Supplemental Digital Content is Available in the Text.
Keywords: GM-CSFR, Multiple sclerosis, Human spinal cord, Microglia, Macrophages, DRG neurons, Injured nerves, Hypersensitivity
Abstract
Introduction:
Granulocyte-macrophage colony-stimulating factor receptor (GM-CSFR) is highly expressed in peripheral macrophages and microglia, and is involved in arthritis and cancer pain in animal models. However, there is limited information on GM-CSFR expression in human central nervous system (CNS), peripheral nerves, or dorsal root ganglia (DRG), particularly in chronic pain conditions.
Objectives:
Immunohistochemistry was used to quantify GM-CSFR expression levels in human tissues, and functional sensory effects of GM-CSF were studied in cultured DRG neurons.
Results:
Granulocyte-macrophage colony-stimulating factor receptor was markedly increased in microglia at lesional sites of multiple sclerosis spinal cords (P = 0.01), which co-localised with macrophage marker CD68 (P = 0.009). In human DRG, GM-CSFR was expressed in a subset of small/medium diameter cells (30%) and few large cells (10%), with no significant change in avulsion-injured DRG. In peripheral nerves, there was a marked decrease in axonal GM-CSFR after chronic painful nerve injury (P = 0.004) and in painful neuromas (P = 0.0043); CD-68–positive macrophages were increased (P = 0.017) but did not appear to express GM-CSFR. Although control synovium showed absent GM-CSFR immunostaining, this was markedly increased in macrophages of painful osteoarthritis knee synovium. Granulocyte-macrophage colony-stimulating factor receptor was expressed in 17 ± 1.7% of small-/medium-sized cultured adult rat DRG neurons, and in 27 ± 3.3% of TRPV1-positive neurons. Granulocyte-macrophage colony-stimulating factor treatment sensitized capsaicin responses in vitro, which were diminished by p38 MAPK or TrkA inhibitors.
Conclusion:
Our findings support GM-CSFR as a therapeutic target for pain and hypersensitivity in clinical CNS and peripheral inflammatory conditions. Although GM-CSFR was decreased in chronic painful injured peripheral nerves, it could mediate CNS neuroinflammatory effects, which deserves study.
1. Introduction
The granulocyte-macrophage colony-stimulating factor (GM-CSF) is a hematopoietic colony-stimulating factor and proinflammatory cytokine11,43 with pleiotropic effects in innate and adaptive immunity. Its biological effects follow binding to the cell surface receptor granulocyte-macrophage colony-stimulating factor receptor α (GM-CSFR), composed of the binding site GM-CSFRα and the signalling β chains.36
Kuner et al. have pioneered studies on the role of GM-CSFR in pain models.5,40,43 Granulocyte-macrophage colony-stimulating factor receptor activation in sensory nerves results in neurite sprouting, sensitization to nociceptive stimuli, and nerve hypertrophy, with altered transcription of several key genes encoding proinflammatory chemokines in mouse dorsal root ganglion (DRG) neurons, including Ccl3, Ccl5, insulin-like growth factor 1 (Igf1), vascular endothelial growth factor A (Vegfa), transcript variant 2, TNFα, and the nociceptor TRPV1, through signalling pathways including JAK/STAT, Src, PLC, PKC, and PKA.5,43 These pathways influence the expression and sensitivity of TRPV1 and Nav1.8, which are key determinants of nociceptor excitability. Granulocyte-macrophage colony-stimulating factor receptor expression by normal pancreatic sensory nerves and in pancreatic carcinoma, and the efficacy of nerve-specific knockdown in attenuating tumour-evoked pain in animal models, identified it as an important novel target in cancer pain,40,43 in addition to inflammatory pain.16
A variety of cells including myeloid cells, T cells, B cells, monocytes, macrophages, dendritic cells, fibroblasts, and endothelial cells secrete GM-CSF, which promotes the survival, proliferation, differentiation, and recruitment of myeloid-lineage cells.17 Thus, GM-CSF is routinely used to treat oncohaematological disorders to replenish bone marrow cells after chemotherapy/radiotherapy,18 albeit with worsened outcomes for individuals with rheumatoid arthritis (RA). Human keratinocytes exposed to IL-1,29,30 and a variety of tumours24 also synthesize GM-CSF.
Granulocyte-macrophage colony-stimulating factor was identified as a potential disease-modification target in several inflammatory conditions, with a role in arthritis, because of its increased expression and of other cytokines in the plasma of patients with RA,47 increased GM-CSFR expression in RA synovium,25 and greater efficacy of anti–GM-CSF than anti-TNF in clinical trials for treating RA.12 The absence of collagen-induced arthritis in GM-CSF−/− mice, and dose-dependent reduction of arthritic scores and macrophages with anti–GM-CSFR antibody treatment in a mouse model of arthritis, further supported the GM-CSFR as a therapeutic target in RA.13 The expression of GM-CSFR in osteoarthritis (OA) synovium is not known.
Granulocyte-macrophage colony-stimulating factor can cross the blood–brain and blood–spinal cord barrier,34 with the potential to mediate pronociceptive and pathogenic effects in central nervous system (CNS) inflammation. Proinflammatory roles for this cytokine are indicated in the CNS, where astrocytes synthesize GM-CSF33 to modulate the function of microglia and neurons. Multiple sclerosis (MS) is a chronic CNS inflammatory and demyelinating disease, with central neuropathic pain affecting 63% of patients with MS, which severely compromises their quality of life.15,22
To elucidate the potential role of GM-CSFR in CNS and peripheral inflammatory and neuropathic pain clinical conditions, we examined its expression in the MS spinal cord, injured DRG/peripheral nerves, and OA synovium. We also determined GM-CSFR expression in cultured rat DRG neurons, co-localisation with TRPV1, and its functional effects through pain signalling pathways.2–4
2. Materials and methods
2.1. Antibodies
List of antibodies used in this study for human tissues is listed in Table 1.
Table 1.
Antibodies used in human tissue studies.
2.2. Human tissues studied
A range of tissues were used in this study. Specimens were snap-frozen in liquid nitrogen and stored at −70°C until use or immersed in Zamboni's fixative (2% wt/vol formalin, 0.1 M phosphate, and 15% vol/vol saturated picric acid) for 2 hours and stored in phosphate-buffered saline (PBS) containing 15% sucrose and 0.01% azide.
2.3. Spinal cords
Data from tissue obtained from patients diagnosed as suffering from clinically and pathologically definite MS (n = 4) were compared with those from a group of subjects with no neurological disorder (n = 9). All tissue specimens, including MS spinal cords, were obtained from UK tissue banks.
2.4. Dorsal root ganglia and limb nerves
Injured DRG (n = 6) were obtained from patients having surgery for brachial plexus avulsion repair (less than 4 weeks after injury). Control, uninjured DRG (n = 3) were obtained postmortem from UK tissue banks. Specimens of injured limb painful nerves (n = 9) and painful neuromas (n = 9) were obtained from patients undergoing nerve repair (median delay was 27 days, range 4–210 days). Uninjured nerves (n = 7), used as grafts in nerve repair during surgery, served as controls.
2.5. Synovium
Synovial joint tissue samples were available from patients with painful knee OA (n = 6) and control postmortem tissues.
All tissue samples used in this study were taken from patients who had given informed consent for use in medical research. Ethical approval was granted by the Riverside Research Ethics Committee, Charing Cross Hospital, London, United Kingdom.
