Abstract
Background:
Coracoid approach brachial plexus block (CABPB) is safe and effective for clinical anesthesia and analgesia. Dual stimulation can enhance the block effect of CABPB when using nerve stimulator. Dexmedetomidine is a highly selective α-adrenoceptor agonist and it can prolong the duration of anesthesia when it is added into local anesthetics. The aim of this study was to assess the effects of dexmedetomidine on the duration of anesthesia and the effective postoperative analgesia time when it was mixed with ropivacaine for CABPB under dual stimulation.
Methods:
A total of 60 patients were randomly assigned into 2 groups (groups D and C), 30 patients in each group. CABPB were guided by nerve stimulator under dual stimulation. Each patient received 40 mL of 0.375% ropivacaine (group C), or 40 mL of 0.375% ropivacaine mixed with 1 μg/kg dexmedetomidine (group D). The duration of anesthesia, the effective postoperative analgesia time, sensory and motor block onset time, visual analog scale (VAS), and the cumulative dose of rescue tramadol were recorded.
Results:
Twenty-eight patients in each group were analyzed. The duration of anesthesia was longer in group D as compared with group C (759 vs 634 minutes, P < .05) and the effective postoperative analgesia time was longer in group D as compared with group C (986 vs 789 minutes, P < .05) too. The onset time of sensory and motor blocks were not significantly different between the 2 groups (P > .05). The VAS was similar in the 2 groups at 6 and 12 hours after block (P > .05), but it was lower in group D at 24 hours after block as compared to group C (P < .05). The cumulative dose of rescue tramadol during the first 48 hours postoperative period was significantly lower in group D as compared to group C (P < .05). No significant changes were observed in vital signs in either group.
Conclusion:
The addition of 1 μg/kg dexmedetomidine to ropivacaine extends the duration of anesthesia and the effective postoperative analgesia time for CABPB under dual stimulation. The VAS at 24 hours after block and the demand for rescue tramadol during the first 48 hours postoperative period are lower as well without side effects in the study group.
(Registered in ClinicalTrials.gov id. NCT02961361).
Keywords: brachial plexus block, coracoid, dexmedetomidine, dual stimulation, onset time, postoperative analgesia, tramadol, visual analog scale
1. Introduction
Brachial plexus block is well suited for upper extremity surgeries. There are several approaches for brachial plexus block. Coracoid approach brachial plexus block (CABPB) is safe and effective for clinical anesthesia and analgesia in patients undergoing distal arm and forearm surgeries.[1] CABPB is performed with the aid of nerve stimulator or ultrasound guidance usually. It has been shown that dual stimulation can strengthen the block effect when using nerve stimulator.[2]
Apart from dual stimulation, various adjuvant agents, including adrenaline, opioids,[3] magnesium sulfate,[4] and clonidine,[5] are coadministered with local anesthetics in an attempt to shorten the onset time, extend the duration, and improve the quality of nerve blocks. Clonidine prolongs the duration of anesthesia and analgesia in brachial plexus block, when it is added to local anesthetics.[5] Dexmedetomidine is a highly selective α-adrenoceptor agonist, and α2 to α1 adrenoceptor selectivity is approximately 8 times greater than that of clonidine.[6]
The aim of this study was to assess the effects of dexmedetomidine on the duration of anesthesia and the effective postoperative analgesia time when it was administered with ropivacaine for CABPB under dual stimulation.
2. Materials and methods
The study was conducted after receiving approval from the medical ethics committee of the first hospital of Qinhuangdao (IRB no. 201601A013). Written informed consent was obtained from each enrolled patient. Inclusion criteria were age 18 to 60 years; American Society of Anesthesiologists physical status I or II; and patients who were to undergo distal arm or forearm surgeries. Exclusion criteria included history of allergy to local anesthetics and dexmedetomidine; neuromuscular, hepatic or renal diseases, coagulation dysfunction, local infection, pregnant women, and psychiatric patients; surgery of extreme length (expected duration longer than the duration of action of the local anesthetic, i.e., 6 hours).
A total of 60 patients were enrolled into this prospective, randomized, double-blind, controlled clinical trial. They were allocated into 2 groups (30 patients in each): control (C) and dexmedetomidine (D) groups. The random number was generated via a computer and sealed in an opaque envelope that was opened immediately before anesthesia. Each patient received either 40 mL of 0.375% ropivacaine (AstraZeneca AB, Goteborg, Sweden) in group C, or 40 mL of 0.375% ropivacaine mixed with 1 μg/kg dexmedetomidine (Jiangsu Hengrui Medicine Co Ltd, Lianyungang, China) in group D. The drug was prepared by a nurse according to the grouping of patients. The block was performed by an experienced anesthesiologist (blinded).
