Supplemental Digital Content is available in the text.
Keywords: canagliflozin; cardiovascular diseases; diabetes mellitus, type 2; glomerular filtration rate; kidney; renal insufficiency, chronic; sodium glucose cotransporter 2; treatment outcome
Abstract
Background:
Canagliflozin is approved for glucose lowering in type 2 diabetes and confers cardiovascular and renal benefits. We sought to assess whether it had benefits in people with chronic kidney disease, including those with an estimated glomerular filtration rate (eGFR) between 30 and 45 mL/min/1.73 m2 in whom the drug is not currently approved for use.
Methods:
The CANVAS Program randomized 10 142 participants with type 2 diabetes and eGFR >30 mL/min/1.73 m2 to canagliflozin or placebo. The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, with other cardiovascular, renal, and safety outcomes. This secondary analysis describes outcomes in participants with and without chronic kidney disease, defined as eGFR <60 and ≥60 mL/min/1.73 m2, and according to baseline kidney function (eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2).
Results:
At baseline, 2039 (20.1%) participants had an eGFR <60 mL/min/1.73 m2, 71.6% of whom had a history of cardiovascular disease. The effect of canagliflozin on the primary outcome was similar in people with chronic kidney disease (hazard ratio, 0.70; 95% CI, 0.55–0.90) and those with preserved kidney function (hazard ratio, 0.92; 95% CI, 0.79–1.07; P heterogeneity = 0.08). Relative effects on most cardiovascular and renal outcomes were similar across eGFR subgroups, with possible heterogeneity suggested only for the outcome of fatal/nonfatal stroke (P heterogeneity = 0.01), as were results for almost all safety outcomes.
Conclusions:
The effects of canagliflozin on cardiovascular and renal outcomes were not modified by baseline level of kidney function in people with type 2 diabetes and a history or high risk of cardiovascular disease down to eGFR levels of 30 mL/min/1.73 m2. Reassessing current limitations on the use of canagliflozin in chronic kidney disease may allow additional individuals to benefit from this therapy.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifiers: NCT01032629, NCT01989754.
Clinical Perspective.
What Is New?
Canagliflozin is currently not approved for the treatment of type 2 diabetes in people with estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2 because glycemic efficacy is dependent on kidney function.
In the CANVAS Program, the effect of canagliflozin on glycohemoglobin was progressively attenuated at lower eGFR levels, but blood pressure and body weight reductions were comparable.
The reduction in risk of major adverse cardiovascular events, hospitalization for heart failure, and progression of kidney disease appeared similar across different levels of kidney function down to eGFR 30 mL/min/1.73 m2.
Safety outcomes were also mostly consistent, but risk of hypoglycemia may increase as eGFR declines.
What Are the Clinical Implications?
People with type 2 diabetes and chronic kidney disease are at high risk of cardiovascular events and progression to end-stage kidney disease.
Canagliflozin could be considered for the management of type 2 diabetes in people at high cardiovascular risk with eGFR down to 30 mL/min/1.73 m2 to reduce the risk of both cardiovascular and renal outcomes.
Reconsidering current eGFR-based limitations on the use of canagliflozin may allow additional individuals to benefit from this therapy.
Excess mortality and morbidity in type 2 diabetes primarily result from cardiovascular and kidney disease.1,2 Sodium glucose cotransporter 2 (SGLT2) inhibitors are a class of medications that promote urinary glucose excretion and natriuresis, and alter glomerular hemodynamics.3 These changes have been noted to result in improvements in glycemic status, blood pressure, weight, and proteinuria in patients with type 2 diabetes,3 and have translated into a reduction in cardiovascular events and preservation of kidney function in large cardiovascular outcome trials.4–6
The glucose-lowering effect of SGLT2 inhibitors is reliant on glomerular filtration. Previous studies have shown that the glycemic efficacy of SGLT2 inhibitors is attenuated in people with chronic kidney disease (CKD), defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2.7,8 As such, these agents are not currently recommended for use in people with significantly reduced kidney function, defined as an eGFR <45 mL/min/1.73 m2 with canagliflozin and empagliflozin or <60 mL/min/1.73 m2 with dapagliflozin and ertugliflozin.9,10 Conversely, the efficacy of SGLT2 inhibitors at reducing blood pressure and proteinuria may be maintained in people with diabetes and CKD.8,11
As individuals with CKD are among the highest-risk groups for cardiovascular disease and progression to end-stage kidney disease,12 it is important to understand whether the benefits of SGLT2 inhibitors for cardiovascular events and progression of renal disease are similar to those in people with normal kidney function.
We undertook a range of post hoc analyses of data from the CANVAS Program to determine the effect of canagliflozin on cardiovascular, renal, and safety outcomes across different levels of kidney function to better understand whether this agent may have a role in people with type 2 diabetes and CKD at high cardiovascular risk, including those with eGFR between 30 and 45 mL/min/1.73 m2 for whom this treatment is not currently approved.
Methods
Data from the CANVAS Program will be made available in the public domain via the Yale University Open Data Access Project (http://yoda.yale.edu/) once the product and relevant indication studied have been approved by regulators in the United States and European Union, and the study has been completed for 18 months. The trial protocols and statistical analysis plans were published along with the primary CANVAS Program manuscript.4
Study Design and Participants
The CANVAS Program comprised 2 multicenter, double-blind, placebo-controlled, randomized trials, CANVAS and CANVAS-R, conducted in comparable populations and designed to collectively assess the cardiovascular safety and efficacy of canagliflozin, as well as its effect on renal and adverse outcomes, in subjects with type 2 diabetes and a history or high risk of cardiovascular disease. Both trials were scheduled for joint closeout and analysis when at least 688 cardiovascular events occurred and the last randomized participant had undergone at least 78 weeks of follow-up.4 Local institutional ethics committees approved the trial protocols at each site, and these are available online (ClinicalTrials.gov NCT01032629 and NCT01989754). All participants provided written informed consent.
The main entry criteria for both trials were identical and included participants with type 2 diabetes (glycohemoglobin [HbA1c] ≥7.0% and ≤10.5%) who were either ≥30 years old with established atherosclerotic vascular disease or ≥50 years old with 2 or more cardiovascular risk factors. These risk factors included duration of diabetes of at least 10 years; systolic blood pressure >140 mm Hg while receiving 1 or more antihypertensive agents; current smoking; microalbuminuria or macroalbuminuria; or high-density lipoprotein cholesterol level <1 mmol/L. Participants with a baseline eGFR <30 mL/min/1.73 m2 were excluded.
Randomization and Masking
All potentially eligible participants underwent a 2-week, single-blind, placebo run-in period before randomization. Participants in CANVAS were randomly assigned in a 1:1:1 ratio to receive canagliflozin 100 mg daily, canagliflozin 300 mg daily, or placebo, while participants in CANVAS-R were randomly assigned in a 1:1 ratio to receive canagliflozin 100 mg daily or matching placebo, with an optional increase to 300 mg or matching placebo daily starting from week 13. Randomization was performed centrally through a web-based response system with the use of a computer-generated randomization schedule with randomly permuted blocks that were prepared by the trial sponsor. All participants and trial staff were blinded to individual treatment allocations until the end of the trial.
