Abstract
Zinc has long been the focus of many biological investigations because of its essential role in biology including a catalytic role in many enzymes, a structural role in the many zinc finger proteins, and a physiological role in many secretory cell processes. Divalent zinc is known to be highly abundant in healthy prostate tissues and lower in prostate cancer (PCa). Given the need for newer diagnostic methods for detection of prostate cancer, zinc-responsive probes of various types have been considered as imaging tools for detecting tissue levels of zinc. Among them, recent zinc-responsive MRI probes show great promise for non-invasive detection of zinc ion secretion from the prostate and other tissues in vivo. In this review, we summarize the need for new diagnostic tools and demonstrate how responsive zinc probes and MRI could satisfy this unmet need.
Keywords: Prostate Cancer, Zinc, Magnetic Resonance Imaging
1. Introduction
Prostate cancer (PCa) is the second most frequently diagnosed cancer and the fifth-leading cause of cancer death in men.[1] PCa development is diverse ranging from slowly growing, indolent intra-prostatic, and castration-resistant PCa (CRPC) that is readily metastasized to other tissues.[2] Ideally, the treatment and prevention of PCa should begin during the early stages of the cancer development. For indolent intra-prostatic PCa, for example, androgen deprivation therapy (ADT) proved to be efficient for the initial assessments. However, 30% of patients eventually experience recurrence of castration-resistant PCa (CRPC) which is then resistant to ADT.[3] Currently, preventative screening for PCa relies on a digital rectal exam (DRE) and a measurement of plasma levels of prostate-specific antigen (PSA). When PSA levels appear abnormally high, a transrectal ultrasound (TRUS) is often ordered. The limitation of using DRE and TRUS screening is the lack of sensitivity and specificity. PSA screening exhibits false-positive rates as high as 75% according to the National Cancer Institute,[4] and this results in over-diagnosis and over-treatment of PCa.[5] As a result, PSA screening provides only a small benefit according to the United States Preventive Services Task Force (USPSTF). Current estimates show that ~25% of men with elevated PSA (2–10 μg/L) results in biopsy-confirmed PCa.[6] At the same time, a biopsy can also provide high rates of false negative results.[7] This lack of specificity in the current screening paradigm increases our overall healthcare financial burden. Hence, identifying new biomarkers of PCa with high specificity is critical to developing personalized therapeutic strategies. Currently, biomarker research for PCa is focused on detecting abnormalities in serum, urine, and tissue samples with three main primary goals: (1) avoiding unnecessary biopsies, (2) reducing the use of interventional strategies, and (3) improving stratification of low-grade tumors. Ultimately, an optimal serum or urine biomarker assay could aid in the detection of micro-metastases that are below the detection limit of traditional imaging techniques. Due to the heterogeneous nature of PCa, developing an effective clinical marker for disease diagnostics and therapeutic evaluation is a huge challenge. Several recent reports of PCa-specific bioassays include the Prostate Cancer Antigen 3 (PCA3) score,[8] the [-2]proPSA (p2PSA) ratio test and Prostate Health Index (phi),[9] and the Four-kallikrein (4 K) panel[10] may prove at some point to improve diagnostic specificity. Further details of such assays can be found in recent reviews.[11] An alternative target that was identified over half a century ago is the tissue concentration of zinc. In healthy prostate, high concentrations of zinc ions (100–300 μg/g wet weight) have been detected in prostatic glands, 3–10 times greater than other soft tissues (Table 1).[2,12] Zinc levels decrease dramatically during the development of PCa, even at an early stage[13] so if one could detect such changes by using some non-invasive imaging marker, this could result in a new type of imaging biomarker. In this review, we highlight the potential of targeting zinc as a useful imaging biomarker for monitoring PCa progression and guide the reader through various multidisciplinary concepts of cancer biology, ex vivo and in vivo imaging, and rational probe design.
Table 1.
Zinc levels in prostate tissue.
| Tissue | Zinc |
|---|---|
| Normal (mixed tissue) | 209 |
| Normal (central zone) | 121 |
| Normal (peripheral zone) | 295 |
| BPH | 589 |
| PCa (mixed tissue) | 55 |
| PCa (malignant) | n.d. |
| Other tissue | 30 |
Zinc values are μg/g wet weight. Data adapted from LC Costello et al.[12]
2. The Role of Zinc in Prostate Epithelial Cells
As a ubiquitous element in biology, zinc is well-known for its structural (e.g., zinc-finger proteins) and catalytic (e.g., hydrolytic enzymes) roles in more than 3,000 metalloproteins.[14] Recently, more attention has been paid to dynamic changes in the pool size of loosely-bound zinc (free, labile, chelatable or histochemically active zinc) in the hippocampus and olfactory bulb of the brain,[15] the pancreas,[16] and the prostate.[17] In the prostate, zinc contributes to key functions in prostatic epithelial cells as (1) an inhibitor of mitochondrial aconitase[17–18] and (2) a regulator of apoptosis.[19] The blockage of m-aconitase activity by zinc results in high accumulation of intracellular citrate[20]. The citrate secretory function of prostatic epithelial cells is prominent especially in the peripheral zone of the prostate (contributing ~70% of the total prostate volume). As a result, epithelial cells in the peripheral zone produce, store, and secrete large amounts of citrate and zinc into the prostatic fluid.[21] Figure 1 summarizes the role of intracellular zinc in modulating Krebs cycle flux in healthy versus cancerous prostate cells. Besides, zinc prevents the abnormal growth of normal prostatic epithelial cells by triggering Bax-induced pore formation, promoting cytochrome c release and mitochondrial apoptogenesis.[19] The dramatic decrease in zinc levels in PCa cells (Table 1) especially in the peripheral zone (~80% of PCa occurs in the peripheral zone[20]) reflects down-regulation of the zinc influx transporter, ZIP1.[22] Consequently, both TCA cycle flux and ATP production increase in PCa, the zinc-induced apoptotic effect is minimized, and the proliferation rate increases in comparison to non-cancerous epithelial cells.[23] Citrate levels have also been suggested as a potential biomarker for PCa aggressiveness.[24]
Figure 1.
