Abstract
Purpose
To define the nature and extent of temporal frequency abnormalities in diabetics who have mild or no nonproliferative diabetic retinopathy (NPDR) by using the flicker electroretinogram (ERG).
Methods
Light-adapted flicker ERGs were recorded from 20 diabetics who have no clinically apparent retinopathy, 20 diabetics who have mild NPDR, and 20 nondiabetic, age-equivalent controls. ERGs were elicited by full-field sinusoidal flicker across the temporal frequency range of 6 to 100 Hz and were recorded using conventional techniques. The amplitude and phase of the fundamental and harmonic response components were derived by Fourier analysis and compared among the groups.
Results
Analysis of variance indicated that compared with the controls, both patient groups had significant amplitude reductions of the fundamental ERG component for temporal frequencies greater than 56 Hz (all P ≤ 0.03). Modeling the amplitude measurements indicated that both patient groups had significant reductions in the high-frequency response cutoff. Response phase, however, did not differ significantly among the groups at any frequency. The amplitude and phase of the high-frequency harmonics (32–96 Hz) of the patients' responses to a low-frequency stimulus (16 Hz) were normal over the temporal frequency range that the fundamental response was abnormal.
Conclusions
Taken together, the diabetics' fundamental amplitude attenuation for rapid flicker combined with their normal high-frequency harmonic responses generated by slow flicker suggest that the likely site of the abnormal temporal filtering occurs prior to the nonlinearity that generates the harmonic components of the ERG, implicating a photoreceptor origin.
Keywords: electroretinogram, flicker electroretinogram, diabetic retinopathy
Diabetic retinopathy (DR) is the leading cause of new cases of blindness among working-age adults,1 and a recent meta-analysis has indicated that the number of individuals who have visual impairment due to DR continues to increase worldwide.2 DR has traditionally been considered a disease of the retinal vasculature, and current international standards classify DR stage based on clinically apparent vascular abnormalities.3,4 However, there has been interest in expanding the defining characteristics of DR to consider retinal neurodegeneration in addition to vascular abnormalities.5 The proposal to consider retinal neurodegeneration in the staging of DR is based on deficits in neural structure and function in these individuals, including thinning of the inner retina, as well as abnormalities in dark adaptation, contrast sensitivity, color vision, and perimetric sensitivity.6–13
Neural abnormalities have also been documented in individuals who have DR by using objective, electrophysiologic measures, such as the pattern electroretinogram (ERG)14 and the multifocal ERG.15–17 The full-field ERG measured under standard clinical conditions is generally thought to be normal in early-stage DR,18 with the exception of the oscillatory potentials that have been shown to be reduced or delayed in some studies (reviewed by Tzekov and Arden19). In later stages of the disease, the photopic single-flash b-wave and flicker ERG can become reduced in amplitude and/or delayed.20–23 Given these findings, as well as the recent availability of a portable instrument for recording the flicker ERG, there has been renewed interest in the flicker ERG as a tool to screen for sight-threatening DR.24–26 Previous studies of the flicker ERG in diabetes have almost exclusively elicited the response by using full-field periodic flashes of light at a flicker rate of approximately 30 Hz, the frequency recommended by international standards.27 However, a recent report from our group has shown value in recording the ERG elicited by higher flicker rates.28 Specifically, diabetic subjects who had no clinically apparent DR (NDR) or mild nonproliferative DR (NPDR) had significant amplitude reductions for the 62.5-Hz flicker ERG, which were not apparent at the international standard flicker rate of approximately 30 Hz. In that study, the mean 62.5-Hz flicker ERG amplitude was reduced by 32% for subjects with NDR and by 41% for subjects with mild NPDR. The source of the high-frequency flicker ERG deficit and the extent to which other flicker frequencies are affected in early-stage DR are presently unknown.
The goal of the present study was to gain a more complete understanding of the nature and extent of the ERG temporal frequency abnormalities of diabetics who have NDR or mild NPDR. To achieve this goal, the flicker ERG was measured across a broad range of temporal frequencies (6–100 Hz). This permitted determining the frequency range over which the flicker ERG was abnormal and also provided an estimate of the high-frequency response cutoff (the frequency beyond which the ERG could not be measured). Additionally, the probable retinal locus of the high-frequency deficit was inferred by analyzing the characteristics of the nonlinear harmonic components of the slow flicker ERG. As described further below and in detail elsewhere,29,30 the probable retinal site that is responsible for the abnormal temporal filtering can be inferred by examining the properties of the nonlinear harmonic components of the flicker response. If the high-frequency harmonic response components generated by slow flicker are normal, then a photoreceptor site of abnormality is likely. Conversely, if the high-frequency harmonic response components generated by slow flicker are abnormal, then a postphotoreceptor site of abnormality is likely. These two alternatives were evaluated by examining the harmonics generated by 16-Hz sinusoidal flicker.
Methods
Subjects
Forty subjects diagnosed with type-2 diabetes mellitus (DM) were recruited from the Retina Clinic and General Eye Clinic of the University of Illinois at Chicago, Department of Ophthalmology and Visual Sciences. Notably, 28 of these subjects participated in a previous study of ERG abnormalities in early-stage DR.28 A comprehensive history was obtained from the medical record, and an examination of each eye was performed by a retina specialist. No subject had systemic disease (other than diabetes) or ocular disease known to affect the retina. Subjects who had sickle cell disease, retinal vascular occlusions, age-related macular degeneration, glaucoma, or high myopia were not recruited. The stage of NPDR was graded, and the subjects were clinically classified as diabetic with no clinically apparent DR (N = 20) or diabetic with mild NPDR (N = 20), according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) scale.3 Subjects classified as mild NPDR had retinal vascular abnormalities, including microaneurysms, hard exudates, cotton-wool spots, and/or mild retinal hemorrhage (equivalent to ETDRS level 35 or less3). Subject characteristics, including age, sex, visual acuity, estimated diabetes duration, and HbA1c percentage, are provided in the Table. With the exception of two mild NPDR subjects who had a history of anti-vascular endothelial growth factor injection, no subject received treatment for DR.