Procedures
Face-to-face follow-up was scheduled at least 3 times in the first year and at intervals of every 6 months thereafter with telephone follow-up between face-to-face assessments. Serum creatinine was measured at least 3 times in the first year, and then once every 26 weeks. Urine albumin/creatinine ratio (UACR) was measured at week 12, and then annually in CANVAS, and every 26 weeks in CANVAS-R. Off-treatment serum creatinine was measured approximately 30 days after cessation of randomized treatment in CANVAS-R participants. Adverse event assessment was performed at each visit. Other glycemic and cardiovascular risk factor management, including renin-angiotensin system (RAS) blockade, was guided by best practice in accordance with local guidelines.
Outcomes
Definitions for the clinical outcomes of the CANVAS Program have been previously published.4
The primary outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. Other secondary cardiovascular outcomes included cardiovascular death, fatal/nonfatal myocardial infarction, fatal/nonfatal stroke, and hospitalization for heart failure.
The main renal outcomes were sustained and independently adjudicated composites of end-stage kidney disease, renal death, and either 40% decrease in eGFR or doubling of serum creatinine. End points of 40% reduction in eGFR and doubling of serum creatinine were sent for adjudication if sustained for 2 consecutive measures of ≥30 days apart or occurring on the last available measure. Further analyses of the adjusted mean eGFR slope difference between canagliflozin and placebo groups were also performed. Central end point adjudication committees blinded to treatment allocation assessed cardiovascular, renal, and key safety outcomes.
The Modification of Diet in Renal Disease Study equation was used to calculate eGFR based on centrally measured serum creatinine collected at study visits. Albuminuria was measured in first-morning void urine specimens and calculated as a UACR.
Adverse events, both serious and nonserious, were collected and reported for the CANVAS trial until January 2014, as mandated by the US Food and Drug Administration and other regulatory bodies as a requirement for initial approval for the use of canagliflozin. After this time, only serious adverse events, adverse events leading to study drug discontinuation, or selected adverse events of interest were collected in the CANVAS trial. We therefore reported all adverse events for the CANVAS trial separately, along with all serious adverse events across the CANVAS Program (CANVAS and CANVAS-R).
Statistical Analysis
Baseline characteristics across eGFR subgroups were compared using χ2 and ANOVA tests for dichotomous and categorical variables.
The effects of canagliflozin on the primary and other cardiovascular, renal, and safety outcomes were analyzed in participants with and without CKD (defined as <60 and ≥60 mL/min/1.73 m2. Analyses were also conducted for all outcomes using more granular eGFR categories (<45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2.
Hazard ratios (HRs) and 95% CIs for primary and other cardiovascular and renal outcomes were estimated with Cox regression models, with stratification according to trial and history of cardiovascular disease (except for renal outcomes) using an intention-to-treat approach, for all canagliflozin groups combined versus placebo. Annualized incidence rates were calculated per 1000 patient-years of follow-up. Sensitivity analyses adjusting for competing risk of death were performed for the main cardiovascular and renal outcomes using the Fine and Gray method.13
The average change in eGFR over time and the differences between canagliflozin and placebo arms were assessed by a piecewise linear mixed-effect model in 2 time periods: baseline to week 13, and week 13 to last available measures during the trial period, using an intention-to-treat approach. A time spline variable measuring the follow-up time from week 13 was introduced in the model to accommodate the nonlinear trends of the eGFR time trajectory. eGFR data collected at the scheduled visits were regressed by the fixed effects with terms for treatment and study, and with linear covariates of time, time spline, and interactions of treatment by time and treatment by the spline variable. Intercept, time, and time spline were included as random effects to allow variation between participants. Time covariates included in the model were calculated in years in order to estimate annualized changes in eGFR. In CANVAS-R, the difference in change from baseline to off-treatment eGFR levels between the canagliflozin and placebo arms was assessed based on serum creatinine measurements approximately 30 days after treatment discontinuation.
The effect of canagliflozin on intermediate markers of cardiovascular risk, including HbA1c, blood pressure, and body weight, were calculated as mean change from baseline across the entire follow-up period. The average change in these continuous outcomes (HbA1c, blood pressure, and body weight) over time, and the difference between canagliflozin and placebo, were analyzed using mixed-effect models for repeated measurements that included all the post-baseline data up to week 338 and the covariates for study, visit, treatment, baseline measures, treatment-by-visit, and baseline-by-visit interactions. Due to the highly skewed distribution of UACR data, UACR were log-transformed, and the geometric mean of post-baseline UACR was estimated using the similar mixed-effect model. Changes in albuminuria were calculated as the ratio of the geometric mean of postrandomization UACR measures with canagliflozin compared to placebo.
Heterogeneity of treatment effect across different levels of kidney function was tested by adding eGFR as a covariate and a term for eGFR by treatment interaction to the relevant model. Terms for eGFR by time interaction were also included in the piecewise linear mixed model. The global P values for heterogeneity across all levels of baseline eGFR were obtained through the likelihood ratio test. For major cardiovascular, renal, and safety outcomes, further analyses were performed, investigating effect modification by eGFR as a continuous variable.
For safety outcomes, on-treatment analysis was performed (with data from participants who had a safety outcome while they were receiving canagliflozin or placebo, or within 30 days after discontinuation of the drug or placebo). The exception was for amputation and fracture outcomes, where analyses included participants who received at least 1 dose of canagliflozin or placebo and had an event at any time during follow-up.
Absolute risk differences for the primary outcome, hospitalization for heart failure, progression to the composite renal outcome, and risk of amputation were estimated by subtracting the incidence rates (per 1000 patient-years) of placebo from those of canagliflozin and multiplying by 5 years. The CIs for these estimates were similarly calculated by multiplying both the lower and upper CI values (which were estimated using the method described by Altman and Andersen14) by 5. The heterogeneity tests for absolute risk differences were performed using a nonlinear mixed-effect model with treatment, subgroup, and treatment-by-subgroup interaction as the covariates.
Analyses were performed with SAS software, version 9.2, and SAS Enterprise Guide, version 7.11.
Role of the Funding Source
The trials were sponsored by Janssen Research & Development, LLC, and were conducted collaboratively by the sponsor, an academic steering committee, and an academic research organization, George Clinical. The sponsor was responsible for study oversight and data collection, and had a representative on the Steering Committee, which was responsible for study design, data analysis, data interpretation, and writing of this report. All authors had full access to all the data and had final responsibility for the decision to submit for publication.