Standard monitoring was applied (electrocardiography, blood pressure, pulse oximetry). Each patient received 2 L/min oxygen through nasal vestibule. Midazolam 0.05 mg/kg and fentanyl 1 μg/kg were administered intravenously as premedication to every patient.
CABPB was guided by the nerve stimulator. A 55 mm and 22-gauge insulated stimulating needle (Stimuplex S, B. Braun Melsungen AG, Melsungen, Germany) was attached to the nerve stimulator (Stimuplex HNS12, Stockert GmbH). The nerve stimulator was set at 1.0 mA, 2.0 Hz, and 0.1 ms originally. The needle insertion point was 2 cm medial and 2 cm caudal to the coracoid process. After injecting 2 mL 1% lidocaine for anesthetizing the skin and subcutaneous tissue, the stimulating needle was inserted vertically. Different motor responses would be obtained, including elbow joint, wrist joint, and fingers’ movements. If there was still a weak muscle movement when the electric current was at 0.3 to 0.5 mA, 20 mL ropivacaine mixed with or without dexmedetomidine was injected slowly. Then the needle was withdrawn back to the subcutaneous tissue, and was adjusted cephalically or caudally in the sagittal plane to elicit a different motor response. Then the rest 20 mL anesthetic was injected slowly.
If the patient complained of pain during the surgery, supplemental infiltration anesthesia with 1% lidocaine or i.v. fentanyl (1 μg/kg) was administered. If this did not come into effect, general anesthesia was necessary.
Postoperative analgesia (tramadol 50 mg) was given on patient request.
Outcome parameters were recorded by blinded observers. Primary outcome measures consisted of the duration of anesthesia and the effective postoperative analgesia time. Secondary outcome measures included sensory and motor block onset time for brachial plexus block and visual analog scale (VAS) after surgery.
The duration of anesthesia was defined as the time interval between the administration of block and the part recovery of algesthesia of the hand and forearm. Effective postoperative analgesia time was defined as the time interval between the administration of block and the first time asking for analgesic drug after surgery. The duration of anesthesia and effective postoperative analgesia time were evaluated every hour after the operation.
Sensory and motor block were assessed every 5 minutes. Sensory and motor block were evaluated with 3-point scale (0–2, 0 = normal pain sensation or movement, 1 = weakened pain sensation or movement, 2 = loss of pain sensation or movement). Sensory block was assessed using the pinprick test in all 5 nerve territories (median, ulnar, radial, medial antebrachial cutaneous nerve, and musculocutaneous nerves). Motor block was assessed by thumb opposition (median nerve), thumb abduction (radial nerve), thumb adduction (ulnar nerve), and flexion of elbow and pronation of forearm (musculocutaneous nerve). The sensory or motor onset time were considered when Scale 2 was achieved for all 5 or 4 nerves. If Scale 2 was not achieved at 30 minutes, the onset time was taken as 30 minutes.
The VAS was used to evaluate the quality of postoperative analgesia: zero (0) represented no pain and 10 meant the worst possible pain. It was measured at 6, 12, and 24 hours after block.
In addition, vital signs (SBP, DBP, MAP, SpO2, and HR) during operation and the cumulative dose of rescue tramadol during the first 48 hours postoperative period were also recorded. Complications including nausea, vomiting, nerve injury, vascular puncture, and pneumothorax were noted. All patients were followed up for 2 days.
Power analysis was performed for a 5% significance with a power of 80%, based on a pilot study involving 10 patients. In the pilot study, dexmedetomidine added to ropivacaine prolonged the duration of anesthesia of brachial plexus block from 640 ± 110 to 750 ± 160 minutes. It was calculated that a minimum of 25 patients in each group would be required to have an 80% power of detecting a 110 minutes difference in the duration of anesthesia at a significance level of 5%. Considering the loss rate, we set sample size to 30 patients in each group.
Statistical analysis was performed by SPSS (version 17.0, SPSS Inc, Chicago, IL). Quantitative data were presented as mean and standard deviation, and qualitative data were presented as frequency and percentage. Normal distribution of data was investigated by Kolmogorov-Smirnov test firstly. Parametric tests were performed using either 2-sample Student t test or Chi-square analysis, according to the type of variable. Nonparametric tests were performed using Mann-Whitney U test. Difference was considered statistically significant if P < .05.
