Abstract
Background:
The medical treatment of periprosthetic joint infection (PJI) involves prolonged systemic antibiotic courses, often with suboptimal clinical outcomes including increased morbidity and health-care costs. Oral and intravenous monotherapies and combination antibiotic regimens were evaluated in a mouse model of methicillin-resistant Staphylococcus aureus (MRSA) PJI.
Methods:
Oral linezolid with or without oral rifampin, intravenous vancomycin with oral rifampin, intravenous daptomycin or ceftaroline with or without oral rifampin, oral doxycycline, or sham treatment were administered at human-exposure doses for 6 weeks in a mouse model of PJI. Bacterial burden was assessed by in vivo bioluminescent imaging and ex vivo counting of colony-forming units (CFUs), and reactive bone changes were evaluated with radiographs and micro-computed tomography (μCT) imaging.
Results:
Oral-only linezolid-rifampin and all intravenous antibiotic-rifampin combinations resulted in no recoverable bacteria and minimized reactive bone changes. Although oral linezolid was the most effective monotherapy, all oral and intravenous antibiotic monotherapies failed to clear infection or prevent reactive bone changes.
Conclusions:
Combination antibiotic-rifampin regimens, including oral-only linezolid-rifampin and the newer ceftaroline-rifampin combinations, were highly effective and more efficacious than monotherapies when used against a preclinical MRSA PJI.
Clinical Relevance:
This study provides important preclinical evidence to better optimize future antibiotic therapy against PJIs. In particular, the oral-only linezolid-rifampin option might reduce venous access complications and health-care costs.
Periprosthetic joint infection (PJI) is a devastating complication following total joint arthroplasty. Bacterial biofilm formation on implanted foreign materials impedes penetration of immune cells and antibiotics, creating chronic and persistent infections1. PJIs are exceedingly difficult to treat as they require reoperations, prolonged systemic antibiotics, and extended rehabilitation due to extended disability, which contribute to worse clinical outcomes2,3. Although rates of PJI have remained at 1% to 2% after primary arthroplasty and 3% to 6% after revision arthroplasty, PJIs are associated with inpatient costs averaging $25,000 to $107,000 per case, corresponding to an annual health-care burden of $3 billion, in the U.S.4.
Management depends on the acuity and complexity of the PJI. According to the Infectious Diseases Society of America (IDSA), acute PJI can be managed with debridement, retention of the prosthesis, and 2 to 6 weeks of pathogen-specific intravenous antibiotics with or without rifampin followed by an appropriate oral antibiotic with or without rifampin for 3 to 6 months5. For complicated or chronic PJI, current American Academy of Orthopaedic Surgeons (AAOS) Clinical Practice Guidelines recommend 2-stage revision arthroplasty with at least 4 to 6 weeks of intervening systemic antibiotics using regimens similar to those described above6. For methicillin-resistant Staphylococcus aureus (MRSA) PJI, first-line therapy is intravenous vancomycin with or without rifampin and second-line therapies include intravenous daptomycin or intravenous or oral linezolid with or without rifampin5. Rifampin is contraindicated as a monotherapy because of the rapid development of antibiotic resistance5. However, including rifampin in a combination therapy has a beneficial effect against PJI, particularly when the implant is being retained7-9 and in preclinical models of S. aureus biofilm infections10,11. If antibiotic therapy for PJI can be optimized to reduce reinfection risk, use of implant retention or 1-stage exchange could be expanded to improve outcomes for patients with complicated or chronic PJI.
There is limited information on the efficacy of newer antibiotics with coverage against MRSA PJI, particularly ceftaroline, daptomycin, or linezolid5, especially since each antibiotic has different efficacy against bacteria in biofilms12-15 and different bone uptake16. In addition, patients with PJI who undergo prolonged intravenous antibiotic therapy are at risk for venous access complications, including central line-associated bloodstream infections17, catheter malposition, thrombosis, and pneumothorax18, as well as drug side effects and toxicities19. The number of these complications will likely increase as the number of total knee and hip arthroplasties performed annually in the U.S. (∼1 million in 2005 and projected to be >4 million by 203020) continues to rise.
We investigated different oral and intravenous antibiotic monotherapies as well as such therapies combined with rifampin in an established preclinical mouse model of MRSA PJI. We focused on an oral-only linezolid-rifampin combination, which if effective could minimize complications of long-term venous access. We hypothesized that rifampin combination therapies would have equivalent or superior efficacy compared with the current standard of care, which is vancomycin plus rifampin. We further hypothesized that antibiotic regimens that decrease bacterial burden most effectively would result in fewer reactive bone changes. Finally, we evaluated whether treatment failures were due to the development of antimicrobial resistance.
Materials and Methods
Bacteria
The bioluminescent USA300 community-acquired MRSA strain SAP231 was previously derived from the clinical isolate NRS38421 and has been used in a preclinical model of an orthopaedic biofilm infection22. SAP231 possesses a stable bioluminescent construct integrated into the bacterial chromosome that is maintained in all progeny without selection, and only live and actively metabolizing bacteria emit light. SAP231 was streaked onto plates containing tryptic soy broth and Bacto agar (1.5%) (BD [Becton, Dickinson and Company]). Colonies of SAP231 were grown overnight at 37°C in a shaking incubator (240 rpm) in tryptic soy broth. Mid-logarithmic-phase bacteria were obtained after a 2-hour subculture of a 1:50 dilution of the overnight culture.
Mouse PJI Model
All procedures were approved by the Johns Hopkins Animal Care and Use Committee. A previously established mouse model of PJI using 6-week to 8-week-old male C57BL/6 mice (Jackson Laboratory) was employed23,24. Briefly, a medial parapatellar approach was performed, and the patella was dislocated laterally to access the distal part of the femur. The femoral medullary canal was reamed with a 25-gauge needle, and a surgical-grade titanium Kirschner wire (0.5 × 9 mm; Modern Grinding) was inserted in a retrograde fashion with 1 mm protruding into the knee joint. An inoculum of 1 × 103 colony-forming units (CFUs) of SAP231 in 2 μL of phosphate-buffered saline solution was pipetted onto the exposed Kirschner wire in the knee joint before closure with absorbable sutures. For pain management, sustained-release buprenorphine (2.5 mg/kg) was administered subcutaneously at the time of surgery.