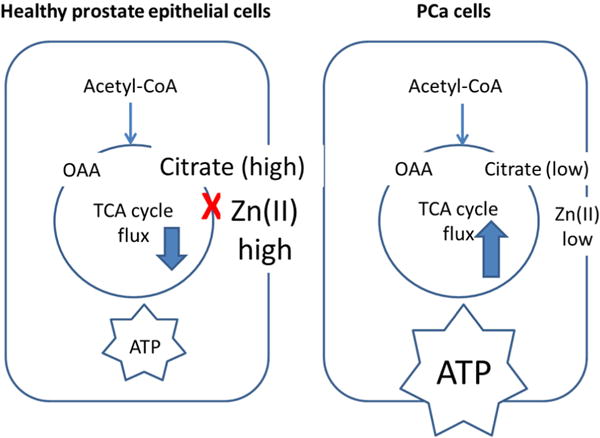
Schematic of metabolic changes known to occur in healthy (left) versus PCa (right) cells. Healthy prostatic epithelial cells accumulate high levels of citrate due to the zinc-dependent inhibition of m-aconitase. This results in reduced ATP production and reduced TCA cycle flux. In PCa, lower intracellular zinc due to the down-regulation of ZIP1 results in higher TCA cycle flux and ATP production rates.
3. Zinc Detection in Prostate
3.1 Ex vivo Detection of Zinc in Prostate
Given the vital role that zinc plays in PCa tumorigenesis, it is reasonable to consider zinc levels as a biomarker of disease progression. Several ex vivo zinc detection techniques including autometallographical (AMG) methods,[25] X-ray fluorescence (XRF) methods,[26] and inductively coupled plasma atomic spectrometry (ICP-MS)[27] have been used to locate or quantify the zinc distribution in normal and malignant prostate tissues.
3.1.1 Autometallographical (AMG) Method
The autometallographic (AMG) approach for detecting prostate zinc requires delivery of a buffered sodium-sulfide solution either to an animal or to post-mortem tissues.[25] This staining method, also called Timm’s sulfide silver staining method,[28] is not accurate for zinc because it also detects other metal ions including gold, mercury, lead, silver, copper, and iron. A zinc-specific staining method was not available until Danscher performed in situ blockage of zinc by injecting dithizone prior to Na2S perfusion and progressively modified the staining procedure to see whether all staining was blocked by in situ chelation (neo-Timm’s method).[29] The modification requires carefully timed perfusion of Na2S at a maximum concentration of 0.1% and the ZnS crystals that form are fixed using silver lactate to avoid the nonspecific silver deposits in tissue. Later, Danscher’s newer silver amplification method, the selenium method, also showed zinc specificity.[30] This general approach was used to detect zinc in the prostate by use of a light microscope (Figure 2). As a control, the tissue is presented with a zinc ion-chelating agent such as dithizone or diethyldithiocarbamate (DEDTC) (Figure 2B) to prevent ZnS staining. This procedure shows that the AMGZnS method provides a highly specific histochemical method for localizing zinc in an ex vivo setting. This method is not easily translated to an in vivo imaging method since the sample needs to be immersed in a sodium-sulfide solution.
Figure 2.
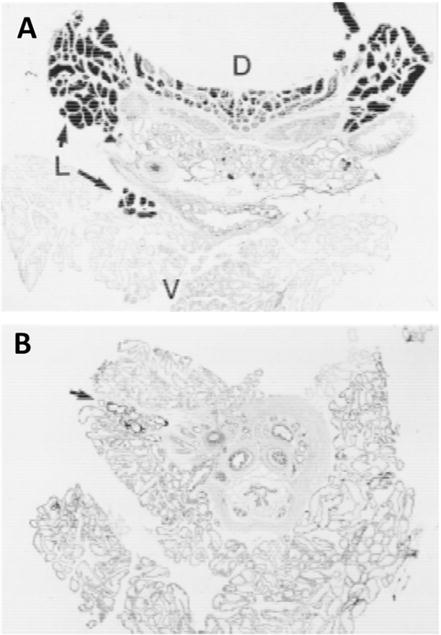
Autometallographic (AMG) imaging of a ZnS-developed cryosection of prostate tissue (30 μm). (A) Prostate from a rat without pretreatment with the zinc chelating agent, DEDTC. A heterogeneous distribution of ZnS was observed in the ventral (V), lateral (L) and dorsal (D) lobes. (B) Prostate from a rat pretreated with DEDTC. This resulted in less development of ZnS. The arrow points to small ZnS crystals remaining in lateral lobe. Reproduced with permission from Wiley-Liss, Inc.[25]
3.1.2 X-ray Fluorescence Method
Although AMG shows the zinc present in situ in histological samples, it cannot provide quantitative zinc information at single-cell level. Synchrotron radiation X-ray fluorescence (SRXRF) uses a tunable energy beam that excites electron scattering from elements in situ and the scattering energies are used as a fingerprint for element identification.[26c,31] This method also shows that zinc levels are somewhat lower in PCa tissue compared to healthy tissue as shown in Figure 3.
Figure 3.
