Abstract
Background:
Arm lymphedema is a well-recognized complication after breast cancer surgery that negatively impacts patients’ quality of life, both physiologically and psychologically. Lymph stasis and inflammation result in excess formation of adipose tissue, which makes removal of the deposited subcutaneous fat necessary to eliminate the excess volume. Liposuction, combined with postoperative controlled compression therapy (CCT), is the only treatment that gives complete reduction of the excess volume. The aim of this study was to evaluate the 5-year results after liposuction in combination with CCT.
Methods:
Patients consecutively operated on between 1993 and 2012 were identified from the lymphedema registry, comprising all patients with nonpitting lymphedema treated with liposuction and CCT in our department. Standardized forms were used to collect pre-, peri-, and postoperative data.
Results:
One hundred five women with nonpitting edema were treated. The mean interval between the breast cancer operation and lymphedema start was 2.9 ± 5.0 years, the mean duration of lymphedema was 10 ± 7.4 years, and the preoperative mean excess volume was 1,573 ± 645 ml. The mean volume aspirated was 1,831 ± 599 ml. Postoperative mean reduction 5 years postoperatively was 117% ± 26% as compared with the healthy arm.
Conclusion:
Liposuction is an effective method for the treatment of chronic, nonpitting, arm lymphedema resistant to conservative treatment. The volume reduction remains complete after 5 years.
INTRODUCTION
Arm lymphedema is a well-known complication after breast cancer treatment affecting the patients’ health-related quality of life, both from a physical and psychological point of view.1
Earlier studies have reported incidences of arm lymphedema of 8–41% following axillary clearance and radiation2–4 and 4–10% following sentinel node biopsy.5,6 After removal of lymph nodes, the lymph collectors become overloaded, and their valves become incompetent. Subsequently, stasis and chronic inflammation7–10 lead to subcutaneous fat hypertrophy and a shift from an edema-dominated state to a more fatty state.11,12 In addition, a magnetic resonance imaging study of arm lymphedema has revealed an increased content of fat in the subfascial compartment, that is in the muscle.13
Initially, patients are treated by conservative methods such as complex decongestive therapy comprising bandaging, physical exercise, manual lymph massage, skin care and self-management, and flat-knitted compression garments.14 Another treatment option in early stages of lymphedema is microsurgical reconstruction of the damaged lymphatic system,15–26 for example, lymphatico-venous or lymphatico-venous-lymphatic shunts15–21,27,28 lymphatic node transfer22,23 and lymph vessel transplantation.24–26 However, there are few studies presenting long-term follow-up after microsurgical procedures using a standardized postoperative treatment protocol, with or without the use of compression garments. In addition, lymph stasis leads to dilatation of the lymphatics with concomitant insufficient valves and irreversible fibrosis of the smooth musculature in the vessel walls, which become rigid and lose their intrinsic contractility (lymphaticosclerosis)29,30, thus making the application of microsurgical techniques difficult.
Neither CDT nor microsurgical reconstruction can be used in later stages of lymphedema as none of the techniques can remove the hypertrophied adipose tissue that occurs in response to lymph stasis and inflammation.31 In later stages of nonpitting lymphedema, not responding to conservative treatment, liposuction, combined with postoperative CCT, gives a complete reduction of the excess volume.32–36
The aim of this study was to evaluate the 5-year results after liposuction in combination with CCT in 105 patients with secondary nonpitting arm lymphedema.
PATIENTS AND METHODS
Patients
Patients consecutively operated on between 1993 and 2012 were identified from the lymphedema registry of our department. All patients with lymphedema treated with liposuction and CCT were entered into the registry at the first consultation, and data were collected at each follow-up. Standardized forms were used to collect pre-, peri-, and postoperative data.
Patients who met the following inclusion criteria were operated on: (1) diagnosis of secondary arm lymphedema following breast cancer treatment; (2) a significant excess volume, that is the volume of the affected arm was at least 10% larger than that of the unaffected arm37 and concomitant subjective discomfort; (3) inability of previous conservative treatment to reduce the excess volume completely; (4) no or minimal pitting (less than 5 mm) as a sign of adipose tissue hypertrophy; and (5) accustomed to the use of compression garments preoperatively. The pitting test has been thoroughly described previously.38,39 Patients with active cancer, wounds, or infections and patients unwilling to undergo continuous postoperative CCT were excluded.32,33,36
Ethics
The study was approved by the Ethics of Human Investigation Committee at Lund University, Sweden in 1998 (697/1998), and by the Regional Ethical Review Board in Lund in 2006 (503/2006) and 2011 (45/2011).
All participants gave their written informed consent to participate. The procedures followed were in accordance with the Declaration of Helsinki of 1964 and its most recent revision 2013.