Results
The CANVAS Program randomized 10 142 participants with a mean follow-up duration of 188.2 weeks. At baseline, 2039 (20.1%) participants had CKD (mean age, 68 years; blood pressure, 137/76 mm Hg; HbA1c, 8.3%; eGFR, 49 mL/min/1.73 m2; median UACR, 22 mg/g), of whom 71.6% had a prior history of cardiovascular disease. This included 554 participants (5.5%) in the eGFR <45 mL/min/1.73 m2 category, among whom 73.3% had a history of cardiovascular disease.
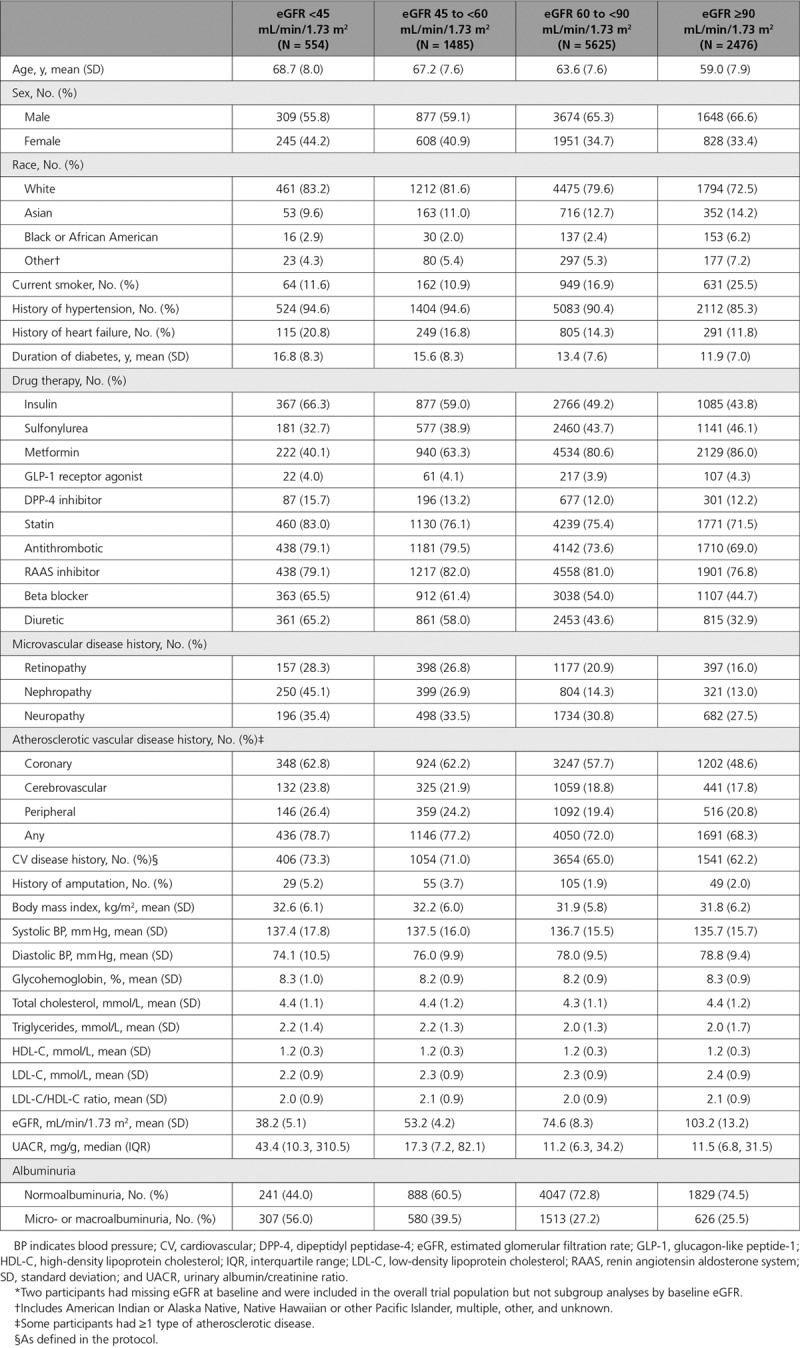
Baseline characteristics of participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 are presented in Table 1. In progressively lower categories of eGFR, participants were older and more likely to be female; be white; have a longer duration of diabetes; have established micro- or macrovascular disease; have a history of heart failure, micro- or macroalbuminuria; and be treated with insulin and cardiovascular protective therapies (all P<0.0001). Baseline characteristics for participants with and without CKD were well balanced across randomized groups and have been previously published.15
Table 1.
Characteristics of Participants With eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at Baseline*
Intermediate Outcomes
Canagliflozin significantly reduced HbA1c, systolic blood pressure, body weight, and albuminuria compared to placebo in participants across all levels of kidney function, although effects on HbA1c were attenuated progressively in lower eGFR subgroups (Figure 1). The placebo-adjusted mean difference in HbA1c in participants with baseline eGFR ≥90, 60 to <90, 45 to <60, and <45 mL/min/1.73 m2 was −0.76%, −0.57%, −0.45%, and −0.35%, respectively (P heterogeneity <0.0001). In contrast, reductions in body weight (−2.45, −2.23, −1.95, and −2.30 kg) and blood pressure (−3.92, −4.06, −3.66, and −3.29 mm Hg) were similar across the respective eGFR subgroups (P heterogeneity = 0.16 and 0.46). The geometric mean ratio of UACR compared to placebo was −17%, −17%, −26%, and −13% for the same eGFR categories (P heterogeneity = 0.01).
Figure 1.