2.6. Immunohistochemistry
Tissues were embedded in optimum cutting tissue medium (RA Lamb Ltd, Eastbourne, United Kingdom). Tissue sections (15-µm thick) were collected onto poly-l-lysine (Sigma, Poole, United Kingdom) coated glass slides and postfixed in 4% wt/vol paraformaldehyde in 0.15-M PBS for 30 minutes. Endogenous peroxidase was blocked by incubation in methanol containing 0.3% wt/vol hydrogen peroxide for thirty minutes. After rehydration with PBS buffer, sections were incubated overnight with primary antibody using a range of dilutions. Sites of primary antibody attachment were revealed using nickel-enhanced, avidin-biotin peroxidase (ABC; Vector Laboratories, Peterborough, United Kingdom) as described.42 Sections were counterstained for nuclei in 0.1% wt/vol aqueous neutral red, dehydrated, and mounted in xylene-based mountant (DPX; BDH/Merck, Poole, United Kingdom), before photomicrography. For immunohistochemical co-localisation (double-staining) studies, serial sections of the human MS spinal cord were double-labelled by overnight incubation with a mixture of primary polyclonal antibody to GM-CSFR (C18, dilution 1:2000) and a monoclonal antibody to CD68 (dilution 1:200). Immunoreaction for GM-CSFR was first revealed using the standard nickel-enhanced ABC peroxidase method as above to give a black product, followed by incubation with ABC alkaline phosphatase for a further 1 hour, and the immunoreactivity developed with Fast Red to give a red product. Double-labelled preparations were mounted in Ultramount medium (Vector Labs).
2.7. Analysis of data
Image analysis was performed for the spinal cord from control subjects and patients with MS collected as previously described48 in affected lesion areas of the dorsolateral white matter, the gray matter (GM), typically the ventral horn and the nonaffected white matter (NAWM), typically the dorsal columns.
Nucleated neurons in sensory ganglia (DRG) showing positive GM-CSFR–immunoreactivity were counted in serial sections for each antibody with the cell body diameter assessed using a calibrated microscope eyepiece graticule and expressed as % total.
Nerve samples were assessed quantitatively using computerised image analysis. Serial sections stained for neurofilaments cocktail and GM-CSFR were used for the analysis. Images were captured using an Olympus DP70 camera mounted to an Olympus BX50 microscope and analysed using analySIS (version 5.0) software. Positive immunostaining was highlighted by setting the gray-level detection limits to threshold, and the area of highlighted immunoreactivity obtained as % area of the field scanned and up to 5 fields per tissue section were scanned at the same magnification (×40). Results were expressed as % immunoreactivity.
The Mann–Whitney test was used for statistical analysis (P values <0.05 were considered statistically significant).
2.8. Antibody characterisation and titration
The 2 antibodies used here, rabbit polyclonal C-18 and mouse monoclonal 4H1, recognise epitopes mapping, respectively, at the C-terminus and α chain of the human GM-CSFR.
To further characterise the 2 GM-CSFR antibodies, they were used to stain a variety of tissues (spinal cord, DRG, nerve, and skin, Table 1). Specimens used to titrate these antibodies were either fixed before embedding and sectioning or postfixed (after sectioning), and both antibodies initially diluted to final concentrations of 1:100, 1:200, 1:500, 1:1000, and 1:2000. The rabbit polyclonal C-18 antibody to GM-CSFR had an optimal working concentration of 1:2000 (supplementary Figure 1, available at http://links.lww.com/PR9/A30). Further titration revealed that concentrations of 1:2000 to 4000 were optimal for the polyclonal antibodies, and 1:10 and 1:25 for the monoclonal 4H1 antibodies. Co-localisation studies of the human MS spinal cord using rabbit polyclonal C-18 antibody to GM-CSFR and a monoclonal antibody to the macrophage marker CD68 showed that all GM-CSFR–positive cells were also positive for CD68 (supplementary Figure 2, available at http://links.lww.com/PR9/A30).
2.9. Preabsorption with granulocyte-macrophage colony-stimulating factor receptor peptide
For immunostaining specificity, rabbit antibody to GM-CSFR C18 (sc-690) was preincubated for 5 hours with GM-CSFR peptide antigen, in the range 5 × 10−2 to 5 × 10−9 mg/mL before immunostaining of the spinal cord or nerve. Preabsorption of GM-CSFR C18 (sc-690) with homologous peptide antigen abolished microglial immunostaining in the human spinal cord at 5 × 10−2 mg/mL and 5 × 10−3 mg/mL antigen. A gradual return of immunostaining and intensity was observed with decreasing concentrations of peptide antigen, until its full return at 5 × 10−6 mg/mL (supplementary Figures 3 and 4, available at http://links.lww.com/PR9/A30). Further dilutions of the peptide from 5 × 10−7 mg/mL up to 5 × 10−9 mg/mL, or PBS alone (vehicle), did not alter staining.
2.10. In vitro studies: preparation of neuronal cultures
Bilateral DRG from all levels were harvested from 6 adult female Wistar rats, 250 g (Charles River UK Ltd, Margate, Kent, United Kingdom), for preparing neuronal cultures as previously described.2,3
2.11. Immunofluorescence
Forty-eight hours after plating, cultures were fixed with 4% paraformaldehyde, for 15 minutes, washed in PBS, permeabilised with methanol (−20°C, 3 minutes), washed in PBS, and incubated with primary antibodies, rabbit anti-PGP9.5 (Ultraclone Ltd, Cambridge, United Kingdom 1:1000), mouse anti–GM-CSFR (1:200, 4H1, SC-21764; Santa Cruz, Dallas, TX), and rabbit anti-TRPV1 (1:500; GSK, Harlow, United Kingdom), for 1 hour at room temperature. This was followed by 3 PBS washes for 5 minutes each before treating with the secondary antibodies, goat anti-mouse Alexa Fluor 488 and goat anti-rabbit Alexa fluor 546 (Molecular probes, Life Technologies, Paisley, United Kingdom, 1:400 each), for 45 minutes at room temperature, washed with PBS, and mounted in Citifluor-containing Dabco (antifade agent; Sigma). Images were acquired with a Photometrics HQ2 Coolsnap CCD camera, using standard wide-field fluorescence optics on an Olympus BX43 upright microscope, after confirming the absence of immunostaining in negative controls where the primary antibody had been omitted. Numbers of cells positive for PGP9.5, GM-CSFR, and TRPV1 were counted, and the data exported to Excel software for analysis.