Antibiotic Therapy
After a 2-week incubation period to allow biofilm formation on the Kirschner wire23, antibiotic treatment (or sham treatment with saline solution) was initiated for 6 weeks with doses that approximate human-exposure doses according to the area under the curve and differences in serum drug protein binding between mice and humans (Table I). There were 10 mice in each treatment group (oral linezolid with or without oral rifampin, intravenous vancomycin with oral rifampin, intravenous daptomycin or ceftaroline with or without oral rifampin, oral doxycycline, or sham treatment), for a total of 90 mice in the study, with each cage of 4 or 5 mice randomly assigned to a treatment group. All subcutaneous injections were performed on the backs of the mice, and all oral doses were administered via gavage. Body weight and mouse behavior, posture, and activity level were used to assess for drug intolerance or toxicity. There were no significant differences in initial body weight among the groups.
TABLE I.
Antibiotic Treatment Groups and Dosages with Comparison with Human Treatment
| Mouse (N = 10/Group) |
Human |
||||
| Antibiotic | Regimen | AUC* (μg·hr/mL) | Regimen | AUC* (μg·hr/mL) | Source |
| Ceftaroline38,39 | 100 mg/kg q12h s.c. | 58.0 | 600 mg q12h i.v. | 56.3 | Forest Pharmaceuticals |
| Daptomycin40-42 | 50 mg/kg q24h s.c. | 595.4 | 6 mg/kg q24h i.v. | 598 | Cubist Pharmaceuticals |
| Doxycycline40,43 | 100 mg/kg q12h p.o. | 12.4 | 600 mg q12h p.o. | 13.0 | West-Ward Pharmaceutical |
| Linezolid40,44-46 | 80 mg/kg q12 p.o. | 160 | 600 mg q12h p.o. or i.v. | 228 | Pfizer |
| Vancomycin40,44,47,48 | 110 mg/kg q12h s.c. | 225 | 1000 mg q12h i.v. | 227 | Fresenius Kabi |
| Rifampin49,50 | 10 mg/kg q12h p.o. | 125.1† | 450 mg q12h p.o. | 48.5† | Lannett |
| Sham treatment (saline solution) | 200 μL p.o. or s.c. | ||||
In Vivo Bioluminescence Imaging
To noninvasively monitor bacterial burden, in vivo bioluminescent imaging using the IVIS Lumina III imaging system (PerkinElmer) with large binning and 5-minute exposure was performed 14, 11, and 7 days before the start of the antibiotic therapy; on the day of the therapy; and 3, 7, 14, 21, 28, 35, and 42 days after the beginning of the therapy. Bioluminescent signals were localized on a grayscale image of the mice and quantified using maximum flux (photons per second per square centimeter per steradian) within an oval 0.5 × 0.75-cm region of interest (level of detection = 2.5 × 103 photons/s/cm2/sr).
Ex Vivo Bacterial Burden
The same cohort of mice was euthanized 1 day after the 6-week antibiotic course. To avoid disturbing the biofilm at the distal end of the implant, the surrounding bone was gently crushed circumferentially with a needle driver and the implant was readily extracted. Bacteria were isolated from the bone and joint tissue by homogenizing tissue from the midpart of the femur through the proximal part of the tibia using a PRO200 Series homogenizer (PRO Scientific). Bacteria adherent to the Kirschner wire implants were isolated by sonication in 0.3% Tween solution (Sigma-Aldrich) for 10 minutes followed by vortexing for 2 minutes. The CFUs were counted after overnight culture with serial dilutions on plates. To further identify any bacteria remaining in the bone or joint tissue or the implants, homogenates and sonicates were cultured for an additional 48 hours (at 37°C in tryptic soy broth at 240 rpm) and the presence or absence of bacterial growth was determined after overnight culture on plates.
Antibiotic Resistance Testing
To examine whether antibiotic resistance developed during the in vivo antibiotic treatment, tissue homogenates and implant sonicates that demonstrated growth in tryptic soy broth were plated and analyzed by the Johns Hopkins Hospital Clinical Microbiology Laboratory using the E-test (for ceftaroline), Phoenix panel (for daptomycin, linezolid, rifampin, and vancomycin), and disk diffusion testing (for doxycycline) according to the guidelines for clinical samples. The minimum inhibitory concentration of the ex vivo CFUs was compared with that of the original stock of SAP231.
Radiographic Imaging and Analysis
After the 6-week antibiotic course and immediately before tissues were harvested for CFU counts, anteroposterior high-resolution radiographs were obtained using the Faxitron MX-20 (Faxitron Bioptics). Ten mice of the same age that had received the same surgery but without bacterial inoculation were used as an additional control. Radiographs were analyzed with ImageJ (https://imagej.nih.gov/ij/; National Institutes of Health) by an observer blinded to the treatment groups. The maximal femoral width (proximal to the fabella) was measured perpendicular to the anatomical axis of the distal part of the femur. The area of the distal 25% of the femur (from the midpoint of a line extending from the intercondylar notch to its intersection with a perpendicular line that bisected the third trochanter) was also calculated. Subsidence, a mechanical phenomenon in which a loose prosthesis migrates from its original surgical position, was identified by assessing for proximal displacement of the implant from the intercondylar notch.