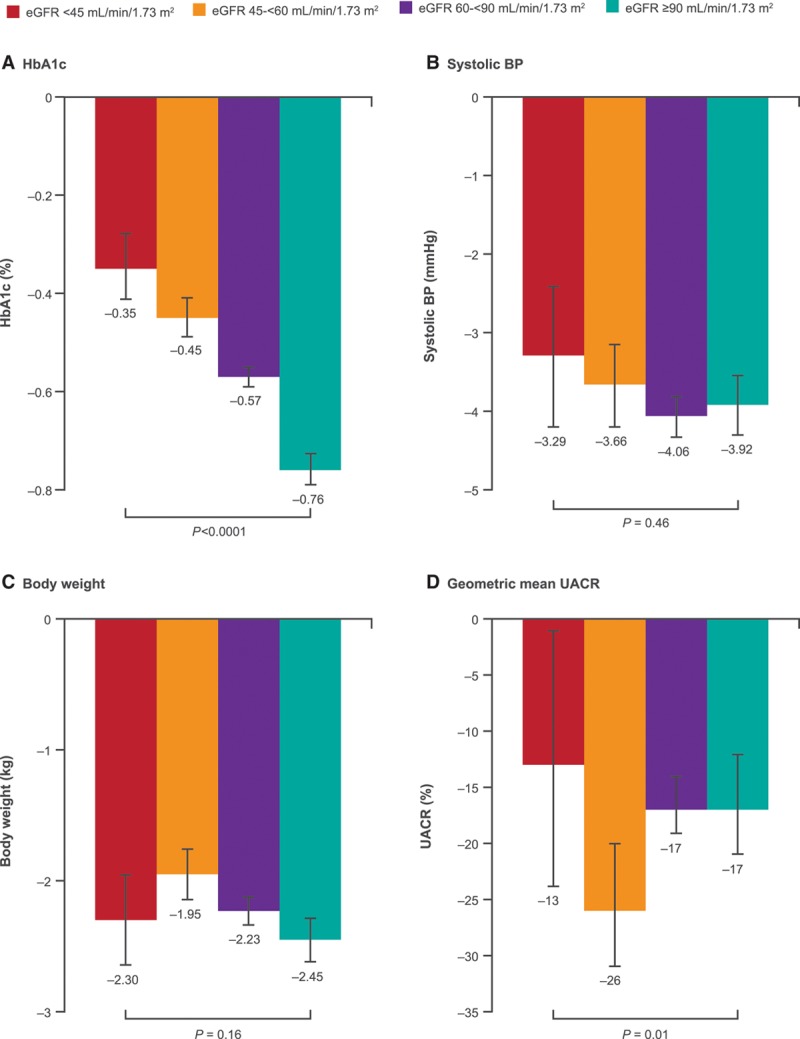
Changes in intermediate outcomes with canagliflozin compared to placebo in participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. Represents the mean difference in change from baseline between canagliflozin and placebo from post-baseline to end of follow-up, except for UACR, where it is percent change in the geometric mean of canagliflozin relative to placebo. BP indicates blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycohemoglobin; and UACR, urinary albumin/creatinine ratio.
When intermediate outcomes were compared in participants with and without CKD (Figure I in the online-only Data Supplement), similar results were observed; however, the effect of canagliflozin on body weight was attenuated in participants with CKD (−1.32 kg versus −1.67 kg; P heterogeneity = 0.0002).
Cardiovascular Outcomes
The effects of canagliflozin on cardiovascular outcomes stratified into 4 eGFR subgroups are summarized in Figure 2. Cardiovascular outcomes in participants with CKD are shown in Figure II in the online-only Data Supplement and compared to those with preserved kidney function in Figures III and IV in the online-only Data Supplement.
Figure 2.
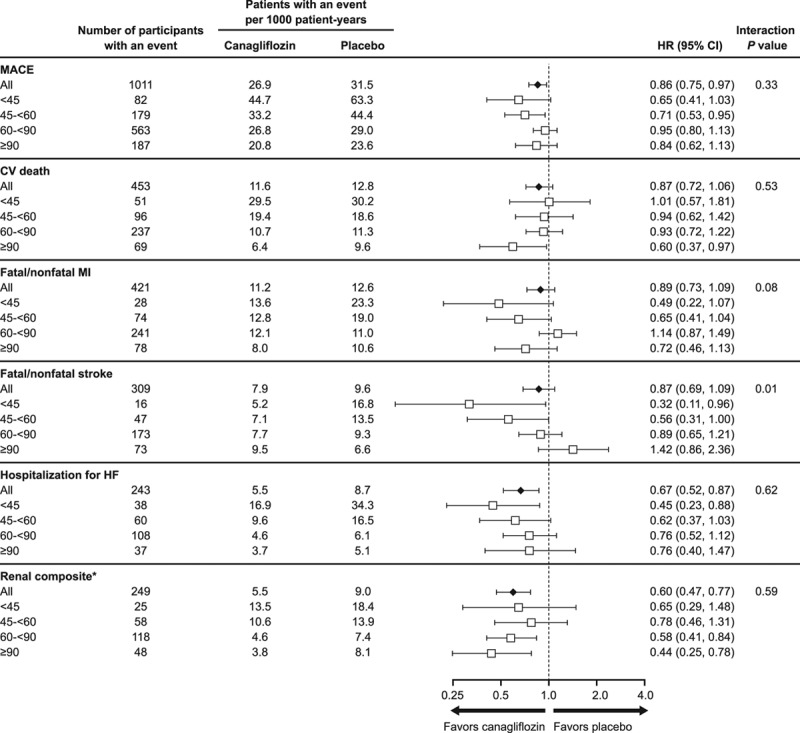
Effects of canagliflozin on cardiovascular and renal outcomes in participants according to baseline eGFR categories <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2. CV indicates cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiovascular event; and MI, myocardial infarction. *Renal composite: 40% decrease in eGFR, end-stage kidney disease, or renal death.
The relative risk reduction in the primary outcome for the overall trial population (HR, 0.86; 95% CI, 0.75–0.97) was similar across 4 eGFR subgroups and for participants with and without CKD (P heterogeneity = 0.33 and 0.08, respectively). Similarly, the effect on cardiovascular death (HR, 0.87; 95% CI, 0.72–1.06) was not modified by baseline kidney function (P heterogeneity >0.50).
While overall effects on fatal/nonfatal myocardial infarction (HR, 0.89; 95% CI, 0.73–1.09) and hospitalization for heart failure (HR, 0.67; 95% CI, 0.52–0.87), were consistent across 4 eGFR subgroups (P heterogeneity = 0.08 and >0.50, respectively), heterogeneity was observed for the effect on fatal/nonfatal stroke (HR, 0.87; 95% CI, 0.69–1.09), with possibly greater benefits with declining kidney function (P heterogeneity = 0.01). The same effect modification was observed for participants with and without CKD (P heterogeneity = 0.01; Figure IV in the online-only Data Supplement). When interaction tests were undertaken using eGFR as a continuous variable, heterogeneity was again found for the effect on stroke (P heterogeneity = 0.004), but not any of the other cardiovascular outcomes (all P heterogeneity >0.20). Results for all cardiovascular outcomes were similar in sensitivity analyses adjusted for competing risk of death.
Renal Outcomes
The reduction in risk of progression to the adjudicated renal composite outcome of sustained 40% decrease in eGFR, end-stage kidney disease, or renal death with canagliflozin in the overall trial population (HR, 0.60; 95% CI, 0.47–0.77) was consistent across 2 and 4 eGFR subgroups (P heterogeneity = 0.28 and >0.50, respectively), and when doubling of serum creatinine was substituted for 40% decrease in eGFR in the renal composite (HR, 0.53; 95% CI, 0.33–0.84 for all participants; P heterogeneity = 0.21 and >0.50, respectively). When interaction tests were undertaken using eGFR as a continuous variable, the renoprotective effect of canagliflozin (for both 40% decrease in eGFR and doubling of serum creatinine–based composite outcomes) continued to suggest benefit at all levels of kidney function, but may be attenuated with declining kidney function (P heterogeneity = 0.02 and 0.01, respectively). Effects on the composite renal outcomes were similar in sensitivity analyses adjusting for competing risk of death.
