Introduction
Carbohydrate recognition is fundamental to a wide variety of interkingdom interactions. For example, bacterial peptidoglycan, an N-acetyl-D-glucosamine (GlcNAc)–N-acetylmuramic acid (MurNAc) polymer [1], and fungal chitin, a GlcNAc polymer [2, 3], are both immunostimulatory to vertebrates. In addition, bacterially produced Nodulation (Nod) factors, which consist of a modified GlcNAc, play a key role in symbiotic interactions with plants [4]. The structural similarity of these GlcNAc-containing molecules (Fig 1) suggests possible overlap in their physiological action, although many of the mechanisms underlying the detection of these molecules in different organisms appear unrelated. However, a single phylogenetically conserved domain, called Lysin (LysM), is found in specific receptors in signaling pathways responsive to one or more of these three related carbohydrates in bacteria, plants, and fungi and possibly mammalian systems. Thus, promiscuous activation could occur when a structurally similar but physiologically inappropriate ligand binds and thereby aberrantly activates an incorrect LysM domain-containing receptor. Here, I will discuss this possibility and its implications for immune pathologies such as asthma in which chitin is relevant.
Fig 1. Bacterial and fungal carbohydrates.
A. Monomer of L-Lys containing peptidoglycan from Staphlycoccus aureus. B. Fungal chitin. C. Sinorhizobium meliloti Nod factor NodSmIV. Lys, Lysin; Nod, Nodulation.
Bacterial carbohydrates: Peptidoglycan
Most bacteria have cell walls containing peptidoglycan, a polymer composed of alternating β(1,4)-linked GlcNAc and MurNAc residues cross-linked by species-specific peptides (Fig 1A). Many proteins are attached noncovalently to the bacterial cell envelope, and the LysM motif is often used to target these proteins to the peptidoglycan. LysM domains are found in a wide range of bacterial proteins that are involved in cell wall metabolism, including peptidoglycan hydrolases and phage lysins [5]. Detailed structural analysis of a LysM domain from a bacterial peptidoglycan hydrolase reveals that it recognizes both the GlcNAc moiety as well as the peptide stem [6]. In plants, LysM domain-containing proteins serve as peptidoglycan receptors and initiate a downstream signaling cascade in response to peptidoglycan binding [7]. While a number of different mammalian proteins are known to recognize peptidoglycan, such as Peptidoglycan recognition proteins (PGRP) [8], they lack LysM motifs. Interestingly, there are recently identified LysM domain-containing proteins in vertebrates, including zebrafish [9] and mice [10], although their physiological function is not known.
Fungal carbohydrates: Chitin
Chitin, a linear β(1,4)-linked polymer of GlcNAc (Fig 1B) derived from a variety of biological sources including the cell walls of fungi and insects, is one of the most abundant polymers in nature. In plants, chitin is sensed by LysM domain-containing proteins that function to mediate an appropriate innate response [11]. In fungi, LysM domain-containing proteins called LysM effectors are important for chitin-triggered immunity, possibly by acting quite generally on processes including spore germination and hyphal growth [12]. Chitin (and its deacetylated form chitosan) are immunostimulatory in mammals, although the mechanism underlying chitin recognition remains elusive, perhaps in part because variables including the size of the physiologically active chitin molecules are not known [3].
Symbiotic carbohydrates: Lipochitin oligosaccharides
The symbiotic relationship between legumes and rhizobial bacteria is central to biological nitrogen fixation and thereby to the global nitrogen cycle. Key to this relationship are Nod factors, short species-specific chito-oligosaccharides containing various substitutions on the reducing and nonreducing termini. One example of a Nod factor is NodSmIV, produced by Sinorhizobium meliloti (Fig 1C), and as can be seen, it is very similar to both peptidoglycan and chitin. The plant Nod factor receptors are receptor kinases that contain one to three extracellular LysM domains [13] with very high (approximately nM) affinity for their specific Nod factor ligand [14].