2.12. Calcium imaging
Functional effects of acute recombinant murine GM-CSF treatment on capsaicin responses were determined as previously described.2–4 Responses to paired capsaicin stimuli were measured in Fura2 AM (Molecular Probes Life Technologies, Paisley, United Kingdom) loaded neurons as a change in the baseline 340/380 λex nm ratio before, during, and after addition. Experiments were conducted at 37°C in a humidified environment on an inverted Nikon microscope (Diaphot 300; Nikon, UK Ltd, Kingston upon Thames, Surrey, United Kingdom) and alternately excited at 340 and 380 nm wavelengths. Neuronal cultures were loaded with the calcium indicator dye Fura 2 AM (Molecular Probes, 2 μM, for 45 minutes at 37°C), in phenol-red–free Hank's balanced salt solution, containing 0.1% bovine serum albumin, washed and incubated in Hank's balanced salt solution containing 0.5% bovine serum albumin for 20 minutes; baseline changes in bound/unbound calcium ratio (340/380 λex nm), in response to capsaicin stimulation were monitored. Acute effects of recombinant murine GM-CSF (PeproTech 315-03, PeproTech, London, United Kingdom) were determined in neurons incubated with 10 and 100 ng/mL GM-CSF for 10 minutes, or in the presence of 1 μMol/L p38 MAP kinase inhibitor (IC50 = 35 nM, 2-(4-chlorophenyl)-4-(4-fluorophenyl)-5-pyridin-4-yl-1,2-dihydropyrazol-3-one, Calbiochem 506126; MerckMillipore, Hertfordshire, United Kingdom), or 12 nMol/L TrkA inhibitor GW 441756 (IC50 = 2 nM, 1,3-dihydro-3-[(1-methyl-1H-indol-3-yl)methylene]-2H-pyrrolo[3,2-b]pyridine-2-one, #2238; Tocris, Bristol, United Kingdom). Neurons were stimulated with a test dose of 200-nM capsaicin to identify capsaicin sensitivity, followed by washout and a second capsaicin stimulus of 1 μM after 40 minutes; the ratio of the second response to the first was calculated to determine the effect of repeat stimulation (tachyphylaxis). A separate group of neurons was preincubated with GM-CSF alone or after 10-minute incubation with p38 MAPK inhibitor or TrkA inhibitor as mentioned above. For each neuron, responses were measured as the difference between baseline and peak ratio change, the second capsaicin response was normalised to the first response, and the average was calculated for each group. The Student t test was used to compare data from the separate groups, with *P < 0.05 considered statistically significant, **P < 0.01 and ***P < 0.001.
3. Results
Human tissue data presented in this study were obtained using the rabbit polyclonal C-18 or the mouse monoclonal 4H1 anti–GM-CSFR antibodies, both immunostained microglia in human spinal cords and neurons in DRG and nerves, optimally at dilutions of 1: 2000 to 4000 and 1:10 to 25, respectively.
3.1. Human spinal cord
Granulocyte-macrophage colony-stimulating factor receptor–immunoreactivity was observed with both antibodies in microglia-like cells in the human spinal cord and was increased at lesion sites in MS spinal cords (Fig. 1A, B and supplementary Figure 2, available at http://links.lww.com/PR9/A30). In the MS spinal cord, image analysis showed a substantial increase at the site of lesion in the dorsolateral white matter compared with controls (*P = 0.012), as did CD68 (**P = 0.009; Fig. 2). There was no difference in GM-CSFR–immunoreactivity in the GM, ie, in the ventral horns, or in the NAWM, ie, in the dorsal columns, compared with controls. CD68-immunostaining had a greater positive % area compared with GM-CSFR (Figs. 1 and 2).
Figure 1.
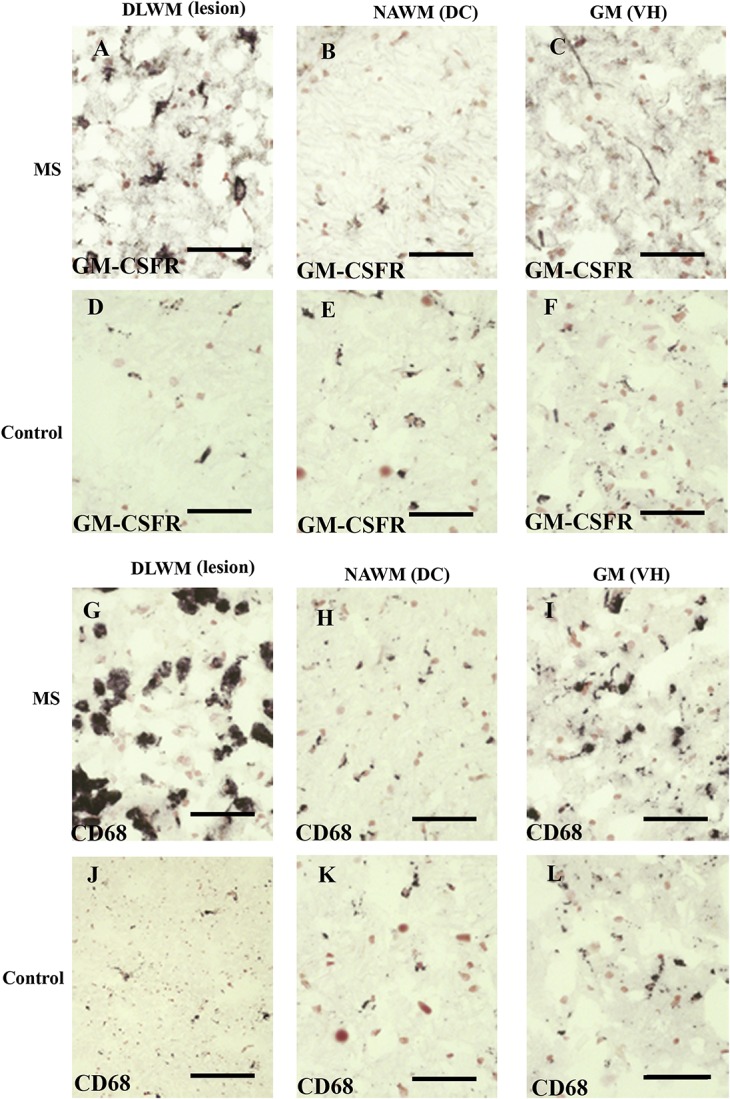
Granulocyte-macrophage colony-stimulating factor and CD68 in the MS spinal cord. Granulocyte-macrophage colony-stimulating factor immunoreactivity (A–F), and CD68 immunoreactivity (G–L), in MS (A–C and G–I) and in the control spinal cord (D–F and J–L). DC, dorsal column; DLWM, dorsolateral white matter, lesional site; GM, gray matter; GM-CSFR, granulocyte-macrophage colony-stimulating factor receptor; MS, multiple sclerosis; NAWM, nonaffected white matter; VH, ventral horn. Scale bar = 100 µm.
Figure 2.
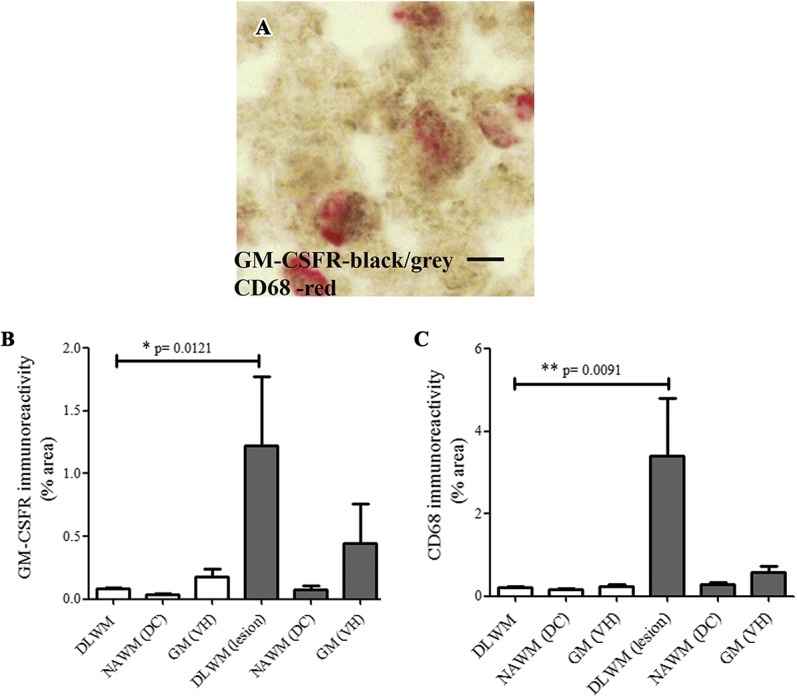
Co-localisation studies of GM-CSFR and CD68 in the spinal cord. Double-staining of GM-CSFR (black/brown) and CD68 cells (red) in (A), the MS spinal cord. Scale bar = 50 µm. Image analysis of GM-CSFR in human spinal cords, bar charts (mean ± SEM) of GM-CSFR (B) and CD68 (C) immunoreactivities, in control (clear bars) and the MS spinal cord. DC, dorsal column; DLWM, dorsolateral white matter, lesional site; GM, gray matter; GM-CSFR, granulocyte-macrophage colony-stimulating factor receptor; MS, multiple sclerosis; NAWM, nonaffected white matter; VH, ventral horn.
