Abstract
To investigate the prognostic value of DHCR24 for patients with bladder cancer (BC). We used public bladder cancer microarray studies to evaluate the expression of DHCR24 between normal bladder tissues and BC cells, to investigate the relationship between the expression of DHCR24 and the clinical features of BC patients. Survival analysis was performed to investigate the correlation between DHCR24 expression and the survivals of BC patients. Gene set enrichment analysis was conducted to identify relevant mechanisms. The results showed that DHCR24 was up-regulated in BC cells compared with that in normal bladder tissues (P = .0389). Results of chi-square test suggested that BC patients in DHCR24 low expression group were proved to have better clinical characteristics (including tumor grade, disease progression, T staging, and N staging) as compared with those in DHCR24 low expression group (P < .0001, P = .002, P = .005, and P = .002, respectively). BC patients in DHCR24 low expression group were associated with better cancer-specific survival and overall survival (P < .0001 and P = .0008, respectively). DHCR24 might promote the proliferation of BC cells through several oncogenesis-associated biological processes (estrogen response, heme metabolism, P53 pathway, cholesterol homeostasis, mTORC1 signaling, peroxisome, xenobiotic metabolism, glycolysis, and protein secretion). Thus, DHCR24 might be a therapeutic target for patients with BC.
Keywords: bladder cancer, DHCR24, prognostic relevance
1. Introduction
Bladder cancer (BC) represents the second most common cancer of the urinary system and the 6th most common cancer in men and the 11th most common in women, respectively.[1,2] Urothelial carcinoma represents the major type of BC—nonmuscle invasive BC (NMIBC)—which represents approximately 70% of newly diagnosed BCs; 70% of NMBICs are pTa BCs, which is confined to bladder epithelium, 20% of NMIBCs are pT1 BCs which invade lamina propria, and 10% of NMIBCs are carcinoma in situ. For the treatment of NMIBC, transurethral resection of the bladder tumor (TURBT) is recommended as the standard surgical option, and 45% to 80% of patients with NMIBC will experience disease recurrence and progression. Meanwhile, muscle invasive bladder cancer (MIBC) represents the remaining newly diagnosed BC. For the treatment of MIBC, radical cystectomy has become a standard of care, and once it becomes metastatic cancer, the 5-year overall survival for patients with invasive BC is dismal.[2–6] Thus, the introduction of novel markers that are associated with the clinical characteristics and predict the prognosis of BC might be of great significance.
DHCR24 is involved in multiple cellular functions, such as oxidative stress, cell differentiation, antiapoptotic function, and anti-inflammatory activity.[7,8] In addition, DHCR24 is dysregulated in various tumors including adrenal, prostate, ovarian, and testicular germ cell tumors. In this study, the relationship between the expression of DHCR24 and the clinical characteristics of BC patients was analyzed to investigate the prognostic value of DHCR24.
2. Methods
2.1. BC microarray studies
The study was approved by the local Ethics Committee (Zhongnan Hospital, Wuhan University). BC gene expression study GSE13507[9,10] and E-MTAB-1803[11] were downloaded from GEO (gene expression omnibus) database (https://www.ncbi.nlm.nih.gov/geo/) and ArrayExpress (https://www.ebi.ac.uk/arrayexpress/). GSE13507 is a gene expression profile of 165 primary BC samples, 23 recurrent nonmuscle invasive tumor tissues, 58 normal looking bladder mucosae surrounding cancer, and 10 normal bladder mucosae, which was annotated with Illumina human-6 v2.0 expression beadchip. The other BC microarray study E-MTAB-1803 was a gene expression profile of 85 primary BCs, which was annotated with Affymetrix Human Genome U133 Plus 2.0 Array. GSE13507 was used to investigate the expression of DHCR24 between normal bladder tissues and BC cells, to characterize the relationship between the expression of DHCR24 and the clinicopathological features of patients with BC, and to evaluate the prognostic relevance of DHCR24 for patients with BC. E-MTAB-1803 (n = 85) was used to perform gene set enrichment analysis (GSEA)[12,13] to identify potentially relevant mechanisms.