Table.
Subject Characteristics (Mean ± SD)
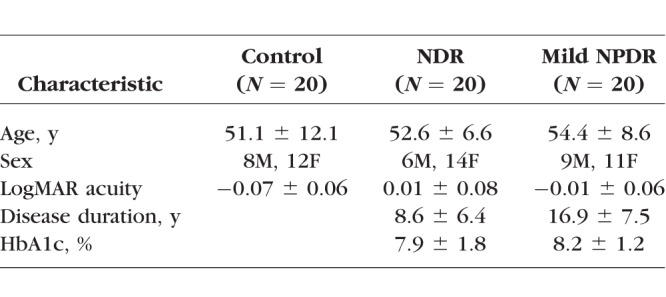
Twenty visually normal, nondiabetic, control subjects also participated; 15 of these subjects served as controls in a previous study of ERG abnormalities in early-stage DR.28 The mean age of the control subjects did not differ significantly from that of the diabetic subjects (F = 0.61, P = 0.55). All control subjects had best-corrected visual acuity of 0.06 logMAR (equivalent to approximately 20/23 Snellen acuity) or better, as assessed with the Lighthouse Distance Visual Acuity Chart and the normal letter contrast sensitivity as measured with a Pelli-Robson chart. The research followed the tenets of the Declaration of Helsinki and was approved by an institutional review board of the University of Illinois at Chicago. All subjects provided written informed consent.
Apparatus, Stimuli, and Procedure
Stimuli were generated by and presented in an LED-driven ganzfeld system (Diagnosys, LLC, Lowell, MA, USA) that we have used previously and described elsewhere.28,31 Prior to testing, the subject adapted for 2 minutes to a uniform field that was composed of 100 cd/m2 of middle-wavelength light (516-nm peak) and 100 cd/m2 of long-wavelength light (632-nm peak). During the ERG recording, the adapting field was modulated sinusoidally at a temporal frequency ranging from 6.3 to 100 Hz (Michelson contrast of 100%). The sinusoidal stimuli used herein are composed of a single temporal frequency. This provides an advantage over the more commonly used pulse stimuli, which are relatively broadband in frequency content, ensuring that the harmonic components of the ERG are not due to high-frequency components contained in the stimulus itself. Each flicker stimulus was presented for approximately 1 second, with the exact duration depending on the stimulus period. Each stimulus frequency was presented a minimum of five times, with the total number of repetitions depending on response quality. Between presentations of the flicker stimulus, the ganzfeld was illuminated uniformly with the steady adapting field. Analyses were based on the mean of the five responses with the fewest eye movement artifacts.
Measurements from all subjects were performed monocularly, with the fellow eye patched. Infrequently, the stage of NPDR differed between the two eyes for the diabetic subjects; the eye with the lower NPDR stage was tested in these rare cases. Prior to the ERG recordings, the pupil of the tested eye was dilated with 2.5% phenylephrine hydrochloride and 1% tropicamide drops. ERGs were recorded with DTL electrodes, and gold-cup electrodes were used as reference (ear) and ground (forehead). Amplifier bandpass settings were 0.30 to 500 Hz and the sampling frequency was 2 kHz.
The amplitude and phase of the mean ERG fundamental and harmonic response components were derived by fast Fourier transform. The “steady-state” response was analyzed by omitting the initial and final few cycles of the waveforms, as these cycles can contain onset and offset transients. In the figures below, phase is given in cosine phase and the responses were ‘‘unwrapped'' to extend beyond 360°, per convention.
Results
Flicker ERG Amplitude and Phase Across Temporal Frequency
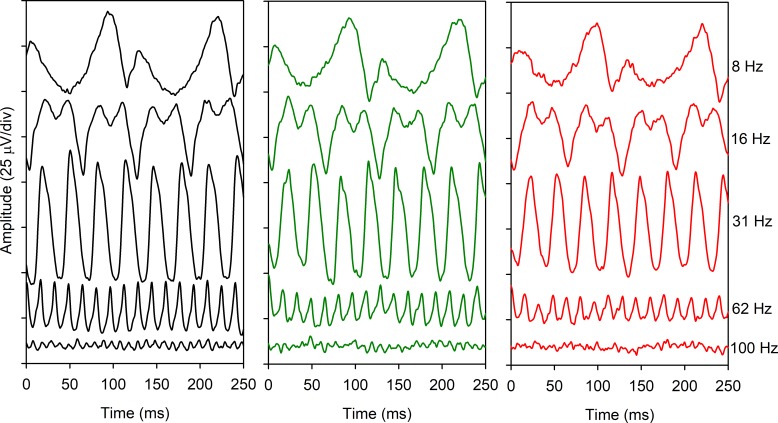
Figure 1 shows ERG traces recorded at five different flicker rates that approximately span the range of frequencies examined. Mean waveforms are shown for the controls (left column), NDR subjects (middle column), and mild NPDR subjects (right column). The shape and timing of the waveforms for the two diabetic groups were highly similar to that of the control group for frequencies from 8 to 62 Hz. Likewise, the trough to peak amplitude for the diabetic and control groups were similar for frequencies between 8 and 31 Hz. For the two highest frequencies shown (62 and 100 Hz), the trough to peak amplitude for the control group was larger than that of both patient groups. In fact, a 100-Hz periodic response was not readily apparent for either patient group, whereas 25 cycles can be seen in the 250-ms window for the control group. The mean ERG traces shown in Figure 1 are intended to provide examples of the waveforms across the range of temporal frequencies examined for the three subject groups; amplitude and timing for the individual subjects are discussed below.
Figure 1.
Mean ERG waveforms recorded at a series of temporal frequencies (indicated at right) for the control subjects (black, left), diabetics who have NDR (green, middle), and diabetics who have mild NPDR (red, right).
