Abstract
Bacterial pneumonia is a leading cause of death late after burn injury due to the severe immune dysfunction that follows this traumatic injury. The Mechanistic/Mammalian Target of Rapamycin (mTOR) pathway drives many effector functions of innate immune cells required for bacterial clearance. Studies have demonstrated alterations in multiple cellular processes in patients and animal models following burn injury in which mTOR is a central component. Goals of this study were to 1) investigate the importance of mTOR signaling in antimicrobial activity by neutrophils and 2) therapeutically target mTOR to promote normalization of the immune response. We utilized a murine model of 20% total body surface area burn and the mTOR-specific inhibitor rapamycin. Burn injury led to innate immune hyper-responsiveness in the lung including recruitment of neutrophils with greater ex vivo oxidative activity compared to neutrophils from sham injured mice. Elevated oxidative function correlated with improved clearance of Pseudomonas aeruginosa, despite downregulated expression of the bacterial-sensing Toll-Like Receptor (TLR) molecules. Rapamycin administration reversed the burn injury-induced lung innate immune hyper-responsiveness and inhibited enhanced bacterial clearance in burn mice compared to untreated burn mice, resulting in significantly higher mortality. Neutrophil ex vivo oxidative burst was decreased by rapamycin treatment. These data indicate that 1) neutrophil function within the lung is more important than recruitment for bacterial clearance following burn injury and 2) mTOR inhibition significantly impacts innate immune hyper-responsiveness, including neutrophil effector function, allowing normalization of the immune response late after burn injury.
Keywords: Pseudomonas aeruginosa, Toll-like Receptor, Reactive Oxygen and Nitrogen Species, Nitric Oxide, Trauma, Macrophage
Introduction
In 2015 almost 500,000 patients sought medical care for a burn injury in the United States, resulting in approximately 40,000 Intensive Care Unit admissions and over 3,000 fatalities. Severe burns often require several weeks of hospitalization, during which patients require careful monitoring to prevent and treat secondary infections [1, 2]. Burn injury leads to immune perturbations that render patients highly susceptible to opportunistic bacterial infections during recovery. Indeed, patients without apparent lung damage at admission often develop bacterial pneumonia during hospitalization, most often due to Pseudomonas aeruginosa. Improved understanding of the complex immune response to burn injury may illuminate treatments to minimize complications, decrease recovery times, and improve long-term prognosis.
The immune response to burn injury is complex and dynamic. For years it was believed that in burns, as in sepsis, patients experience an initial Systemic Inflammatory Response Syndrome (SIRS) marked by pro-inflammatory cytokines and other inflammatory mediators. In sepsis, SIRS is followed by a later, Compensatory Anti-Inflammatory Response Syndrome (CARS) where immunosuppression renders the patient vulnerable to infection. Recent studies indicate that the SIRS/CARS paradigm may not accurately represent the complex immune response in burn patients because pro- and anti- inflammatory mediators are often detected simultaneously [1–6] and patients experience a mixed antagonist response syndrome (MARS), typified by an early hyper-inflammatory phase, resulting in immune pathophysiology and ultimately multiple organ dysfunction syndrome. Several animal models of burn injury have been described, including scalding, contact burns, and flame burns in pigs, rats, and mice [7–10]. While the preponderance of animal studies have focused on the early (“SIRS”) immune response following injury, animal studies focused on the late immune response (the former CARS phase) have been infrequent. In addition, these studies have demonstrated significantly enhanced, immunopathogenic hyper- innate immune responses [11–14] rather than the clinical picture of general immunosuppression.
Burn injury generates numerous inflammatory stimuli including Danger-Associated Molecular Patterns (DAMPs) that active innate immune cells through Pattern Recognition Receptors (PRR) such as Toll-Like Receptors (TLR), which results in the activation of the Mechanistic/Mammalian Target of Rapamycin (mTOR) pathway that drives many effector functions of immune cells [15]. mTOR nucleates a major eukaryotic signaling network that plays a fundamental role in cell and organismal physiology, where many aspects of mTOR function have only recently been elucidated, and multiple questions still remain [16]. With that said, it is appreciated that the mTOR axis acts as a molecular switch to regulate inflammatory and metabolic functions. To this end, in addition to immune dysfunction, burn patients also experience severe metabolic dysregulation, characterized by elevated basal metabolic rate and a paradoxical comingling of hyperglycemia and hyperinsulinemia [17–21]. Similarly, several animal studies have demonstrated altered metabolism in animal models of burn injury, including insulin resistance and hyperglycemia [21–23]. The relationship between metabolism and immunity has been focus of much recent research activity [24, 25]. Insulin receptor signaling suppresses TLR expression in human granulocytes [26], and hyperglycemia perturbs both cytokine secretion and TLR signaling in myeloid cells [27–30]. Insulin receptor signaling leads to mTOR activation, allowing cells to interpret nutrient availability to support translational and metabolic processes that lie downstream [31].
Thus, we hypothesize that dysregulation of the mTOR axis following burn injury is a driver of immunological dysfunction observed and that targeting mTOR at the appropriate stage post-burn injury holds promise for clinical intervention. Therefore, the goals of this study were to 1) investigate the importance of mTOR signaling in antimicrobial activity by neutrophils and 2) therapeutically target mTOR during burn injury to promote normalization of the immune response. Using systemic administration of rapamycin in a murine model of burn injury, we observed an mTOR-dependent decrease in neutrophil function in the lung following injury that resulted in decreased bacterial clearance and survival following a pulmonary P. aeruginosa infection.