Potential cross-reactivity in peptidoglycan and chitin recognition
In fungi, LysM domain-containing proteins called LysM effectors play a role in regulating host colonization, possibly by sequestering fungal-derived chitin [12]. It has been proposed that the ability of LysM domains to also bind peptidoglycan may allow fungi to affect potential bacterial competitors during infection [12]. This could occur because cell wall binding even in the absence of muralytic activity can be sufficient for antibacterial function [15]. In plants, the LysM domains that mediate Nod factor recognition are also found in other proteins that serve as receptors for pathogenic signals including chitin and/or peptidoglycan [16, 17]. This has led to the intriguing hypothesis that Nod factors may have evolved as a mechanism to suppress innate immunity by interfering with the recognition of chitin and/or peptidoglycan [18]. In support of this idea, Nod factors suppress an innate immune reaction in Arabidopsis thaliana, even though this species lacks a Nod receptor [14]. Since this effect depends on the presence of the LYK3 LysM-containing receptor kinase that functions as an innate immune receptor, the Nod factor could therefore act as a competitive antagonist of LYK3.
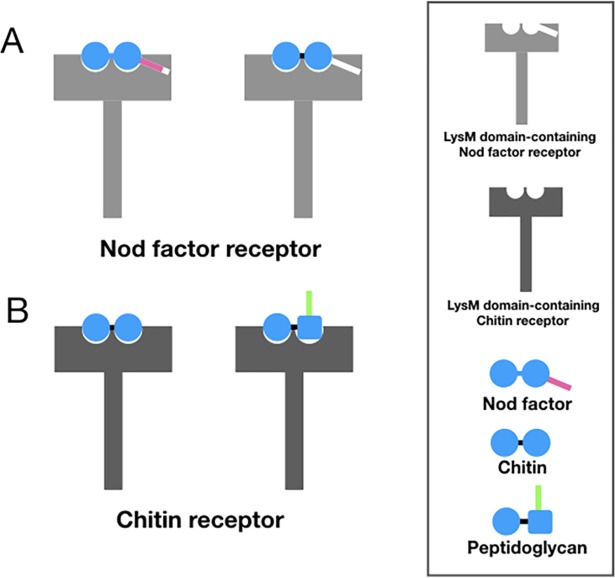
In the case of plants, the ability of LysM domain-containing proteins to bind both chitin and Nod factors presents a potential problem, given the heterogeneous mixture of microbially produced carbohydrates present in the soil. Specificity of the physiological response could be maintained through distinct receptor sets, which have a greater affinity for one class of saccharide. Recently, such a mechanism has been shown to operate in the legume Lotus japonicus, in which a LysM domain-containing kinase that mediates the response to pathogens exhibits much higher sensitivity to chitin as compared to Nod factors [19]. In mammalian cells, the ability of Nod factors (lipochitin oligosaccharides) to stimulate mammalian angiogenesis [20], taken with the previously mentioned observation that a Nod factor is able to suppress a plant innate immune reaction in species that lack the Nod receptor [14], suggests that physiologically relevant cross-activation is possible. For example, a chitin derived molecule could bind a LysM domain that normally binds Nod factor (Fig 2, left). Conversely, a peptidoglycan-derived muropeptide could bind a LysM domain that normally binds chitin (Fig 2, right). Presumably, these noncognate bindings would occur at relatively lower affinity, but if environmental concentrations of the heterologous ligands are in excess to the normal ligand, then noncognate binding could be physiologically relevant.
Fig 2. Potential cross-activation of LysM domain-containing carbohydrate receptors.
A. The LysM domain of a Nod factor receptor normally binds Nod factor (left) but could also bind a structurally related chitin molecule (right). B. The LysM domain of a chitin receptor that normally binds chitin (left) could also bind a structurally similar peptidoglycan muropeptide (right). Lys, Lysin, Nod; Nodulation.