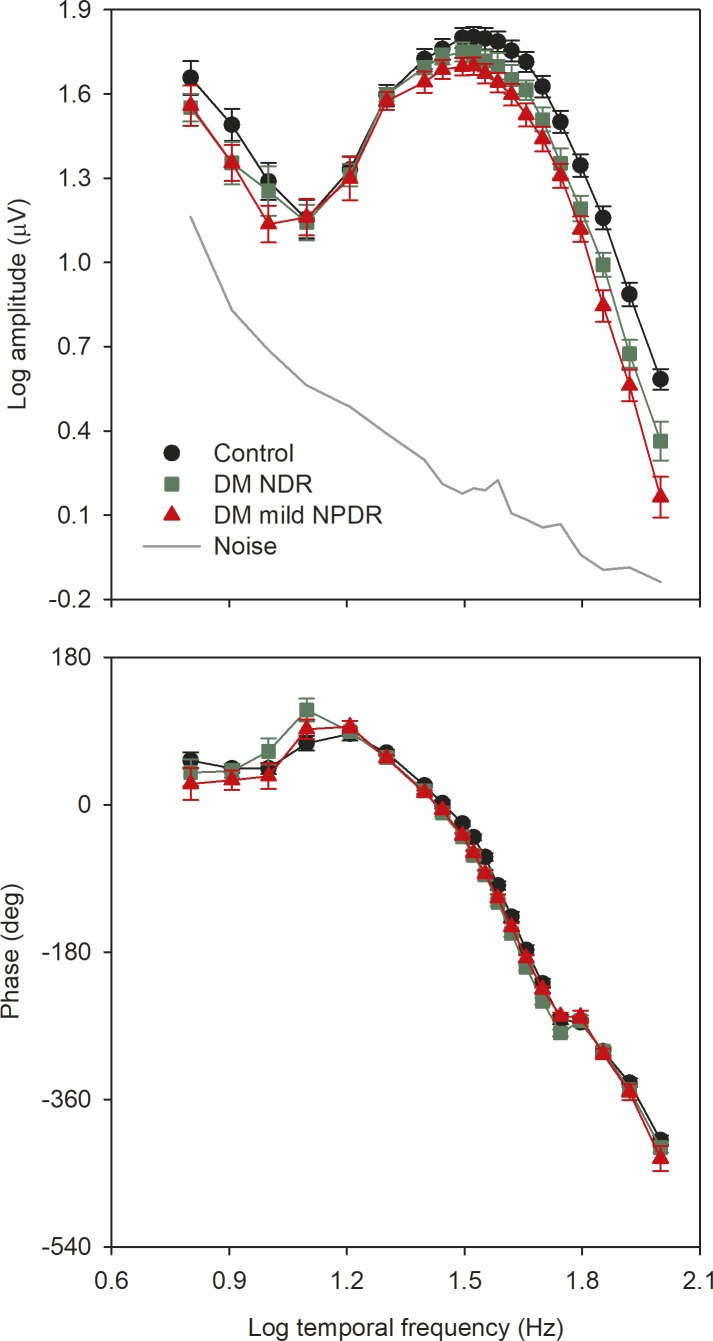
Figure 2 shows the mean (±SEM) log fundamental amplitude (top) and phase (bottom) as a function of log temporal frequency for the three subject groups. The solid gray line represents the noise level, defined as the mean of the amplitudes measured at frequencies that neighbor the stimulus frequency (approximately 1.5 Hz above and below the stimulus frequency). There were small differences among the three groups for the three lowest temporal frequencies examined (6–10 Hz). However, individual recordings have substantial noise contamination, as indicated by the gray line. The data points for the three groups were superimposed for frequencies between 12 and 20 Hz. For higher temporal frequencies, systematic differences among the groups become apparent, particularly across the high temporal frequency range (greater than approximately 50 Hz). In contrast to the amplitude findings, the phase of the fundamental response was similar for the three groups for all temporal frequencies.
Figure 2.
Mean (±SEM) log fundamental amplitude (top) and phase (bottom) as a function of log stimulus temporal frequency. Control subjects are shown in black, diabetics with NDR are shown in green, and diabetics with mild NPDR are shown in red. The gray line represents the noise level, as described in the text.
A repeated measures 2-way analysis of variance (ANOVA), with main effects of group (control, NDR, mild NPDR) and stimulus frequency, was performed to compare the amplitudes among the groups. There were significant effects of group (F = 6.10, P = 0.004) and stimulus frequency (F = 339.00, P < 0.001), as well as a significant interaction between these main effects (F = 2.50, P < 0.001). Holm-Sidak pairwise comparisons indicated a statistically significant reduction in mean amplitude for the mild NPDR group for frequencies of 38.5 Hz and greater (all t > 2.29, P < 0.05). For the NDR group, pairwise comparisons indicated a statistically significant reduction in mean amplitude for frequencies of 55.6 Hz and greater (all t > 3.10, P ≤ 0.004). The NDR and mild NPDR amplitudes differed significantly for frequencies of 71 Hz and above (all t > 2.15, P < 0.05). Thus, both patient groups had statistically significant amplitude reductions across the high-frequency range, but the frequency at which the amplitude loss became statistically significant differed for the two groups.
A repeated measures 2-way ANOVA, with main effects of group (control, NDR, mild NPDR) and stimulus frequency was performed to compare the phases among the groups. There was no significant effect of group (F = 3.00, P = 0.06), but there was a significant effect of stimulus frequency (F = 1391.95, P < 0.001). The interaction between these effects was not significant (F = 1.26, P = 0.14). Thus, there were no significant phase differences between the control and diabetic groups.