Materials and Methods
Burn injury and rapamycin treatment
Nine week old female C57/BL6 mice (Taconic Farms; Hudson, NY) were housed in a specific pathogen free environment. All procedures were carried out in accordance with guidelines from the National Institutes of Health regarding use of vertebrate animals in research. Protocols were approved by the University of North Carolina at Chapel Hill’s Institutional Animal Care and Use Committee. Numbers of mice used in each experiment are given in the Figure Legends. Experiments were repeated to confirm findings and representative data are shown. There are sex differences in patient and mouse response to burn [32], but we have not observed any no significant differences in the immune response to burn injury with this model. We utilize female mice to be consistent with our previous studies using this mouse model [8, 10, 33–36].
The 20% total body surface area full-thickness contact burn procedure was carried out under isoflurane sedation as previously described [10, 36], including prophylactic subcutaneous morphine prior to injury and oral morphine ad libitum in drinking water following injury. Rapamycin was administered (4 mg/kg/d) daily starting 7 d before the burn injury via an intraperitoneal injection.
Infection and sample collection
A wildtype strain (PAK) of Pseudomonas aeruginosa was utilized for all infections as previously described [10] . Bacteria from frozen cultures were grown overnight in Luria Broth (LB). The following morning, cultures were diluted 50x and grown for approximately two hours until mid-log phase growth was achieved (OD600=0.6–1.2). Cultures were then centrifuged at 14000 rpm for 30 seconds, and the pellet was washed using 1mL PBS+1% Protease Peptone solution (PBS+1%PP). Bacterial pellets were re-suspended and diluted to the desired concentration and verified by optical density at 600nm. Bacterial concentration was then confirmed by serial dilution and plating on LB agar plates. Mice were sedated with tribromoethanol (475mg/kg body weight. Sigma-Aldrich; St. Louis, MO), sedation was confirmed by toe-pinch, and mice were mounted on an intubation platform. A MicroSprayer® Aerosolizer Model 1A-1C and FMJ-250 High-Pressure Syringe (Penn Century, Inc.; Wyndmoor, PA) nozzle was inserted into the trachea and 50μL of aerosolized inoculum or vehicle control (1% PP-PBS) was instilled into the airway.
Levels of nitric oxide (NO) were measured directly in blood as previously described [35]. To determine bacterial burden, lung and liver tissue were collected at time of sacrifice and serial dilutions of tissue homogenate in PBS were plated on LB-agar plates and grown overnight at 37˚C.
To prepare tissue for histology, lungs were inflated with paraformaldehyde (4% by weight in PBS) to a constant pressure and fixed overnight. Lungs were embedded in paraffin, sectioned, and hematoxylin and eosin (H&E) stained at the Lineberger Comprehensive Cancer Center Animal Histopathology Core. Images were taken with an Olympus BX61 Upright Wide Field Microscope at the UNC Microscopy Services Laboratory.
Flow cytometric analysis
Lung tissue was minced and digested with collagenase I (4.5 mg) and DNAse (2μg) with shaking for 1 h at 37˚C. Filtered samples were subjected to ammonium-chloride-potassium lysis and treated with a blocking immunoglobulin against CD16/CD32 (eBioscience; San Diego, CA) prior to staining with fluorescently conjugated antibodies against CD45, CD11b, CD11c, Ly6C, Ly6G and F4/80 (BD Biosciences; San Jose, CA). To quantify reactive oxygen and nitrogen species (RONS) production, stained cells were incubated with 0.75 mg Dihydrorhodamine 123 (DHR; Thermo-Fisher; Waltham, MA). Duplicate samples were either stimulated with 62.5 ng phorbol 12-myristate 13-acetate (PMA) or left unstimulated. After 30 min, reactions were quenched with a fixation buffer containing 1% paraformaldehyde and mean fluorescence intensity (MFI) was measured. To quantify S6 phosphorylation (pS6), cells were surface stained as above and then intracellularly stained with anti-pS6 antibody (eBioscience; San Diego, CA). Samples were analyzed at the UNC Flow Cytometry Core Facility on a CyAn and Summit 5.3 software was used to analyze data (Beckmann-Coulter; Indianapolis, IN). Cells were initially gated on FSC-A/FSC-H “singlets” to exclude groups of cells. Neutrophils were defined as CD45+CD11b+CD11c-Ly6G+ and macrophages were defined as CD45+CD11c+Ly6C+F4/80+Ly6G-. For some experiments, cells from whole lung tissue were collected at 14 d post injury and stimulated with fetal bovine serum (FBS) in vitro with or without 20nM Rapamycin for 30 minutes. Cells were stained for neutrophils and macrophages with intracellular pS6 staining as above.
Statistical analysis
GraphPad Prism Version 5.0 for Windows was used to analyze data by Student’s t-Test, One-Way Analysis of Variance (ANOVA) with Tukey post-test, or Two-Way ANOVA with Bonferroni post-test, as appropriate. Data are represented as mean +/− standard error of the mean (SEM).
Results and Discussion
Burn injury leads to sustained recruitment of neutrophils to the pulmonary vasculature.