Micro-Computed Tomographic Imaging and Analysis
Micro-computed tomographic (μCT) imaging was performed on anesthetized mice on day 42 using the nanoScan PET/CT Small Animal Imager (Mediso; tube potential = 50 kVp, 720 projections) and the NanoSPECT/CT Small Animal Imager (BioScan; tube potential = 45 kVp, 360 projections). Images were reconstructed and analyzed using VivoQuant 2.50 (inviCRO) by an observer blinded to the treatment groups. The femur was reoriented vertically in sagittal and coronal planes and the distal 25% of the femur was identified. A semi-automated approach of connected thresholding was used, and regions of interest were added for the titanium Kirschner wire implant and the bone, with voxels ranging from connected thresholding of 5,000 to 50,000 Hounsfield units (HU) and 700 to 4,999 HU25, respectively. Three-dimensional (3D) images were acquired to quantify distal femoral bone density.
Statistical Analysis
GraphPad Prism 5 (GraphPad Software) was used to compare data among groups. These data included in vivo bioluminescent imaging signals, compared using 2-way analysis of variance (ANOVA); ex vivo CFU counts, radiographic findings, and μCT findings, compared using the unpaired 1-tailed Mann-Whitney test; and the presence or absence of bacterial growth and radiographic subsidence, compared using the 1-tailed Fisher exact test. Values of p < 0.05 were considered significant.
Results
Efficacy of Antibiotic Therapy
In Vivo Bioluminescent Imaging Signals
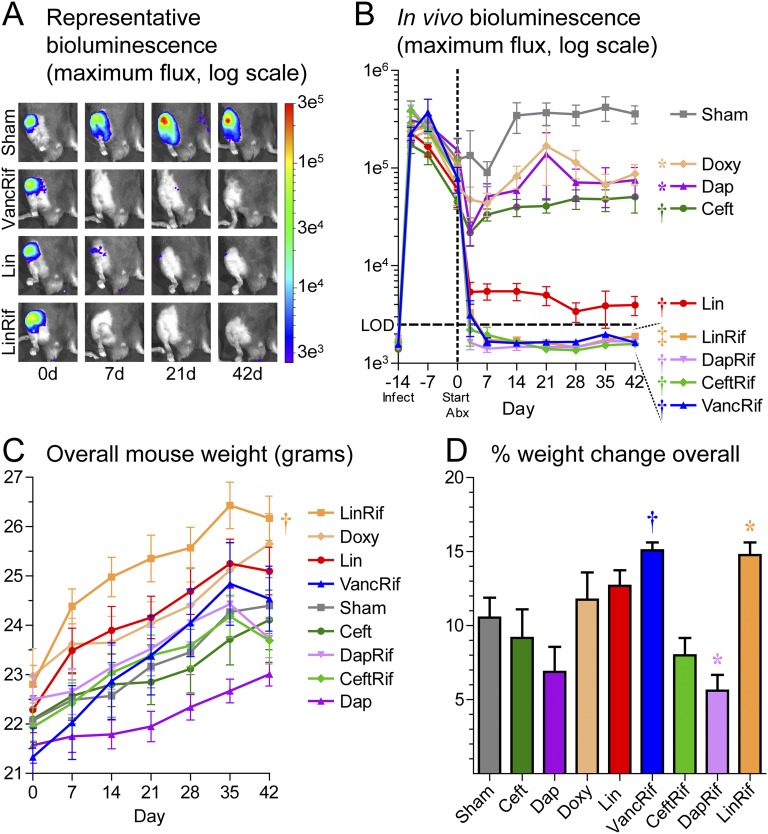
In vivo bioluminescent imaging signals, which were previously shown to closely correlate with ex vivo CFUs10,23, showed a similar bacterial burden in all groups 14, 11, and 7 days prior to, and on the day of, initiation of the antibiotics (Figs. 1-A and 1-B). When assessed 3 and 7 days after the start of the antibiotics (or sham treatment) and weekly thereafter for 6 weeks (day 42), in vivo bioluminescent signals were found to have plateaued at 3.6 ± 0.8 × 105 in the sham-treatment group. Notably, the signals in all groups treated with an antibiotic-rifampin combination (vancomycin-rifampin, ceftaroline-rifampin, daptomycin-rifampin, or linezolid-rifampin) decreased below the level of detection by day 7 (Fig. 1-B) and remained below the level of detection for the entire 6-week therapy course. In contrast, ceftaroline, daptomycin, and doxycycline monotherapies decreased the in vivo bioluminescent imaging signals modestly compared with sham treatment. Linezolid was the most effective single antibiotic therapy. In all groups, body weights increased over time, suggesting that there was no systemic antibiotic toxicity (Fig. 1-C); notably, mice treated with vancomycin plus rifampin or linezolid plus rifampin gained the most weight (Fig. 1-D).
Fig. 1.
Effect of antibiotic therapy on in vivo bioluminescent imaging signals and body weight in a mouse model of PJI in which different antibiotic regimens or sham treatment were initiated (day 0) 2 weeks after surgery and MRSA inoculation, and were continued for 6 weeks (n = 10/group). Fig. 1-A Representative in vivo bioluminescent images. Fig. 1-B Mean maximum flux (photons/s/cm2/sr) (and SEM). LOD = level of detection (2.5 × 103 photons/s/cm2/sr). Figs. 1-C and 1-D Mean weekly body weights and mean percent change in body weight at 42 days (and SEM). *p < 0.05, †p < 0.01, and ‡p < 0.001 for the difference between the antibiotic and sham treatment as shown by 2-way ANOVA (Figs. 1-B and 1-C) or the Mann-Whitney 1-tailed test (Fig. 1-D).