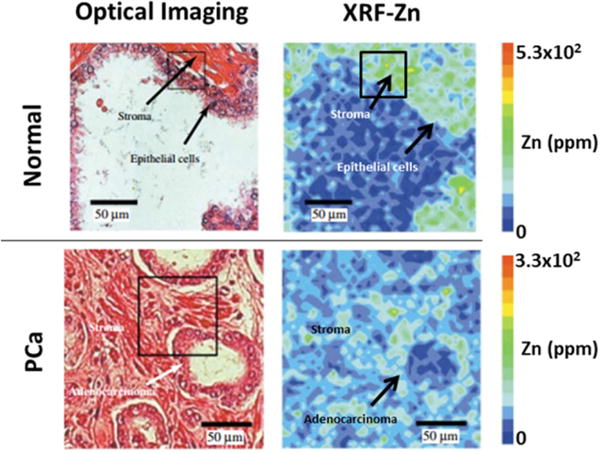
XRF imaging of the tissue distribution of zinc in normal and PCa tissues. Optical imaging (left) shows the tissue morphology measurement area by hematoxylin and eosin stainning (H/E) between normal and PCa tissues. XRF imaging of zinc (right) with color scale of 0–5.3 × 102 ppm (top) and 0–3.3 × 102 ppm (bottom). Reproduced with permission from Wiley-Liss, Inc. [35]
Currently, prostate cancer aggressiveness can only be determined by histopathology, and it involves evaluation of needle biopsy samples by an experienced pathologist to categorize tissue using a Gleason scoring system. Briefly, samples are classified into five grading tiers (1–5) that range from well-differentiated to poorly differentiated tissue.[32] The overall Gleason score may range from 2–10, where each score is the sum of the primary and secondary dominant grades in a sample (e.g. 3+4, primary+secondary=7). These scores correspond to a prognostic scale where a higher score corresponds to more aggressive prostate cancer.[33] Cortesi and colleagues measured zinc levels by XRF on tissue samples collected from needle biopsies. The data showed that depletion of zinc strongly correlated with an increased Gleason score (Figure 4).[26c,31] Unfortunately, needle biopsies are invasive and may increase the risk for prostatitis or sepsis.[34] There are also concerns about seeding cancer cells during the biopsy collection procedure. Despite these risks, these XRF data show the potential of developing a zinc-based imaging method to follow PCa progression.
Figure 4.
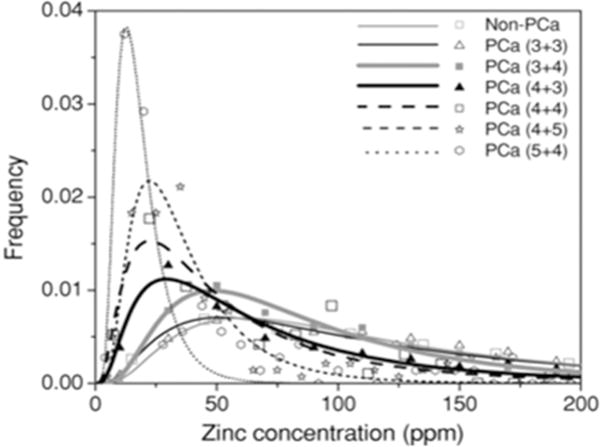
Distribution of zinc concentration in non-PCa and in PCa tissues with low to high Gleason scores. Zinc concentration was measured by X-ray fluorescence of the biopsy samples. Reproduced with permission from IOP publishing [31]
While these methods can provide accurate localization in tissue and sensitive quantitative measurements of zinc and other elements, they can only be performed on tissue biopsies or tissue collected during radical prostatectomy. These data illustrate that a non-destructive, non-invasive method for detecting zinc levels in vivo will be required to capitalize on differential zinc as a biomarker of prostatic disease state.
3.2 In Vivo Detection of Zinc in Prostate
Several agents have been reported for selective detection of zinc in vitro but only a few have been used in vivo. The most notable examples have been probes that detect zinc and insulin co-secretion from pancreatic β-cells in response to an increase in plasma glucose,[36] a cell-permeable porphyrin-based MR probe that highlights zinc-rich tissues such as the hippocampus in mouse brain,[37] and probes that detect zinc secretion from the prostate.[38]
3.2.1 In Vivo Zinc-targeted Optical Imaging
In a recent report,[41] a new cell-permeable fluorescent sensor (ZPP1) that becomes highly fluorescent (~500 nm excitation and ~540 nm emission) only after binding of two Zn(II) ions (Figure 5A) has been used to longitudinally monitor the zinc content of tissues in vivo by optical imaging. ZPP1 has been used to detect zinc levels ex vivo in urine samples of PCa patients[42] and in vivo using whole-body intravital fluorescence confocal imaging of the mouse prostate.[40] The in vivo feasibility of ZPP1 as a zinc optical sensor was performed in healthy C57Bl6 mice and a clear whole-body optical imaging signal was observed in the mouse prostate after intravenous injection of ZPP1. Conversely, no signal was observed after co-injection of ZPP1 and a second zinc chelator, tris[(2-pyridyl)methyl]amine (TPA). These promising results led to further investigations of zinc levels in the prostate of a transgenic adenocarcinoma of the mouse prostate model (TRAMP). TRAMP hemizygotes exhibit prostatic intraepithelial neoplasia (PIN) as early as 10 weeks of age, and well-differentiated adenocarcinoma by 18–24 weeks of age.[43] This animal model was deemed histopathologically relevant as it develops progressive forms of prostate cancer comparable to that seen in the human. Longitudinal optical imaging was conducted on TRAMP mice and C57BL/6J mice as controls (Figure 5B). A significant reduction in fluorescence signal intensity was observed in TRAMP mice as early as 19 weeks (Figure 5C) and a strong correlation to disease state was confirmed by histology and fluorescence microscopy (Figure 5D). The progressive glandular disorganization was studied by histological examinations, as well as the ZPP1-Zn2 fluorescence loss due to glandular zinc depletion. This method demonstrated that total tissue zinc might be useful as a biomarker of prostate cancer progression. However, optical approaches may be limited for studies in small animal models of disease because due to limited tissue penetration of light and the high hemoglobin absorptivity at 540 nm.[44]
Figure 5.