3. Results
One patient refused regional anesthesia (in group D), whereas 2 patients in group C and 1 patient in group D were excluded from the analysis because they were discharged home on the same day after surgery. Therefore, 28 patients in each group were analyzed. Intraoperatively, supplemental local infiltration anesthesia was necessary for 2 patients in group D and 2 patients in group C. Intravenous fentanyl and general anesthesia were not required.
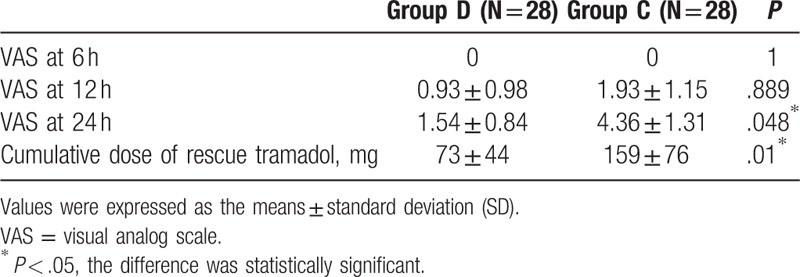
The demographic characteristics such as sex, age, weight, surgical region, surgery type, and duration of surgery were comparable in the 2 groups. There were no significant differences between the 2 groups (Table 1).
Table 1.
Demographic characteristics and the surgical characteristics.
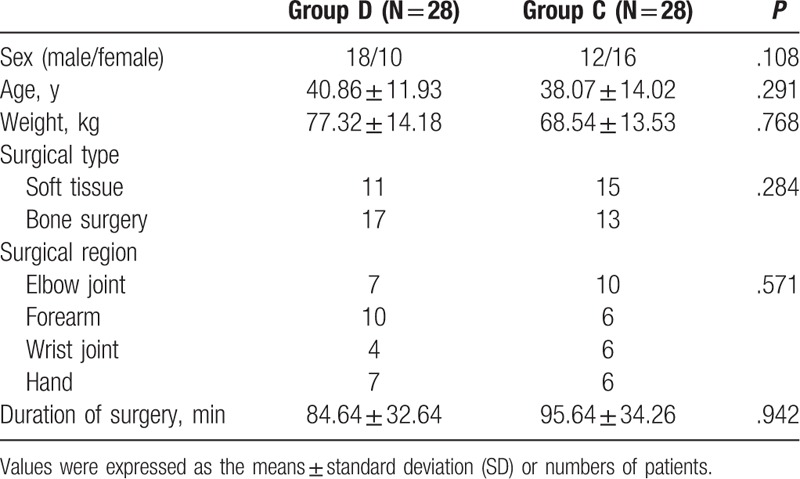
The differences of sensory and motor onset time were not significant between the 2 groups (P > .05). The duration of anesthesia was longer in group D (759 ± 161 minutes) as compared to group C (634 ± 110 minutes) (P < .05). The effective postoperative analgesia time was also longer in group D (986 ± 206 minutes) as compared to group C (789 ± 126 minutes) (P < .05) (Table 2).
Table 2.
Comparison of block effect between the 2 groups (min).
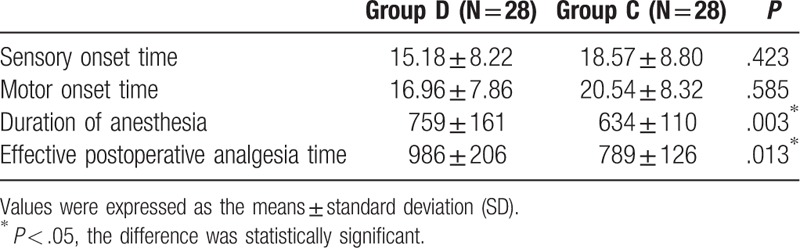
The VAS was similar in the 2 groups at 6 and 12 hours after block (P > .05), but it was lower in group D at 24 hours after block as compared to group C (P < .05). The cumulative dose of rescue tramadol during the 48 hours postoperative period was significantly lower in group D as compared to group C (P < .05) (Table 3).
Table 3.
Comparison of postoperative analgesia between the 2 groups.
No statistically and clinically significant changes were observed in vital signs in either group. Unexpected vascular puncture occurred in 1 patient in group D and 2 patients in group C without further sequelae. No other serious complications were observed during this study.
4. Discussion
In the present study, we found that dexmedetomidine added into ropivacaine prolonged the duration of anesthesia, as well as the effective postoperative analgesia time for CABPB under dual stimulation. Dexmedetomidine decreased both the VAS at 24 hours and the postoperative analgesic consumption during the first 48 hours. Sensory and motor onset times for brachial plexus block were analogous in the 2 groups.