2.2. Statistical analysis
Statistical analyses were conducted from October 10 to November 1. Firstly, we evaluated the expression levels of DHCR24 in normal bladder tissues and BC cells. The expression of DHCR24 (the corresponding probe ID is ILMN_1725510 in GSE13507) was presented as mean ± standard deviation (SD), and unpaired t test was conducted to evaluate the difference between normal bladder tissue and BC cells. Secondly, the primary BC samples in GSE13507 (n = 165) were classified into DHCR24 low expression group (n = 83) and DHCR24 high expression group (n = 82) based on the median of DHCR24 expression levels in these primary BC samples, and then chi-square test was conducted to investigate the relationship between the expression of DHCR24 and clinical characteristics (including age, sex, tumor grade, recurrence, progression, and TNM staging) of patients with BC. Meanwhile, we conducted logistical regression analysis to analyze the relationship between the expression of DHCR24 and the above clinical characteristics of BC patients. Finally, log-rank based survival analysis and Cox proportional-hazards regression model were conducted to investigate the prognostic relevance of DHCR24 for BC patients in GSE13507 (BC patients were categorized into DHCR24 low expression group and DHCR24 high expression using the same methods as mentioned above). Cancer-specific survival and overall survival were defined as Kim et al introduced.[9,10]P value less than .05 was regarded as statistical significance for chi-square test and survival analysis. Finally, GSEA was performed to investigate the relevant mechanisms involved in the regulation of DHCR24 on BC cells using E-MTAB-1803. BC samples in E-MTAB-1803 were also divided into DHCR24 low expression group and DGCR24 high expression group based on the median of DHCR24 expression in E-MTAB-1803. The gene set ‘h.all.v5.2.symbols.gmt’ was used as a reference. Differences were considered statistically significant at nominal P value <.05 and false discovery rate <25%.
3. Results
Our results showed that DHCR24 was increased in BC cells compared with that in normal bladder tissues. BC patients in DHCR24 low expression group were associated with better clinical characteristics (including tumor grade, disease progression, T staging, and N staging) than those in DHCR24 low expression group. BC patients in DHCR24 low expression group were associated with better cancer-specific survival and overall survival. The results of GSEA suggested that DHCR24 might promote the proliferation of BC cells through several oncogenesis-associated biological processes.
3.1. DHCR24 was up-regulated in BC cells
GSE13507 was used to evaluate the expression of DHCR24 between normal bladder tissues and BC cells. As shown in Fig. 1, DHCR24 (the corresponding probe ID is ILMN_1725510) was significantly up-regulated in BC cells compared with that in normal bladder tissues (9.364 ± 1.007 vs 9.74 ± 1.367; P = .0389).
Figure 1.

The expression of DHCR24 in normal bladder tissue and bladder cancer.
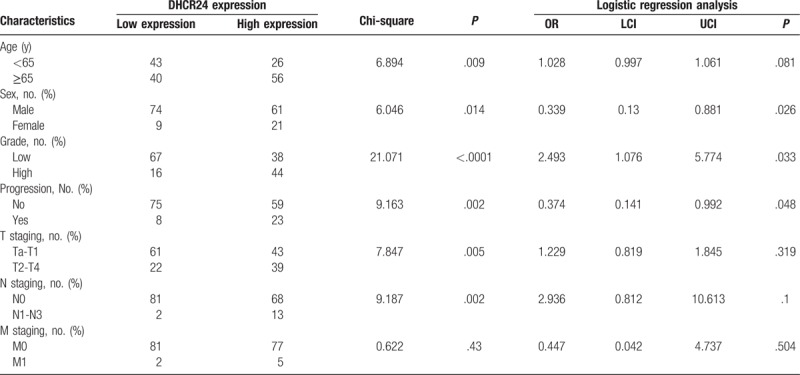
3.2. The relationship between the expression of DHCR24 and the clinical characteristics of BC
The primary BC samples in GSE13507 were categorized into DHCR24 high expression group and DHCR24 low expression group according to the median of DHCR24 expression. As shown in Table 1, BC patients in DHCR24 low expression group were associated with better clinical characteristics (including sex, age, tumor grade, disease progression, T staging, and N staging) as compared with those in DHCR24 high expression group (P = .014, P = .009, P < .0001, P = .002, P = .005, and P = .002, respectively). Moreover, the results of logistic regression analysis suggested that the expression of DHCR24 was associated with the sex (P = .026), grade (P = .033), and progression (P = .048) of BC patients.
Table 1.
The clinical characteristics of patients with bladder cancer in DHCR24 low expression group and DHCR24 high expression group.