Measurement of Excess Volume and Reduction
Plethysmography was used to record arm volumes. Both arms were always measured at each visit, and the difference was defined as the excess volume.40 The displaced water volume was weighed on a scale to the nearest 5 g, corresponding to 5 mL.32,33,41 The excess volume reduction was also measured in percentage of the preoperative excess volume,
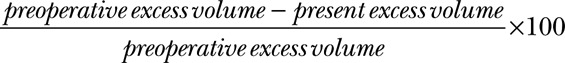 |
All volumes were measured by the same physiotherapist and occupational therapist until the patient’s excess volume was reduced as much as possible. The whole arm was measured, which is equivalent to 40–44 cm from the wrist. Thereafter, they were followed up by the lymph therapist they had before surgery, who reported back to our lymphedema team every year. In most cases, these volumes were based on circumference measurements taken every 4 cm along the arm and transferred into volumes using a computer program based on the formula of the truncated cone, which has a high correlation with plethysmography.41 The mean ratio between the volume of the edematous and healthy arm was also calculated.
Compression Garments
Initially, arms were bandaged with elastic rolls, but it was difficult to get even compression. Therefore, application of a sleeve and gauntlet was used at the time of surgery. Thus, compression garments (3 sleeves and 2 gloves/gauntlets) were measured for and ordered 2 weeks before surgery, using the healthy arm and edematous hand as a template. One of the sleeves was sterilized and was put on at the time of surgery. Sterilization decreases the compression, and this garment was thus only used for 2 days until the first change of garments was carried out.
Liposuction
For the majority of patients, power-assisted liposuction (Lipomatic, Nutational Infrasonic Liposculpture, Euromi, Andrimont, Belgium) was performed to facilitate liposuction. During the period 1993–1997, the “dry technique” was used. During the period 1997–2012, a tourniquet was utilized in combination with the tumescence technique to minimize blood loss.42,43
Around ten, 3- to 4-mm long incisions were made, and liposuction was performed using 15- and 25-cm long cannulas with diameters of 3 and 4 mm. Initially, the hand was also treated, but since no fat could be aspirated, we ceased to treat this area. Circumferential liposuction was performed from wrist to shoulder, and as much of the hypertrophied fat was removed as possible using previously measured circumferences of the healthy arm as a control (Fig. 1).
Fig. 1.
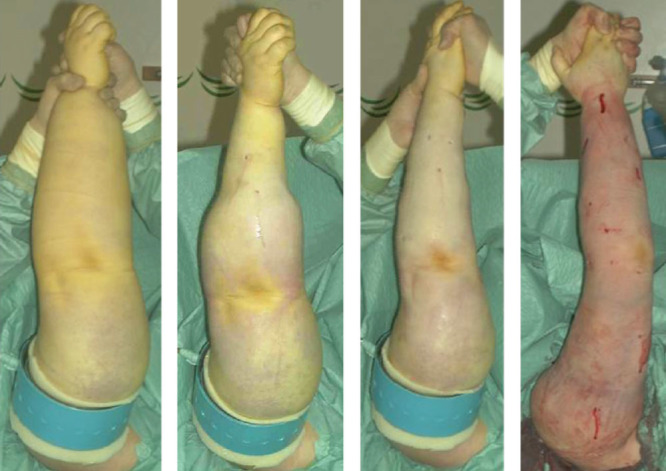
Liposuction of arm lymphedema combined with the use of tourniquet and tumescence technique. The procedure takes about 2 hours. From preoperative to postoperative state (left to right). (Published with permission from J Lymphoedema 2008; 1: 38–47).
When the arm distal to the tourniquet had been treated, a sterilized custom-made compression sleeve was applied (Jobst Elvarex, compression class 2) to the arm to minimize bleeding and reduce postoperative edema. A sterilized, standard interim glove (Cicatrex interim, Thuasne Begat, France) was put on the hand. The tourniquet was then removed, and the most proximal part of the upper arm was treated using the tumescent technique, where 1,000 ml saline mixed with 1 mg adrenaline and 40 ml lidocaine 2% (Xylocaine, AstraZeneca PLC, London, United Kingdom) was infused subcutaneously. Finally, the proximal part of the compression sleeve was pulled up to compress the proximal part of the upper arm (Fig. 2), and the position was secured with the strap that came with the sleeve. The incisions were left open to drain through the sleeve.
Fig. 2.