Brachial plexus block is extensively used for upper extremity surgery, and can be performed from various approaches. Since described by Whiffler,[7] the coracoid approach has been used widely. In order to increase success rate, dual or triple stimulation can be applied. Rodriguez et al[2] concluded that success rate was higher under dual or triple stimulation compared with single stimulation, but success rate was not significantly different between dual and triple stimulation. Based on this, we decided to use the dual stimulation technique.
Adjuvants to local anesthetics has been in the focus of the research for a long time.[8] α2-Adrenergic receptor agonists get a lot of attention for their extensive advantages including sedative, analgesic, perioperative sympatholytic, and cardiovascular stabilizing effects.[9]
Dexmedetomidine is a highly selective α-adrenoceptor agonist, and its α2 to α1 adrenoceptor selectivity is approximately 8 times greater than that of clonidine. It is a potential adjuvant agent for peripheral nerve blocks. The safety of dexmedetomidine mixed with local anesthetic used for peripheral nerve block has been well proven in previous studies. In an animal study,[10] it was found that high-dose (up to 40 μg/kg) dexmedetomidine administered through sciatic nerve block impaired neither axons nor myelin sheaths, and might even alleviate perineural inflammation. In a single-center, prospective, randomized, triple-blind, controlled trial,[11] 150 μg dexmedetomidine administered through interscalene block did not increase the incidence of adverse events. Furthermore, the plasma concentration of dexmedetomidine was relatively low.
The mechanism by which dexmedetomidine strengthens the effect of peripheral nerve block is likely to be complicated, including central and peripheral factors. In central nervous system, dexmedetomidine inhibits the release of substance P in dorsal root neuron and activates α2-adrenoceptors in the locus coeruleus.[12] In peripheral nervous system, dexmedetomidine blocks action potential in Aδ and C fibers,[13] and thus intensifying the conduction block of local anesthetic. In addition, dexmedetomidine modulates the perioperative inflammatory response that is involved in the occurrence of postoperative pain.[14] Dexmedetomidine also can act on ion channel. Dexmedetomidine produces a dose-dependent analgesic effect due to inhibiting the current of hyperpolarization-activated cyclic nucleotide-gated channels.[15] Brummett et al[16] showed that the analgesic effect of perineural dexmedetomidine was likely mediated by blocking the hyperpolarization-activated cation current (Ih current), but the analgesic effect was irrelevant to agonism of the α2-adrenoceptor, and could not be reversed by an α2-adrenoceptor antagonist.
In addition to peripheral nerve block, dexmedetomidine administered peripherally can engender central sedative action. Kwon et al[17] found that dexmedetomidine added to ropivacaine for brachial plexus block induced natural sedation and decreased the BIS value to 60, and patients were in a state of moderate-to-deep sedation or hypnosis, but they were easily awakened and memory function was unaffected.
The most significant adverse effects of perineural dexmedetomidine are hypotension and bradycardia (both are dose dependent).[18] If bradycardia is defined as a heart rate of <50 bpm, its incidence is as high as 23%, when adding 100 μg dexmedetomidine to levobupivacaine for axillary block.[19] When lower dose (1 μg/kg, an average of 50–60 μg) is administered, the incidence of hypotension and bradycardia is not significant.[20] This is consistent with our findings.
In this present study, dexmedetomidine added into ropivacaine does not shorten the sensory and motor onset time for brachial plexus block. This is in contradiction with the conclusion of Chinnappa et al.[21] This might be associated with the volume of local anesthetics. We used a larger volume of local anesthetics, which could accelerate the onset time.
This study had some limitations. Firstly, we fixed the dose of dexmedetomidine on 1 μg/kg, and did not set up gradient doses. Our aim was to assess the effect of dexmedetomidine on the block characteristics without increasing the incidence of side effects such as hypotension and bradycardia. Secondly, in some patients, the block effect was poor in the nonoperative area, without affecting patient comfort during the surgery. In these cases onset time was taken as 30 minutes. In fact, the onset time could be much longer.
5. Conclusion
The addition of 1 μg/kg of dexmedetomidine to ropivacaine extends the duration of anesthesia and the effective postoperative analgesia time for CABPB under dual stimulation. The VAS at 24 hours after block and the demand for rescue tramadol during the first 48 hours postoperative period are decreased without severe side effects. Further pharmacokinetic and dose finding studies are needed.