3.3. BC patients in DHCR24 low expression group were associated with better survivals
Next, we analyzed the prognostic significance of DHCR24 for patients with DHCR24. BC patients were classified into DHCR24 low expression group and DHCR24 high expression group as mentioned above. The mean follow-up period for these BC patients was 48 months (median 37 months; range 1–137 months). As shown in Fig. 2, both the cancer-specific survival and overall survival favored BC patients in DHCR24 low expression group over BC patients in DHCR24 high expression group (for cancer-specific survival: hazard ratio [HR] 6.018, 95% confidence interval [CI] 2.979–12.16, P < .0001; for overall survival: HR 2.299, 95% CI 1.412–3.743, P = .0008). Meanwhile, the results of multivariable Cox proportional-hazards regression analysis suggested that DHCR24 was an independent prognostic factor for patients with BC in terms of overall survival (HR 1.483, 95% CI 1.062–2.071, P = .021).
Figure 2.

The correlation between the expression of DHCR24 and cancer-specific survival (A) and overall survival (OS) of patients with bladder cancer.
3.4. Gene set enrichment analysis
We conducted GSEA based on the expression of DHCR24. As shown in Table 2, BC samples in DHCR24 high expression group were mostly enriched gene sets like estrogen response, heme metabolism, P53 pathway, cholesterol homeostasis, mTORC1 signaling, peroxisome, xenobiotic metabolism, glycolysis, and protein secretion, indicating that DHCR24 might promote the proliferation of BC cells through these oncogenesis associated biological processes.
Table 2.
Gene sets enriched in BC samples with higher expression of DHCR24.

4. Discussion
Bladder cancer is 1 of the most common malignancies worldwide.[1] Although the risks of metastasis and death of patients with noninvasive BC remain relatively low, 40% to 80% of noninvasive BC cases can develop invasive BC.[1–3] Tumors that progress to invasive diseases result in a dismal 6% of 5-year overall survival for patients with invasive BC.[2,5] Currently, there is no highly effective standard management for both invasive and noninvasive BC. Therefore, it is of great significance to identify biomarkers that could predict the clinical prognosis of patients with BC.[14]
In the present study, 3 independent BC microarray studies were analyzed, demonstrating that DHCR24 was increased in BC cells. Moreover, patients with lower expression of DHCR24 displayed better clinicopathological features, and cancer-specific survival and overall survival, and DHCR24 might promote the proliferation of BC cells through several oncogenesis-associated biological processes (estrogen response, heme metabolism, P53 pathway, cholesterol homeostasis, mTORC1 signaling, peroxisome, xenobiotic metabolism, glycolysis, and protein secretion).
DHCR24, also known as 24-dehydrocholesterol reductase, locates on 1p32.3 and comprises 8 introns and 9 exons.[8] Seladin-1, encoded by DHCR24, participates in the late steps of cholesterol biosynthesis l.[15] Moreover, several studies demonstrated that DHCR24 was involved in the tumor progression of multiple human malignancies. Dai et al demonstrated that DHCR24 was significantly increased in endometrial cancer cells, and that the DHCR24 elevation was involved in advanced clinical stage, histological grading, vascular invasion, lymphatic metastasis, and reduced overall survival of patients with endometrial cancer. Simi et al[16] demonstrated that the promoter methylation regulated the expression of DHCR24 in adrenal cancer. Battista et al[17] demonstrated that DHCR24 was down-regulated in prostate cancer, and the up-regulation of DHCR24 could inhibit the proliferation of prostate cancer cells. Meanwhile, Cecchi et al[18] suggested that up-regulation of DHCR24 enhanced the resistance to Abeta toxicity of neuroblastoma cells.[18] Di Stasi et al[19] demonstrated that DHCR24 was increased in metastatic melanoma. Our results indicated that DHCR24 was up-regulated in BC cells (both MIBC and NMIBC cells), which was similar with the conclusions of literature review that DHCR24 might be associated with the carcinogenesis of BC.