3.2. Avulsed and control human dorsal root ganglia
Granulocyte-macrophage colony-stimulating factor receptor-like immunoreactivity was noted in both postmortem control DRG and avulsion-injured DRG (Fig. 3). The GM-CSFR–immunoreactivity was observed mainly in neurons of ≤50 µm diameter (c. 30%) and also in some larger neurons (c. 10%). Avulsion-injured DRG were not significantly different from control DRG.
Figure 3.
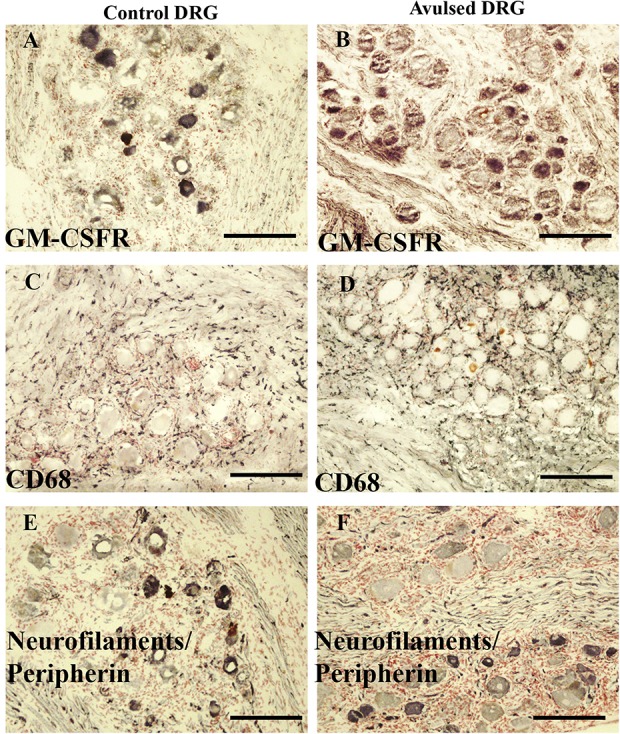
Granulocyte-macrophage colony-stimulating factor receptor is expressed in DRG neurons. Control (panels on left) and avulsed DRG (panels on right) immunostained for GM-CSFR (A and B), macrophage marker CD68 (C and D), and neurofilaments/peripherin cocktail (E and F). Scale bar = 200 µm. DRG, dorsal root ganglion; GM-CSFR, granulocyte-macrophage colony-stimulating factor receptor.
3.3. Nerves
Granulocyte-macrophage colony-stimulating factor receptor staining was observed in the control nerves, painful injured nerves, and neuromas. Neurofilaments cocktail staining was also performed in serial sections, along with CD68 (Fig. 4). Image analysis (Fig. 4J–M) showed that there was a substantial and highly significant decrease of GM-CSFR–positive axons in the injured nerve and neuroma groups. Granulocyte-macrophage colony-stimulating factor receptor–positive immunoreactivity was decreased by c. 80% in injured painful nerves (**P = 0.0043) and by c. 90% in the painful neuroma group (**P = 0.0043). The ratios of GM-CSFR:neurofilaments cocktail (Fig. 4L) were also markedly reduced in the injured nerve group (**P = 0.004) and neuroma group (**P = 0.004).
Figure 4.
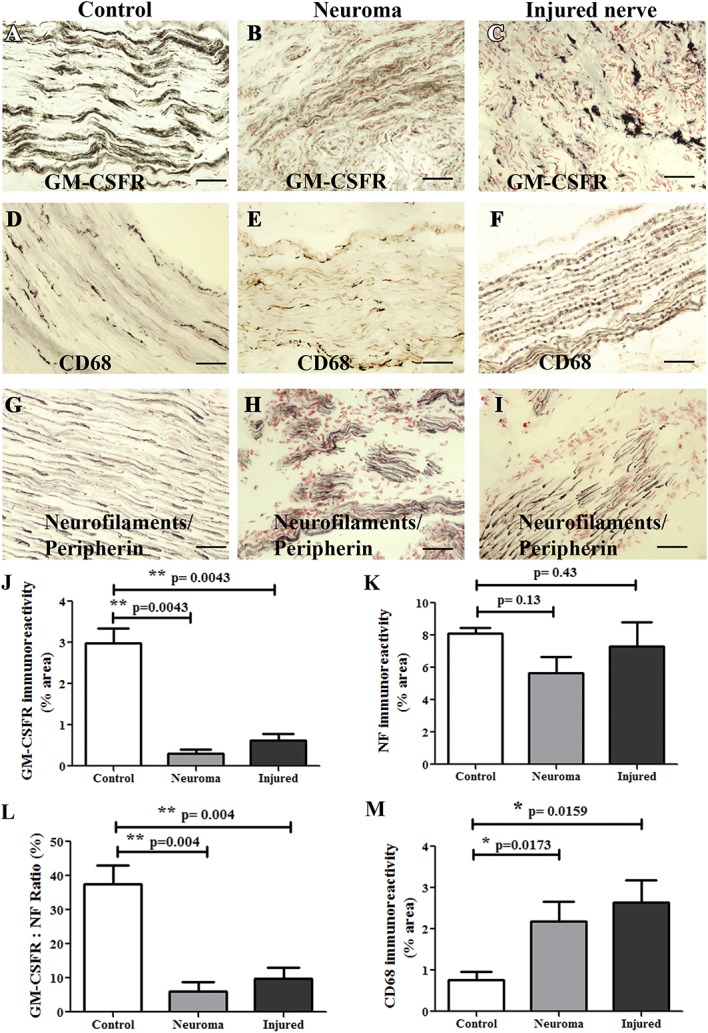
Granulocyte-macrophage colony-stimulating factor receptor expression in peripheral nerves. Control nerves (panels on left), neuromas (middle panels), or injured nerve (panels on right) immunostained for GM-CSFR (A–C), macrophage marker CD68 (D–F), and with neurofilaments/peripherin cocktail (G–I). Scale bar = 100 µm. Image analysis of nerve immunostaining. Bar charts (mean ± SEM) for GM-CSFR (J), neurofilaments/peripherin cocktail (NF) (K), ratios of GM-CSFR:NF (L), and CD68 (M). GM-CSFR, granulocyte-macrophage colony-stimulating factor receptor.