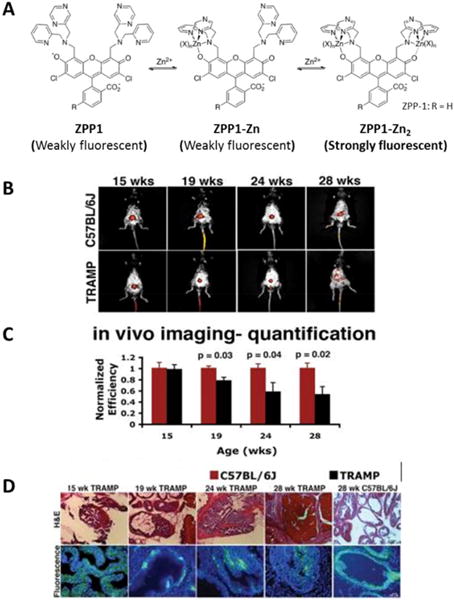
In vivo detection and monitoring of prostate cancer by epi-fluorescence optical imaging with ZPP1 (A) Schematic illustration of zinc binding to ZPP1. An intense fluorescent signal is generated only when ZPP1 binds to two zinc ions. (B) Epi-fluorescence imaging of 15-, 19-, 24-, and 28-wk old TRAMP mice (bottom) and C57BL/6J (top) control mice 30 min after tail-vein injection of ZPP1. (C) Histopathology (top) and fluorescence microscopy (bottom) of prostate tissue sections derived from the same animals. The fluorescence microscopy images are shown as an overlay of the green (ZPP1) and blue (DAPI) channels. Reproduced with permission from American Chemical Society[39] and American Association for Cancer Research.[40]
3.2.2 In Vivo Zinc-sensing MRI Agents
MR imaging offers a combination of high spatial and temporal resolution with unlimited tissue penetration. These advantages make MRI the ideal imaging modality for developing agents for detection of zinc ions. A manganese porphyrin-derivative, Mn(III)-(DPA-C2)2-TPPS3, was first used to detect contrast enhancement (T1-weighted images) in zinc-rich brain regions such as the hippocampus and the caudate–putamen.[45,50] Even though the T1 characteristics of this agent are not favorable for detecting zinc based solely on changes in relaxivity, Mn(III)-(DPA-C2)2-TPPS3 (Table 2) is unique in that it is cell-permeable and collects largely in zinc-rich cells. Inspired by this study, we have been investigating a series of gadolinium-based zinc sensors based upon the structure of GdDOTA-diBPEN. In the presence of zinc ions, GdDOTA-diBPEN shows only a modest increase in r1 but in the presence of both zinc ions and human serum albumin (HSA), a ternary complex is formed and r1 subsequently increases from 5.6 mM−1s−1 to 9.4 mM−1s−1 at 9.4 T.[46] Even though the r1 difference between bound and free forms is even higher at 0.47 T, the difference at 9.4 T was sufficient to detect release of zinc from pancreatic β-cells in vivo by MRI.[51]
Table 2.
Relaxivities and KD values for the different sensors measured at 9.4 T and 37°C (100 mM Tris buffer).
| CAs | r1 (s−1 mM−1) No Zn2+ |
With Zn2+ | r1 (s−1 mM−1) 0.6 mM HSA alone |
Zn2+ +0.6 mM HSA | KD(GdLZn) (nM)e | Reference |
|---|---|---|---|---|---|---|
| Mn-(DPA-C2)2-TPPS3 | 8.7a | 6.6a | – | – | 12 | [45] |
| GdDOTA-diBPEN | 4.0 | 4.9 | 4.0 | 6.6 | n.d | [46] |
| GdDO2A-diBPEN-propionate | 5.6 | n.d | n.d | 9.4a | n.d | [47] |
| GdDO2A-diBPEN-propylamide | 5.8 | n.d | n.d | 12.0b | n.d | [47] |
| GdDOTA-diBPYREN | nd | nd | 3.2 | 4.0 | 339 × 103 | [48] |
| GdDOTA | 3.7 | – | – | 2.7b | – | [49] |
CA: Contrast agent,
25 C, 25 mM PIPES, pH 7.0 and 4.7 T.
0.47 T
The most common strategy in the design of Zn(II) sensors for MRI is either to alter the number of coordinated water molecules (q), the water exchange rates (kex), or the molecular tumbling rate (τR) of the molecule.[48] Given that the first generation agent, GdDOTA-diBPEN, was known to have a relatively slow water exchange rate that limits the increase in r1 that accompanies formation of a ternary complex with albumin, we set out to develop a family of derivatives designed to increase the rate of water exchange rate by simple chemical manipulations (Figure 6).[46–47] Some of these new derivatives displayed a two-fold increase in r1 in the presence of Zn(II) and HSA at 9.4 T, and even greater enhancements at lower fields (Table 2). The GdDO2A-diBPEN-propylamide derivative also showed improved kinetic stability over Magnevist® and other agents identified to undergo transmetalation in the presence of excess Zn(II) ions.[48] Furthermore, the application of these agents in vivo in healthy mice showed pancreatic enhancement only during secretion of insulin and zinc initiated by an increase in blood glucose. Interestingly, the glucose challenge elicited a significant signal increase in both the pancreas and, unexpectedly, the prostate. Further in vitro studies confirmed that prostate epithelial cells respond to glucose by secreting Zn(II) ions similar to that seen in pancreatic β-cells (Figure 7A).[38a] The non-permeable nature of these Gd-based zinc sensors indicates that glucose-stimulated zinc secretion (GSZS) from the prostate must detect an increase in extracellular Zn(II) levels rather than intracellular Zn(II) detected by the fluorescent agent, ZPP1. A longitudinal study of GSZS from the prostate was performed in TRAMP mice using a zinc-sensitive MRI sensor and reduction in Zn(II) secretion was evident in small tumors during early PCa development as reflected by a hypointense signals in tissue regions later characterized by histology as PCa. Although a detection paradigm that relies on identifying regions of signal decrease rather than increase may at first be thought of as a negative feature, this imaging paradigm appears to be highly sensitive and specific for detection of PCa. At least in the TRAMP mouse precisely because one can detect small hypointense regions (PCa) rather easily on a background of hyperintense regions (healthy tissue) (Figure 7B).