So what direct evidence exists that particular LysM domains, which mediate detection of either peptidoglycan or chitin in a particular physiological context, could promiscuously interact with both ligands? That is, could chitin or peptidoglycan interact with a LysM domain of a protein that is normally the receptor for the other molecule? The structure of Enterococcus faecalis AtlA, a LysM domain-containing peptidoglycan hydrolase, indicates that specific, well-conserved residues in the AtlA LysM domain mediate binding to GlcNAc-(GlcNAc/MurNAc)-GlcNAc. This structure implies that further specificity is determined by secondary interactions between hydrophobic LysM residues such as leucine that interacts with hydrophobic substitutions of the nonreducing terminal sugar in the case of Nod factor recognition or the interaction mediated by a nonaromatic residue at the reducing end of the ligand in the case of peptidoglycan [6].
Both in vitro and in vivo evidence supporting some promiscuity exists (Table 1). In vitro, E. faecalis AtlA binds chitin with a higher affinity than peptidoglycan [6]. LysM domains of bacterial origin bind peptidoglycan fragments and chitin polymers with similar affinity, although plant LysM demonstrate a greater affinity for chitin [21]. Similarly, a protein consisting of the three LysM domains from the Lactococcus lactis autolysin AcaA binds both bacterial-derived peptidoglycan sacculus as well as chitin and the cell wall of the fungus Psilocybe cubensis [22]. In vivo, the LysM domain-containing A. thaliana kinase CERK1 responds to both peptidoglycan and chitin [23] and the rice LysM receptor-like kinase CERK1 [24] and LysM-domain containing proteins LYP4 and LYP6 [25] respond to chitin as well as to peptidoglycan.
Table 1. Chitin/PG biding of LysM-domain containing proteins.
When the specific comparative specificity has been determined, it is noted; otherwise ligands with approximately comparable affinities observed are listed.
| Organism | LysM-domain proteins | Specificity |
|---|---|---|
| E. faecalis | AtlA | Chitin>PG [6] |
| L. lactis | AcaA | Chitin, PG [22] |
| M. smegmatis | MSL | Chito-oligosaccharide, Chitin, PG [39]. |
| B. subtilis | NlpC/P60 | Chitin, PG [21] |
|
T. atroviride |
TAL6 |
Chitin, PG [40] |
| A. thaliana | CERK1 | PG [23], chitin [23] |
| O. sativa (rice) | OsCERK1, OsLYP4, OsLYP6 | Chitin/PG [24], [25] |
| M. japonicus (shrimp) | MjLPBP |
Chitin, PG [41] |
Abbreviations: PG, peptidoglycan.
So how is specificity in signaling maintained? One possibility is some kind of post-translational modification to the LysM domain (e.g., N-glycosylation [26]) that differentially affects binding of different GlcNAc-containing molecules. Alternatively, modifications could be targeted to the ligands. Peptidoglycan is often modified, and these modifications can affect its interaction with the innate immune system [27]. For example, wall teichoic acids of Staphylococcal aureus affect binding to an important peptidoglycan recognition protein in Drosophila melanogaster [28], although whether these or other modifications affect LysM binding is not known. Structural studies of chitin binding to a protein containing multiple LysM domains suggest that cooperative binding to the GlcNAc strand could be important [21, 29]. And in the case of peptidoglycan, multiple LysM domains increase the affinity, at least as compared to chito-oligosaccharides [6]. However, while these and other studies implicate the length of the saccharide polymer as an important factor in specificity, the size of the chitin or peptidoglycan fragments that are physiologically active is not known [30, 31]. While very small peptidoglycan fragments derived from the gut flora can exogenously induce the genesis of lymphoid follicles in mice [32], similar molecules do not stimulate immunity-associated defenses in Arabidopsis [23]. Thus, polymer chain length is likely a critical but not well-understood parameter for specificity of activation.