3.4. Synovium
In normal joint synovium, little or no GM-CSFR immunoreactivity was observed, and few CD68-positive bipolar resting macrophages were noted (Fig. 5A, B). By contrast, in painful OA synovium, the GM-CSFR immunoreactive cells (Fig. 5C, E) resembled larger activated CD68-positive macrophages (Fig. 5D) and not T cells marked with CD3 (Fig. 5F). Image analysis comparison of GM-CSFR in control and painful OA synovium was not performed, given the little or no immunostaining in control synovium.
Figure 5.
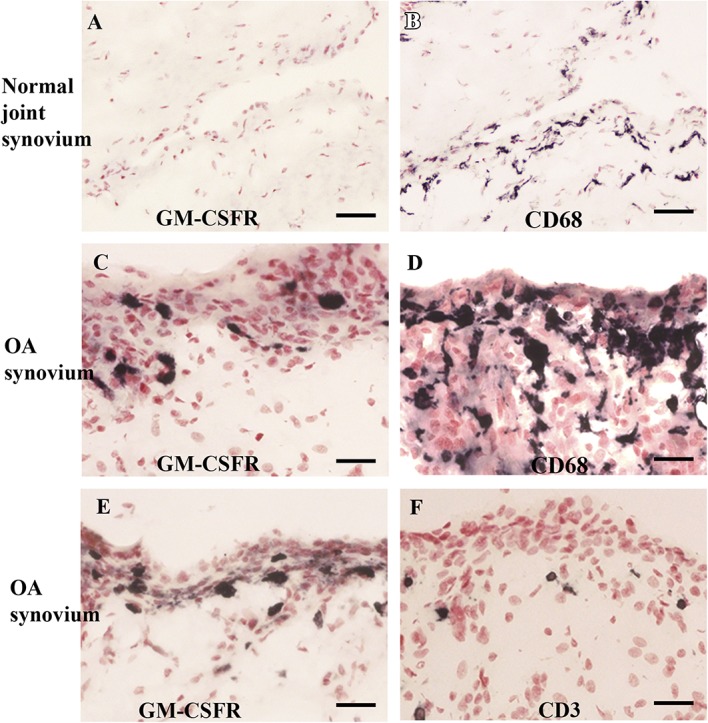
Granulocyte-macrophage colony-stimulating factor receptor–immunoreactivity (A) and CD68-immunoreactivity (B) in normal joint synovium. Scale bar = 100 µm. Granulocyte-macrophage colony-stimulating factor receptor immunoreactivity in painful OA synovium (C and E), macrophage marker CD68 (D), and T-cell marker CD3 (F). Scale bar = 50 µm. GM-CSFR, granulocyte-macrophage colony-stimulating factor receptor; OA, osteoarthritis.
3.5. In vitro studies
Double immunofluorescence studies showed that adult rat cultured DRG neurons were uniformly positive for PGP9.5 expression, with a subset of neurons coexpressing GM-CSFR in mostly small diameter neurons and some large diameter neurons (Fig. 6A–C). Of 1143 PGP9.5-positive neurons, 193 neurons were GM-CSFR–positive (17 ± 1.7%). Similarly, double immunofluorescence showed that out of 1384 TRPV1-positive neurons, 354 were GM-CSFR–positive (27 ± 3.3%). A small proportion of GM-CSFR–positive neurons was not positive for TRPV1 (Fig. 7D–F).
Figure 6.
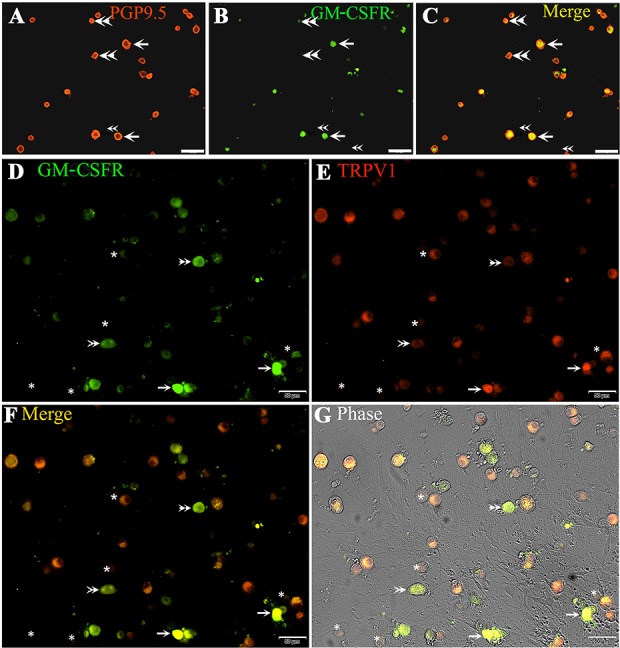
Granulocyte-macrophage colony-stimulating factor receptor expression in rat DRG neurons. Immunofluorescence in cultured adult rat DRG neurons showing co-localization of PGP9.5 (A, red) and GM-CSFR (B, green) appearing yellow in the merged image (C, yellow, single arrows); Double arrowheads indicate neurons positive for PGP9.5 (red in A and C) but negative for GM-CSFR (B, green). Co-localization of GM-CSFR (D, green, single arrows) and TRPV1 (E, red), appearing yellow in the merged image (F), and phase contrast image (G). Asterisks indicate neurons that are negative for GM-CSFR and TRPV1. Double arrowheads indicate GM-CSFR–positive neurons (D and F), but TRPV1 negative (E). Scale bar in (A–C = 100 µm and D–G = 50 µm). DRG, dorsal root ganglion; GM-CSFR, granulocyte-macrophage colony-stimulating factor receptor.
Figure 7.
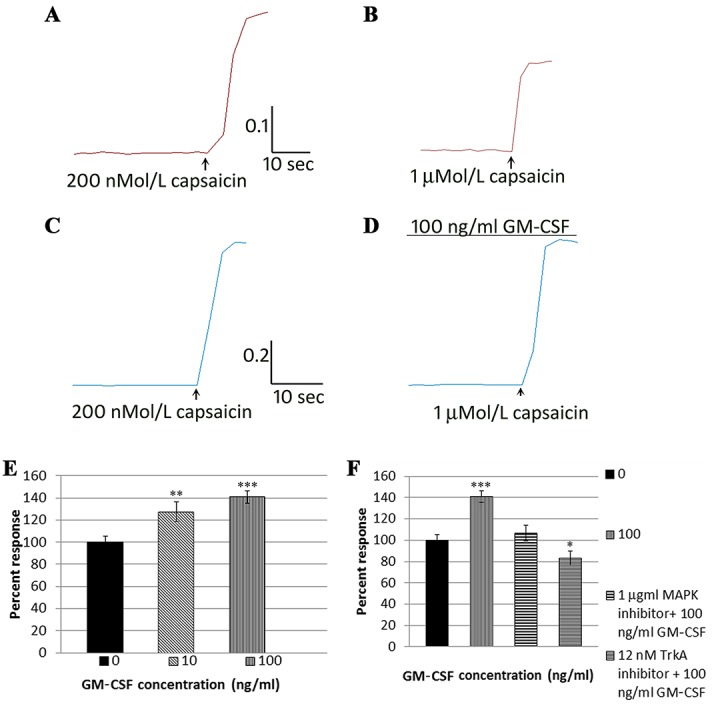
Sample trace of response to 200 nMol/L capsaicin (A), followed by washout and smaller response to 1 μMol/L capsaicin (B) in a vehicle-treated DRG neuron. Similar trace of response to 200 nMol/capsaicin in another neuron (C) and enhanced response to 1 μMol/L capsaicin in the presence of 100 ng/mL GM-CSF (D). The scale bar in (A–D) indicates the time in seconds on the x-axis and the 340/380 ratio change on the y-axis. Graph of dose-related enhancement of capsaicin responses due to GM-CSF (E) and its reversal in the presence of the MAPK inhibitor and TrkA inhibitor (F). DRG, dorsal root ganglion; GM-CSF, granulocyte-macrophage colony-stimulating factor. *P<0.05, **P<0.01, ***P<0.001.