The most common cause of mortality amongst burn patients late after injury is bacterial pneumonia. In order to characterize the immune environment of the lung, we performed cutaneous burn or sham injury on wildtype C57BL/6 mice. Using flow cytometry, we observed that neutrophils are recruited to the lung within hours of the injury (Figure 1A), with absolute numbers significantly higher for up to two weeks (Figure 1B). Figure 1C shows a representative flow plot of Ly6g-Ly6C+F4/80+ macrophages and Ly6g+Ly6C-F4/80- neutrophils after gating on CD45+ and excluding CD11b-CD11c- cells. The gates were used to calculate the frequency of neutrophils, which increased approximately five-fold in the lung compared to sham injured mice at 14 days post injury (dpi). We did not observe an increase in macrophage numbers or frequency in the lung following burn injury (Figure 1D). Histology revealed that the neutrophils are sequestered in the lung vasculature, with no appearance of neutrophils, edema, or other damage in the airway or alveolar space (Figure 1E; three representative images are shown for sham and burn; neutrophils marked with an arrow).
Figure 1: Neutrophils are recruited to the lung following burn injury.
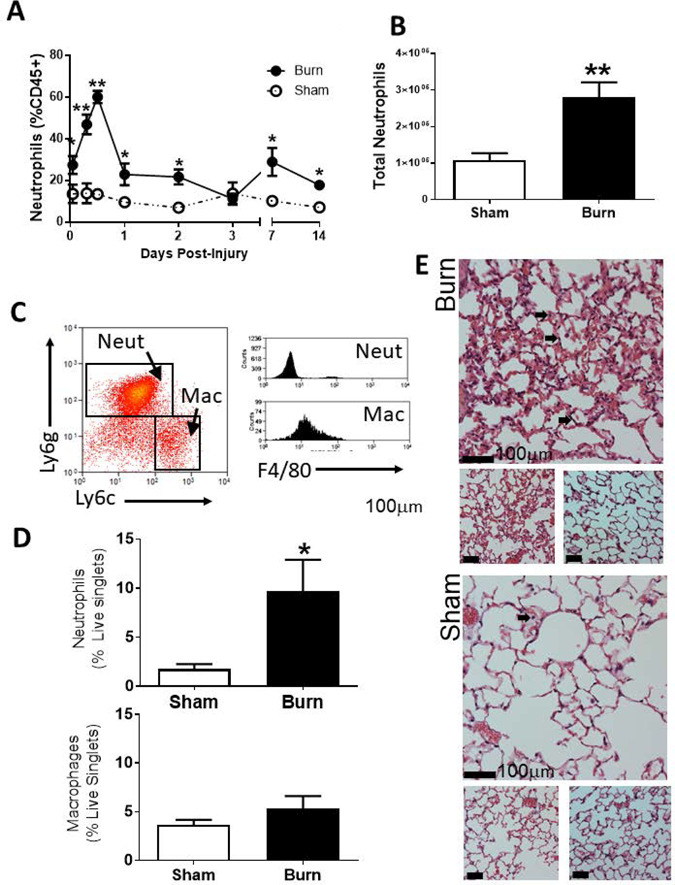
A) Neutrophils are shown as a percentage of CD45+ cells from whole lung tissue as measured by flow cytometry. B) Total number of neutrophils in the lung is determined as (%neutrophils as live cells x live cells counted at harvest). C) Representative Ly6g-Ly6C+F4/80+ macrophages and Ly6g+Ly6C-F4/80- neutrophils flow plots after gating on singlet cells that are CD45+ and excluding CD11b-CD11c- cells. D) Neutrophils and macrophages are shown as a fraction of live singlet cells as measured by flow cytometry. E) Representative specimens of H&E stained distal airway are shown from sham and burn mice at 12 h after treatment. Bar represents 100µm. Arrows indicate neutrophils sequestered in the vasculature. Data shown are +/−SEM (*p<0.05, **p<0.01) and representative of three repeated experiments. Numbers of mice in representative figures; A) sham, n=4; burn, n=5 (day 1 for sham, n=3); B) sham, n=5; burn, n=6; D) sham, n=5; burn, n=6.
Neutrophils that accumulate in the pulmonary vasculature after burn have greater oxidative activity ex vivo.
We hypothesized that neutrophils that accumulate late after burn injury were hyper-activate and contribute to the dysfunctional immune response observed after burn injury. We quantified reactive oxygen and nitrogen species (RONS) production by cells isolated from whole lung 14 d after cutaneous burn injury and utilized Dihydrorhodamine-123 (DHR) to detect the production of reactive oxygen and nitrogen species (RONS) in cells [37]. In burned mice compared to sham we observed a significant increase in the ability of neutrophils to oxidize DHR without further stimulation ex vivo (Figure 2A). Stimulation with PMA revealed that the maximal RONS production was equivalent in burn and sham neutrophils, resulting in a significantly reduced stimulation index (stimulated MFI/unstimulated MFI; Figure 2B). Neither resting nor maximal RONS production was altered in lung macrophages after burn injury. These data suggest that pulmonary neutrophils, which accumulate late after burn injury, likely have hyper-responsive oxidative activity in vivo.
Figure 2: Burn injury impacts reactive oxygen and nitrogen species (RONS) production in neutrophils.