Author contributions
Data curation: Wen-sheng He, Zhen-yu Wu.
Formal analysis: Wen-sheng He, Zhuo Liu.
Funding acquisition: Xiu-li Wang.
Investigation: Xiao-chun Yang.
Methodology: Wen-sheng He, Xiu-li Wang.
Software: Zhen-yu Wu, Hai-jun Sun Sun.
Supervision: Zhen-yu Wu, Hai-jun Sun Sun.
Validation: Xiao-chun Yang.
Writing – original draft: Wen-sheng He, Zhen-yu Wu.
Writing – review and editing: Xiu-li Wang.
Footnotes
Abbreviations: CABPB = coracoid approach brachial plexus block, VAS = visual analog scale.
The authors have no conflicts of interest to disclose.
References
- [1].Trehan V, Srivastava U, Kumar A, et al. Comparison of two approaches of infraclavicular brachial plexus block for orthopaedic surgery below mid-humerus. Indian J Anaesth 2010;54:210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rodriguez J, Barcena M, Taboada-Muniz M, et al. A comparison of single versus multiple injections on the extent of anesthesia with coracoid infraclavicular brachial plexus block. Anesth Analg 2004;99:1225–30. [DOI] [PubMed] [Google Scholar]
- [3].Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 2009;9:3–8. [DOI] [PubMed] [Google Scholar]
- [4].Mukherjee K, Das A, Basunia SR, et al. Evaluation of Magnesium as an adjuvant in Ropivacaine-induced supraclavicular brachial plexus block: a prospective, double-blinded randomized controlled study. J Res Pharm Pract 2014;3:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gabriel JS, Gordin V. Alpha 2 agonists in regional anesthesia and analgesia. Curr Opin Anaesthesiol 2001;14:751–3. [DOI] [PubMed] [Google Scholar]
- [6].Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia 1999;54:146–65. [DOI] [PubMed] [Google Scholar]
- [7].Whiffler K. Coracoid block—a safe and easy technique. Br J Anaesth 1981;53:845–8. [DOI] [PubMed] [Google Scholar]
- [8].Swain A, Nag DS, Sahu S, et al. Adjuvants to local anesthetics: current understanding and future trends. World J Clin Cases 2017;5:307–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Popping DM, Elia N, Marret E, et al. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: a meta-analysis of randomized trials. Anesthesiology 2009;111:406–15. [DOI] [PubMed] [Google Scholar]
- [10].Brummett CM, Norat MA, Palmisano JM, et al. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology 2008;109:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med 2014;39:37–47. [DOI] [PubMed] [Google Scholar]
- [12].Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth 1993;71:447–9. [DOI] [PubMed] [Google Scholar]
- [13].Zhang Y, Wang CS, Shi JH, et al. Perineural administration of dexmedetomidine in combination with ropivacaine prolongs axillary brachial plexus block. Int J Clin Exp Med 2014;7:680–5. [PMC free article] [PubMed] [Google Scholar]
- [14].Grosu I, Lavand’Homme P. Continuous regional anesthesia and inflammation: a new target. Minerva Anestesiol 2015;81:1001–9. [PubMed] [Google Scholar]
- [15].Yang YC, Meng QT, Pan X, et al. Dexmedetomidine produced analgesic effect via inhibition of HCN currents. Eur J Pharmacol 2014;740:560–4. [DOI] [PubMed] [Google Scholar]
- [16].Brummett CM, Hong EK, Janda AM, et al. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology 2011;115:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kwon Y, Hwang SM, Lee JJ, et al. The effect of dexmedetomidine as an adjuvant to ropivacaine on the bispectral index for supraclavicular brachial plexus block. Korean J Anesthesiol 2015;68:32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gurajala I, Thipparampall AK, Durga P, et al. Effect of perineural dexmedetomidine on the quality of supraclavicular brachial plexus block with 0.5% ropivacaine and its interaction with general anaesthesia. Indian J Anaesth 2015;59:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Esmaoglu A, Yegenoglu F, Akin A, et al. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg 2010;111:1548–51. [DOI] [PubMed] [Google Scholar]
- [20].Rancourt MP, Albert NT, Cote M, et al. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg 2012;115:958–62. [DOI] [PubMed] [Google Scholar]
- [21].Chinnappa J, Shivanna S, Pujari VS, et al. Efficacy of dexmedetomidine with ropivacaine in supraclavicular brachial plexus block for upper limb surgeries. J Anaesthesiol Clin Pharmacol 2017;33:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