Promiscuity of carbohydrate ligands in pathologies
While the mechanisms underlying specificity discussed above may be sufficient for normal physiological function, the presence of aberrantly higher levels of one class of carbohydrate could interfere with these mechanisms. That is, during a bacterial infection or environmental exposure to fungal-derived [33] or insect-derived chitin [2], the relative concentrations of ligands may undergo significant changes with potential pathological consequences. Chitin is thought to be an important trigger leading to the development of asthma. Chitin administered to murine airways induces infiltration of eosinophils and basophils and drives stimulation of both resident and recruited macrophages [34]. In addition, mammalian lung epithelial cells secrete a chitinase, and mice lacking this enzyme exhibit premature morbidity and mortality, concomitant with significant airway accumulation of environmentally derived chitin polymers [35]. One possible explanation for this pathology is that increased chitin levels result in aberrant activation of the peptidoglycan receptors in the lung, thereby disturbing the normal balance of ligand concentration required to achieve proper specificity of recognition. Although the specific peptidoglycan receptors in the lung are not known, LysM domain-containing proteins of unknown function are found in mammals [10]. Promiscuous activation could also be relevant to the intriguing but not well-understood role of the microbiota in the pathogenesis of asthma [36]. Recent observations that transient early life microbial dysbiosis is an important factor influencing asthma development [37] suggest that bacterial products may have a role in mediating these effects. Specifically, peptidoglycan is released by growing bacterial cells, and the substantial levels of peptidoglycan fragments generated by the microbiota have important systemic immunological effects mediated by key innate immune proteins like Nod1 [30]. Thus, perhaps the levels of these molecules relative to their receptors is affected by the presence of chitin, which changes the normal ligand:receptor stoichiometry. This would be analogous to the situation in plants in which exogenous Nod factors can act as a competitive agonist and suppress the normal plant innate immune reaction to chitin [14]. Finally, inflammatory bowel disease is another example in which interactions between chitin- and peptidoglycan-signaling systems could be relevant. Recent work has demonstrated that chitin microparticles administered to colitis models greatly affected the bacterial community in the colon, both in overall number as well as overall species composition [38].
Acknowledgments
I thank Howard Shuman for helpful comments.
Funding Statement
The work in my laboratory is supported by NIH GM114213-03 and by a Burroughs-Welcome Fund Investigators in the Pathogenesis of Infectious Disease award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wolf AJ, Underhill DM. Peptidoglycan recognition by the innate immune system. Nat Rev Immunol. 2018;18(4):243–54. 10.1038/nri.2017.136 . [DOI] [PubMed] [Google Scholar]
- 2.Elieh Ali Komi D, Sharma L, Dela Cruz CS. Chitin and Its Effects on Inflammatory and Immune Responses. Clin Rev Allergy Immunol. 2018;54(2):213–23 10.1007/s12016-017-8600-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bueter CL, Specht CA, Levitz SM. Innate sensing of chitin and chitosan. PLoS Pathog. 2013;9(1):e1003080 10.1371/journal.ppat.1003080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oldroyd GE, Murray JD, Poole PS, Downie JA. The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet. 2011;45:119–44. 10.1146/annurev-genet-110410-132549 [DOI] [PubMed] [Google Scholar]
- 5.Buist G, Steen A, Kok J, Kuipers OP. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol. 2008;68(4):838–47. 10.1111/j.1365-2958.2008.06211.x . [DOI] [PubMed] [Google Scholar]