Actually, Lee et al[20] suggested that DHCR24 was associated with tumor progression of patients with NMIBC by detecting mRNA expression of DGCR24 using RT-PCR and performing immunohistochemical (IHC). In their study, a total of 162 NMIBC patients were included, and the median follow-up period of these NMIBC patients was 60.0 months (range 12.1–219.9 months). During the period of follow-up, 59 (36.4%) NMIBC patients developed disease recurrence and 19 (11.7%) developed disease progression. Our results extended their conclusions to both NMIBC and MIBC patients, and proved that BC patients in DHCR24 low expression group were proved to have better clinical characteristics (including tumor grade, disease progression, T staging, and N staging) as compared with those in DHCR24 high expression group, indicating that DHCR24 was associated the progression of patients with NMIBC and MIBC. Moreover, the survival analysis of DHCR24 proved that both cancer-specific survival and overall survival favored BC patients in DHCR24 low expression group over BC patients in DHCR24 high expression group, indicating that DHCR24 was associated with poor survival of patients with BC.
The results of GSEA suggested that DHCR24 promoted the proliferation of BC cells through biological processes that had been reported to be involved in carcinogenesis, such as estrogen response,[21] heme metabolism,[22] P53 pathway,[23,24] cholesterol homeostasis,[25] mTORC1 signaling,[26] peroxisome,[27] xenobiotic metabolism,[28] glycolysis,[29] and protein secretion.
Actually, our study has limitations. Firstly, this is a retrospective study and the conclusions are not as robust as prospective studies. Second, the size of patients included in our study is not large (n = 256 in GSE13507 and n = 85 in E-MTAB-1803), which might not exclude selection bias. Thus, the conclusions of our study should be interpreted with caution and large-scale prospective studies are required to characterize the prognostic role of DHCR24 in bladder cancer.
5. Conclusions
Taken together, this study demonstrated that DHCR24 was up-regulated in BC cells, and up-regulation of DHCR24 was associated with worse clinical features for patients with BC, and BC patients in DHCR24 low expression group was proved to live longer than those in DHCR24 high expression group. DHCR24 affected the proliferation of BC cells through several biological processes involved in carcinogenesis.
Author contributions
Conceptualization: Xing-Huan Wang.
Data curation: Xiao-Ping Liu, Xiao-Hong Yin, Xing-Huan Wang.
Formal analysis: Xian-Tao Zeng.
Funding acquisition: Xing-Huan Wang.
Investigation: Xiao-Ping Liu, Xing-Huan Wang.
Methodology: Xiao-Ping Liu, Xiang-Yu Meng.
Project administration: Xiao-Ping Liu, Yue Cao.
Resources: Xiao-Ping Liu, Xin-Hui Yan, Xian-Tao Zeng.
Software: Xiao-Hong Yin.
Validation: Xin-Hui Yan, Xing-Huan Wang.
Visualization: Xiao-Ping Liu.
Writing – original draft: Xiao-Ping Liu.
Writing – review & editing: Xiang-Yu Meng, Yue Cao.
Footnotes
Abbreviations: BC = bladder cancer, DHCR24: 24-dehydrocholesterol reductase, GEO = gene expression omnibus, GSEA = gene set enrichment analysis, TURBT = transurethral resection of the bladder tumor.
Funding: The National Key research and development program of China (2016YFC0106300).
The authors declare no conflicts of interest.
References
- [1].Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- [2].Clark PE, Spiess PE, Agarwal N, et al. NCCN Guidelines Insights: Bladder Cancer, Version 2.2016. J Natl Compr Canc Netw 2016;14:1213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int 2017;119:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reis LO, Moro JC, Ribeiro LF, et al. Are we following the guidelines on non-muscle invasive bladder cancer? Int Braz J Urol 2016;42:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].So A. Bladder cancer, ESMO 2016. Can Urol Assoc J 2016;10(11-12 suppl 6):S224–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dorkin TJ, Robson CN, Neal DE. The molecular pathology of urological malignancies. J Pathol 1997;183:380–7. [DOI] [PubMed] [Google Scholar]
- [7].Nuti F, Luciani P, Marinari E, et al. Seladin-1 and testicular germ cell tumours: new insights into cisplatin responsiveness. J Pathol 2009;219:491–500. [DOI] [PubMed] [Google Scholar]