Bacterial Burden
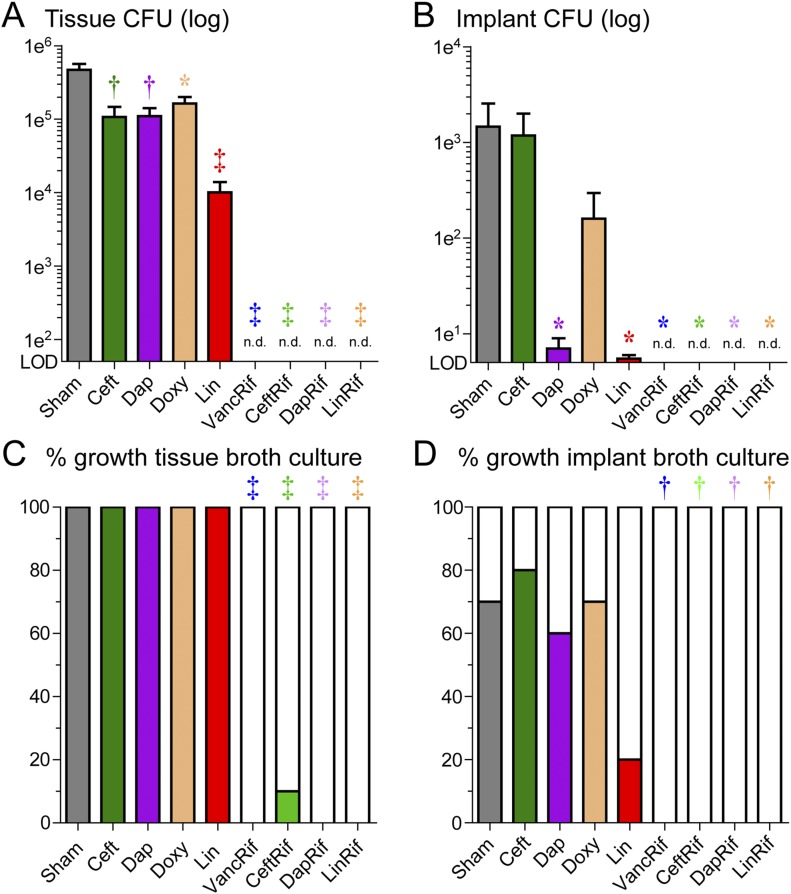
One day after the 6 weeks of treatment, ex vivo CFUs from homogenized bone and joint tissue specimens and sonicated Kirschner wire implants were enumerated (Figs. 2-A and 2-B). Sham treatment resulted in a mean (and standard error of the mean [SEM]) of 4.7 ± 1.0 × 105 CFUs from the tissue homogenates and 1.5 ± 1.1 × 103 CFUs from the implants. All rifampin combination therapies resulted in no CFUs detected from either the tissue homogenates or implants. In contrast, ceftaroline, daptomycin, doxycycline, and linezolid monotherapies resulted in recoverable bacteria in all tissue homogenates and most implants, but the bacterial burden was significantly decreased compared with that associated with sham treatment; linezolid monotherapy led to the most substantial reduction in bacterial burden.
Fig. 2.
Effect of antibiotic therapy on ex vivo CFU counts. Figs. 2-A and 2-B After 6 weeks of antibiotic or sham treatment, peri-implant joint and bone tissue were homogenized, implants were sonicated, and CFUs were isolated and enumerated ex vivo (n = 10/group). Data are presented as the mean number of CFUs (and SEM) isolated from the peri-implant bone and joint tissue (Fig. 2-A) and implants (Fig. 2-B). LOD = level of detection and n.d. = not detected. Figs. 2-C and 2-D To evaluate whether the antibiotic therapy eradicated infection, tissue homogenates and implants were cultured for an additional 48 hours in broth followed by overnight plate culture and the presence or absence of CFUs was determined. Data are presented as the percentage of tissue samples (Fig. 2-C) or implants (Fig. 2-D) with CFUs present out of the total number of samples assayed. *p < 0.05, †p < 0.01, and ‡p < 0.001 for the difference between the antibiotic and sham treatment as shown by the Mann-Whitney 1-tailed test (Figs. 2-A and 2-B) or the Fisher exact 1-tailed test (Figs. 2-C and 2-D).
To determine whether the antibiotic therapies eradicated the infection, the homogenized tissue specimens and sonicated implants were cultured ex vivo in broth for 48 hours at 37°C followed by overnight culture on plates, and the presence or absence of bacterial growth was determined (Figs. 2-C and 2-D). Bacterial growth was present in all 10 tissue specimens and 7 of the 10 implants in the sham-treatment group. All antibiotic-rifampin combinations resulted in bacterial growth in none (vancomycin plus rifampin, daptomycin plus rifampin, and linezolid plus rifampin) or 1 (ceftaroline plus rifampin) of the tissue specimens and in none of the 10 implant samples. In contrast, CFUs were present in all tissue specimens in all antibiotic monotherapy groups. The implant bacterial growth in the ceftaroline, daptomycin, and doxycycline groups (seen in 6, 7, or 8 tissue specimens) was similar to that in the sham-treatment specimens. Only 2 of 10 implants in the linezolid monotherapy group had bacterial growth, with the difference compared with the sham-treatment group approaching significance (p = 0.0698).
To determine whether the development of antibiotic resistance contributed to treatment failure, the minimum inhibitory concentrations for bacteria isolated from tissues and implants on day 42 were assessed according to clinical guidelines (Table II). In all cases, the minimum inhibitory concentrations for ceftaroline, daptomycin, doxycycline, and linezolid were at or below the minimum inhibitory concentration for the original SAP231 stock, indicating that antibiotic resistance did not develop during the therapy. As mentioned above, ceftaroline-rifampin was the only antibiotic-rifampin combination group with bacterial growth—in a single tissue sample—and no resistance to either antibiotic was detected for bacteria isolated from that sample.
TABLE II.
Testing for Development of Antibiotic Resistance*
| Minimum Inhibitory Concentration (μg/mL) |
||||
| No. of Samples with CFUs After 48-Hr Broth Culture (Tissue/Implant) | CFUs from Tissue/Implant | Original SAP231 | Resistance Breakpoint† According to Clinical Guidelines (μg/mL) | |
| Ceftaroline | 18 (10/8) | 0.5-1 | 1 | >1 |
| Daptomycin | 16 (10/6) | 0.5 | 0.5 | >1 |
| Doxycycline | 16 (10/6) | 30 | 30 | >30 |
| Linezolid | 12 (10/2) | 1-2 | 2 | >4 |
| Ceftaroline-rifampin‡ | 1 (1/0) | 0.5 (ceftaroline), 0.5 (rifampin) | 1 (ceftaroline), 0.5 (rifampin) | >1, >0.5 |
After the 6-week antibiotic treatment, tissue homogenates and sonicated implant solutions with positive bacterial growth underwent minimum inhibitory concentration testing according to guidelines for clinical microbiology specimens.