The difference in eGFR slope between canagliflozin and placebo arms varied during follow-up. Within the first 13 weeks, participants who received canagliflozin experienced a decline in eGFR, which was similar in participants with eGFR ≥90, 60 to <90, 45 to <60, and <45 mL/min/1.73 m2 at baseline (placebo-subtracted differences of −1.89, −2.33, −2.85, and −2.75 mL/min/1.73 m2, respectively; P heterogeneity = 0.09). From week 13 to the end of follow-up (ie, the chronic eGFR slope), canagliflozin significantly slowed the annual decline in kidney function in all subgroups (Figure 3), with placebo-subtracted mean slope differences of 1.47, 1.09, 1.05, and 1.35 mL/min/1.73 m2 per year for respective eGFR subgroups (P heterogeneity = 0.21). The overall eGFR slope during follow-up for canagliflozin- and placebo-treated participants in each eGFR subgroup is shown in Figure V in the online-only Data Supplement. In participants who were re-evaluated approximately 30 days after treatment discontinuation (as part of the CANVAS-R protocol), the differences in change from baseline to off-treatment eGFR levels between canagliflozin and placebo arms across 2 and 4 eGFR subgroups are summarized Figures VI and VII in the online-only Data Supplement, respectively.
Figure 3.
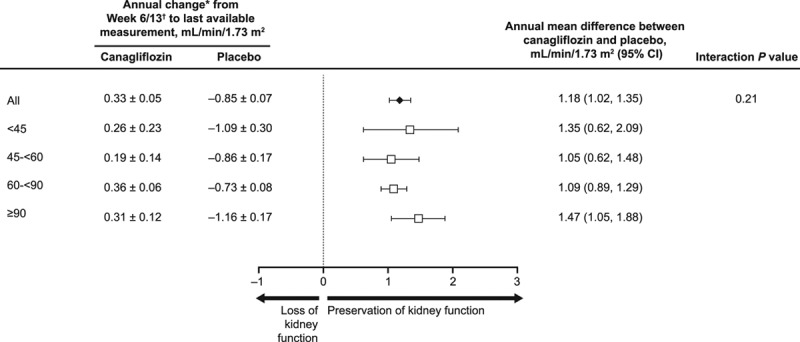
Effect on eGFR slope from week 6/13 until end of follow-up in participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. eGFR indicates estimated glomerular filtration rate; and SE, standard error. *Data are mean±SE. †Data are reported for week 6 in CANVAS and week 13 in CANVAS-R.
Adverse Outcomes
Effects of canagliflozin on safety outcomes were consistent across eGFR subgroups, including for serious renal safety outcomes (Figures 4 and 5). The exception was a borderline significant interaction test observed for hypoglycemia across 4 eGFR subgroups (P heterogeneity = 0.06), which persisted when assessed using eGFR as a continuous variable (P heterogeneity = 0.004), although participants were more likely to be receiving concomitant insulin therapy as kidney function declined. Relative effects on other safety outcomes were broadly consistent when interaction tests were applied across 4 eGFR subgroups or were undertaken using eGFR as a continuous variable.
Figure 4.
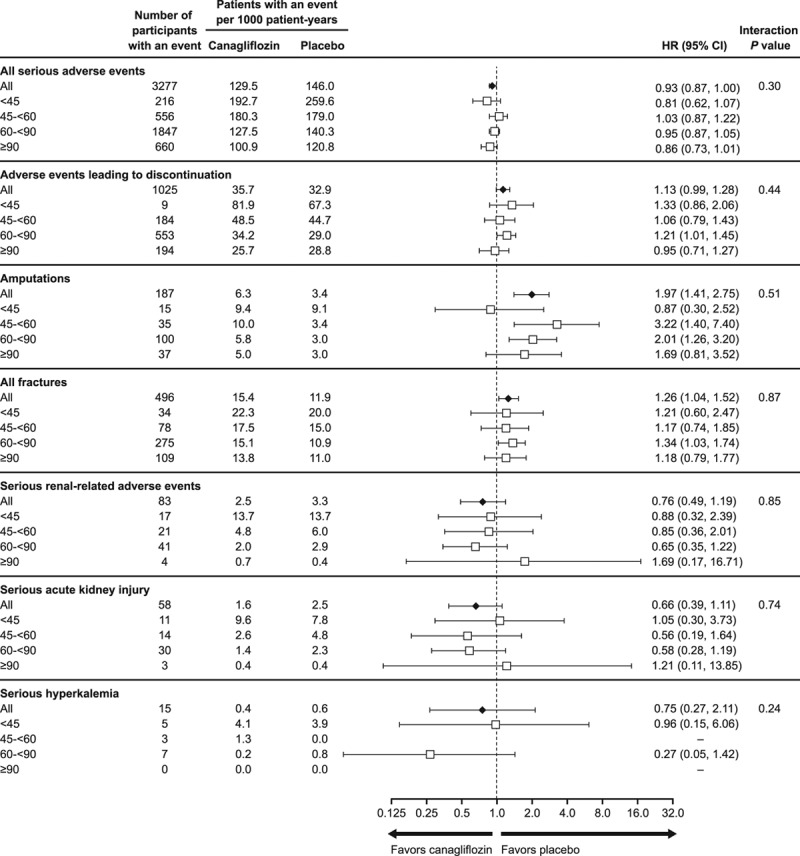
Adverse events across the CANVAS Program in participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. eGFR indicates estimated glomerular filtration rate; and HR, hazard ratio.
Figure 5.