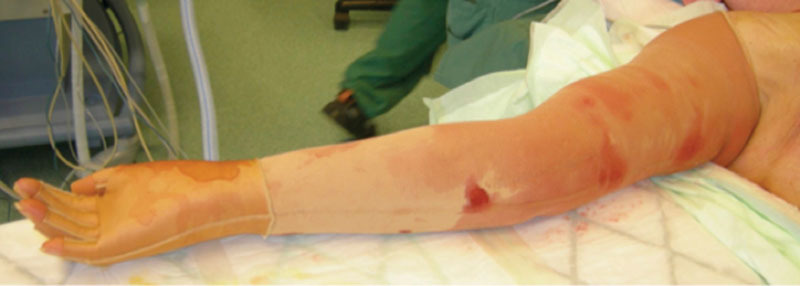
The compression garment and interim glove at the end of surgery.
The arm was wrapped with a large absorbent compress covering the arm (60 × 60 cm, Cover-Dri, www.attends.co.uk), and this was changed when needed. The following day, the gauntlet (Jobst Elvarex, compression class 2) was put over the interim glove (Fig. 3). In severe hand swelling, a glove was used instead.
Fig. 3.
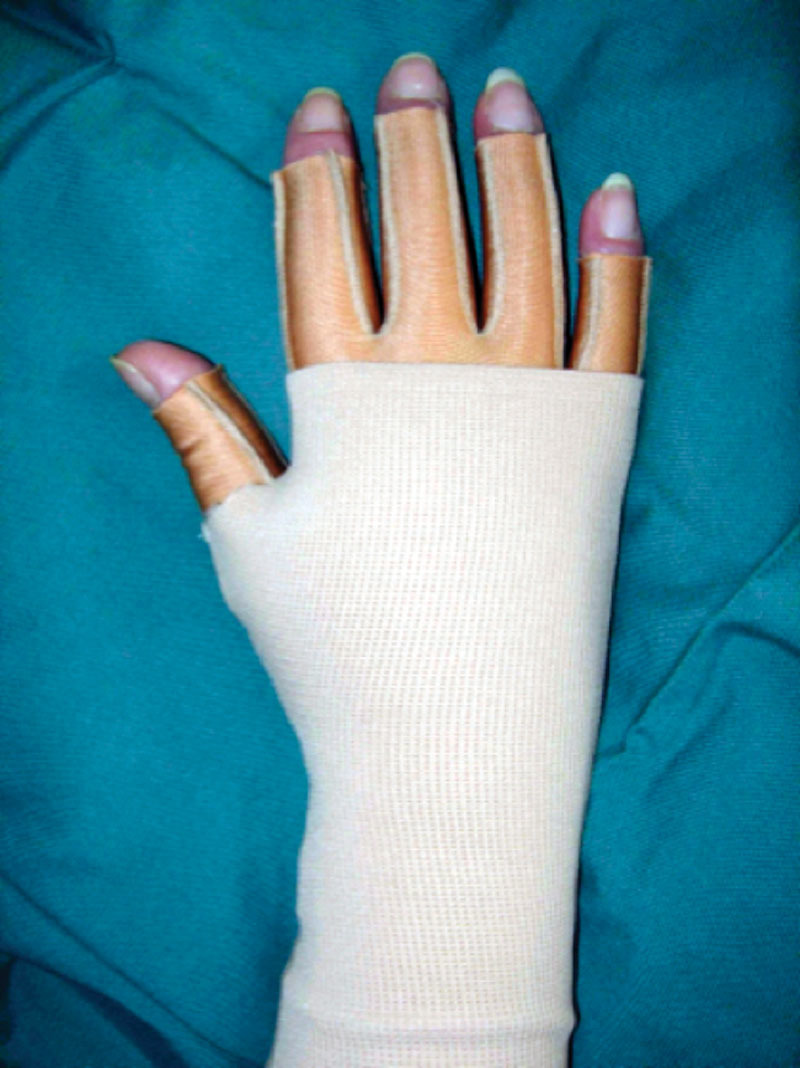
Interim glove with gauntlet.
An isoxazolyl penicillin was given intravenously for the first 24 hours and then orally until the incisions were healed, approximately during 10 days after surgery. In the case of penicillin allergy, clindamycin was used instead.
Volume of Removed Fat
Aspirate volumes were collected in graded 2,000 ml canisters, and the total volume was calculated with an accuracy of 10 ml. When the “dry technique” was used, the total amount of aspirate was noted. When a tourniquet and tumescence was used, the proportion of free fat, the supernatant, was calculated. The aspirate was collected in 2 portions: 1 from the forearm and the distal part of the upper arm below the tourniquet, and 1 from the tumescent portion including the area under the tourniquet and the remaining proximal part of the upper arm.
Postoperative Management
The garments were removed 2 days after surgery by the patient herself and she then took a shower. The skin was lubricated with lotion, and the other set of garments was applied by the patient under supervision. The used sleeve and glove/gauntlet were washed. On the fourth postoperative day, the second change was performed and the patient was discharged. Thereafter, the patient changed the garments every other day during the first week at home, and then daily so that a clean set was always put on after showering and lubricating the arm. Used garments were washed to be used at the next change after drying, and so on. Thus, garments were worn continuously, night and day.