Resistance breakpoints were obtained from the European Committee on Antimicrobial Susceptibility Testing, version 6.0, 2016 (http://www.eucast.org/).
The ceftaroline-rifampin group was the only antibiotic-rifampin-combination group that had bacterial growth for resistance testing.
Reactive Bone Changes
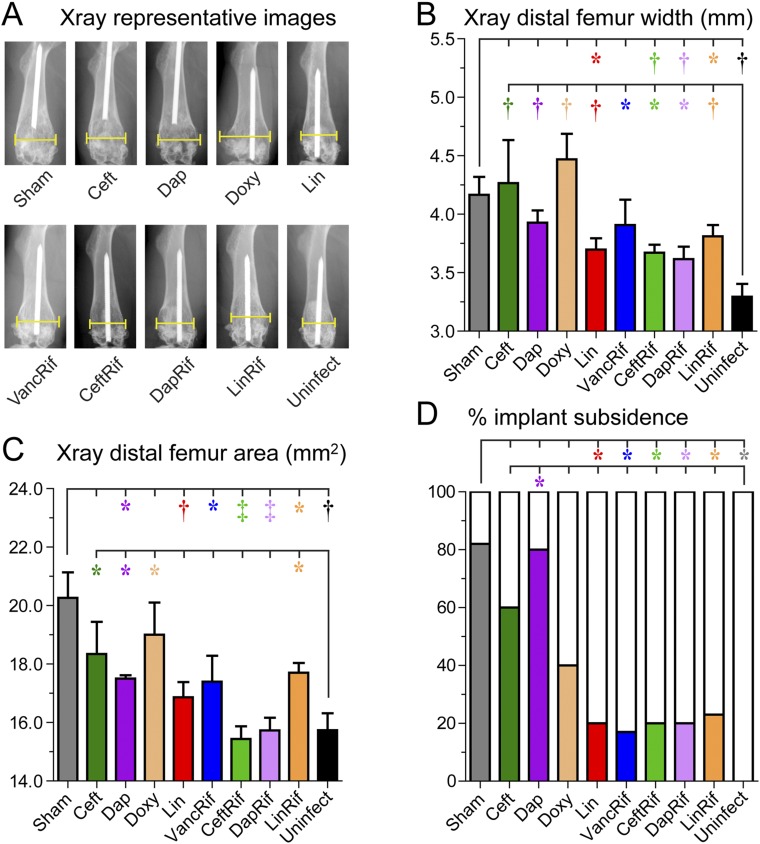
PJI can cause reactive bone changes, including periprosthetic osteolysis, cortical expansion, and/or prosthetic subsidence, all of which can be detrimental to clinical outcomes and necessitate revision arthroplasty. The impacts of the different antibiotic therapies in terms of minimizing pathologic bone changes were evaluated by assessing high-resolution anteroposterior radiographs of the distal part of the femur after the 6-week antibiotic course by an observer blinded to the treatment groups (Fig. 3-A). All antibiotic-rifampin combinations except vancomycin plus rifampin were associated with significantly less femoral width, femoral area, and implant subsidence than the sham treatment; vancomycin plus rifampin significantly differed from sham treatment in terms of femoral area and implant subsidence but not femoral width (Figs. 3-B, 3-C, and 3-D). Of the antibiotic monotherapies, linezolid but not ceftaroline, daptomycin, or doxycycline resulted in less femoral width, area, and subsidence compared with the sham treatment. All antibiotic treatment groups developed increased femoral width compared with that of the mice that had had placement of a Kirschner wire implant without introduction of infection (uninfected mice), and the ceftaroline, daptomycin, doxycycline, and linezolid-rifampin groups had significantly increased femoral area. None of the uninfected mice exhibited implant subsidence whereas some percentage of the implants subsided in all antibiotic-treated groups regardless of the effectiveness of the antibiotic.
Fig. 3.
Effect of antibiotic therapy on bone changes and implant subsidence seen on radiographs made after 6 weeks of antibiotic or sham treatment (n = 5 to 10/group). Fig. 3-A Representative anteroposterior radiographs with brackets denoting maximal femoral width. Fig. 3-B Mean maximum width of the distal 25% of the femur in millimeters (and SEM). Fig. 3-C Mean area of the distal 25% of the femur in square millimeters (and SEM). Fig. 3-D Percentage of femora with proximal displacement of the implant (subsidence) out of the total number of femora in each group (and SEM). *p < 0.05, †p < 0.01, and ‡p < 0.001 for the difference between the antibiotic and sham treatment or uninfected control mice as shown by the Mann-Whitney 1-tailed test (Figs. 3-B and 3-C) or the Fisher exact 1-tailed test (Fig. 3-D).
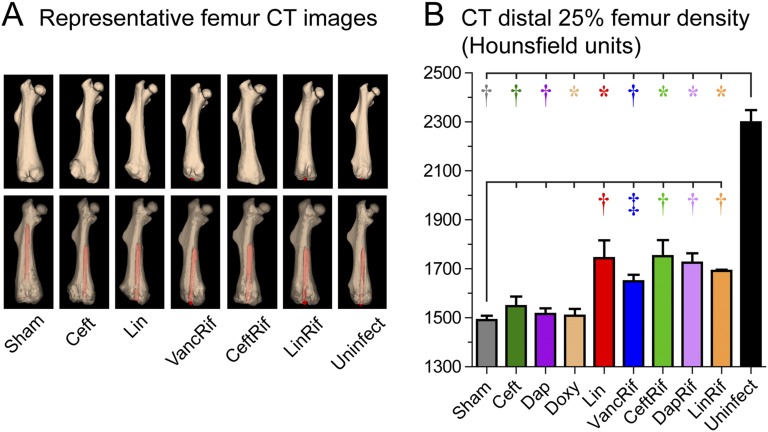
Distal femoral bone density was also measured with μCT imaging (Fig. 4-A). All antibiotic-rifampin combinations led to higher femoral density than that seen in the sham-treatment group. Of the monotherapies, linezolid but not ceftaroline, daptomycin, or doxycycline was associated with higher density compared with that in the sham-treatment group (Fig. 4-B). Of note, all antibiotic-rifampin combinations and all monotherapies led to decreased femoral density compared with that in the uninfected group.