Figure 3 presents the flicker ERG amplitude for each NDR subject (green squares) and each mild NPDR subject (red triangles) for select frequencies ranging from 31.25 to 100 Hz. The gray boxes indicate the range of amplitude obtained from the control subjects (i.e., maximum and minimum control values). The horizontal bars indicate the mean of the control group (black), NDR group (green), and mild NPDR group (red). It is clear that as stimulus temporal frequency increased, the number of patients falling outside of the control range also increased. At the International Society for Clinical Electrophysiology of Vision (ISCEV) standard flicker rate of 31.25 Hz, 10% of the NDR and 20% of the mild NPDR subjects were slightly below the lower limit of normal. In comparison, at 100 Hz, 20% of the NDR and 65% of the mild NPDR subjects were below the lower limit of normal. Several of these subjects had amplitudes that did not exceed the noise level (dashed line; calculated as described in Fig. 2).
Figure 3.
Log fundamental amplitude measured at select stimulus temporal frequencies for each subject. The gray regions represent the range of the visually normal control subjects. Amplitude for each diabetic subject with NDR is indicated by the green squares and each diabetic subject with mild NPDR is indicated by the red triangles. The means of the control subjects, NDR subjects, and mild NPDR subjects are shown by the black, green, and red horizontal lines, respectively. The dashed gray lines represent the noise level, as described in the text.
Flicker ERG High-Frequency Cutoff
Figures 1 through 3 include data from all subjects tested, regardless of their individual signal-to-noise ratios (SNRs). At high temporal frequencies, several of the patients, particularly in the mild NPDR group, had small amplitude responses that were not statistically different from noise. As an additional approach to quantify the high-frequency response attenuation in the diabetic subjects, the high-frequency cutoff of the amplitude function was calculated after excluding individual responses that did not exceed the noise, as follows. The SNR was calculated as described above, and the response at a given temporal frequency was considered distinguishable from noise if the SNR was 2.82 or greater. This criterion has been used previously and, as discussed elsewhere,32 represents the SNR that corresponds to a significance level of P = 0.05. Log amplitudes for temporal frequencies with SNRs >2.82 were plotted as a function log temporal frequency and were fit with a function described elsewhere33,34,
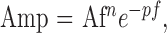 |
where Amp is the response amplitude at temporal frequency f, n governs the attenuation at low temporal frequencies, and A and p are vertical and horizontal scaling parameters, respectively, on logarithmic coordinates. A, n, and p were adjusted to minimize the mean squared error between the data and the fitted function. The temporal frequency at which the flicker ERG was 1 μV was determined from the fits (i.e., the frequency at which the x-axis is crossed in Fig. 4).
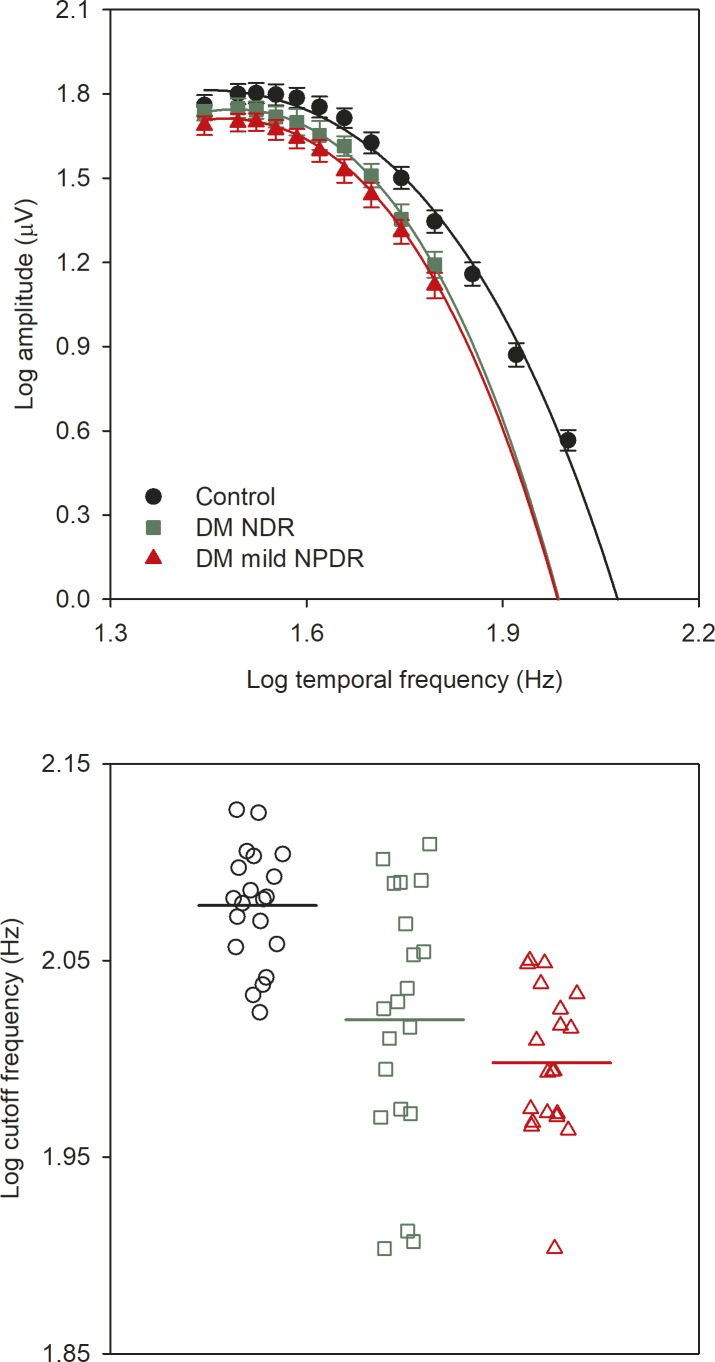
Figure 4.
Mean (±SEM) log fundamental amplitude as a function of log stimulus temporal frequency. The lines represent fits of the equation to the data. Control subjects are shown in black, NDR subjects are shown in green, and mild NPDR subjects are shown in red. The lower panel shows the high-frequency cutoff derived from the fit for each subject. The means of the control subjects, NDR subjects, and mild NPDR subjects are shown by the black, green, and red horizontal lines, respectively.