Controlled Compression Therapy and Follow-up
Patients were followed up regularly at 0.5, 1, 3, 6, 9 months, and at 1 year after surgery, and then every year. If complete reduction was not reached at 1 year, 3-month visits were scheduled. Patients with complete reduction at 2 years were followed up by their previous lymph therapist, who reported arm volumes yearly. Following surgery CCT was started with custom-made compression garments: a sleeve and glove or gauntlet, that is, a glove without fingers, but with a thumb (Jobst Elvarex) with compression in the range 32–40 mm Hg (class 2). If the hand showed pitting, a glove was initially used, but in most cases a gauntlet was sufficient. Two weeks before liposuction, measurements for garments were taken using the healthy arm as a template. These initial sleeves were ordered with a shoulder cap and strap to prevent them from sliding down (Fig. 4). When complete reduction was achieved, sleeves without a strap (stay-up sleeves) were used.
Fig. 4.
Compression garment secured with a strap.
At the 3-month visit, the arm was measured for 2 new sets of custom-made garments; 1 set comprised 1 sleeve and 1 glove or gauntlet. The circumferential measurements were reduced. The custom-made sleeve was sometimes taken in, when needed, at each visit using a sewing machine, to make up for reduced elasticity and reduced arm volume while waiting for the new compression to arrive. This procedure was repeated at 6, 9, and 12 months. The 9-month visit could be omitted if complete reduction was achieved at 6 months. In that case, compression garments for the following 6 months were prescribed. When the excess volume had decreased as much as possible and a steady state had been achieved, new garments were prescribed using the latest measurements. The garments were renewed 3 or 4 times during the first year. Two sets of garments were always at hand; 1 being worn while the other was washed. Thus, garments were worn permanently, and treatment was disrupted only briefly when showering or when attending specific social occasions. Hygiene and skin care are important as patients with lymphedema are susceptible to infections.32,33 The life span of 2 sets of garments worn alternately is usually 3–6 months. Complete reduction is usually achieved after 3–6 months, although this can often occur earlier. If complete reduction was achieved at 1 year, a visit at 1.5 years and 2 years was performed, followed by yearly visits when new garments were prescribed for the next year, usually 4 sleeves and 4 gloves/gauntlets. For very active patients, 6–8 garments and the same number of gloves/gauntlets per year are needed. Patients without preoperative swelling of the hand can usually stop using the glove/gauntlet after 6–12 months postoperatively.
Statistical Analysis
Data are presented as mean values, standard deviation (SD), and ranges. The normality of all data points was tested and confirmed using the Shapiro-Wilk test. A parametric Student’s paired t test (2-tailed) was used to analyze differences between pre- and postoperative outcome of surgery. Linear regression was used to determine the relationships between outcomes. The cumulative sum control chart was used to show any possible learning curve. The outcome of the significance tests was considered to show exploratory results, and therefore nominal P values are presented without any adjustment for multiple comparisons. A P value less than 0.05 was considered statistically significant. All statistical analyses were carried out using IBM SPSS Statistics for Mac OSX, Armonk, NY, USA. (Version 23.0, SPSS Inc.).
RESULTS
Between 1993 and 2012, a total of 127 consecutive women were operated on. Twenty-two could not be followed for 5 years: 18 died before the last follow-up (10 because of breast cancer and 8 of other causes), 1 had recurrence of breast cancer, 1 stopped using CCT, 1 moved abroad, and in 1 case, data from the therapist was missing. Thus, 105 women with nonpitting lymphedema remained in the study. The characteristics of the study population (mean, range) are shown in Table 1.
Table 1.
Characteristics of the Study Population Mean ± SD (Range)

Ninety-eight patients (93%) had irradiation therapy, 81 patients (77%) had axillary clearance, and 74 patients (70%) had both. Fifty-seven patients (54%) were affected in their dominant arm.
Liposuction Techniques and Complications
With the first 27 patients the “dry” liposuction technique without a tourniquet was used (blood transfusions, 15/27, 56%). In the following 35 patients, the “dry” technique in combination with a tourniquet was used (blood transfusions, 5/35, 14%). With the remaining 43 patients, the tumescence technique and a tourniquet was used. Following the introduction of tourniquet in combination with tumescence, no patient needed a blood transfusion (0/43, 0%).32,33,43 No other major or minor peri- or postoperative complications were recorded. The skin retracted nicely in all patients, and there was no need for skin excision.