Fig. 4.
Effect of antibiotic therapy on bone density seen on μCT imaging performed after 6 weeks of antibiotic or sham therapy (n = 4 to 8/group). Fig. 4-A Representative 3D reconstructed μCT images (opaque [top] and translucent [bottom] femora with implants in red). Fig. 4-B Mean bone density (and SEM) of the distal 25% of the femur as measured in HU. *p < 0.05, †p < 0.01, and ‡p < 0.001 for the difference between the antibiotic and sham treatment or uninfected control mice as shown by the Mann-Whitney 1-tailed test.
Discussion
The current treatment of chronic PJI in the U.S. typically involves 2-stage revision arthroplasty with prolonged antibiotic courses, resulting in substantial morbidity and mortality and increased health-care costs. Improvement of antibiotic therapy could expand indications for 1-stage revision or implant retention and improve clinical outcomes7,26. We used in vivo bioluminescent imaging in an established preclinical mouse model of PJI23,24 to noninvasively and longitudinally evaluate different antibiotics as monotherapy and in combination with rifampin.
All rifampin-based combination therapies were highly effective in (1) decreasing in vivo bioluminescent imaging signals; (2) eradicating the infection in that no viable bacteria remained in the homogenized bone or joint tissue or the implants; and (3) minimizing osteolysis, the loss of bone density, and implant subsidence. The efficacy of the antibiotic-rifampin combinations in our study is consistent with prior studies of PJIs in humans and mice and supports the IDSA recommendation of rifampin combination therapy in their guidelines for treating PJI5. These guidelines noted concerns about the long-term use of rifampin, including potential toxicities (e.g., hepatitis and drug interactions) and rifampin-induced osteomalacia5, and although these were not observed in our study they need to be considered when treating patients.
An effective oral-only alternative for treating PJI would eliminate the risk of central line-associated bloodstream infections and other venous access complications and would reduce patient discomfort and health-care costs associated with central catheter insertion and long-term maintenance27, which can exceed $13,000 for an outpatient antibiotic course28. Notably, the efficacy of the oral-only linezolid-rifampin combination was equivalent to that of intravenous combination antibiotics in terms of a lack of bacterial growth and minimization of reactive bone changes, suggesting that oral-only linezolid-rifampin therapy might be a highly effective option for treating PJI in patients. Consistent with our findings, linezolid plus rifampin was highly effective in preclinical models of osteomyelitis and foreign-body infections29,30 as well as in salvage treatment of PJI in patients with implant retention31. The risk of linezolid-induced myelosuppression32 needs to be considered when using linezolid in patients.
Ceftaroline is a novel 5th-generation cephalosporin with activity against MRSA. The efficacy of the ceftaroline-rifampin combination was similar to that of the other rifampin combinations, a finding consistent with the efficacy of ceftaroline-rifampin treatment in a rabbit model of PJI33. The toxicity profile of ceftaroline is similar to that of other cephalosporins, which are generally well tolerated, and does not require serum-level testing as required for vancomycin34. Thus, future studies of humans might be warranted to evaluate ceftaroline plus rifampin as a potential second-line option for treating PJI.
Although all antibiotic monotherapies decreased the bacterial burden compared with that associated with sham treatment, all resulted in recoverable bacteria from tissues and implants. Vancomycin monotherapy was previously evaluated in this same model of PJI, and its efficacy was similar to that of ceftaroline, daptomycin, or doxycycline10. Importantly, linezolid alone was the best-performing monotherapy in our study as it resulted in the greatest reduction of in vivo bioluminescent imaging signals, ex vivo isolated CFUs, reactive bone changes, and implant subsidence, suggesting that future studies of linezolid monotherapy to treat PJI might be warranted, especially in cases in which rifampin combinations cannot be used or when venous access is problematic.
Lastly, the minimum inhibitory concentrations for the bacteria isolated from all tissue homogenates and implants remained unchanged from that for the original bacterial stock, indicating that in vivo antibiotic resistance did not develop. Thus, the treatment failures of the monotherapies were likely due to inadequate drug levels at the site of the infection and/or poor penetration of the antibiotics into bacterial biofilms or bone or joint tissue35.
The current study has some limitations. First, the genetic engineering to insert the bioluminescent construct might have influenced the biofilm-producing ability of the S. aureus strain used. Although this was not formally tested, the strain that we used was similar to multiple other biofilm-producing S. aureus strains in terms of the ex vivo CFUs obtained from the tissue and implants24. Second, we did not perform in vitro assays to evaluate the activity of the antibiotics against biofilms. However, a strength of this study is that the antibiotics were assessed in an in vivo model of PJI, which recapitulates infection of the bone and joint tissue in addition to biofilm formation. Third, bacterial growth from clinical orthopaedic implants involves static plate cultures for ≥14 days, especially to identify slow-growing organisms (e.g., Propionibacterium acnes)36. In this study, tissue homogenates and sonicated implants were cultured in shaking broth cultures for 48 hours followed by overnight culture on plates, which has been shown to be sufficient to detect S. aureus growth, including slow-growing and small-colony variants associated with S. aureus biofilms37. Fourth, C57BL/6 mice have lower baseline bone density than other mouse strains, and the reactive bone changes might have differed in mouse strains with higher bone density. Finally, mice may not fully recapitulate human PJI, and clinical studies need to be conducted to confirm these findings.