Figure 4 plots mean log amplitude versus log temporal frequency for the controls (black circles), NDR subjects (green squares), and mild NPDR subjects (red triangles). Data points are not shown for the NDR and mild NPDR subjects for frequencies higher than 71 Hz because several DM subjects had responses that were not significantly different from noise at these high temporal frequencies. The solid lines are fits of the equation to the mean data. It is clear from the fitted functions that the control retina is capable of responding to higher flicker frequencies than the diabetic retina. The lower panel plots the cutoff temporal frequency for each subject. As in Figure 3, the horizontal bars represent the mean of each group. A 1-way ANOVA indicated that there were statistically significant differences among the groups in the cutoff frequency (F = 15.77, P < 0.001). Holm-Sidak pairwise comparisons indicated that the mean cutoff frequency was significantly lower for the NDR and mild NPDR groups compared with the controls (both t > 3.91, P < 0.001). However, there was no significant difference in mean cutoff frequency for the two patient groups (t = 1.54, P = 0.13).
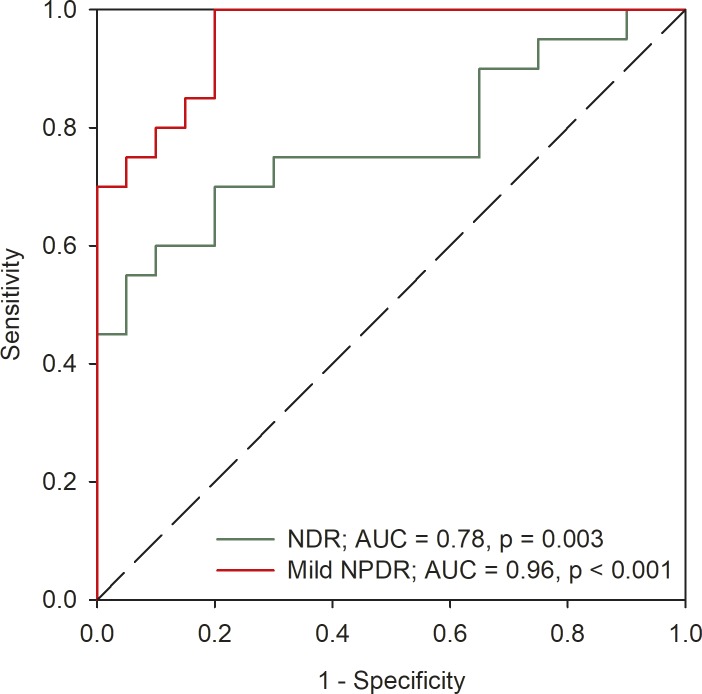
The extent to which the two patient groups can be separated from the control group based on the high-frequency cutoff was determined from receive operating characteristic (ROC) analysis. Figure 5 plots the proportion of the NDR subjects (green) and mild NPDR subjects (red) classified as abnormal (sensitivity) as a function of the proportion of the controls classified as abnormal (1-specificity; false positives). The area under the ROC curves (AUC) was 0.78 (P = 0.003) and 0.96 (P < 0.001) for the NDR and mild NPDR subjects, respectively. The optimal cutoff frequency was approximately 110 Hz for both patient groups. This cutoff frequency resulted in a sensitivity of 0.60 with a specificity of 0.90 for the NDR group. For the mild NPDR group, the sensitivity was 1.00 and the specificity was 0.80.
Figure 5.
Receiver operating characteristic curves for the NDR (green) and mild NPDR (red) subjects. The proportion of the DM subjects classified as abnormal (sensitivity) is plotted as a function of the proportion of the controls classified as abnormal (1-specificity; false positives).
Harmonic Analysis of the Slow Flicker Response
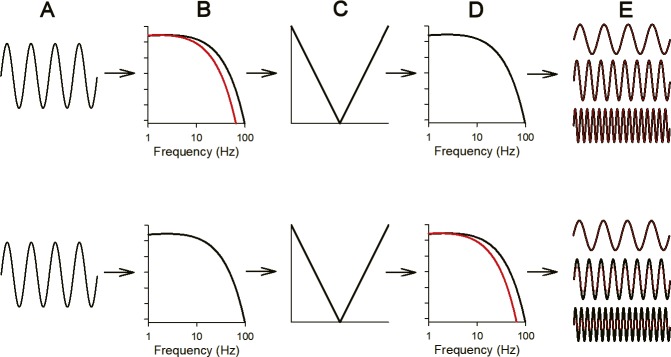
Despite the use of sinusoidal stimulation, the ERG was not a simple sinusoid, which is most apparent in responses to slow flicker (see Fig. 1; 8 and 16 Hz). The nonlinear distortion of the response can be quantified based on the harmonic components (i.e., responses that occur at multiples of the stimulus frequency). In addition to quantifying response nonlinearities, the harmonics can be used to infer the retinal locus of the high-frequency ERG attenuation. The logic underlying this approach is discussed in detail elsewhere.29,30 In brief, two sites of temporal filtering have been proposed that govern the flicker ERG: one before the retinal nonlinearity that generates the harmonic response components and a second that follows the retinal nonlinearity.29,30 The retinal nonlinearity is thought to occur early in the visual pathway, before the signals from different cone types converge at the photoreceptor-bipolar cell synapse.35 As illustrated by the schematic (Fig. 6), the high-frequency harmonics will be affected differently depending on whether the abnormal temporal filtering occurs before or after the retinal nonlinearity. The response to 16-Hz sinewave stimulation (Fig. 6A) is expected to be passed through the initial linear filter (Fig. 6B) without substantial attenuation and then through a retinal nonlinearity (Fig. 6C) that generates the harmonic response components. The fundamental and harmonics are then passed through a second linear filter (Fig. 6D), with the results shown in Figure 6E. If diabetes acts only at the first filter (Fig. 6B; red), then the 16-Hz fundamental and harmonic response components will be unaffected (red and black waveforms are superimposed in Fig. 6E, top). Conversely, if diabetes acts to alter only the second filter (Fig. 6D; red), then the 16 Hz fundamental will be unaffected, but the high-frequency harmonic response components will be attenuated, because they are passed through a low-pass filter with a reduced corner frequency (Fig. 6D; red). Figure 6E (bottom) shows that the harmonics in red are smaller than the harmonics in black.