Aspirated Volumes
Total aspirate mean volume was 1,831 ± 599 ml (range, 650–3,780) for all patients (n = 105). The mean volume of aspirate removed when a tourniquet was applied (n = 76) was 951 ± 405 ml (range, 310–2,060) and contained 94% ± 11% fat (range, 58–100; Fig. 5).
Fig. 5.
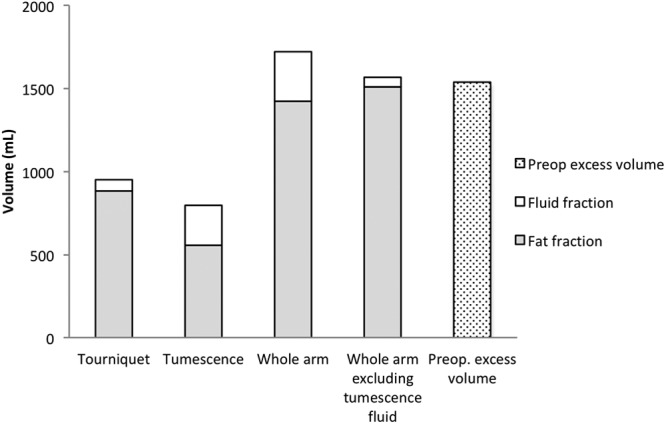
Aspirate analysis in 76 patients when tourniquet was used. Mean peroperative aspirate (fat and fluid fractions) and excess volumes. The excess volume was 1,525 ± 645 ml (range, 570–3,520). Tourniquet: Fractions removed while using the tourniquet. Tumescence: Fractions removed from the area where the tourniquet was applied. Whole arm: Fractions of the total aspirate. Whole arm excluding tumescence fluid: The volume of all fat + fluid fraction when using tourniquet. Preoperative excess volume: Volume of swollen arm minus volume of normal arm. The mean volume of aspirate removed when a tourniquet was applied (n = 76) was 951 ± 405 ml (range, 310–2,060) and contained 94 ± 11% fat (range, 58–100). The mean volume of aspirate removed with tumescence* was 795 ± 275 ml (range, 210–1,700), and the mean proportion of fat was 72 ± 12% (range, 42–100). The high proportion of fluid in the tumescence fraction was due to the aspirated tumescent fluid. Thus, excluding the fluid in the tumescence fraction gives an aspirate fat content of 96 ± 7.0% (range, 74–100). *One patient has no swelling in the proximal part of the upper arm, thus no liposuction was made here resulting in 0 aspirate and was excluded in the analysis of the tumescent fraction.
Excess Volume Reduction
The preoperative mean excess volume (±SD) was 1,573 ± 645 ml (range, 570–3,520), and the ratio between the lymphedematous and healthy arm was 1.5 ± 0.2 (range, 1.2–2.1). A successive postoperative reduction was seen, and this continued at 6 months when the reduction was 107% ± 22% (range, 73–179) with an excess volume of −51 ± 273 ml (range, from −760 to 730), ratio 1.0 ± 0.1 (range, 0.8–1.2). During the remaining follow-ups, it was more than 100%, and at 5 years, it was 117% ± 26% (range, 25–191) with an excess volume of −188 ± 300 ml (range, from −920 to 1,010) and a ratio of 0.9 ± 0.1 (range, 0.8–1.4), that is, the lymphedematous arm was somewhat smaller than the healthy one (Figs. 6, 7).
Fig. 6.
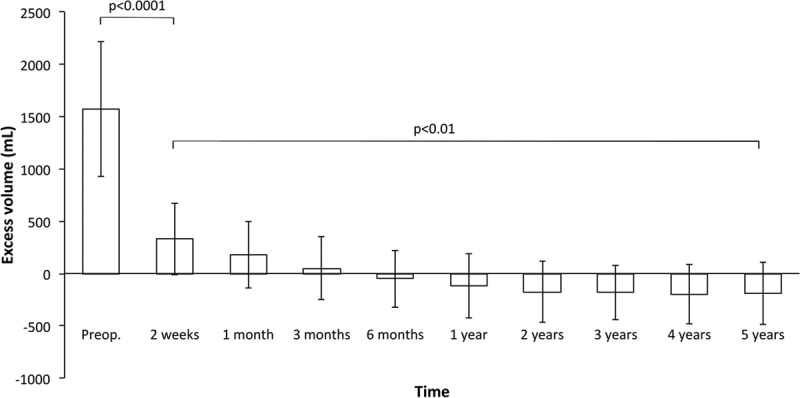
Pre- and postoperative excess volume reduction (mean ± SD). Following surgery a significant reduction was seen at 2 weeks and continued during the follow-up with a complete reduction at 6 months that was maintained during 5 years’ follow-up.
Fig. 7.