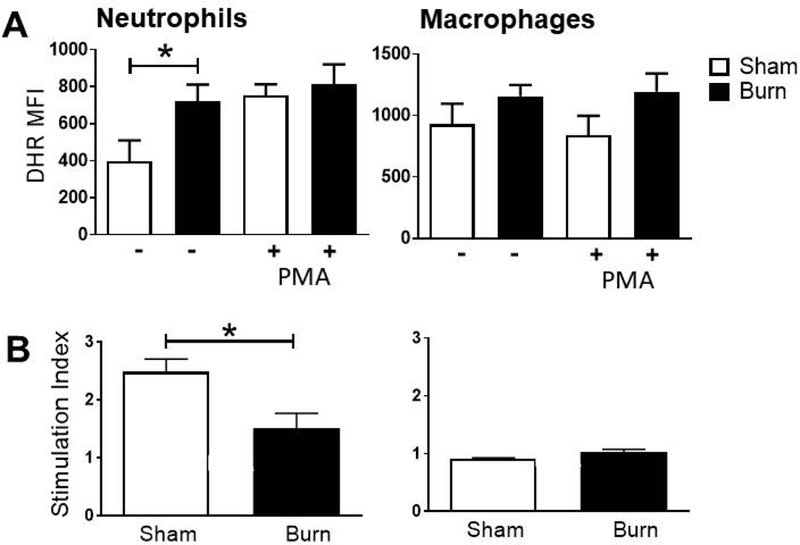
Cells from whole lung tissue were collected at 14 d post-injury and analyzed by flow cytometry. A) DHR MFI of gated neutrophils or macrophages was measured in unstimulated samples. B) DHR MFI was measured in gated neutrophils and macrophages from paired stimulated and unstimulated samples. Stimulation index was calculated by dividing the DHR MFI of stimulated samples by that of unstimulated samples. Data shown are +/−SEM (*p<0.05) and representative of three repeated experiments. Numbers of mice in representative figures; A-B) sham, n=4; burn, n=6.
Burn mice clear bacterial infection more effectively than sham injured mice despite reduced TLR expression.
We hypothesized that the increased oxidative capacity of neutrophils late after injury accounts for the ability of burned mice to clear a pulmonary bacterial infection. We inoculated burn or sham injured mice 14 d after injury with aerosolized Pseudomonas aeruginosa (strain PAK), a clinically relevant opportunistic pathogen that frequently causes fatal pneumonia in burn patients. We observed significantly greater clearance of PAK in burn mice compared to sham mice, despite comparable increases in neutrophil numbers in the airway (Figure 3A). On examination of the innate cell activity in the lung, we found that both burn injury alone and burn injury plus infection resulted in elevated RONS production by pulmonary neutrophils ex vivo; however, we did not observe an infection-dependent increase in neutrophil RONS production in either burn or sham animals (Figure 3B). In contrast, only bacterial infection significantly increased RONS by pulmonary macrophages. These findings suggest that the increased oxidative capacity of neutrophils late after injury is maintained during bacterial infection.
Figure 3: Changes in immune function, but not recruitment, lead to improved bacterial clearance in the lung following burn injury.
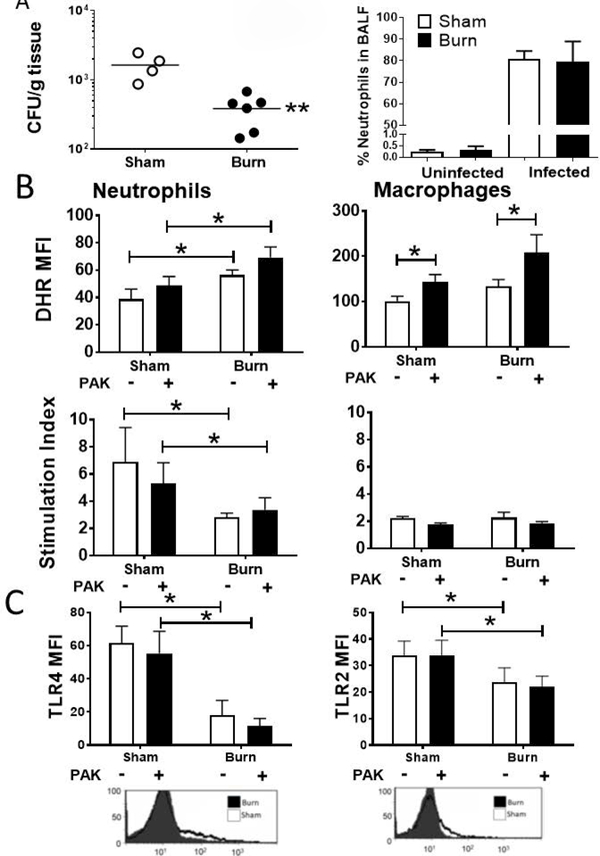
Mice were infected intratracheally with 1× 106 CFU of PAK at 14 dpi and samples were collected 48 h later. A) Bacterial burden in whole lung homogenate was observed in burn mice compared with sham mice. B) DHR MFI of gated pulmonary neutrophils or macrophages from both stimulated and unstimulated samples was measured by flow cytometry. Unstimulated values are shown, as well as the stimulation index (DHR MFI stimulated / DHR MFI unstimulated) C) MFI of PE-labeled TLR4 on gated neutrophils and TLR2 on gated macrophages was determined by flow cytometry in samples from lung. Representative flow cytometry plots used to derive MFI are shown under each graph of untreated burn and sham injured mice. Data shown are +/−SEM. *p<0.05, **p<0.01 and representative of three repeated experiments. Numbers of mice in representative figures; A) sham, n=4; burn, n=6; B) sham, n=4; burn, n=5; C) sham, n=5; burn, n=6.
Toll-like receptor (TLR) signaling is an important sensing mechanism for antimicrobial responses. We have previously described reduced TLR expression by innate cells late after burn injury [8]. Other studies have shown that TLR2 and TLR4 signaling impact neutrophil chemotaxis, activation, and lifespan, with the latter effects being especially important for prolonged responses to stimulus as in the case of our model [38]. Herein, using flow cytometry, we observe significantly reduced TLR2 and TLR4 expression in neutrophils from burn mice compared to sham at 14 d after injury (Figure 3C). These changes in TLR expression occurred with and without infection. The ability of burn mice to clear pulmonary bacteria therefore appears more tightly correlated with oxidative burst than TLR expression.