Figure 6.
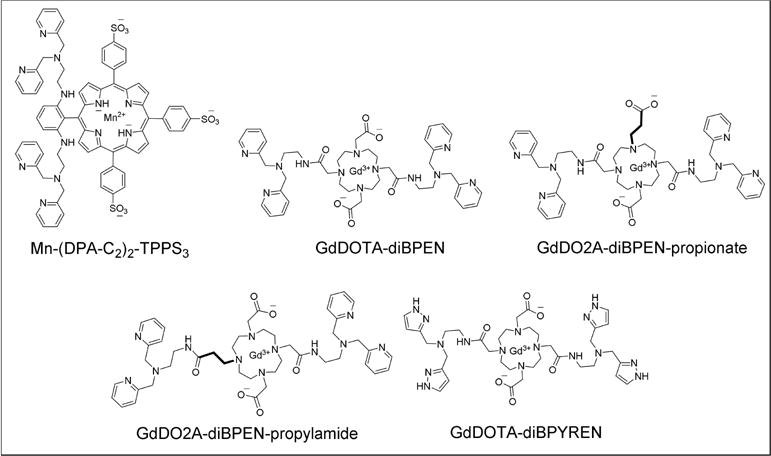
Chemical structures of some zinc-responsive MRI contrast agents.
Figure 7.
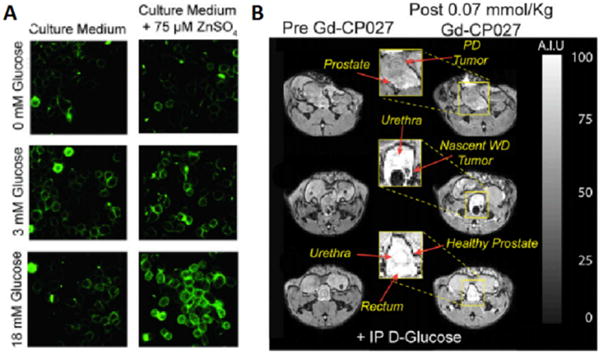
(A) In vitro fluorescence images of prostate epithelial cells (RWPE-1) cultured in a low-glucose medium without (Left) or with (Right) 75 μM ZnSO4. 1 μM ZIMIR was added to label the outer surface of the cells for detection of zinc release from intracellular stores. Only cells that had accumulated substantial zinc during incubation released zinc in response to glucose in a concentration-dependent manner. (B) Glucose-stimulated contrast enhanced T1-weighted 3D MR images at 9.4 T of the TRAMP mouse prostate during various stages of tumor development. (Bottom) A typical glucose stimulated zinc secretion pattern of the healthy prostate in a young TRAMP mouse (age: 10 wk). (Middle) A nascent tumor in dorsal lobe of the prostate is hypointense presumably reflecting a reduction in the release of intracellular zinc (age: 17 wk). (Top) A poorly differentiated (PD) tumor that originated in lateral lobe and extending to ventral lobe. No glucose stimulated zinc secretion was detected in the tumor, and significant loss of noncancerous prostate was observed (age: 19 wk). GdDO2A-diBPEN-propionate as shown in Figure 6 was used in this study. Reproduced with permission from Proceedings of the National Academies of Science.[38a]
Although this MRI method proved successful in detecting zinc-release from the prostate via MR imaging, there is still much to improve in terms regarding optimization of new prostate-specific zinc sensors for in vivo applications. For example, the understanding of the questions such as: 1) what is the optimal zinc binding affinity for a sensor to minimize the interference with normal physiological functions, 2) which pools of labile zinc (intracellular or extracellular) can we target for MR imaging, 3) how important is the cellular permeability when designing zinc sensors for PCa detection 4) does secretion of Zn(II) ions stimulated by glucose reflect the actual zinc content of the tissue or are the two measures independent of one another? An initial step towards answering these questions was recently taken by the design of GdDOTA–diBPYREN as a zinc sensor. GdDOTA-diBPYREN was designed with the goal of determining the role of the binding affinity for zinc (KDzn) in obtaining MR images with better contrast.[49] In fact, when the agent affinity for zinc is decreased, the impact of the agent itself on physiological levels of zinc is less, and the background MRI signal that results from binding of the agent with freely available zinc ions in all extracellular spaces is also reduced. The net result is that when additional zinc ions are released due to GSZS, the percent increase in MR signal intensity is magnified. Initial imaging studies of GSZS in mice have shown that both phase I and phase II insulin secretion from the pancreas can be detected for the first time using a weaker zinc binding agent (unpublished results).