In conclusion, rifampin-based combinations proved to be highly effective in this preclinical mouse model of MRSA PJI, and linezolid plus rifampin might represent a highly effective oral-only regimen to reduce venous-access-related complications and associated health-care costs for patients. Future studies of larger animals and humans are needed to translate these findings to the treatment of PJI in patients.
Acknowledgments
Note: The authors thank Tsigereda Tekle at the Johns Hopkins Clinical Microbiology Laboratory for performing the antibiotic resistance testing.
Investigation performed at the Johns Hopkins University School of Medicine, Baltimore, Maryland
Disclosure: This work was supported by DP2-OD-006492 (S.K.J.), R01AR069502 (L.S.M.), and T32 AR07708-01 (J.M.T.) from the National Institutes of Health. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work; “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work; and “yes” to indicate that the author had other relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work (http://links.lww.com/JBJS/C260).
John M. Thompson and Vikram Saini contributed equally to the writing of this article, and Sanjay K. Jain and Lloyd S. Miller jointly directed this work.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999. May 21;284(5418):1318-22. [DOI] [PubMed] [Google Scholar]
- 2.Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med. 2009. August 20;361(8):787-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf BR, Lu X, Li Y, Callaghan JJ, Cram P. Adverse outcomes in hip arthroplasty: long-term trends. J Bone Joint Surg Am. 2012. July 18;94(14):e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012. September;27(8)(Suppl):61-5.e1. Epub 2012 May 2. [DOI] [PubMed] [Google Scholar]
- 5.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013. January;56(1):e1-25. Epub 2012 Dec 6. [DOI] [PubMed] [Google Scholar]
- 6.Parvizi J, Della Valle CJ. AAOS clinical practice guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010. December;18(12):771-2. [DOI] [PubMed] [Google Scholar]
- 7.Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu JM, Rico A, Palomino J, Rodríguez-Pardo D, Horcajada JP, Benito N, Bahamonde A, Granados A, del Toro MD, Cobo J, Riera M, Ramos A, Jover-Sáenz A, Ariza J; REIPI Group for the Study of Prosthetic Infection. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013. January;56(2):182-94. Epub 2012 Aug 31. [DOI] [PubMed] [Google Scholar]
- 8.Senneville E, Joulie D, Legout L, Valette M, Dezèque H, Beltrand E, Roselé B, d’Escrivan T, Loïez C, Caillaux M, Yazdanpanah Y, Maynou C, Migaud H. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis. 2011. August;53(4):334-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE; Foreign-Body Infection (FBI) Study Group. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA. 1998. May 20;279(19):1537-41. [DOI] [PubMed] [Google Scholar]
- 10.Niska JA, Shahbazian JH, Ramos RI, Francis KP, Bernthal NM, Miller LS. Vancomycin-rifampin combination therapy has enhanced efficacy against an experimental Staphylococcus aureus prosthetic joint infection. Antimicrob Agents Chemother. 2013. October;57(10):5080-6. Epub 2013 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saleh-Mghir A, Muller-Serieys C, Dinh A, Massias L, Crémieux AC. Adjunctive rifampin is crucial to optimizing daptomycin efficacy against rabbit prosthetic joint infection due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011. October;55(10):4589-93. Epub 2011 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cafiso V, Bertuccio T, Spina D, Purrello S, Stefani S. Tigecycline inhibition of a mature biofilm in clinical isolates of Staphylococcus aureus : comparison with other drugs. FEMS Immunol Med Microbiol. 2010. August;59(3):466-9. Epub 2010 May 19. [DOI] [PubMed] [Google Scholar]
- 13.Smith K, Perez A, Ramage G, Gemmell CG, Lang S. Comparison of biofilm-associated cell survival following in vitro exposure of meticillin-resistant Staphylococcus aureus biofilms to the antibiotics clindamycin, daptomycin, linezolid, tigecycline and vancomycin. Int J Antimicrob Agents. 2009. April;33(4):374-8. Epub 2008 Dec 19. [DOI] [PubMed] [Google Scholar]
- 14.Rose WE, Poppens PT. Impact of biofilm on the in vitro activity of vancomycin alone and in combination with tigecycline and rifampicin against Staphylococcus aureus. J Antimicrob Chemother. 2009. March;63(3):485-8. Epub 2008 Dec 24. [DOI] [PubMed] [Google Scholar]
- 15.Raad I, Hanna H, Jiang Y, Dvorak T, Reitzel R, Chaiban G, Sherertz R, Hachem R. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob Agents Chemother. 2007. May;51(5):1656-60. Epub 2007 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet. 2009;48(2):89-124. [DOI] [PubMed] [Google Scholar]
- 17.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011. May;39(4)(Suppl 1):S1-34. [DOI] [PubMed] [Google Scholar]
- 18.Eisen LA, Narasimhan M, Berger JS, Mayo PH, Rosen MJ, Schneider RF. Mechanical complications of central venous catheters. J Intensive Care Med. 2006. Jan-Feb;21(1):40-6. [DOI] [PubMed] [Google Scholar]
- 19.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009. January 1;66(1):82-98. [DOI] [PubMed] [Google Scholar]
- 20.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007. April;89(4):780-5. [DOI] [PubMed] [Google Scholar]
- 21.Plaut RD, Mocca CP, Prabhakara R, Merkel TJ, Stibitz S. Stably luminescent Staphylococcus aureus clinical strains for use in bioluminescent imaging. PLoS One. 2013;8(3):e59232 Epub 2013 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brady RA, Mocca CP, Prabhakara R, Plaut RD, Shirtliff ME, Merkel TJ, Burns DL. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of Staphylococcus aureus infection. PLoS One. 2013. April 29;8(4):e63040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010. September 07;5(9):e12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pribaz JR, Bernthal NM, Billi F, Cho JS, Ramos RI, Guo Y, Cheung AL, Francis KP, Miller LS. Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J Orthop Res. 2012. March;30(3):335-40. Epub 2011 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gittoes N. Osteoporosis: pathophysiology and clinical management. Clin Endocrinol (Oxf). 2003. December;59(6):826-7. [DOI] [PubMed] [Google Scholar]