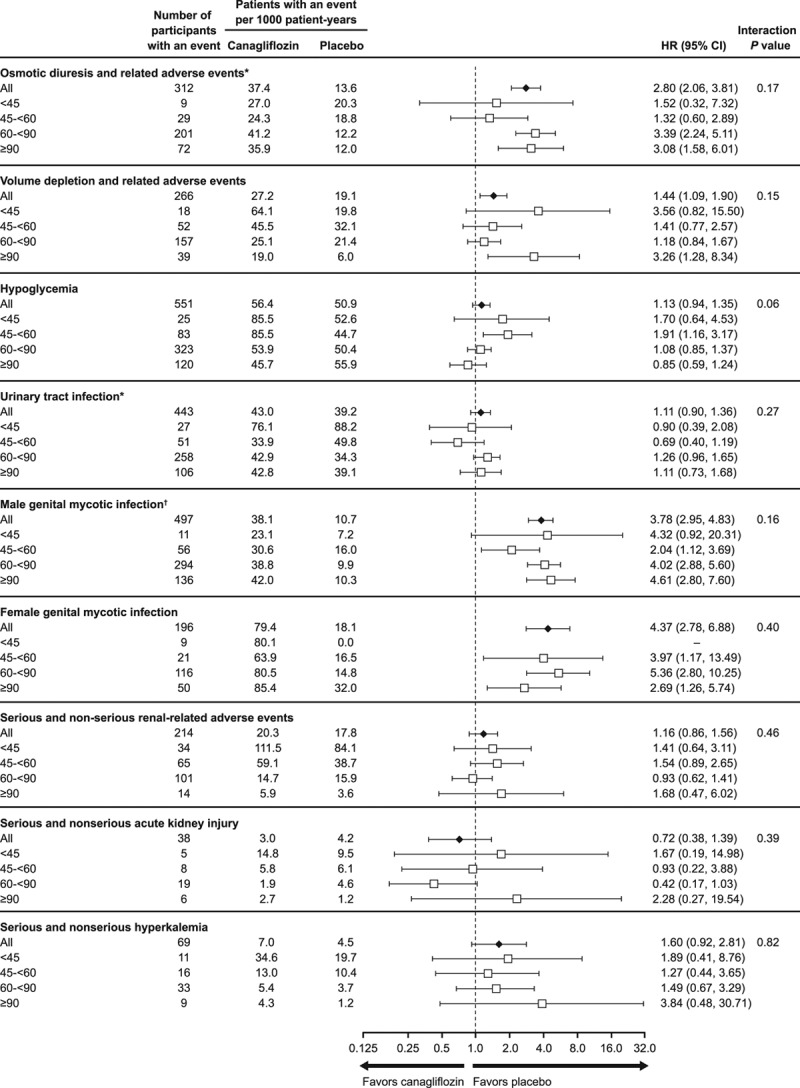
Adverse events collected in CANVAS alone in participants with eGFR ≤45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. The annualized incidence rates, estimates for HRs, and 95% CIs are reported for the CANVAS study alone through January 7, 2014, because after this time, only serious adverse events or adverse events leading to study drug discontinuation, or selected adverse events of interest were collected. eGFR indicates estimated glomerular filtration rate; and HR, hazard ratio. *Note that 1 patient in the placebo group who experienced an event had a missing baseline eGFR value; therefore, this patient is only counted in the overall total. †Data collected in CANVAS and CANVAS-R. Includes infections of male genitalia and phimosis and excludes circumcision.
Absolute Risk Reduction
The absolute differences in risk between canagliflozin and placebo across 4 eGFR subgroups and among participants with and without CKD are shown in Figure 6 and Figure VIII in the online-only Data Supplement. Absolute effects were consistent across 4 eGFR subgroups, with the exception of a borderline possibly greater absolute reduction in risk of hospitalization for heart failure with declining kidney function (P heterogeneity = 0.06; Figure 6). Similar possible heterogeneity for the absolute effect on heart failure was also observed when comparing participants with and without CKD (P heterogeneity = 0.02; Figure VIII in the online-only Data Supplement).
Figure 6.
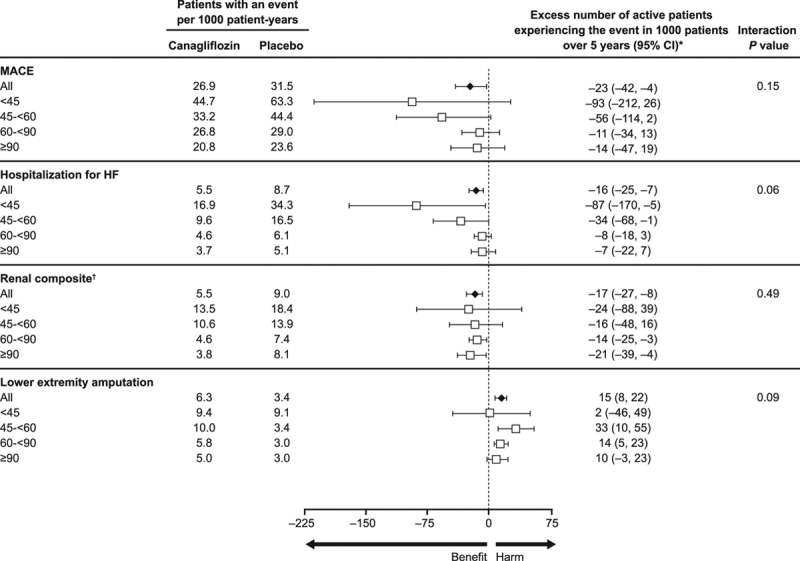
Absolute benefits and risks per 1000 patients over 5 years with canagliflozin versus placebo in the overall population and in participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. eGFR indicates estimated glomerular filtration rate; HF, heart failure; and MACE, major adverse cardiovascular event. *Excess number is relative to the placebo group. If the number is negative, then fewer participants in the canagliflozin group experienced the event compared to the placebo group. †Renal composite: 40% decrease in eGFR, end-stage kidney disease, or renal death.
Discussion
In this secondary analysis of the CANVAS Program, the relative effects of canagliflozin on the primary and most other cardiovascular outcomes were consistent across different levels of kidney function with possibly heterogeneity observed only for the outcome of fatal/nonfatal stroke. Absolute effects were also similar, with the exception of a possibly greater absolute benefit with respect to heart failure across progressively lower eGFR subgroups. These data also suggest the renoprotective effects of canagliflozin are not likely to be modified by baseline eGFR, with slower rates of kidney function loss at all levels of baseline kidney function and similar effects on the composite renal outcomes in all eGFR strata, while also raising the possibility that the magnitude of benefit might be somewhat attenuated in participants with lower baseline eGFR levels. Taken together, these data suggest that the cardiovascular and renal effects of canagliflozin are consistent across different levels of kidney function in people with type 2 diabetes with or at high risk of cardiovascular disease down to eGFR levels of 30 mL/min/1.73 m2.
One of the hallmark characteristics of this class of agents is their lesser effect on urinary glucose excretion with decreasing kidney function,16,17 which has been demonstrated with a number of agents in the class,8,18,19 and is likely to be mediated by reduced available nephron mass, and therefore, diminished glucose reabsorption capacity. In contrast, while effects on sodium reabsorption and natriuresis are equally likely to be dependent on kidney function, the blood pressure–lowering effects of canagliflozin were similar across eGFR subgroups. A synergistic hemodynamic effect with other blood pressure–lowering agents or diuretics could potentially explain these findings, given that participants were more likely to be taking these drugs in progressively lower eGFR categories. Another possibility may be that patients with CKD exhibit higher sensitivity to changes in renal sodium and glucose handling,8 or that there are other as yet unrecognized mechanisms involved. The variations in UACR reduction across eGFR subgroups were not explained by the use of RAS blockade, which was similar at baseline and during follow-up for the canagliflozin and placebo arms across all levels of kidney function, highlighting the need for further mechanistic insights into SGLT2 inhibition.