- 6.Mesnage S, Dellarole M, Baxter NJ, Rouget JB, Dimitrov JD, Wang N, et al. Molecular basis for bacterial peptidoglycan recognition by LysM domains. Nat Commun. 2014;5:4269 10.1038/ncomms5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gust AA. Peptidoglycan Perception in Plants. PLoS Pathog. 2015;11(12):e1005275 10.1371/journal.ppat.1005275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziarski R, Gupta D. Review: Mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity. Innate Immun. 2010;16(3):168–74. 10.1177/1753425910366059 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Laroche FJ, Tulotta C, Lamers GE, Meijer AH, Yang P, Verbeek FJ, et al. The embryonic expression patterns of zebrafish genes encoding LysM-domains. Gene Expr Patterns. 2013;13(7):212–24. 10.1016/j.gep.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama CC, Baldridge MT, Leung DW, Zhao G, Desai C, Liu TC, et al. LysMD3 is a type II membrane protein without an in vivo role in the response to a range of pathogens. J Biol Chem. 2018;293(16):6022–38. 10.1074/jbc.RA117.001246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka K, Nguyen CT, Liang Y, Cao Y, Stacey G. Role of LysM receptors in chitin-triggered plant innate immunity. Plant Signal Behav. 2013;8(1):e22598 10.4161/psb.22598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kombrink A, Thomma BP. LysM effectors: secreted proteins supporting fungal life. PLoS Pathog. 2013;9(12):e1003769 10.1371/journal.ppat.1003769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fliegmann J, Bono JJ. Lipo-chitooligosaccharidic nodulation factors and their perception by plant receptors. Glycoconj J. 2015;32(7):455–64. 10.1007/s10719-015-9609-3 [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Cao Y, Tanaka K, Thibivilliers S, Wan J, Choi J, et al. Nonlegumes respond to rhizobial Nod factors by suppressing the innate immune response. Science. 2013;341(6152):1384–7. 10.1126/science.1242736 [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim HR, Matsuzaki T, Aoki T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001;506(1):27–32. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Vallet A, Mesters JR, Thomma BP. The battle for chitin recognition in plant-microbe interactions. FEMS Microbiol Rev. 2015;39(2):171–83. 10.1093/femsre/fuu003 [DOI] [PubMed] [Google Scholar]
- 17.Antolin-Llovera M, Ried MK, Binder A, Parniske M. Receptor kinase signaling pathways in plant-microbe interactions. Annual review of phytopathology. 2012;50:451–73. 10.1146/annurev-phyto-081211-173002 [DOI] [PubMed] [Google Scholar]
- 18.Liang Y, Toth K, Cao Y, Tanaka K, Espinoza C, Stacey G. Lipochitooligosaccharide recognition: an ancient story. New Phytol. 2014;204(2):289–96. 10.1111/nph.12898 [DOI] [PubMed] [Google Scholar]
- 19.Bozsoki Z, Cheng J, Feng F, Gysel K, Vinther M, Andersen KR, et al. Receptor-mediated chitin perception in legume roots is functionally separable from Nod factor perception. Proc Natl Acad Sci U S A. 2017;114(38):E8118–E27. 10.1073/pnas.1706795114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djordjevic MA, Bezos A, Susanti, Marmuse L, Driguez H, Samain E, et al. Lipo-chitin oligosaccharides, plant symbiosis signalling molecules that modulate mammalian angiogenesis in vitro. PLoS ONE. 2014;9(12):e112635 10.1371/journal.pone.0112635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong JE, Alsarraf HM, Kaspersen JD, Pedersen JS, Stougaard J, Thirup S, et al. Cooperative binding of LysM domains determines the carbohydrate affinity of a bacterial endopeptidase protein. FEBS J. 2014;281(4):1196–208. 10.1111/febs.12698 [DOI] [PubMed] [Google Scholar]
- 22.Visweswaran GR, Dijkstra BW, Kok J. A genetically engineered protein domain binding to bacterial murein, archaeal pseudomurein, and fungal chitin cell wall material. Appl Microbiol Biotechnol. 2012;96(3):729–37. 10.1007/s00253-012-3871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willmann R, Lajunen HM, Erbs G, Newman MA, Kolb D, Tsuda K, et al. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(49):19824–9. 10.1073/pnas.1112862108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ao Y, Li Z, Feng D, Xiong F, Liu J, Li JF, et al. OsCERK1 and OsRLCK176 play important roles in peptidoglycan and chitin signaling in rice innate immunity. Plant J. 2014;80(6):1072–84. 10.1111/tpj.12710 [DOI] [PubMed] [Google Scholar]