Figure 6.
Schematic illustration of the “sandwich model” of retinal processing that has been proposed to underlie the flicker ERG. Column A represents a 16-Hz sinewave stimulus. Column B represents an early linear low-pass filter; the red (diabetic model) and black (control model) filters differ in their corner frequencies. Column C represents the nonlinearity that generates the harmonic components of the flicker ERG. The fundamental and harmonics are passed through a second linear filter (column D); the red (diabetic model) and black (control model) filters differ in their corner frequencies. The result of passing the fundamental and harmonics through the filters is shown in column E. An abnormality in the initial linear filter (column B, top) has no effect on the response; the red (diabetic) and black (control) waveforms shown in column E (top) are identical. Conversely, an abnormality in the second linear filter (column D, bottom) attenuates the high-frequency harmonics; the red (diabetic) harmonic waveforms are smaller than the black (control) harmonic waveforms (shown in column E, bottom).
These two alternatives were examined by comparing the amplitude and phase of the harmonics generated by 16-Hz sinusoidal flicker. Figure 7 shows data for the 16-Hz fundamental (F) and for each of the first six harmonics. In general, the fundamental and harmonic response components were within the range of the controls for both the NDR and mild NPDR subjects. Small amplitude losses of the 3F component were apparent for five NDR subjects and for two mild NPDR subjects. Of the nine patients who had a fundamental response amplitude at 45.45 Hz that was below the control range, only one had 3F amplitude (equivalent to 48 Hz) that was below the control range. Small amplitude losses were also observed for one NDR and two mild NPDR subjects for the 5F component. Of the 12 patients who had a fundamental response amplitude at 83.33 Hz that was below the control range (Fig. 3), none had 5F amplitude (equivalent to 80 Hz) that was below the control range. A repeated measures 2-way ANOVA with main effects of group (control, NDR, and mild NPDR) and harmonic frequency was performed to compare the amplitudes among the groups for each harmonic. There was no significant effect of group (F = 3.02, P = 0.06), but there was an effect of harmonic frequency (F = 290.97, P < 0.001). The interaction between group and harmonic frequency was not significant (F = 0.97, P = 0.47). Likewise, a repeated measures 2-way ANOVA with main effects of group (control, NDR, and mild NPDR) and harmonic frequency was performed to compare the phases among the groups. There was no significant effect of group (F = 1.40, P = 0.25), but there was a significant effect of harmonic frequency (F = 662.11, P < 0.001); the interaction between these main effects was not significant (F = 1.65, P = 0.10). Thus, neither the mean harmonic amplitude nor phase differed significantly among the groups.
Figure 7.
Log fundamental and harmonic response amplitude elicited by 16-Hz sinusoidal flicker. Other conventions are as in Figure 3.
Discussion
The purpose of the present study was to define the nature and extent of flicker ERG abnormalities in patients who have NDR or mild NPDR. Although both patient groups had significantly attenuated high-frequency flicker ERGs, the temporal frequency at which the amplitude loss became statistically significant differed for the two diabetic groups: significant amplitude reductions were apparent at 55.6 Hz and greater for the NDR subjects and at 38.5 Hz and greater for the mild NPDR subjects. Of note, the mean amplitude of the ISCEV standard flicker ERG, which is recorded near 30 Hz, did not differ significantly from the controls for either patient group. Likewise, the results also showed no statistical differences in mean fundamental phase at any temporal frequency for either diabetic group, compared with the controls. Thus, the flicker ERG abnormalities appear to be restricted to amplitude attenuation elicited by flicker in the range of approximately 40 to 50 Hz and higher.
The amplitude loss for both diabetic groups became greater as temporal frequency increased. This indicates that very rapid flicker (e.g., 100 Hz) would be optimal for identifying ERG amplitude abnormalities in diabetics. However, many of the patients had extinguished flicker ERGs at this frequency, which complicates statistical comparisons between the patient and control groups. To overcome this limitation, the high-frequency ERG cutoff was derived based only on amplitudes that were significantly greater than noise. The high-frequency flicker ERG cutoff is a measure of the highest temporal frequency that elicits an ERG of a criterion amplitude. For our control subjects, the mean cutoff was estimated to be 119 Hz, whereas the cutoffs for the NDR and mild NPDR groups were estimated to be 104 Hz and 98 Hz, respectively. Although large-scale clinical trials are needed to define the usefulness of the high-frequency flicker ERG for detecting neural dysfunction in diabetics, the ROC analysis of the present sample indicated good sensitivity and specificity for separating both diabetic groups from the controls. Thus, the high-frequency cutoff provides a useful index of the high-frequency amplitude attenuation in diabetic subjects.
Analysis of the harmonic components of the ERG recorded in response to slow flicker indicated that the abnormal temporal filtering responsible for the attenuated high-frequency flicker ERG likely arises at the photoreceptor level. This inference is based on a “sandwich model” of retinal processing that has been used to model the flicker ERG.29,30 Specifically, a retinal nonlinearity, which can be characterized as a rectifier, is sandwiched between two linear filters. The initial linear filter likely occurs at the photoreceptor level and is believed to have a corner frequency of approximately 50 Hz.30 Frequencies below approximately 50 Hz are passed without attenuation, whereas the responses to higher frequencies are attenuated. A possible explanation for the high-frequency ERG amplitude loss in our diabetics is that the corner frequency of this initial filter is shifted to a lower temporal frequency. A change in the corner frequency of this filter, however, will not affect the high-frequency nonlinear components of the ERG elicited by slow flicker, as these are generated after the initial linear filter. This is the pattern of abnormality that was generally observed in the diabetic groups: amplitude attenuation for the fundamental component elicited by high-frequency flicker, but normal high-frequency harmonics elicited by slow flicker. For example, the mean fundamental response elicited by 62-Hz flicker was reduced by 34% for the NDR subjects, but their fourth harmonic response to 16-Hz flicker (equivalent to 64 Hz) was only reduced by 1%, on average. Taken together, the findings suggest that the dominant source of the high-frequency flicker ERG attenuation occurs at the photoreceptors for most of our diabetic subjects.