The reason that the relative cardiovascular benefits are at least as large in participants with CKD is therefore unclear, and the results of this analysis require confirmation and clarification in dedicated, separately powered trials in people with diabetic kidney disease. Our findings are broadly consistent with a similar analysis of empagliflozin.18 While it is unclear why treatment heterogeneity was observed for the outcome of stroke, qualitatively similar findings have been reported with empagliflozin,18 supporting the need for better understanding of this finding. Given the attenuated effect on HbA1c in patients with CKD, as well as the inconsistent evidence for glucose lowering for the prevention of macrovascular complications in type 2 diabetes,20–22 these data suggest that the cardiovascular benefits in patients with CKD are not likely to be driven by glucose excretion alone.22 The preserved blood pressure–lowering effect in this population highlights sodium and volume overload as critical contributors to the increased cardiovascular and renal burden in people with CKD.23 Other mechanisms may also contribute; for example, there is evidence that SGLT2 inhibition modestly increases the production of circulating ketones, thus providing an alternative energy substrate that might improve myocardial cell function in the setting of hypoxic or ischemic stress.22,24–26 The strength of these findings is supported by the consistency of the results when eGFR is further subdivided into a greater number of categories and analyzed as a continuous variable.
It is likely that SGLT2 inhibitors confer kidney benefits through a direct renal mechanism. Head-to-head trials with other glucose-lowering agents have shown that canagliflozin slows decline in kidney function independent of glycemic control.19 An increasingly cited physiological explanation for the renoprotective properties of this class of agents is their ability to enhance afferent arteriolar tone by manipulating tubuloglomerular feedback,3 thereby reducing intraglomerular pressure via mechanisms that parallel and are complementary to those of RAS blockade.27 Clinically this is reflected in the acute dose-dependent decline in eGFR on initiation of SGLT2 inhibition, followed by stabilization and preservation of kidney function, which has been demonstrated in trials of this and other agents in the class.6,15 The data from this analysis suggest that these effects on renal hemodynamics (as measured by changes in albuminuria and eGFR), and the likely kidney protection that results, might be similar across different levels of kidney function. The ongoing CREDENCE trial (NCT02065791) will specifically study the effects of SGLT2 inhibition in 4401 participants with established kidney disease and macroalbuminuria, almost 60% of whom have eGFR <60 mL/min/1.73 m2 at baseline, and will provide additional data in this regard.28 Other dedicated CKD outcome trials for empagliflozin (EMPA-KIDNEY) and dapagliflozin (DAPA-CKD) have also been announced or are underway.29,30 Given the unique renal hemodynamic effects of SGLT2 inhibition, there is considerable interest as to whether the potential benefits may extend to CKD patients without diabetes. As such, both trials aim to recruit patients with and without diabetes, and will be powered to detect benefits in the nondiabetic cohort.
Across all outcomes, event rates increased with declining kidney function, underscoring the fact that CKD is a cause, consequence, and risk multiplier of cardiovascular disease.31,32 The absolute risk reductions in these outcomes among participants with CKD tended to be larger than those observed in the overall trial population and are likely to be greater than the increase in risk of amputations, especially major amputations. These benefits are also likely to be clinically important, especially as they occurred in addition to the standard of care that included RAS blockade in approximately 80% of participants. Importantly, there appears to be no increased risk of renal adverse events, including acute kidney injury or hyperkalemia, when SGLT2 inhibitors are used in combination with RAS blockade in participants with CKD, including those with eGFR between 30 and 45 mL/min/1.73 m2.
This study has a number of strengths. Data were derived from a large, multicenter, placebo-controlled trial program that was conducted to an extremely high standard. The cardiovascular and renal outcomes are clinically meaningful and were adjudicated by expert committees. While not explicitly powered to assess results in participants with established kidney disease, this represents one of the largest analyses to date of the effects of SGLT2 inhibition on cardiovascular and renal outcomes in this high cardiovascular and renal risk population.
This secondary analysis of the CANVAS Program is limited by drawbacks inherent to all post hoc analyses of randomized trials. The interaction P values reported for eGFR subgroups are nominal in nature, and no correction was applied for multiple comparisons. The relatively small number of participants with eGFR <45 mL/min/1.73 m2 precludes our ability to draw definitive conclusions about the effects of canagliflozin in participants with significantly reduced kidney function, but underscores the importance of CREDENCE and other planned or ongoing CKD outcome trials for dapagliflozin and empagliflozin.29,30 The high proportion of participants with a history of cardiovascular disease limits the generalizability of these findings to the broader CKD population. However, the magnitude and consistency of effect size on a range of outcomes, as well as concordance with subgroup data from the EMPA-REG OUTCOME trial, support the likely beneficial effects of SGLT2 inhibitors in high cardiovascular risk patients with type 2 diabetes and eGFR levels down to 30 mL/min/1.73 m2. The number of events for some outcomes, particularly progression to end-stage kidney disease, were too few to draw definitive conclusions. Finally, participants with an eGFR below 30 mL/min/1.73 m2 were excluded, so the effects in this population remain to be determined.
In conclusion, despite smaller effects on HbA1c with declining kidney function, the effects of canagliflozin on cardiovascular and renal outcomes were not modified by baseline eGFR in people with type 2 diabetes and a history or high risk of cardiovascular disease. Reassessing current limitations on the use of canagliflozin in CKD may allow additional individuals to benefit from this therapy.
Acknowledgments
The paper is presented on behalf of the CANVAS Program collaborative group. The authors thank all investigators, study teams, and patients for participating in these studies. The authors thank the following individuals for their contributions to the statistical monitoring/analyses and the protocol development, safety monitoring, and operational implementation over the duration of both studies: Lyndal Hones, Sharon Dunkley, Tao Sun, Gordon Law, George Capuano, Severine Bompoint, Laurent Billot, Mary Lee, Joan Lind, Roger Simpson, Mary Kavalam, Ed Connell, Jacqueline Yee, Dainius Balis, Frank Vercruysse, Elisa Fabbrini, Richard Oh, Nicole Meyers, Wayne Shaw, and Gary Meininger. B.L.N., T.O., H.D., and Q.L. contributed to the analysis and interpretation of data. B.N., D.R.M., D.d.Z., K.W.M., G.F., M.D., N.R., M.J.J., G.B., and V.P. contributed to the design and conduct of the study and the interpretation of the data. B.L.N. and V.P. wrote the first draft of the manuscript, and all authors contributed to subsequent drafts and approved the final version for submission. V.P. and M.D. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
This work was supported by Janssen Research & Development, LLC. The sponsor was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. Medical writing support was provided by Kimberly Dittmar, PhD, of MedErgy, and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corp.