Calcium imaging showed that the second capsaicin response (1 μMol/L) in vehicle-treated neurons was reduced compared with the first response, due to repeat stimulation (tachyphylaxis) as expected (Fig. 7A, B) (69.3 ± 3.7%, n = 18 neurons). However, the second capsaicin responses were significantly enhanced after 10-minute incubation with GM-CSF, in a dose-dependent manner, to 127.5 ± 8.7% with 10 ng/mL GM-CSF (n = 15 neurons, **P < 0.01), and to 141 ± 4.2% with 100 ng/mL (n = 19 neurons, ***P < 0.001) normalized to vehicle-treated controls (Fig. 7C–E). The sensitization due to 100-ng/mL GM-CSF was abolished in the presence of 1 μMol/L p38 MAPK inhibitor, which reduced the second capsaicin response to 106 ± 7.3% (n = 19 neurons, Fig. 7F), and 12 nMol/L TrkA inhibitor GW441756, which reduced the capsaicin responses to 83 ± 6.7% (n = 18 neurons, *P < 0.05, Fig. 7F).
4. Discussion
This study examined the expression of GM-CSFR as a pain treatment target in a range of human CNS and peripheral pain conditions. The tissues studied included MS spinal cord, injured DRG, peripheral nerves, and OA synovium, with characterized antibodies including their specificity. We also determined the expression of GM-CSFR in cultured adult rat DRG neurons, and the functional effects of its ligand GM-CSF on neuronal sensitivity to capsaicin, with elucidation of the signalling pathways involved.
Our findings demonstrate the expression of GM-CSFR in infiltrating peripheral macrophage and/or microglia-like cells in the gray and white matter of control human spinal cords, which was significantly elevated in the areas of white matter lesions in MS spinal cords, corresponding with CD68 labelling, but not in neurons or nerve fibres. The CD68 antibody (clone EBM11) stains mainly macrophages in a wide variety of human tissues and has been reported to also stain antigen-presenting cells, peripheral blood monocytes, large lymphocytes, and basophils and mast cells. Thus, further work is required to confirm the inflammatory cell(s) of GM-CSFR in the MS spinal cord. Although we48 and others15 have previously described CD68-positive macrophages in the demyelinating lesions in MS, this is the first report of GM-CSFR expression in the macrophages/microglia in the human MS spinal cord. Classic neuroinflammatory diseases such as MS are characterized by the focal influx of inflammatory cells into the CNS, and the cytokines secreted by these cells lead to further inflammation, tissue destruction, and neurological impairment.15 Macrophages are a known source of GM-CSF and may contribute to pathogenesis and pain.
In brain MS lesions, GM-CSFR was reported in activated microglia and perivascular macrophages45 but not by T cells, in contrast to murine studies where the GM-CSF produced by T cells was important in the development of experimental autoimmune encephalomyelitis.35 Widespread GM-CSFR expression, co-localized with GM-CSF, was also reported in the rat, mouse, and human brain, which was upregulated after ischaemia.39 In the human brain, the expression of GM-CSF and its receptor has been reported in neurons, astrocytes, and ependymal cells, with marked reduction in the diseased hippocampus of Alzheimer's disease brain.37
Granulocyte-macrophage colony-stimulating factor is associated with neuroinflammation and degeneration, as GM-CSF overexpressing mice demonstrate macrophage accumulation and severe inflammation with widespread degenerative changes in a variety of tissues, including the retina, cornea, pleural and peritoneal cavities, and premature death with muscle wasting due to accumulation of macrophages.31 The very high levels of CD68- and GM-CSFR–expressing macrophages/microglia in white matter lesions of spinal cords observed in our study and others, combined with elevated GM-CSF levels in the CSF of patients with MS,46 could contribute to the degenerative changes and associated pain in MS. The efficacy of GM-CSF antagonism in several autoimmune and inflammatory preclinical disease models, including inflammatory arthritis,16 is expected to extend to inflammatory diseases of the CNS.46 Lymphocytes and myeloid cells mediate tissue damage by secreting cytokines; interleukin-1 β (IL-1β) and IL-6 can be sensed by cells in the inflamed CNS and are central to the inflammatory process; IL-23 is sensed by tissue-damaging T cells and promoted by GM-CSF.7 A GM-CSFRα–specific monoclonal antibody was well tolerated in early-phase clinical trials in RA, with impressive clinical responses including pain relief46; mavrilimumab, an anti–GM-CSFR mAb, is now in phase IIb clinical trials for RA.12 This is the first report of GM-CSFR immunoreactivity in human DRG neurons, mainly in the small diameter nociceptive neurons of normal and avulsed DRG, and in a small proportion of large diameter neurons. These findings are similar to GM-CSFR immunoreactivity in mouse DRG neurons.40 We found a small decrease in DRG expression levels after avulsion injury (similar to central axotomy), but this was not statistically significant. Granulocyte-macrophage colony-stimulating factor receptor expression is reported to be negatively regulated by receptor internalization after ligand binding in normal human neutrophils, as GM-CSF, tumor necrosis factor, formyl-Met-Leu-Phe, and agonists of PKC downregulated neutrophil GM-CSFR expression after 2 hours of exposure at biologically active concentrations.14 A similar mechanism may explain the mildly reduced GM-CSFR expression observed in avulsed DRG neurons, due to the presence of inflammatory cytokines after DRG and associated nerve root injury.
Our observation of GM-CSFR expression in peripheral nerves is in agreement with previous reports on sensory nerves in normal pancreas and pancreatic carcinoma. Injured nerve–derived fibroblasts but not Schwann cells produce significant amounts of GM-CSF, from 4 hours after injury until at least 2 weeks in mice,38 activating macrophages and Schwann cells, and inducing phagocytosis of myelin to regulate Wallerian degeneration.8,23 However, marked decreases in GM-CSFR expression were observed in the axons of chronically injured nerves and painful neuromas compared with uninjured nerves in our study, and while CD-68–positive macrophages were increased, these did not appear to express increased GM-CSFR. This may reflect differences between acute models of injury in mice with contrast to chronic nerve injury in humans. We have previously reported differential expression by macrophages over time in injured human nerves; while macrophages infiltrated injured nerves within days of injury, they expressed increased Cox-2-immunoreactivity only from 2 to 3 weeks after injury, which reached a peak at 4 to 6 weeks, and was then persistently increased in painful neuromas for years.20,21 A similar time course of changes was found in the rat chronic constriction injury model, both in the injured nerves and ipsilateral dorsal spinal cord. Using [11C](R)-PK11195, a sensitive in vivo marker of microglial cell activation and positron emission tomography, we provided first evidence in patients that limb nerve injury induces an increase of activated microglia in the contralateral thalamus for years.6 The expression of GM-CSFR in the activated CNS microglia is not known in patients with peripheral nerve injury and could be a therapeutic target for neuroinflammation and pain.