3. Summary
Zinc levels in the prostate are known to decrease dramatically during development of PCa due to down-regulation of zinc transporters. This differential tissue biomarker makes zinc an attractive imaging target for early detection of PCa by molecular imaging. One surprising result observed in our lab during MRI studies of zinc and insulin secretion from the mouse pancreas was that zinc is also released from healthy prostate tissues in response to glucose (GSZS). Given that total zinc in isolated PCa tissues has been reported to parallel pathology-assigned Gleason scores (Figure 4), further development of imaging methods to detect total tissue zinc are warranted. The zinc-responsive MRI agents described herein detect secretion of zinc from cells and not necessarily total tissue zinc, so it remains to be seen whether the MRI method can be used to grade PCa without collection of a tissue biopsy. Although it is well established that prostatic epithelial cells lose their ability to accumulate zinc ions in PCa, they may also lose their ability to secrete zinc ions in response to glucose during this biotransformation. Nonetheless, given that most benign prostatic hyperplasia (BPH) develops in the transition zone and that zinc levels between PCa and BPH markedly differ, this MRI method could prove useful in differentiating PCa from BPH in patients where multi-parametric MR images (mpMRI) of the transition zone remains inconclusive. We envision that one may be able to inject a single bolus of glucose plus a zinc-responsive MR agent just prior to imaging the prostate in a fasted patient previously diagnosed with an abnormally high level of PSA to rule out or confirm PCa without a tissue biopsy. Non-invasive zinc imaging by MRI is just beginning to emerge, but these early examples illustrate that responsive imaging sensors that can highlight the biological differences in zinc homeostasis in pathological tissues are indeed possible.
Supplementary Material
Acknowledgments
Financial support from the National Institutes of Health (R01-DK095416, and in part by the Harold C. Simmons Cancer Center through an NCI Cancer Center Support Grant, 1P30-CA142543), the American Diabetes Association (7-12-MN-76 and 7-12-IN-42), and the Robert A. Welch Foundation (AT-584) is gratefully acknowledged.
Biographies

Su-Tang Lo obtained his bachelor’s degree in 2001 from National Central University (Taiwan) in Chemistry, MSc degree in 2004 from National Taiwan University (Taiwan) in Biochemistry and PhD degree in 2013 from the UT Southwestern Medical Center where he worked on the development of theranostic agents for prostate cancer and multimodality imaging techniques (PET/CT, SPECT/CT, and MRI) for noninvasive assessment of diabetes. Currently, he is working as a Post-Doctoral fellow in Prof. A. Dean Sherry’s laboratory at UTSW with research interest in noninvasive imaging assessments for the progression of diabetes and prostate cancer.

André F Martins was born in Aveiro and studied Biochemistry at the University of Coimbra, Portugal. In 2013, he earned a joint Ph. D degree in Chemistry and Biochemistry from U. Coimbra, Portugal and U. Orleans, France. His thesis was honored with the Foundation for Science and Technology Ph.D. Fellowship entitled “Targeted Multimodal Imaging Agents for the Detection of Aβ Plaques in Alzheimer’s Disease”. In November 2013, he joined the group of Prof. A. D. Sherry at UT Dallas and UT Southwestern Medical Center, as a Research Associate scientist. He has been since then studying responsive imaging agents for magnetic resonance, lanthanide-based MRI contrast agents and multimodal probes for molecular imaging and clinical translation.

Veronica Clavijo Jordan obtained her bachelor’s degree in Bioengineering and her Ph.D in Biomedical Engineering from Arizona State University where she worked on the design of apoferritin-based nanoparticle MRI contrast agents through controlled metal deposition. In November of 2013 she joined the Advanced Imaging Research Center at UT Southwestern as a Post-Doctoral Fellow where she works under the mentorship of Prof. A. Dean Sherry. Her interests involve using MRI to understand animal and human physiology by imaging organ function in vivo. Since her arrival at UT Southwestern she has been working on the translation of novel MRI metal responsive contrast agents using small and large animal models of disease.

A. Dean Sherry received a PhD in Inorganic Chemistry from Kansas State University in 1971 on Lewis acid–base theory and thermodynamics of hydrogen bonding. He was awarded a National Institutes of Health Postdoctoral Fellowship (1971–72) to study lanthanide cations as spectroscopic probes of protein structure. He joined Chemistry faculty at UT Dallas in 1972 and was promoted to Professor in 1982. He served as Chairman of the department from 1979–1990. In 1990, he accepted an appointment as Professor of Radiology at UT Southwestern Medical Center in Dallas where he studies intermediary metabolism in animal models and develops metabolic imaging agents. He currently serves as Director of the Advanced Imaging Research Center on that campus. He has been honored with the W. T. Doherty Award (1990), a Chancellor’s Outstanding Teaching Award (1994), and was awarded Gold Metal Awards from the International Society for Magnetic Resonance in Medicine and the World Molecular Imaging Society for his contributions to science. Dr Sherry was the Scientific Founder of Macrocyclics, Inc. and VitalQuan, LLC.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Franz MC, Anderle P, Burzle M, Suzuki Y, Freeman MR, Hediger MA, Kovacs G. Mol Aspects Med. 2013;34:735–741. doi: 10.1016/j.mam.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MB, Rokhlin OW. J Cell Biochem. 2009;106:363–371. doi: 10.1002/jcb.22022. [DOI] [PubMed] [Google Scholar]