- 26.Callaghan JJ, Katz RP, Johnston RC. One-stage revision surgery of the infected hip. A minimum 10-year followup study. Clin Orthop Relat Res. 1999. December;369:139-43. [DOI] [PubMed] [Google Scholar]
- 27.Stephens JM, Gao X, Patel DA, Verheggen BG, Shelbaya A, Haider S. Economic burden of inpatient and outpatient antibiotic treatment for methicillin-resistant Staphylococcus aureus complicated skin and soft-tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res. 2013. September 16;5:447-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010. September 15;51(Suppl 2):S198-208. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen NP, Skovdal SM, Meyer RL, Dagnæs-Hansen F, Fuursted K, Petersen E. Rifampicin-containing combinations are superior to combinations of vancomycin, linezolid and daptomycin against Staphylococcus aureus biofilm infection in vivo and in vitro. Pathog Dis. 2016. June;74(4):ftw019 Epub 2016 Apr 1. [DOI] [PubMed] [Google Scholar]
- 30.Baldoni D, Haschke M, Rajacic Z, Zimmerli W, Trampuz A. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection. Antimicrob Agents Chemother. 2009. March;53(3):1142-8. Epub 2008 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gómez J, Canovas E, Baños V, Martínez L, García E, Hernández-Torres A, Canteras M, Ruiz J, Medina M, Martínez P, Canovas A, Soriano A, Clavel M. Linezolid plus rifampin as a salvage therapy in prosthetic joint infections treated without removing the implant. Antimicrob Agents Chemother. 2011. September;55(9):4308-10. Epub 2011 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B, Kuter DJ. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002. August;46(8):2723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatin L, Saleh-Mghir A, Tasse J, Ghout I, Laurent F, Crémieux AC. Ceftaroline-fosamil efficacy against methicillin-resistant Staphylococcus aureus in a rabbit prosthetic joint infection model. Antimicrob Agents Chemother. 2014. November;58(11):6496-500. Epub 2014 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stryjewski ME, Jones RN, Corey GR. Ceftaroline: clinical and microbiology experience with focus on methicillin-resistant Staphylococcus aureus after regulatory approval in the USA. Diagn Microbiol Infect Dis. 2015. March;81(3):183-8. Epub 2014 Dec 3. [DOI] [PubMed] [Google Scholar]
- 35.del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007. August;82(2):204-9. Epub 2007 May 30. [DOI] [PubMed] [Google Scholar]
- 36.Schwotzer N, Wahl P, Fracheboud D, Gautier E, Chuard C. Optimal culture incubation time in orthopedic device-associated infections: a retrospective analysis of prolonged 14-day incubation. J Clin Microbiol. 2014. January;52(1):61-6. Epub 2013 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh R, Ray P, Das A, Sharma M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol. 2009. August;58(Pt 8):1067-73. Epub 2009 Jun 15. [DOI] [PubMed] [Google Scholar]
- 38.Andes D, Craig WA. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob Agents Chemother. 2006. April;50(4):1376-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riccobene T, Jakate A, Rank D. A series of pharmacokinetic studies of ceftaroline fosamil in select populations: normal subjects, healthy elderly subjects, and subjects with renal impairment or end-stage renal disease requiring hemodialysis. J Clin Pharmacol. 2014. July;54(7):742-52. Epub 2014 Jan 22. [DOI] [PubMed] [Google Scholar]
- 40.LaPlante KL, Leonard SN, Andes DR, Craig WA, Rybak MJ. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob Agents Chemother. 2008. June;52(6):2156-62. Epub 2008 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domínguez-Herrera J, Docobo-Pérez F, López-Rojas R, Pichardo C, Ruiz-Valderas R, Lepe JA, Pachón J. Efficacy of daptomycin versus vancomycin in an experimental model of foreign-body and systemic infection caused by biofilm producers and methicillin-resistant Staphylococcus epidermidis. Antimicrob Agents Chemother. 2012. February;56(2):613-7. Epub 2011 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodworth JR, Nyhart EH, Jr, Brier GL, Wolny JD, Black HR. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob Agents Chemother. 1992. February;36(2):318-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother. 2006. August;58(2):256-65. Epub 2006 Jul 1. [DOI] [PubMed] [Google Scholar]
- 44.Hegde SS, Reyes N, Skinner R, Difuntorum S. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J Antimicrob Chemother. 2008. January;61(1):169-72. Epub 2007 Nov 9. [DOI] [PubMed] [Google Scholar]
- 45.Andes D, van Ogtrop ML, Peng J, Craig WA. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother. 2002. November;46(11):3484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meagher AK, Forrest A, Rayner CR, Birmingham MC, Schentag JJ. Population pharmacokinetics of linezolid in patients treated in a compassionate-use program. Antimicrob Agents Chemother. 2003. February;47(2):548-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crandon JL, Kuti JL, Nicolau DP. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob Agents Chemother. 2010. December;54(12):5115-9. Epub 2010 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Healy DP, Polk RE, Garson ML, Rock DT, Comstock TJ. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob Agents Chemother. 1987. March;31(3):393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Steenwinkel JE, Aarnoutse RE, de Knegt GJ, ten Kate MT, Teulen M, Verbrugh HA, Boeree MJ, van Soolingen D, Bakker-Woudenberg IA. Optimization of the rifampin dosage to improve the therapeutic efficacy in tuberculosis treatment using a murine model. Am J Respir Crit Care Med. 2013. May 15;187(10):1127-34. [DOI] [PubMed] [Google Scholar]
- 50.Ruslami R, Nijland HM, Alisjahbana B, Parwati I, van Crevel R, Aarnoutse RE. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother. 2007. July;51(7):2546-51. Epub 2007 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]