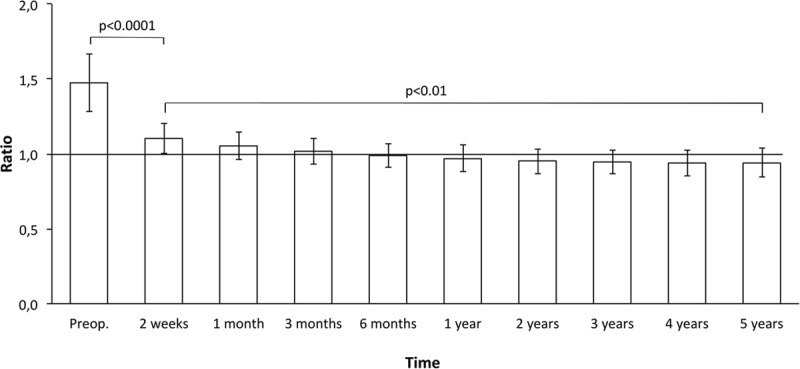
Pre- and postoperative excess ratio (affected/nonaffected arm) reduction (mean ± SD). After 6 months, the ratio was less than 1.
Typical postoperative outcomes are shown in Figs. 8, 9.
Fig. 8.
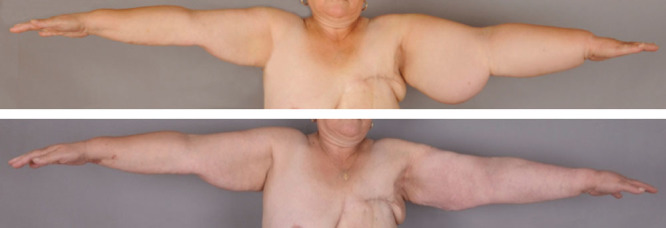
A, A 57-year-old woman with an excess volume of 4,325 ml that had existed for 5 years. B, Complete reduction at 1 year after liposuction.
Fig. 9.
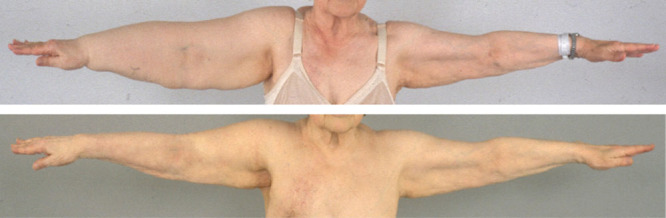
A, A 77-year-old woman with an excess volume of 2,480 ml that had lasted for 9 years. B, Complete reduction at 5 years after liposuction.
The excess volume reduction in percent after 5 years was linearly related to the preoperative excess volume with a coefficient of regression (slope) of −0.02 and a Pearson coefficient of correlation of −0.47 (P < 0.0001; Fig. 10).
Fig. 10.
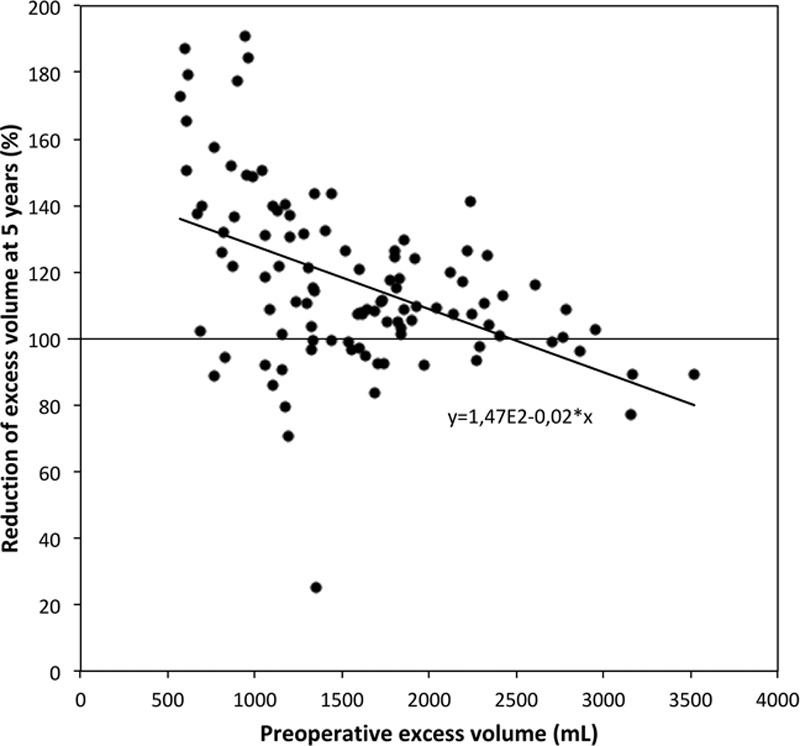
Percentage reduction of the excess volume at 5 years compared with the preoperative excess volume.