Granulocyte-macrophage colony-stimulating factor receptor immunostaining was absent in normal synovium, but greatly increased in OA synovium, and associated with activated macrophages. Similar findings have been previously reported in synovial tissue from patients with RA and psoriatic arthritis, with increased expression of CD68-positive macrophages co-localized with GM-CSFRα.25 Locally acting GM-CSF reportedly upregulated macrophage-/monocyte-derived dendritic cell numbers through GM-CSFR signalling, and administration of a neutralizing anti–GM-CSF mAb ameliorated antigen-induced arthritis and inflammatory responses.12,16 The presence of GM-CSFR in macrophages of OA synovium provides a potential treatment target.
Granulocyte-macrophage colony-stimulating factor receptor expression and neurite-promoting effects of its ligand GM-CSF have been described in cultured mouse DRG neurons40 and superior cervical ganglion neurons through a nerve growth factor (NGF)-independent MAPK activation pathway.28 Our findings of GM-CSFR expression in nociceptive small-/medium-sized cultured rat DRG neurons are in agreement with reports in mouse DRG neurons.40 Although most but not all GM-CSFR–positive neurons coexpressed the TRPV1 receptor, some were negative for TRPV1, representing the nonpeptidergic IB4-positive neuronal population; we have previously shown increased TRPV1 expression in DRG neurons due to the presence of increased neurotrophic factors, used in this study, and as observed in conditions of chronic pain.2 TRPV1 is the noxious pain receptor activated by H+, temperature above 43°C, capsaicin, the hot ingredient of chilli peppers,27 and is modulated by inflammatory mediators and growth factors.1,2,9,32 Capsaicin stimulation results in sustained calcium influx in TRPV1-expressing neurons, which underlies pain perception and signalling.19
Although GM-CSF did not directly activate calcium influx in cultured DRG neurons, it caused TRPV1 sensitization in a dose-dependent manner, similar to GM-CSF–mediated sensitization of mechanical and capsaicin responses in mice.40 These effects are rapidly induced through activation of the MAPK pathway. Granulocyte-macrophage colony-stimulating factor–mediated sensitization was reversed by the p38 MAPK and TrkA inhibitors. Our results suggest that GM-CSF effects are additive or synergistic to those of NGF and glial cell line–derived neurotrophic factor, which are included in our in vitro model of hypersensitivity,2–4 through activation of p38 MAPK and TrkA signalling pathways, similar to the effects of angiotensin II–mediated neuronal sensitization through the AT2 receptor.4 TRPV1 and p38 MAPK activation both require serine/threonine phosphorylation,10,41 and as GM-CSF phosphorylates p38 MAPK,44 this mechanism is likely to contribute to GM-CSF–mediated neuronal sensitization seen in our study, being sensitive to p38 MAPK inhibition. Nerve growth factor is also known to sensitize TRPV1 by p38 MAPK activation,26 providing convergence in the TrkA and GM-CSF signalling pathways. Inhibition of GM-CSF–mediated neuronal sensitization by the TrkA inhibitor, observed in our study, is in agreement with previous reports of GM-CSF signal transduction involving activation of a tyrosine kinase.24 Thus, in inflammatory conditions, the presence of abundant GM-CSF producing macrophages potentially contributes to tissue damage and ongoing pain caused by neuronal sensitization. The co-localization of GM-CSFR with TRPV1 in small nociceptive neurons, combined with GM-CSF–mediated hypersensitivity, implicates this receptor as an important target for neuroinflammatory pain.
In conclusion, we provide novel information about the differential expression of GM-CSFR in macrophages in MS spinal cord lesions, injured human DRG and peripheral nerves, and OA synovium. The coexpression of GM-CSFR with TRPV1 provides a mechanistic basis for neuronal sensitization through p38 MAPK and TrkA pain signalling pathways.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgements
The authors thank colleagues at MedImmune/AstraZeneca UK for funding support and discussions.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A30.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth 2011;107:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anand U, Otto WR, Casula MA, Day NC, Davis JB, Bountra C, Birch R, Anand P. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett 2006;399:51–6. [DOI] [PubMed] [Google Scholar]
- [3].Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, Birch R, Benham CD, Bountra C, Chessell IP, Anand P. Cannabinoid receptor CB2 localisation and agonist mediated inhibition of capsaicin responses in human sensory neurons. PAIN 2008;138:667–80. [DOI] [PubMed] [Google Scholar]
- [4].Anand U, Yiangou Y, Sinisi M, Fox M, MacQuillan A, Quick T, Korchev YE, Bountra C, McCarthy T, Anand P. Mechanisms underlying clinical efficacy of Angiotensin II type 2 receptor (AT2R) antagonist EMA401 in neuropathic pain: clinical tissue and in vitro studies. Mol Pain 2015;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bali KK, Venkataramani V, Satagopam VP, Gupta P, Schneider R, Kuner R. Transcriptional mechanisms underlying sensitization of peripheral sensory neurons by granulocyte-/granulocyte-macrophage colony stimulating factors. Mol Pain 2013;9:48–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T, Birch R, Anand P. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport 2001;12:3439–42. [DOI] [PubMed] [Google Scholar]
- [7].Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol 2017;17:49–59. [DOI] [PubMed] [Google Scholar]
- [8].Be'eri H, Reichert F, Saada A, Rotshenker S. The cytokine network of Wallerian degeneration: IL-10 and GM-CSF. Eur J Neurosci 1998;10:2707–13. [PubMed] [Google Scholar]
- [9].Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci 1995;75:4918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., IV cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 2002;35:721–31. [DOI] [PubMed] [Google Scholar]
- [11].Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem 1977;252:1998–2003. [PubMed] [Google Scholar]
- [12].Burmester GR, Weinblatt ME, McInnes IB, Porter D, Barbarash O, Vatutin M, Szombati I, Esfandiari E, Sleeman MA, Kane CD, Cavet G, Wang B, Godwood A, Magrini F. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. EARTH Study Group. Ann Rheum Dis 2013;72:1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Campbell IK, Rich MJ, Bischof RJ, Dunn AR, Grail D, Hamilton JA. Protection from collagen-induced arthritis in granulocyte macrophage colony-stimulating factor-deficient mice. J Immunol 1998;161:3639–44. [PubMed] [Google Scholar]
- [14].Cannistra SA, Groshek P, Garlick R, Millers J, Griffin JD. Regulation of surface expression of the granulocyte/macrophage colony-stimulating factor receptor in normal human myeloid cells. Proc Natl Acad Sci U S A 1990;87:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–17. [DOI] [PubMed] [Google Scholar]