Disclosures
Dr Neuen is supported by the John Chalmers PhD Scholarship from The George Institute for Global Health and a University Postgraduate Award from University of New South Wales Sydney. Dr Ohkuma is supported by the John Chalmers Clinical Research Fellowship of the George Institute. Q. Li reports being full-time employees of The George Institute for Global Health. Dr Neal has received research support from the Australian National Health and Medical Research Council Principal Research Fellowship and from Janssen, Roche, Servier, and Merck Schering-Plough; and has served on advisory boards and/or has been involved in continuing medical education programs for Abbott, Janssen, Novartis, Pfizer, Roche, and Servier, with any consultancy, honoraria, or travel support paid to his institution. Dr Matthews has received research support from Janssen; served on advisory boards and as a consultant for Novo Nordisk, Novartis, Sanofi-Aventis, Janssen, and Servier; and has given lectures for Novo Nordisk, Servier, Sanofi-Aventis, Novartis, Janssen, Mitsubishi Tanabe, and Aché Laboratories. Dr de Zeeuw has served on advisory boards and/or as a speaker for AbbVie, Astellas, Fresenius, Janssen, Boehringer Ingelheim, Bayer, and Mitsubishi Tanabe, with all consultancy honoraria paid to his institution. Dr Mahaffey’s disclosures can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey. Dr Fulcher has received research support from Novo Nordisk, and served on advisory boards and as a consultant for Janssen, Novo Nordisk, Boehringer Ingelheim, and Merck Sharp & Dohme. Drs Desai and Rosenthal and H. Deng are full-time employees of Janssen Research & Development, LLC, and hold stock in Johnson & Johnson. Dr Jardine is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship; is responsible for research projects that have received unrestricted funding from Gambro, Baxter, CSL, Amgen, Eli Lilly, and Merck; has served on advisory boards sponsored by Akebia, Baxter, and Boehringer Ingelheim; and has spoken at scientific meetings sponsored by Janssen, Amgen, and Roche with any consultancy, honoraria, or travel support paid to her institution. Dr Bakris has received research funding paid to the University of Chicago for Bayer, Janssen, and Vascular Dynamics; has served as a consultant for Merck and Relypsa; and has served as Editor-in-Chief for American Journal of Nephrology, as the Nephrology and Hypertension Section Editor for UpToDate, as Section Editor ofHypertension, and as associate editor of Diabetes Care and Hypertension Research. Dr Perkovic has received research support from the Australian National Health and Medical Research Council (Senior Research Fellowship and Program Grant); served on Steering Committees for AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, and Pfizer; and served on advisory boards and/or spoken at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier, and Vitae, with all honoraria paid to his employer.
Supplementary Material
Footnotes
Sources of Funding, see page 1549
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.118.035901.
References
- 1.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. doi 10.1016/S0140-6736(10)60484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR on behalf of the CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. doi 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 5.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 6.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. doi 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 7.Petrykiv S, Sjostrom CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12:751–759. doi: 10.2215/CJN.10180916. doi 10.2215/CJN.10180916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherney DZI, Cooper ME, Tikkanen I, Pfarr E, Johansen OE, Woerle HJ, Broedl UC, Lund SS. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93:231–244. doi: 10.1016/j.kint.2017.06.017. doi: 10.1016/j.kint.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Hinnen D. Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab. 2015;6:92–102. doi: 10.1177/2042018815575273. doi: 10.1177/2042018815575273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015;54:691–708. doi: 10.1007/s40262-015-0264-4. doi: 10.1007/s40262-015-0264-4. [DOI] [PubMed] [Google Scholar]
- 11.Seidu S, Kunutsor SK, Cos X, Gillani S, Khunti K for and on behalf of Primary Care Diabetes Europe. SGLT2 inhibitors and renal outcomes in type 2 diabetes with or without renal impairment: a systematic review and meta-analysis. Prim Care Diabetes. 2018;12:265–283. doi: 10.1016/j.pcd.2018.02.001. doi: 10.1016/j.pcd.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi 10.1080/01621459.1999.10474144. [Google Scholar]
- 14.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Rosenthal N, Desai M, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: data from the CANVAS randomized clinical trial program. Lancet Diabetes Endocrinol. 2018 doi: 10.1016/S2213-8587(18)30141-4. In press. doi 10.1016/S2213-8587(18)30141–4. [DOI] [PubMed] [Google Scholar]
- 16.Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, Shalayda K, Demarest K, Rothenberg P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–672. doi: 10.1111/j.1463-1326.2011.01406.x. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, Du F, Liu Y, Xu J, Conway B, Conway J, Polidori D, Ways K, Demarest K. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS ONE. 2012;7:e30555. doi: 10.1371/journal.pone.0030555. doi 10.1371/journal.pone.0030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B EMPA-REG OUTCOME Investigators. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137:119–129. doi: 10.1161/CIRCULATIONAHA.117.028268. doi: 10.1161/CIRCULATIONAHA.117.028268. [DOI] [PubMed] [Google Scholar]
- 19.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368–375. doi: 10.1681/ASN.2016030278. doi: 10.1681/ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. doi 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 22.Plutzky J, Bakris G. Sodium/glucose cotransporter 2 inhibitors in patients with diabetes mellitus and chronic kidney disease: turning the page. Circulation. 2018;137:130–133. doi: 10.1161/CIRCULATIONAHA.117.031422. doi: 10.1161/CIRCULATIONAHA.117.031422. [DOI] [PubMed] [Google Scholar]
- 23.Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, Tarng DC. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014;85:703–709. doi: 10.1038/ki.2013.336. doi 10.1038/ki.2013.336. [DOI] [PubMed] [Google Scholar]
- 24.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39:1115–1122. doi: 10.2337/dc16-0542. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 25.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. doi 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 26.Bell RM, Yellon DM. SGLT2 inhibitors: hypotheses on the mechanism of cardiovascular protection. Lancet Diabetes Endocrinol. 2018;6:435–437. doi: 10.1016/S2213-8587(17)30314-5. doi: 10.1016/S2213-8587(17)30314-5. [DOI] [PubMed] [Google Scholar]
- 27.Cherney DZI, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, von Eynatten M, Wanner C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 28.Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, Bull S, Cannon CP, Charytan DM, de Zeeuw D, Edwards R, Greene T, Heerspink HJL, Levin A, Pollock C, Wheeler DC, Xie J, Zhang H, Zinman B, Desai M, Perkovic V CREDENCE study investigators. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017;46:462–472. doi: 10.1159/000484633. doi: 10.1159/000484633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pecoits-Filho R, Perkovic V. Are SGLT2 inhibitors ready for prime time for CKD? Clin J Am Soc Nephrol. 2018;13:318–320. doi: 10.2215/CJN.07680717. doi: 10.2215/CJN.07680717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boehringer Ingelheim. Boehringer Ingelheim and Lilly announce an academic collaboration with University of Oxford to investigate the effects of empagliflozin in people with chronic kidney disease. https://www.boehringer-ingelheim.com/EMPA-KIDNEY. Accessed May 22, 2018.
- 31.Tonelli M, Agarwal S, Cass A, Garcia Garcia G, Jha V, Naicker S, Wang H, Yang CW, O’Donoghue D. How to advocate for the inclusion of chronic kidney disease in a national noncommunicable chronic disease program. Kidney Int. 2014;85:1269–1274. doi: 10.1038/ki.2012.488. doi 10.1038/ki.2012.488. [DOI] [PubMed] [Google Scholar]
- 32.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]


