and Prevention (CDC): Karrie-Ann Toews, Emily Weston, Londell McGlone, Gayle Langley, Melissa Arvay, Olivia Almendares, Huong Pham, and the Bacterial Meningitis Laboratory.
Financial support. This work was supported by a cooperative agreement with the Emerging Infections Program of the CDC (CDC-RFA-CK12–120205CONT16).
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest. R. L. coedited a book on infectious disease surveillance and donated received royalties to the Minnesota Department of Health. W. S. has served as a consultant and member of a data safety monitoring board for various pharmaceutical companies. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.MacNeil JR, Cohn AC, Farley M, et al. Current epidemiology and trends in invasive Haemophilus influenzae disease—United States, 1989–2008. Clin Infect Dis 2011; 53:1230–6. [DOI] [PubMed] [Google Scholar]
- 2.Blain A, MacNeil J, Wang X, et al. Invasive Haemophilus influenzae disease in adults ≥65 years, United States, 2011. Open Forum Infect Dis 2014; 1:ofu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briere EC, Jackson M, Shah SG, et al. Haemophilus influenzae type b disease and vaccine booster dose deferral, United States, 1998–2009. Pediatrics 2012; 130:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams WG, Deaver KA, Cochi SL, et al. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA 1993; 269:221–6. [PubMed] [Google Scholar]
- 5.Bisgard KM, Kao A, Leake J, Strebel PM, Perkins BA, Wharton M. Haemophilus influenzae invasive disease in the United States, 1994–1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis 1998; 4:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children—United States, 1998–2000. MMWR Morb Mortal Wkly Rep 2002; 51:234–7. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1987–1995. MMWR Morb Mortal Wkly Rep 1996; 45:901–6. [PubMed] [Google Scholar]
- 8.Healthy People 2020. Immunization and Infectious Diseases. Available at: www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 28, 2015.
- 9.Centers for Disease Control and Prevention. Healthy People 2020 Objectives Related to ABCs Pathogens. Available at: http://www.cdc.gov/abcs/reports-findings/healthy-people-2020.html. Accessed October 28, 2015.
- 10.Bender JM, Cox CM, Mottice S, et al. Invasive Haemophilus influenzae disease in Utah children: an 11-year population-based study in the era of conjugate vaccine. Clin Infect Dis 2010; 50:e41–6. [DOI] [PubMed] [Google Scholar]
- 11.Tsang RS, Bruce MG, Lem M, Barreto L, Ulanova M. A review of invasive Haemophilus influenzae disease in the Indigenous populations of North America. Epidemiol Infect 2014; 142:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urwin G, Krohn JA, Deaver-Robinson K, Wenger JD, Farley MM. Invasive disease due to Haemophilus influenzae serotype f: clinical and epidemiologic characteristics in the H. influenzae serotype b vaccine era. The Haemophilus influenzae Study Group. Clin Infect Dis 1996; 22:1069–76. [DOI] [PubMed] [Google Scholar]
- 13.Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis 2014; 14:1281–92. [DOI] [PubMed] [Google Scholar]
- 14.Livorsi DJ, Macneil JR, Cohn AC, et al. Invasive Haemophilus influenzae in the United States, 1999–2008: epidemiology and outcomes. J Infect 2012; 65:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langley G, Schaffner W, Farley MM, et al. Twenty years of Active Bacterial Core surveillance. Emerg Infect Dis 2015; 21:1520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. Bridged-Race Population Estimates, United States. Available at: http://wonder.cdc.gov/bridged-race-v2015.html. Accessed December 15, 2017.
- 17.Wang X, Mair R, Hatcher C, et al. Detection of bacterial pathogens in Mongolia meningitis surveillance with a new real-time PCR assay to detect Haemophilus influenzae. Int J Med Microbiol 2011; 301:303–9. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. 2nd ed. Geneva, Switzerland: World Health Organization, 2011. [Google Scholar]
- 19.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 1997; 16:791–801. [DOI] [PubMed] [Google Scholar]
- 20.Collins S, Litt DJ, Flynn S, Ramsay ME, Slack MP, Ladhani SN. Neonatal invasive Haemophilus influenzae disease in England and Wales: epidemiology, clinical characteristics, and outcome. Clin Infect Dis 2015; 60:1786–92. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker R, Economopoulou A, Dias JG, et al. Epidemiology of invasive Haemophilus influenzae disease, Europe, 2007–2014. Emerg Infect Dis 2017; 23:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theodore MJ, Anderson RD, Wang X, et al. Evaluation of new biomarker genes for differentiating Haemophilus influenzae from Haemophilus haemolyticus. J Clin Microbiol 2012; 50:1422–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Wessel K, Rodenburg GD, Veenhoven RH, Spanjaard L, van der Ende A, Sanders EA. Nontypeable Haemophilus influenzae invasive disease in the Netherlands: a retrospective surveillance study 2001–2008. Clin Infect Dis 2011; 53:e1–7. [DOI] [PubMed] [Google Scholar]
- 24.Singleton R, Holve S, Groom A, et al. Impact of immunizations on the disease burden of American Indian and Alaska Native children. Arch Pediatr Adolesc Med 2009; 163:446–53. [DOI] [PubMed] [Google Scholar]
- 25.Bruce MG, Deeks SL, Zulz T, et al. Epidemiology of Haemophilus influenzae sero-type a, North American Arctic, 2000–2005. Emerg Infect Dis 2008; 14:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McConnell A, Tan B, Scheifele D, et al. Invasive infections caused by Haemophilus influenzae serotypes in twelve Canadian IMPACT centers, 1996–2001. Ped Infect Dis J 2007; 26:1025–31. [DOI] [PubMed] [Google Scholar]
- 27.Bruce MG, Zulz T, DeByle C, et al. Haemophilus influenzae serotype a invasive disease, Alaska, USA, 1983–2011. Emerg Infect Dis 2013; 19:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plumb ID, Lecy D, Singleton R, et al. Invasive Haemophilus influenzae serotype a infection in children: clinical description of an emerging pathogen—Alaska, 2002–2014. Pediatr Infect Dis J 2018; 37:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulanova M, Tsang RSW. Haemophilus influenzae serotype a as a cause of serious invasive infections. Lancet Infect Dis 2014; 14:70–82. [DOI] [PubMed] [Google Scholar]
- 30.Kapogiannis BG, Satola S, Keyserling HL, Farley MM. Invasive infections with Haemophilus influenzae serotype a containing an IS1016-bexA partial deletion: possible association with virulence. Clin Infect Dis 2005; 41:e97–103. [DOI] [PubMed] [Google Scholar]
- 31.Murphy TF. Vaccines for nontypeable Haemophilus influenzae: the future is now. Clin Vaccine Immunol 2015; 22:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerquetti M, Giufrè M. Why we need a vaccine for non-typeable Haemophilus influenzae. Hum Vaccin Immunother 2016; 12:2357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammitt LL, Akech DO, Morpeth SC, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health 2014; 2:e397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandileone MC, Zanella RC, Almeida SCG, et al. ; Pneumococcal Carriage Study Group. Effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae among children in São Paulo, Brazil. Vaccine 2016; 34:5604–11. [DOI] [PubMed] [Google Scholar]
- 35.Barreto L, Cox AD, Ulanova M, Bruce MG, Tsang RSW. The emerging Haemophilus influenzae serotype a infection and a potential vaccine: implementation science in action. Can Commun Dis Rep 2017; 43:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