Inhibition of mTOR reverses burn injury-induced innate immune hyper-responsiveness in the lung.
It has been proposed that mTOR activity impacts neutrophil recruitment, RONS production and TLR signaling [24]. mTOR can act downstream of the insulin receptor, making it a target of hyperinsulinemia observed in burn patients [20]. Furthermore, processes downstream of mTOR activation, such as oxidative burst and protein translation, are enhanced by hyperglycemia [39–41].
Because of the importance of mTOR and its signaling partners in insulin signaling and immune regulation, we hypothesize that mTOR promotes granulocyte hyper-responsiveness following trauma and infection by boosting TLR sensitivity and RONS production. To test this hypothesis, we inhibited mTOR activity with systemic administration of rapamycin (daily, starting 7 d before the injury) and measured bacterial clearance as well as TLR expression and RONS production by pulmonary granulocytes following injury. We observed increased bacterial burden in burn mice treated with rapamycin compared to rapamycin treated sham-injured mice (Figure 4A). At a high infectious dose, we observed significant mortality within 72 h of infection only in burn injury and rapamycin treated mice (Figure 4B).
Figure 4: mTOR activity is restricted to neutrophils, and is required for survival and efficient bacterial clearance in burned mice following infection.
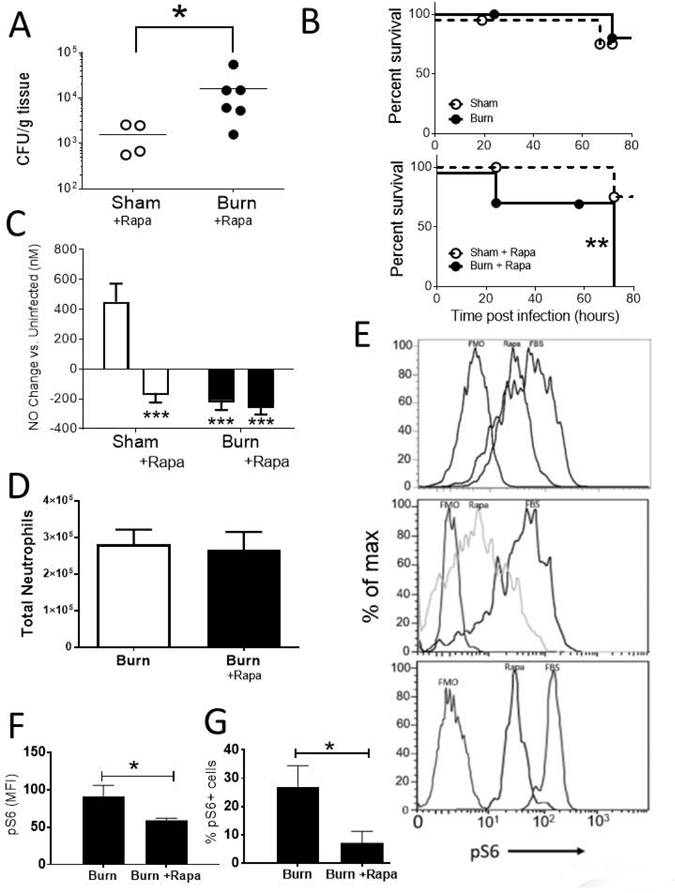
Burn or sham mice treated with rapamycin (4mg/kg/day) underwent an intra-tracheal inoculation with PAK at 14 dpi. A) 48 hours following infection with 1 × 106 CFU of PAK, bacterial burden was determined in whole lung homogenate. B) Following infection with 5 × 106 CFU of PAK, mice were monitored for morbidity for 72h, and moribund or deceased animals were recorded. C) Blood from the submandibular vein was collected and NO levels were directly measured. D) Total number of neutrophils in the lung is determined as (%neutrophils as live cells x live cells counted at harvest). E) Cells from whole lung tissue were collected at 14 d post burn injury and stimulated with FBS in vitro with (Rapa) or without (FBS) Rapamycin for 30 minutes. After gating on neutrophils, we show intracellular pS6 staining in three representative replicates from three repeat experiments. FMO refers to the Fluorescence Minus One control, which is the fluorescence signal using every antibody apart from anti-pS6. E-F) pS6 staining of gated neutrophils were measured and used to calculate F) MFI of cellular pS6 and G) pS6+ percentage of gated neutrophils. Data shown are +/−SEM. *p<0.05, **p<0.01 and representative of three repeated experiments. Numbers of mice in representative figures; A, C) sham, n=4; burn, n=6; B) sham, n=8; burn, n=8; D) sham, n=6; burn, n=6; F,G) sham, n=5; burn, n=5.
We have previously reported that increased blood nitric oxide (NO) marks a robust innate response to bacterial infection [35]. Using a novel microfluidics based electrode to rapidly detect blood NO, we found that sham injured mice were able to mount a significantly increased NO response after PAK infection. In contrast, burn mice infected 14 d after injury had a significant reduction in the concentration of blood NO compared to infected sham mice; mTOR inhibition suppressed systemic NO production following infection in both sham and burn mice (Figure 4C).