- 25.Liu B, Li JF, Ao Y, Qu J, Li Z, Su J, et al. Lysin motif-containing proteins LYP4 and LYP6 play dual roles in peptidoglycan and chitin perception in rice innate immunity. Plant Cell. 2012;24(8):3406–19. 10.1105/tpc.112.102475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fliegmann J, Uhlenbroich S, Shinya T, Martinez Y, Lefebvre B, Shibuya N, et al. Biochemical and phylogenetic analysis of CEBiP-like LysM domain-containing extracellular proteins in higher plants. Plant Physiol Biochem. 2011;49(7):709–20. 10.1016/j.plaphy.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 27.Davis KM, Weiser JN. Modifications to the peptidoglycan backbone help bacteria to establish infection. Infection and immunity. 2011;79(2):562–70. 10.1128/IAI.00651-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atilano ML, Yates J, Glittenberg M, Filipe SR, Ligoxygakis P. Wall teichoic acids of Staphylococcus aureus limit recognition by the drosophila peptidoglycan recognition protein-SA to promote pathogenicity. PLoS Pathog. 2011;7(12):e1002421 10.1371/journal.ppat.1002421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Vallet A, Saleem-Batcha R, Kombrink A, Hansen G, Valkenburg DJ, Thomma BP, et al. Fungal effector Ecp6 outcompetes host immune receptor for chitin binding through intrachain LysM dimerization. Elife. 2013;2:e00790 10.7554/eLife.00790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16(2):228–31. doi: nm.2087 [pii] 10.1038/nm.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gendrin M, Welchman DP, Poidevin M, Herve M, Lemaitre B. Long-range activation of systemic immunity through peptidoglycan diffusion in Drosophila. PLoS Pathog. 2009;5(12):e1000694 10.1371/journal.ppat.1000694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–10. 10.1038/nature07450 [DOI] [PubMed] [Google Scholar]
- 33.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, et al. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol. 2011;187(5):2261–7. 10.4049/jimmunol.1100972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447(7140):92–6. 10.1038/nature05746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Dyken SJ, Liang HE, Naikawadi RP, Woodruff PG, Wolters PJ, Erle DJ, et al. Spontaneous Chitin Accumulation in Airways and Age-Related Fibrotic Lung Disease. Cell. 2017;169(3):497–509 e13. 10.1016/j.cell.2017.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17(5):592–602. 10.1016/j.chom.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152 10.1126/scitranslmed.aab2271 [DOI] [PubMed] [Google Scholar]
- 38.Nagatani K, Wang S, Llado V, Lau CW, Li Z, Mizoguchi A, et al. Chitin microparticles for the control of intestinal inflammation. Inflamm Bowel Dis. 2012;18(9):1698–710. 10.1002/ibd.22874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patra D, Mishra P, Vijayan M, Surolia A. Negative Cooperativity and High Affinity in Chitooligosaccharide Binding by a Mycobacterium smegmatis Protein Containing LysM and Lectin Domains. Biochemistry. 2016;55(1):49–61. 10.1021/acs.biochem.5b00841 [DOI] [PubMed] [Google Scholar]
- 40.Seidl-Seiboth V, Zach S, Frischmann A, Spadiut O, Dietzsch C, Herwig C, et al. Spore germination of Trichoderma atroviride is inhibited by its LysM protein TAL6. FEBS J. 2013;280(5):1226–36. 10.1111/febs.12113 [DOI] [PubMed] [Google Scholar]
- 41.Shi XZ, Feng XW, Sun JJ, Yang MC, Lan JF, Zhao XF, et al. Involvement of a LysM and putative peptidoglycan-binding domain-containing protein in the antibacterial immune response of kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2016;54:489–98. 10.1016/j.fsi.2016.04.134 [DOI] [PubMed] [Google Scholar]