- 4.a) Smith DS, Humphrey PA, Catalona WJ. Cancer. 1997;80:1852–1856. [PubMed] [Google Scholar]; b) Carraway RE, Dobner PR. Biochim Biophys Acta. 2012;1823:544–557. doi: 10.1016/j.bbamcr.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 5.a) http://www.screeningforprostatecancer.org, 2017;; Bibbins-Domingo K, Grossman DC, Curry SJ. Jama. 2017 doi: 10.1001/jama.2017.4413. [DOI] [PubMed] [Google Scholar]
- 6.Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, Richie JP, deKernion JB, Walsh PC, Scardino PT, Lange PH, Subong EN, Parson RE, Gasior GH, Loveland KG, Southwick PC. Jama. 1998;279:1542–1547. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 7.Serefoglu EC, Altinova S, Ugras NS, Akincioglu E, Asil E, Balbay MD. Can Urol Assoc J. 2013;7:E293–298. doi: 10.5489/cuaj.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groskopf J, Aubin SMJ, Deras IL, Blase A, Bodrug S, Clark C, Brentano S, Mathis J, Pham J, Meyer T, Cass M, Hodge P, Macairan ML, Marks LS, Rittenhouse H. Clin Chem. 2006;52:1089–1095. doi: 10.1373/clinchem.2005.063289. [DOI] [PubMed] [Google Scholar]
- 9.a) Filella X, Alcover J, Molina R, Gimenez N, Rodriguez A, Jo J, Carretero P, Ballesta AM. Int J Cancer. 1995;63:780–784. doi: 10.1002/ijc.2910630605. [DOI] [PubMed] [Google Scholar]; b) Roddam AW, Duffy MJ, Hamdy FC, Ward AM, Patnick J, Price CP, Rimmer J, Sturgeon C, White P, Allen NE, N. H. S. P. C. R. M. Programme Eur Urol. 2005;48:386–399. doi: 10.1016/j.eururo.2005.04.015. discussion 398–389. [DOI] [PubMed] [Google Scholar]
- 10.Stattin P, Vickers AJ, Sjoberg DD, Johansson R, Granfors T, Johansson M, Pettersson K, Scardino PT, Hallmans G, Lilja H. Eur Urol. 2015;68:207–213. doi: 10.1016/j.eururo.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Filella X, Foj L. Clin Chem Lab Med 2015. 53:963–973. doi: 10.1515/cclm-2014-0988. [DOI] [PubMed] [Google Scholar]; b) Martin NE. Curr Opin Oncol. 2016;28:248–252. doi: 10.1097/CCO.0000000000000279. [DOI] [PubMed] [Google Scholar]; c) Loeb S, Catalona WJ. Ther Adv Urol. 2014;6:74–77. doi: 10.1177/1756287213513488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello LC, Feng P, Milon B, Tan M, Franklin RB. Prostate Cancer Prostatic Dis. 2004;7:111–117. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mawson CA, Fischer MI. Can J Med Sci. 1952;30:336–339. doi: 10.1139/cjms52-043. [DOI] [PubMed] [Google Scholar]
- 14.a) Vallee BL, Falchuk KH. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]; b) Maret W. Biometals. 2009;22:149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- 15.Sensi SL, Paoletti P, Bush AI, Sekler I. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 16.Taylor CG. Biometals. 2005;18:305–312. doi: 10.1007/s10534-005-3686-x. [DOI] [PubMed] [Google Scholar]
- 17.Costello LC, Franklin RB. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costello LC, Franklin RB, Liu Y, Kennedy MC. J Inorg Biochem. 2000;78:161–165. doi: 10.1016/s0162-0134(99)00225-1. [DOI] [PubMed] [Google Scholar]
- 19.Feng P, Li T, Guan Z, Franklin RB, Costello LC. Mol Cancer. 2008;7:25. doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costello LC, Franklin RB. Prostate Cancer Prostatic Dis. 2009;12:17–24. doi: 10.1038/pcan.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teahan O, Bevan CL, Waxman J, Keun HC. Int J Biochem Cell Biol. 2011;43:1002–1009. doi: 10.1016/j.biocel.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 22.a) Costello LC, Franklin RB, Zou J, Feng P, Bok R, Swanson MG, Kurhanewicz J. Cancer Biol Ther. 2011;12:1078–1084. doi: 10.4161/cbt.12.12.18367. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Costello LC, Franklin RB, Zou J, Naslund MJ. Chemotherapy (Los Angel) 2015;4 doi: 10.4172/2167-7700.1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P, Costello LC. J Inorg Biochem. 2003;96:435–442. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.a) Costello LC, Franklin RB. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]; b) Costello LC, Franklin RB, Feng P, Tan M, Bagasra O. Cancer Causes Control. 2005;16:901–915. doi: 10.1007/s10552-005-2367-y. [DOI] [PubMed] [Google Scholar]
- 24.Giskeodegard GF, Bertilsson H, Selnas KM, Wright AJ, Bathen TF, Viset T, Halgunset J, Angelsen A, Gribbestad IS, Tessem MB. PLoS One. 2013;8:e62375. doi: 10.1371/journal.pone.0062375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen MB, Stoltenberg M, Juhl S, Danscher G, Ernst E. Prostate. 1997;31:125–130. doi: 10.1002/(sici)1097-0045(19970501)31:2<125::aid-pros8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.a) Zaichick V, Sviridova TV, Zaichick SV. Int Urol Nephrol. 1997;29:565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]; b) Cortesi M, Fridman E, Volkov A, Shilstein S, Chechik R, Breskin A, Vartsky D, Kleinman N, Kogan G, Moriel E, Gladysh V, Huszar M, Ramon J, Raviv G. Prostate. 2008;68:994–1006. doi: 10.1002/pros.20766. [DOI] [PubMed] [Google Scholar]; c) Cortesi M, Fridman E, Volkov A, Shilstein SS, Chechik R, Breskin A, Vartsky D, Kleinman N, Kogan G, Moriel E, Gladysh V, Huszar M, Ramon J, Raviv G. Prostate. 2008;68:994–1006. doi: 10.1002/pros.20766. [DOI] [PubMed] [Google Scholar]