In other models characterized by neutrophil accumulation, particularly models of Acute Lung Injury (ALI), rapamycin can impede neutrophil accumulation to the lungs [42]. TLR4 signaling is required for neutrophil accumulation to occur in ALI models, whereas complement activation and upregulation of integrin are the primary mechanisms that drive neutrophil accumulation in pulmonary vasculature following cutaneous burn. Rapamycin did not impact neutrophil recruitment to the lungs late after burn injury (Figure 4D). Further study will be required to clearly enumerate and differentiate the chemotactic signals in acute, direct lung injury models compared to chronic, distal injuries with lung involvement.
To measure direct control of the mTOR pathway by burn injury and rapamycin treatment in pulmonary innate immune cells, we quantified intracellular phosphorylation of the downstream molecule S6 via flow cytometry. In an ex vivo experiment to determine if immune cells from the lung respond directly to rapamycin, we observed that pS6 signal was decreased by rapamycin compared with positive control FBS stimulation (Figure 4E; three representative replicates from three repeat experiments). Moreover, mTOR-pathway activation observed in neutrophils after burn injury was blocked by in vivo treatment with rapamycin, exhibited by less pS6 per neutrophil (Figure 4F) accompanied by a significant decrease in the number of PS6+ neutrophils (Figure 4G). We therefore propose that the effect of systemic rapamycin on bacterial clearance and inflammation may be attributable to a direct effect on innate immune cells in the lung although we cannot exclude indirect impacts of Rapamycin on other cell types. To investigate the independent roles of mTOR in immune dysfunction after injury, studies are ongoing using an inducible and conditional mTOR gene knockout strategy using the Cre-loxP and interferon-α-inducible promoter system. We will also investigate the role of other cell types by utilizing cell-specific targeting strategies.
We explored the cellular mechanism of the decreased bacterial control and increased mortality following burn plus rapamycin treatment. Neutrophil and macrophage TLR expression was reduced by rapamycin treatment in both sham and burn injured mice (Figure 5A). Future studies should determine whether signal transduction downstream of TLR ligation is similarly impaired by rapamycin treatment. Moreover, we found that in neutrophils (Figure 5B) and macrophages (Figure 5C), increased RONS production after burn and infection was dependent on mTOR; however, there was a further burn-dependent decrease of RONS production by neutrophils, evidenced by a reduced stimulation index, which was independent of mTOR activity (Figure 5B).
Figure 5: mTOR inhibition impacts innate immune function following burn injury and infection.
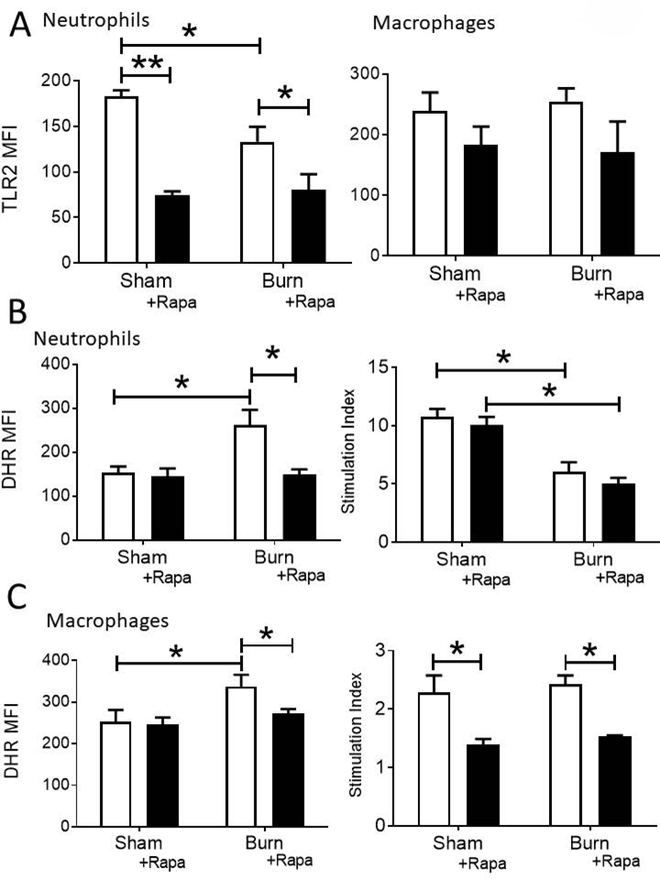
Mice underwent an intra-tracheal inoculation with 1 × 106 CFU PAK at 14 dpi., with or without Rapamycin treatment. A) MFI of PE-labeled TLR2 on neutrophils and macrophages from whole lung cell suspensions was determined by flow cytometry. B-C), DHR MFI of gated pulmonary B) neutrophils and C) macrophages from both stimulated and unstimulated samples was measured by flow cytometry. Unstimulated values are shown, as well as the stimulation index (DHR MFI stimulated / DHR MFI unstimulated). Data shown are +/−SEM. *p<0.05, **p<0.01, ***p<0.001 and representative of three repeated experiments. Numbers of mice in representative figures; A-C) sham, n=4; burn, n=6.