To evaluate a potential learning curve, the excess volume reduction in percentage and the operation serial number was analyzed, which showed a coefficient of regression (slope) of 0.25 and a Pearson coefficient of correlation of 0.29 (P < 0.002). No linearity was found when analyzing the excess volume reduction in percentage with duration of lymphedema. This can also be described as in Figure 11, which shows the cumulative sum control chart of the operation serial number with expected 90% success at 5 years with a 95% confidence interval. The curve thus shows the accumulated percentage of patients who did not get 90% reduction, that is 9 out of 105 patients (8.6%).
Fig. 11.
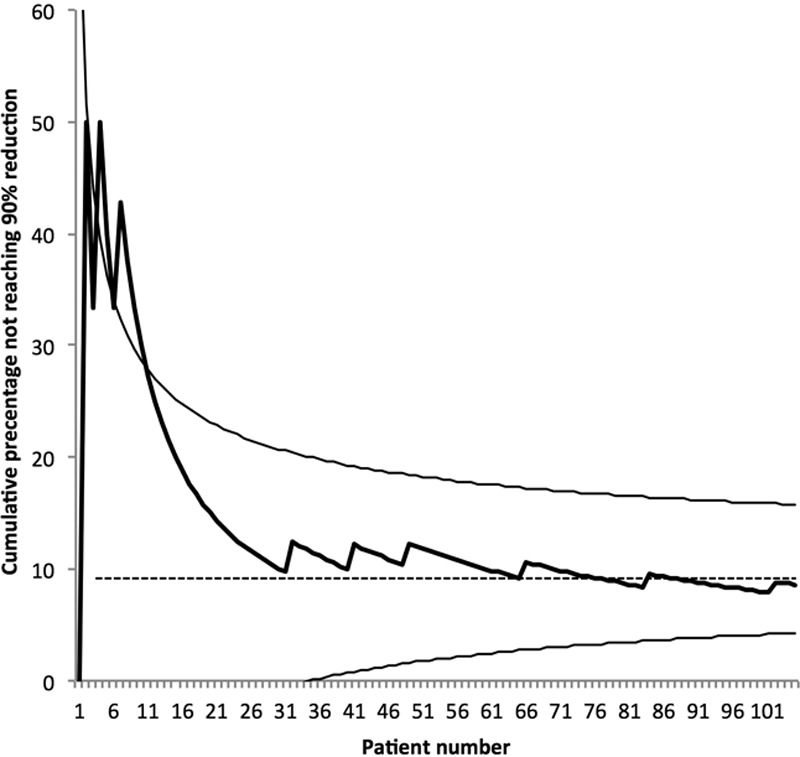
The cumulative sum control chart of the operation serial number with expected 90% success at 5 years with a 95% confidence interval. Thus, 9 out of 105 patients (8.6%) did not get 90% reduction.
DISCUSSION
Our first liposuction was undertaken in 1987, but it was not until 1993 that a more detailed treatment protocol was established. We have previously reported stable results over time33 with a 1-year overall edema reduction of 106% for the method.32 In the present study, the overall edema reduction was increased to 117% at the 5-year follow-up, further strengthening the beneficial long-term effects of the treatment protocol. In addition, several teams have adopted our techniques and have reported no signs of recurrence.44–46 In brief, our treatment protocol gives a permanent reduction of lymphedema.
All patients were accustomed to the use of compression garments before surgery, thus their use meant no change in the regimen following surgery except regarding the need, for most patients, to wear the garments also during the night. Patient compliance is therefore of the utmost importance for the treatment success. The patients’ quality of life after liposuction and CCT is improved even though they need to wear compression garments continuously for life.1,47
The high content of adipose tissue and the low portion of fluid in the aspirate confirm that the pitting test is a valuable tool for selecting patients for surgery. There was an inverse correlation between the excess volume reduction in percentage at 5 years and the preoperative excess volume after 1 year. Thus, normalization is easier to achieve when the lymphedema is less pronounced (Fig. 10). Despite variations between patients in outcomes, the results show that complete reduction of the excess volume is possible in patients with an excess volume up to around 3,000 ml (Fig. 10).
Although our early patients achieved adequate clinical results, there was a significant correlation between the operation serial number and excess volume reduction in percentage after 5 years. It is therefore possible that increased skill with the operative technique will improve outcome, which also can be seen in the cumulative sum control chart (Fig. 11).