- 27.Ogunlewe JO, Osegbe DN. Cancer. 1989;63:1388–1392. doi: 10.1002/1097-0142(19890401)63:7<1388::aid-cncr2820630725>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.a) Timm F. Dtsch Z Gesamte Gerichtl Med. 1958;47:428–431. [PubMed] [Google Scholar]; b) Timm F. Dtsch Z Gesamte Gerichtl Med. 1958;46:706–711. [PubMed] [Google Scholar]
- 29.Danscher G. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- 30.Danscher G. Histochemistry. 1982;76:281–293. doi: 10.1007/BF00543951. [DOI] [PubMed] [Google Scholar]
- 31.Cortesi M, Chechik R, Breskin A, Vartsky D, Ramon J, Raviv G, Volkov A, Fridman E. Phys Med Biol. 2009;54:781–796. doi: 10.1088/0031-9155/54/3/020. [DOI] [PubMed] [Google Scholar]
- 32.Kumar Nelso VF, Abbas Abul. Robbins & Cotran Pathologic Basis of Disease. (Seventh) 2004 [Google Scholar]
- 33.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. BJU Int. 2013;111:753–760. doi: 10.1111/j.1464-410X.2012.11611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson E, Leahy O, Cheng AC, Grummet J. BMC Infect Dis. 2015;15:580. doi: 10.1186/s12879-015-1328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ide-Ektessabi A, Fujisawa S, Sugimura K, Kitamura Y, Gotoh A. X-Ray Spectrometry. 2002;31:7–11. [Google Scholar]
- 36.a) Lubag AJ, De Leon-Rodriguez LM, Burgess SC, Sherry AD. Proc Natl Acad Sci USA. 2011;108:18400–18405. doi: 10.1073/pnas.1109649108. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yu J, Martins AF, Preihs C, Clavijo Jordan V, Chirayil S, Zhao P, Wu Y, Nasr K, Kiefer GE, Sherry AD. J Am Chem Soc. 2015;137:14173–14179. doi: 10.1021/jacs.5b09158. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Esqueda AC, Lopez JA, Andreu-de-Riquer G, Alvarado-Monzon JC, Ratnakar J, Lubag AJ, Sherry AD, Leon-Rodriguez LM De. J Am Chem Soc. 2009;131:11387–11391. doi: 10.1021/ja901875v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee T, Zhang XA, Dhar S, Faas H, Lippard SJ, Jasanoff A. Chem Biol. 2010;17:665–673. doi: 10.1016/j.chembiol.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.a) Clavijo Jordan MV, Lo S-T, Chen S, Preihs C, Chirayil S, Zhang S, Kapur P, Li W-H, De Leon-Rodriguez LM, Lubag AJM, Rofsky NM, Sherry AD. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5464–5471. doi: 10.1073/pnas.1609450113. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ghosh SK, Kim P, Zhang XA, Yun SH, Moore A, Lippard SJ, Medarova Z. Cancer Res. 2010;70:6119–6127. doi: 10.1158/0008-5472.CAN-10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buccella D, Horowitz JA, Lippard SJ. Journal of the American Chemical Society. 2011;133:4101–4114. doi: 10.1021/ja110907m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh SK, Kim P, Zhang X-a, Yun S-H, Moore A, Lippard SJ, Medarova Z. Cancer Research. 2010;70:6119–6127. doi: 10.1158/0008-5472.CAN-10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang XA, Hayes D, Smith SJ, Friedle S, Lippard SJ. Journal of the American Chemical Society. 2008;130:15788–15789. doi: 10.1021/ja807156b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medarova Z, Ghosh SK, Vangel M, Drake R, Moore A. Am J Cancer Res. 2014;4:385–393. [PMC free article] [PubMed] [Google Scholar]
- 43.a) Gelman IH. Cancer research. 2016;76:6137–6139. doi: 10.1158/0008-5472.CAN-16-2636. [DOI] [PubMed] [Google Scholar]; b) Gingrich JR, Greenberg NM. Toxicol Pathol. 1996;24:502–504. doi: 10.1177/019262339602400414. [DOI] [PubMed] [Google Scholar]
- 44.Liu K, Wang Y, Kong X, Liu X, Zhang Y, Tu L, Ding Y, Aalders MCG, Buma WJ, Zhang H. Nanoscale. 2014;6:9257–9263. doi: 10.1039/c4nr02090a. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XA, Lovejoy KS, Jasanoff A, Lippard SJ. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10780–10785. doi: 10.1073/pnas.0702393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esqueda AC, Lopez JA, Andreu-de-Riquer G, Alvarado-Monzon JC, Ratnakar J, Lubag AJM, Sherry AD, De Leon-Rodriguez LM. Journal of the American Chemical Society. 2009;131:11387–11391. doi: 10.1021/ja901875v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J, Martins AF, Preihs C, Clavijo Jordan V, Chirayil S, Zhao P, Wu Y, Nasr K, Kiefer GE, Sherry AD. Journal of the American Chemical Society. 2015;137:14173–14179. doi: 10.1021/jacs.5b09158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helm L, Merbach AE, Tóth Ev. The chemistry of contrast agents in medical magnetic resonance imaging. Second. John Wiley & Sons Inc.; Hoboken, NJ: 2013. [Google Scholar]
- 49.De Leon-Rodriguez LM, Lubag AJM, Lopez JA, Andreu-de-Riquer G, Alvarado-Monzon JC, Sherry AD. Medchemcomm. 2012;3:480–483. doi: 10.1039/C2MD00301E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee T, Zhang X-a, Dhar S, Faas H, Lippard SJ, Jasanoff A. Chemistry& biology. 2010;17:665–673. doi: 10.1016/j.chembiol.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lubag AJM, De Leon-Rodriguez LM, Burgess SC, Sherry AD. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18400–18405. doi: 10.1073/pnas.1109649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