In conclusion, we report substantial neutrophil accumulation in the lung following burn injury, accompanied by increased RONS production and minimal extravasation into the airway. In contrast, macrophages were not “primed” by burn injury in this way. When challenged with a pulmonary bacterial infection, we observe enhanced bacterial clearance in burn mice compared to uninjured controls, accompanied by increased RONS production by both neutrophils and macrophages even in the face of reduced TLR expression. Augmented RONS production and S6 phosphorylation, but not granulocyte recruitment, were abolished by mTOR inhibition after burn injury. The absence of the innate “priming” effect of burn injury in rapamycin-treated mice led to increased bacterial susceptibility in burn mice compared to uninjured controls. We hypothesize that after burn injury, accelerated metabolism increases mTOR signaling, priming innate immune activity to protect the airway from infection by driving up innate cell RONS production. It may be more therapeutically beneficial to target innate immune effector function rather than recruitment in trauma patients. Future studies will determine whether systemic agonists of AMPK and HIF1α, which act up- and downstream of mTOR, respectively [15, 31], are effective in offsetting pneumonia after burn injury in mice. Additional studies are underway to quantify factors that impact mTOR signaling in trauma patients.
Summary sentence:
After burn injury, mTOR signaling promotes a pro-inflammatory response to protect the airway from bacterial infection by increasing innate cell RONS production.
Acknowledgements
All flow cytometry studies were carried out with support of the UNC Flow Cytometry Core Facility, which is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center (LCCC). Animal histopathology was performed in the LCCC Animal Histopathology Core Facility at the University of North Carolina at Chapel Hill. The LCCC Animal Histopathology Core is supported in part by an NCI Center Core Support Grant (2P30CA016086–40) to the UNC Lineberger Comprehensive Cancer Center. MHS acknowledges research support from the National Institutes of Health (AI097539).
Abbreviations
- CARS
Compensatory anti-inflammatory response syndrome
- CFU
Colony Forming Units
- DHR
Dihydrorhodamine 123
- dpi
Days post-injury
- FBS
Fetal bovine serum
- ICU
Intensive Care Unit
- LB
Luria Broth
- MFI
Mean Fluorescence Intensity
- mTOR
Mammalian target of rapamycin
- NO
Nitric Oxide
- PAK
Pseudomonas aeruginosa strain PAK
- RONS
Reactive oxygen and nitrogen species
- SIRS
Systemic inflammatory response syndrome
- TBSA
Total Body Surface Area
- TLR
Toll-like Receptor
Footnotes
Conflict of Interest
Mark Schoenfisch is the founder and a member of the board of directors, and maintains a financial interest in Clinical Sensors, Inc, a company that is commercializing microfluidic nitric oxide sensors.
References
- 1.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D, Tompkins RG, Investigators of the, I., the Host Response Glue, G. (2008) Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Molecular medicine 14, 553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN (2011) Long-term persistance of the pathophysiologic response to severe burn injury. PloS one 6, e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones SW, Zhou H, Ortiz-Pujols SM, Maile R, Herbst M, Joyner BL Jr., Zhang H, Kesic M, Jaspers I, Short KA, Meyer AA, Peden DB, Cairns BA, Noah TL (2013) Bronchoscopy-derived correlates of lung injury following inhalational injuries: a prospective observational study. PloS one 8, e64250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maile R, Jones S, Pan Y, Zhou H, Jaspers I, Peden DB, Cairns BA, Noah TL (2015) Association between early airway damage-associated molecular patterns and subsequent bacterial infection in patients with inhalational and burn injury. American journal of physiology. Lung cellular and molecular physiology 308, L855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendoza AE, Maile LA, Cairns BA, Maile R (2013) Burn injury induces high levels of phosphorylated insulin-like growth factor binding protein-1. International journal of burns and trauma 3, 180–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG, Inflammation, Host Response to Injury Large-Scale Collaborative Research, P. (2011) A genomic storm in critically injured humans. The Journal of experimental medicine 208, 2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdullahi A, Amini-Nik S, Jeschke MG (2014) Animal models in burn research. Cellular and molecular life sciences : CMLS 71, 3241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns BA, Barnes CM, Mlot S, Meyer AA, Maile R (2008) Toll-like Receptor 2 and 4 Ligation Results in Complex Altered Cytokine Profiles Early and Late After Burn Injury. Journal of Trauma and Acute Care Surgery 64, 1069–1078. [DOI] [PubMed] [Google Scholar]
- 9.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, Jeschke MG (2008) Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock 30, 503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neely CJ, Kartchner LB, Mendoza AE, Linz BM, Frelinger JA, Wolfgang MC, Maile R, Cairns BA (2014) Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12-neutrophil polarization. PloS one 9, e85623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner JC, Noel JG, Nikolaidis NM, Karns R, Aronow BJ, Ogle CK, McCormack FX (2014) G-CSF drives a posttraumatic immune program that protects the host from infection. J Immunol 192, 2405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noel JG, Guo X, Wells-Byrum D, Schwemberger S, Caldwell CC, Ogle CK (2005) Effect of thermal injury on splenic myelopoiesis. Shock 23, 115–22. [DOI] [PubMed] [Google Scholar]
- 13.Noel JG, Osterburg A, Wang Q, Guo X, Byrum D, Schwemberger S, Goetzman H, Caldwell CC, Ogle CK (2007) Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock 28, 684–93. [DOI] [PubMed] [Google Scholar]