Although an inferred photoreceptor source of the high-frequency flicker ERG attenuation is somewhat unexpected, given the more commonly reported effects of diabetes on inner-retina structure and function,10–12,36,37 the finding is not entirely without precedent. That is, Holopigian et al.38 reported cone sensitivity loss as derived from cone a-wave measurements in a small sample of diabetics who had different stages of retinopathy. They suggested that the ERG sensitivity loss might stem from transduction abnormalities, possibly due to retinal hypoxia. Future work is needed to determine the relationship between response parameters derived from analysis of the cone a-wave (i.e., sensitivity and maximum response amplitude) and the high-frequency flicker ERG attenuation. Nevertheless, the presumed photoreceptor source of the high-frequency flicker ERG attenuation is consistent with the findings of Holopigian et al.38
Electrophysiologic measures of inner-retina function, such as the PhNR39 and pERG,40 were not obtained in this study, as the focus was on photoreceptor and bipolar cell changes in early-stage diabetes. It would be of interest to determine the relationship between the previously reported abnormalities of inner-retina structure and function (e.g., retinal ganglion cell layer thinning and dysfunction)10,11,41–43 and the outer-retina abnormalities observed in this study. An intriguing possibility is that early-stage DR preferentially affects photoreceptor function; then, as the disease progresses, inner-retina dysfunction becomes more apparent. Future longitudinal studies are needed to evaluate this speculation and to define the sequence of events that lead to neural dysfunction at different sites throughout the diabetic retina. Along these lines, a recent review that summarized diabetes-induced alterations in photoreceptor structure and function emphasized the need for further studies of the role of photoreceptors in the pathogenesis of DR.44
In summary, the high-frequency flicker ERG is useful for evaluating neural dysfunction in diabetics who have NDR or mild NPDR, as the response amplitude across the high-frequency range can be significantly abnormal. The high-frequency attenuation in diabetes is reflected in a reduced high-frequency cutoff, such that the maximum flicker rate that elicits a measurable ERG is lower, on average, in the diabetic retina compared with visually normal subjects. The source of the high-frequency attenuation appears, in large part, to occur early in the visual pathway, likely at the photoreceptor level. Notably, marked high-frequency flicker ERG abnormalities have also been reported in patients who have inherited retinal diseases, including retinitis pigmentosa45 and juvenile X-linked retinoschisis.46 Taken together, previous findings and the current data set support the use of higher flicker rates than are traditionally used in ERG recordings to identify and understand temporal processing abnormalities. This approach may also have value as a noninvasive, objective outcome measure for future clinical trials.
Acknowledgments
Supported by National Institutes of Health research grants R01EY026004 (JJM), P30EY001792 (core grant), an unrestricted departmental grant, and a Dolly Green Scholar award (JJM) from Research to Prevent Blindness.
Disclosure: J.J. McAnany, None; J.C. Park, None
References
- 1.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 2.Leasher JL, Bourne RR, Flaxman SR, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39:1643–1649. doi: 10.2337/dc15-2171. [DOI] [PubMed] [Google Scholar]
- 3.Davis MD, Fisher MR, Gangnon RE, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci. 1998;39:233–252. [PubMed] [Google Scholar]
- 4.Wilkinson CP, Ferris FL, III,, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 5.Abramoff MD, Fort PE, Han IC, Jayasundera KT, Sohn EH, Gardner TW. Approach for a clinically useful comprehensive classification of vascular and neural aspects of diabetic retinal disease. Invest Ophthalmol Vis Sci. 2018;59:519–527. doi: 10.1167/iovs.17-21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams AJ, Bearse MA., Jr Retinal neuropathy precedes vasculopathy in diabetes: a function-based opportunity for early treatment intervention? Clin Exp Optom. 2012;95:256–265. doi: 10.1111/j.1444-0938.2012.00733.x. [DOI] [PubMed] [Google Scholar]
- 7.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 8.Henson DB, North RV. Dark adaptation in diabetes mellitus. Br J Ophthalmol. 1979;63:539–541. doi: 10.1136/bjo.63.8.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAnany JJ, Park JC. Reduced contrast sensitivity is associated with elevated equivalent intrinsic noise in type 2 diabetics who have mild or no retinopathy. Invest Ophthalmol Vis Sci. 2018;59:2652–2658. doi: 10.1167/iovs.18-24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dijk HW, Kok PH, Garvin M, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dijk HW, Verbraak FD, Kok PH, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:3660–3665. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk HW, Verbraak FD, Kok PH, et al. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012;53:2715–2719. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dijk HW, Verbraak FD, Stehouwer M, et al. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011;51:224–228. doi: 10.1016/j.visres.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falsini B, Porciatti V, Scalia G, et al. Steady-state pattern electroretinogram in insulin-dependent diabetics with no or minimal retinopathy. Doc Ophthalmol. 1989;73:193–200. doi: 10.1007/BF00155037. [DOI] [PubMed] [Google Scholar]
- 15.Han Y, Adams AJ, Bearse MA, Jr,, Schneck ME. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch Ophthalmol. 2004;122:1809–1815. doi: 10.1001/archopht.122.12.1809. [DOI] [PubMed] [Google Scholar]
- 16.Chan HH, Chu PH, Lung JC, et al. Detection of early functional changes in diabetic retina using slow double-stimulation mfERG paradigm. Br J Ophthalmol. 2011;95:1560–1563. doi: 10.1136/bjo.2010.192476. [DOI] [PubMed] [Google Scholar]