- [16].Cook AD, Louis C, Robinson MJ, Saleh R, Sleeman MA, Hamilton JA. Granulocyte macrophage colony-stimulating factor receptor α expression and its targeting in antigen-induced arthritis and inflammation. Arth Res Ther 2016;18:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cousins DJ, Staynov DZ, Lee TH. Regulation of interleukin-5 and granulocyte-macrophage colony-stimulating factor expression. Am J Resp Crit Care Med 1994;150(5 pt 2):S50–3. [DOI] [PubMed] [Google Scholar]
- [18].de Vries EG, Willemse PH, Biesma B, Stern AC, Limburg PC, Vellenga E. Flare-up of rheumatoid arthritis during GM-CSF treatment after chemotherapy. Lancet 1991;338:517–18. [DOI] [PubMed] [Google Scholar]
- [19].Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition calcineurin inhibits the desensitization capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch 1996;431:828–37. [DOI] [PubMed] [Google Scholar]
- [20].Durrenberger PF, Facer P, Gray RA, Chessell IP, Naylor A, Bountra C, Banati RB, Birch R, Anand P. Cyclooxygenase-2 (Cox-2) in injured human nerve and a rat model of nerve injury. J Peripher Nerv Syst 2004;9:15–25. [DOI] [PubMed] [Google Scholar]
- [21].Durrenberger PF, Facer P, Casula MA, Yiangou Y, Gray RA, Chessell IP, Day NC, Collins SD, Bingham S, Wilson AW, Elliot D, Birch R, Anand P. Prostanoid receptor EP1 and Cox-2 in injured human nerves and a rat model of nerve injury: a time-course study. BMC Neurol 2006;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Foley PL, Vesterinen HM, Laird BJ, Sena ES, Colvin LA, Chandran S, MacLeod MR, Fallon MT. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. PAIN 2013;154:632–42. [DOI] [PubMed] [Google Scholar]
- [23].Franzen R, Bouhy D, Schoenen J. Nervous system injury: focus on the inflammatory cytokine “granulocyte-macrophage colony stimulating factor”. Neurosci Lett 2004;361:76–8. [DOI] [PubMed] [Google Scholar]
- [24].Griffin JD, Cannistra SA, Sullivan R, Demetri JD, Ernst TJ, Kanakura Y. The biology of GM-CSF: regulation of production and interaction with its receptor. Int J Cell Cloning 1990;8:35–44. [DOI] [PubMed] [Google Scholar]
- [25].Greven DEA, Cohen ES, Gerlag DM, Campbell J, Woods J, Davis N, van Nieuwenhuijze A, Lewis A, Heasmen S, McCourt M, Corkill D, Dodd A, Elvin J, Statache G, Wicks IP, Anderson IK, Nash A, Sleeman MA, Tak PP. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Ann Rheum Dis 2015;74:1924–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ji RR, Samad TA, Jin S-X, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002;36:57–68. [DOI] [PubMed] [Google Scholar]
- [27].Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001;413:203–10. [DOI] [PubMed] [Google Scholar]
- [28].Kannan Y, Moriyama M, Sugano T, Yamate J, Kuwamura M, Kagaya A, Kiso Y. Neurotrophic action of interleukin 3 and granulocyte-macrophage colony-stimulating factor on murine sympathetic neurons. Neuroimmunomodulation 2000;8:132–41. [DOI] [PubMed] [Google Scholar]
- [29].Kupper TS, Lee F, Coleman D, Chodakewitz J, Flood P, Horowitz M. Keratinocyte derived T-cell growth factor (KTGF) is identical to granulocyte macrophage colony stimulating factor (GM-CSF). J Invest Derm 1988;91:185–8. [DOI] [PubMed] [Google Scholar]
- [30].Kupper TS, Dower S, Birchall N, Clark S, Lee F. Interleukin 1 binds to specific receptors on keratinocytes and induces granulocyte/macrophage colony stimulating factor (GM-CSF) mRNA and protein: a potential autocrine role for IL-1 in epidermis. J Clin Invest 1988;82:1787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lang RA, Metcalf D, Cuthbertson RA, Lyons I, Stanley E, Kelso A, Kannourakis G, Williamson DJ, Klintworth GK, Gonda TJ, Dunn AR. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell 1987;51:675–86. [DOI] [PubMed] [Google Scholar]
- [32].Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci 1993;13:2136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Malipiero UV, Frei K, Fontana A. Production of hemopoietic colony-stimulating factors by astrocytes. J Immunol 1990;144:3816–21. [PubMed] [Google Scholar]
- [34].McLay RN, Kimura M, Banks WA, Kastin AJ. Granulocyte macrophage colony-stimulating factor crosses the blood–brain and blood–spinal cord barriers. Brain 1997;120:2083–91. [DOI] [PubMed] [Google Scholar]
- [35].McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CCA. Granulocyte macrophage colony-stimulating factor a new putative therapeutic target in multiple sclerosis. J Exp Med 2001;194:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miyajima A. Molecular structure of the IL-3, GM-CSF and IL-5 receptors. Int J Cell Cloning 1992;10:126–34. [DOI] [PubMed] [Google Scholar]
- [37].Ridwan S, Bauer H, Frauenknecht K, von Pein H, Sommer CJ. Distribution of granulocyte-monocyte colony-stimulating factor and its receptor α-subunit in the adult human brain with specific reference to Alzheimer's disease. J Neural Transm 2012;119:1389–406. [DOI] [PubMed] [Google Scholar]
- [38].Saada A, Reichert F, Rotshenker S. Granulocyte macrophage colony stimulating factor produced in lesioned peripheral nerves induces the up-regulation of cell surface expression of MAC-2 by macrophages and Schwann cells. J Cell Biol 1996;133:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schäbitz W-R, Krüger C, Pitzer C, Weber D, Laage R, Gassler N, Aronowski J, Mier W, Kirsch F, Dittgen T, Bach A, Sommer C, Schneider A. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J Cereb Blood Flow Metab 2008;28:29–43. [DOI] [PubMed] [Google Scholar]
- [40].Schweizerhof M, Stösser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, Schmelz M, Bali KK, Michalski CW, Brugger S, Dickenson A, Simone DA, Kuner R. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med 2009;15:802–7. [DOI] [PubMed] [Google Scholar]
- [41].Seger R, Krebs EG. The MAPK signalling cascade. FASEB J 1995;9:726–35. [PubMed] [Google Scholar]
- [42].Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett 1988;85:169–71. [DOI] [PubMed] [Google Scholar]
- [43].Stösser S, Schweizerhof M, Kuner R. Hematopoietic colony-stimulating factors: new players in tumor-nerve interactions. J Mol Med (Berl) 2011;89:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Suzuki K, Hino M, Kutsuna H, Hato F, Sakamoto C, Takahashi T, Tatsumi N, Kitagawa S. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1β. J Immunol 2001;167:5940–7. [DOI] [PubMed] [Google Scholar]
- [45].Vogel DYS, Kooij G, Heijnen PDAM, Breur M, Peferoen LAN, van der Valk P, de Vries HE, Amor S, Dijkstra CD. GM-CSF promotes migration of human monocytes across the blood brain barrier. Eur J Immunol 2015;45:1808–19. [DOI] [PubMed] [Google Scholar]
- [46].Wicks IP, Roberts AW. Targeting GM-CSF in inflammatory diseases. Nat Rev Rheumatol 2016;12:37–48. [DOI] [PubMed] [Google Scholar]
- [47].Wright HL, Bucknall RC, Moots RJ, Edwards SW. Analysis of SF and plasma cytokines provides insights into the mechanisms of inflammatory arthritis and may predict response to therapy. Rheumatology (Oxford) 2012;51:451–9. [DOI] [PubMed] [Google Scholar]
- [48].Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol 2006;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]