- 14.Tschop J, Martignoni A, Reid MD, Adediran SG, Gardner J, Noel GJ, Ogle CK, Neely AN, Caldwell CC (2009) Differential immunological phenotypes are exhibited after scald and flame burns. Shock 31, 157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weichhart T, Hengstschlager M, Linke M (2015) Regulation of innate immune cell function by mTOR. Nature reviews. Immunology 15, 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxton RA and Sabatini DM (2017) mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mecott GA, Al-Mousawi AM, Gauglitz GG, Herndon DN, Jeschke MG (2010) The role of hyperglycemia in burned patients: evidence-based studies. Shock 33, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy CV, Coffey R, Cook CH, Gerlach AT, Miller SF (2011) Early glycemic control in critically ill patients with burn injury. Journal of burn care & research : official publication of the American Burn Association 32, 583–90. [DOI] [PubMed] [Google Scholar]
- 19.Williams FN, Jeschke MG, Chinkes DL, Suman OE, Branski LK, Herndon DN (2009) Modulation of the hypermetabolic response to trauma: temperature, nutrition, and drugs. J Am Coll Surg 208, 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin-Long C, Zhao-Fan X, Dao-Feng B, Wei D (2011) mTOR partly mediates insulin resistance by phosphorylation of insulin receptor substrate-1 on serine(307) residues after burn. Burns 37, 86–93. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Yu YM, Ma H, Carter EA, Fagan S, Tompkins RG, Fischman AJ (2013) Glucose metabolism during the early “flow phase” after burn injury. The Journal of surgical research 179, e83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugita H, Kaneki M, Sugita M, Yasukawa T, Yasuhara S, Martyn JA (2005) Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle. American journal of physiology. Endocrinology and metabolism 288, E585–91. [DOI] [PubMed] [Google Scholar]
- 23.Carter EA, Paul KW, Barrow SA, Fischman AJ, Tompkins RG (2012) Previous burn injury predisposes mice to lipopolysaccharide-induced changes in glucose metabolism. Journal of burn care & research : official publication of the American Burn Association 33, 683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keating R and McGargill MA (2016) mTOR Regulation of Lymphoid Cells in Immunity to Pathogens. Front Immunol 7, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsalikis J, Croitoru DO, Philpott DJ, Girardin SE (2013) Nutrient sensing and metabolic stress pathways in innate immunity. Cell Microbiol 15, 1632–41. [DOI] [PubMed] [Google Scholar]
- 26.Ghanim H, Mohanty P, Deopurkar R, Sia CL, Korzeniewski K, Abuaysheh S, Chaudhuri A, Dandona P (2008) Acute modulation of toll-like receptors by insulin. Diabetes care 31, 1827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devaraj S, Venugopal SK, Singh U, Jialal I (2005) Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta}. Diabetes 54, 85–91. [DOI] [PubMed] [Google Scholar]
- 28.Devaraj S and Jialal I (2009) Increased secretion of IP-10 from monocytes under hyperglycemia is via the TLR2 and TLR4 pathway. Cytokine 47, 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanmugam N, Reddy MA, Guha M, Natarajan R (2003) High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 52, 1256–64. [DOI] [PubMed] [Google Scholar]
- 30.Shanmugam N, Gaw Gonzalo IT, Natarajan R (2004) Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes 53, 795–802. [DOI] [PubMed] [Google Scholar]
- 31.Hay N and Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18, 1926–45. [DOI] [PubMed] [Google Scholar]
- 32.Plackett TP, Gamelli RL, Kovacs EJ (2010) Gender-based differences in cytokine production after burn injury: a role of interleukin-6. J Am Coll Surg 210, 73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchanan IB MR, Frelinger JA, Fair JH, Meyer AA, Cairns BA (2006) The effect of burn injury on CD8+ and CD4+ T cells in an irradiation model of homeostatic proliferation. J Trauma 61, 1062–8. [DOI] [PubMed] [Google Scholar]
- 34.Cairns B MR, Barnes CM, Frelinger JA, Meyer AA. (2006) Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. J Trauma 61, 293–8. [DOI] [PubMed] [Google Scholar]
- 35.Dunn JL, Hunter RA, Gast K, Maile R, Cairns BA, Schoenfisch MH (2016) Direct detection of blood nitric oxide reveals a burn-dependent decrease of nitric oxide in response to Pseudomonas aeruginosa infection. Burns [DOI] [PMC free article] [PubMed]
- 36.Maile R, Barnes CM, Nielsen AI, Meyer AA, Frelinger JA, Cairns BA (2006) Lymphopenia-induced homeostatic proliferation of CD8+ T cells is a mechanism for effective allogeneic skin graft rejection following burn injury. J Immunol 176, 6717–26. [DOI] [PubMed] [Google Scholar]
- 37.Crow JP (1997) Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1, 145–57. [DOI] [PubMed] [Google Scholar]
- 38.Sabroe I, Dower SK, Whyte MK (2005) The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 41 Suppl 7, S421–6. [DOI] [PubMed] [Google Scholar]
- 39.Fejfarova V, Jirkovska A, Lupinkova J, Kovar J, Kalanin J, Striz I, Skibova J, Boucek P, Pelikanova T (2006) Effect of acute hyperglycemia and/or hyperinsulinemia on polymorphonuclear functions in healthy subjects. Metabolism 55, 811–8. [DOI] [PubMed] [Google Scholar]
- 40.Stegenga ME, van der Crabben SN, Dessing MC, Pater JM, van den Pangaart PS, de Vos AF, Tanck MW, Roos D, Sauerwein HP, van der Poll T (2008) Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med 25, 157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hand WL, Hand DL, Vasquez Y (2007) Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract 76, 44–50. [DOI] [PubMed] [Google Scholar]
- 42.Lorne E, Zhao X, Zmijewski JW, Liu G, Park YJ, Tsuruta Y, Abraham E (2009) Participation of mammalian target of rapamycin complex 1 in Toll-like receptor 2- and 4-induced neutrophil activation and acute lung injury. Am J Respir Cell Mol Biol 41, 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