- [8].Zerenturk EJ, Sharpe LJ, Ikonen E, et al. Desmosterol and DHCR24: unexpected new directions for a terminal step in cholesterol synthesis. Prog Lipid Res 2013;52:666–80. [DOI] [PubMed] [Google Scholar]
- [9].Kim WJ, Kim EJ, Kim SK, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee JS, Leem SH, Lee SY, et al. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J Clin Oncol 2010;28:2660–7. [DOI] [PubMed] [Google Scholar]
- [11].El Behi M, Krumeich S, Lodillinsky C, et al. An essential role for decorin in bladder cancer invasiveness. EMBO Mol Med 2013;5:1835–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–73. [DOI] [PubMed] [Google Scholar]
- [14].Sanguedolce F, Brunelli M, D’amuri A, et al. Evolving concepts and use of immunohistochemical biomarkers in flat non-neoplastic urothelial lesions: WHO 2016 classification update with diagnostic algorithm. Biomarkers 2018;23:305–14. [DOI] [PubMed] [Google Scholar]
- [15].Fuller PJ, Alexiadis M, Jobling T, et al. Seladin-1/DHCR24 expression in normal ovary, ovarian epithelial and granulosa tumours. Clin Endocrinol (Oxf) 2005;63:111–5. [DOI] [PubMed] [Google Scholar]
- [16].Simi L, Malentacchi F, Luciani P, et al. Seladin-1 expression is regulated by promoter methylation in adrenal cancer. BMC Cancer 2010;10:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Battista MC, Guimond MO, Roberge C, et al. Inhibition of DHCR24/seladin-1 impairs cellular homeostasis in prostate cancer. Prostate 2010;70:921–33. [DOI] [PubMed] [Google Scholar]
- [18].Cecchi C, Rosati F, Pensalfini A, et al. Seladin-1/DHCR24 protects neuroblastoma cells against Abeta toxicity by increasing membrane cholesterol content. J Cell Mol Med 2008;12(5B):1990–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Di Stasi D, Vallacchi V, Campi V, et al. DHCR24 gene expression is upregulated in melanoma metastases and associated to resistance to oxidative stress-induced apoptosis. Int J Cancer 2005;115:224–30. [DOI] [PubMed] [Google Scholar]
- [20].Lee GT, Ha YS, Jung YS, et al. DHCR24 is an independent predictor of progression in patients with non-muscle-invasive urothelial carcinoma, and its functional role is involved in the aggressive properties of urothelial carcinoma cells. Ann Surg Oncol 2014;21Suppl 4:S538–45. [DOI] [PubMed] [Google Scholar]
- [21].Shen SS, Smith CL, Hsieh JT, et al. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer 2006;106:2610–6. [DOI] [PubMed] [Google Scholar]
- [22].Miyata Y, Kanda S, Mitsunari K, et al. Heme oxygenase-1 expression is associated with tumor aggressiveness and outcomes in patients with bladder cancer: a correlation with smoking intensity. Transl Res 2014;164:468–76. [DOI] [PubMed] [Google Scholar]
- [23].Pan CW, Liu H, Zhao Y, et al. JNK2 downregulation promotes tumorigenesis and chemoresistance by decreasing p53 stability in bladder cancer. JNK2 downregulation promotes tumorigenesis and chemoresistance by decreasing p53 stability in bladder cancer. Oncotarget 2016;7:35119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Du J, Wang SH, Yang Q, et al. Yao X. p53 status correlates with the risk of progression in stage T1 bladder cancer: a meta-analysis. World J Surg Oncol 2016;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hamm R, Chen YR, Seo EJ, et al. Induction of cholesterol biosynthesis by archazolid B in T24 bladder cancer cells. Biochem Pharmacol 2014;91:18–30. [DOI] [PubMed] [Google Scholar]
- [26].Becker MN, Wu KJ, Marlow LA, et al. The combination of an mTORc1/TORc2 inhibitor with lapatinib is synergistic in bladder cancer in vitro. Urol Oncol 2014;32:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tseng CH, Tseng FH. Peroxisome proliferator-activated receptor agonists and bladder cancer: lessons from animal studies. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2012;30:368–402. [DOI] [PubMed] [Google Scholar]
- [28].Martone T, Vineis P, Malaveille C, et al. Impact of polymorphisms in xeno (endo)biotic metabolism on pattern and frequency of p53 mutations in bladder cancer. Mutat Res 2000;462:303–9. [DOI] [PubMed] [Google Scholar]
- [29].Wan W, Peng K, Li M, et al. Histone demethylase JMJD1A promotes urinary bladder cancer progression by enhancing glycolysis through coactivation of hypoxia inducible factor 1α. Oncogene 2017;36:3868–77. [DOI] [PubMed] [Google Scholar]