- 17.Lung JC, Swann PG, Chan HH. Early local functional changes in the human diabetic retina: a global flash multifocal electroretinogram study. Graefes Arch Clin Exp Ophthalmol. 2012;250:1745–1754. doi: 10.1007/s00417-012-2010-z. [DOI] [PubMed] [Google Scholar]
- 18.Bresnick GH. Diabetic Retinopathy. St. Louis: Mosby Year Book;; 1991. [Google Scholar]
- 19.Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44:53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- 20.Bresnick GH, Palta M. Temporal aspects of the electroretinogram in diabetic retinopathy. Arch Ophthalmol. 1987;105:660–664. doi: 10.1001/archopht.1987.01060050078042. [DOI] [PubMed] [Google Scholar]
- 21.Jansson RW, Raeder MB, Krohn J. Photopic full-field electroretinography and optical coherence tomography in type 1 diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:989–997. doi: 10.1007/s00417-015-3034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satoh S, Iijima H, Imai M, Abe K, Shibuya T. Photopic electroretinogram implicit time in diabetic retinopathy. Jpn J Ophthalmol. 1994;38:178–184. [PubMed] [Google Scholar]
- 23.Tahara K, Matsuura T, Otori T. Diagnostic evaluation of diabetic retinopathy by 30-Hz flicker electroretinography. Jpn J Ophthalmol. 1993;37:204–210. [PubMed] [Google Scholar]
- 24.Al-Otaibi H, Al-Otaibi MD, Khandekar R, et al. Validity, usefulness and cost of RETeval system for diabetic retinopathy screening. Trans Vis Sci Tech. 2017;6(3):3. doi: 10.1167/tvst.6.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuo M, Kondo M, Hirose A, et al. Screening for diabetic retinopathy using new mydriasis-free, full-field flicker ERG recording device. Sci Rep. 2016;6:36591. doi: 10.1038/srep36591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maa AY, Feuer WJ, Davis CQ, et al. A novel device for accurate and efficient testing for vision-threatening diabetic retinopathy. J Diabetes Complications. 2016;30:524–532. doi: 10.1016/j.jdiacomp.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 28.McAnany JJ, Park JC. Amplitude loss of the high-frequency flicker electroretinogram in early diabetic retinopathy. Retina. doi: 10.1097/IAE.0000000000002262. [published online ahead of print July 16, 2018] [DOI] [PMC free article] [PubMed]
- 29.Alexander KR, Fishman GA, Grover S. Temporal frequency deficits in the electroretinogram of the cone system in X-linked retinoschisis. Vision Res. 2000;40:2861–2868. doi: 10.1016/s0042-6989(00)00119-x. [DOI] [PubMed] [Google Scholar]
- 30.Burns SA, Elsner AE, Kreitz MR. Analysis of nonlinearities in the flicker ERG. Optom Vis Sci. 1992;69:95–105. doi: 10.1097/00006324-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Park JC, Cao D, Collison FT, Fishman GA, McAnany JJ. Rod and cone contributions to the dark-adapted 15-Hz flicker electroretinogram. Doc Ophthalmol. 2015;130:111–119. doi: 10.1007/s10633-015-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meigen T, Bach M. On the statistical significance of electrophysiological steady-state responses. Doc Ophthalmol. 1999;98:207–232. doi: 10.1023/a:1002097208337. [DOI] [PubMed] [Google Scholar]
- 33.McAnany JJ, Alexander KR. Contrast sensitivity for letter optotypes vs. gratings under conditions biased toward parvocellular and magnocellular pathways. Vision Res. 2006;46:1574–1584. doi: 10.1016/j.visres.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Rohaly AM, Owsley C. Modeling the contrast-sensitivity functions of older adults. J Opt Soc Am A. 1993;10:1591–1599. doi: 10.1364/josaa.10.001591. [DOI] [PubMed] [Google Scholar]
- 35.Chang Y, Burns SA, Kreitz MR. Red-green flicker photometry and nonlinearities in the flicker electroretinogram. J Opt Soc Am A. 1993;10:1413–1422. doi: 10.1364/josaa.10.001413. [DOI] [PubMed] [Google Scholar]
- 36.Barber AJ, Baccouche B. Neurodegeneration in diabetic retinopathy: potential for novel therapies. Vision Res. 2017;139:82–92. doi: 10.1016/j.visres.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Lynch SK, Abramoff MD. Diabetic retinopathy is a neurodegenerative disorder. Vision Res. 2017;139:101–107. doi: 10.1016/j.visres.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holopigian K, Greenstein VC, Seiple W, Hood DC, Carr RE. Evidence for photoreceptor changes in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38:2355–2365. [PubMed] [Google Scholar]
- 39.Frishman L, Sustar M, Kremers J, et al. ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol. 2018;136:207–211. doi: 10.1007/s10633-018-9638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bach M, Brigell MG, Hawlina M, et al. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol. 2013;126:1–7. doi: 10.1007/s10633-012-9353-y. [DOI] [PubMed] [Google Scholar]
- 41.Caputo S, Di Leo MA, Falsini B, et al. Evidence for early impairment of macular function with pattern ERG in type I diabetic patients. Diabetes Care. 1990;13:412–418. doi: 10.2337/diacare.13.4.412. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Zhang M, Huang S, Wu D. The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol. 2008;117:129–135. doi: 10.1007/s10633-008-9114-0. [DOI] [PubMed] [Google Scholar]
- 43.Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D. Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol. 2006;50:367–373. doi: 10.1007/s10384-006-0326-0. [DOI] [PubMed] [Google Scholar]
- 44.Kern TS, Berkowitz BA. Photoreceptors in diabetic retinopathy. J Diabetes Investig. 2015;6:371–380. doi: 10.1111/jdi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seiple WH, Siegel IM, Carr RE, Mayron C. Objective assessment of temporal modulation transfer functions using the focal ERG. Am J Optom Physiol Opt. 1986;63:1–6. doi: 10.1097/00006324-198601000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Alexander KR, Barnes CS, Fishman GA. High-frequency attenuation of the cone ERG and ON-response deficits in X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2001;42:2094–2101. [PubMed] [Google Scholar]