An objection to liposuction has been that the lymphatics might be further damaged by the procedure. However, studies have shown no further decrease of the already decreased transport capacity,48,49 as liposuction is unlikely to cause major lesions of epifascial lymph49 or even improve lymphatic flow.50 Hence, the risk for further damage to the lymphatics seems to be very small. So far, no complications, such as thromboembolism or skin necrosis, have occurred. The introduction of use of a tourniquet and tumescence has made liposuction a safe procedure, and the need of blood transfusions has been reduced significantly.43 In addition, the incidence of erysipelas decreases with 87% following liposuction.51
CONCLUSIONS
The results of this prospective long-term study of 105 patients demonstrate that liposuction combined with CCT is an effective and safe method for treatment of chronic, nonpitting arm lymphedema resistant to conservative treatment. A mean reduction of 117% was achieved, and such normalization can be anticipated in patients with an excess volume of around 3,000 ml. So far, it is the only known method that completely reduces the excess volume at all stages of arm lymphedema, as the removal of hypertrophied adipose tissue is a prerequisite for complete reduction. The complete reduction is maintained through continuous use of compression garments postoperatively.
Footnotes
Published online 16 August 2018.
Supported by the Swedish Cancer Society, Stockholm, Skåne County Council’s Research, and the Development Foundation, and Blekinge County Council’s Research and Development Foundation.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the supporting sources.
REFERENCES
- 1.Hoffner M, Bagheri S, Hansson E, et al. SF-36 shows increased quality of life following complete reduction of postmastectomy lymphedema with liposuction. Lymphat Res Biol. 2017;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazeron JJ, Otmezguine Y, Huart J, et al. Conservative treatment of breast cancer: results of management of axillary lymph node area in 3353 patients. Lancet. 1985;1:1387. [DOI] [PubMed] [Google Scholar]
- 3.Kissin MW, Querci della Rovere G, Easton D, et al. Risk of lymphoedema following the treatment of breast cancer. Br J Surg. 1986;73:580. [DOI] [PubMed] [Google Scholar]
- 4.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279. [DOI] [PubMed] [Google Scholar]
- 5.Lucci A, McCall LM, Beitsch PD, et al. ; American College of Surgeons Oncology Group. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657. [DOI] [PubMed] [Google Scholar]
- 6.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen OA, Lassen NA, Quaade F. Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand. 1966;66:337. [DOI] [PubMed] [Google Scholar]
- 8.Zampell JC, Aschen S, Weitman ES, et al. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg. 2012;129:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aschen S, Zampell JC, Elhadad S, et al. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg. 2012;129:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockson SG. The lymphatics and the inflammatory response: lessons learned from human lymphedema. Lymphat Res Biol. 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199. [DOI] [PubMed] [Google Scholar]
- 12.Brorson H, Ohlin K, Olsson G, et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol. 2009;7:3. [DOI] [PubMed] [Google Scholar]
- 13.Hoffner M, Peterson P, Månsson S, et al. Lymphedema leads to fat deposition in muscle and decreased muscle/water volume after liposuction: a magnetic resonance imaging study. Lymphat Res Biol. 2018;16:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockson SG, Miller LT, Senie R, et al. American Cancer Society Lymphedema Workshop. Workgroup III: diagnosis and management of lymphedema. Cancer. 1,998;83:2882. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg. 1977;60:197. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien BM, Mellow CG, Khazanchi RK, et al. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast Reconstr Surg. 1990;85:562. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Sugihara T. Microsurgical lymphaticovenous implantation for the treatment of chronic lymphedema. Plast Reconstr Surg. 1998;101:157. [DOI] [PubMed] [Google Scholar]
- 18.Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg. 2000;16:437. [DOI] [PubMed] [Google Scholar]
- 19.Campisi C, Davini D, Bellini C, et al. Is there a role for microsurgery in the prevention of arm lymphedema secondary to breast cancer treatment? Microsurgery. 2006;26:70. [DOI] [PubMed] [Google Scholar]
- 20.Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010;126:752. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa H, Osawa M, Saito A, et al. Microsurgical lymphaticovenous implantation targeting dermal lymphatic backflow using indocyanine green fluorescence lymphography in the treatment of postmastectomy lymphedema. Plast Reconstr Surg. 2011;127:1804. [DOI] [PubMed] [Google Scholar]
- 22.Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg. 2009;123:1265. [DOI] [PubMed] [Google Scholar]
- 24.Ho LC, Lai MF, Kennedy PJ. Micro-lymphatic bypass in the treatment of obstructive lymphoedema of the arm: case report of a new technique. Br J Plast Surg. 1983;36:350. [DOI] [PubMed] [Google Scholar]
- 25.Kleinhans E, Baumeister RG, Hahn D, et al. Evaluation of transport kinetics in lymphoscintigraphy: follow-up study in patients with transplanted lymphatic vessels. Eur J Nucl Med. 1985;10:349. [DOI] [PubMed] [Google Scholar]
- 26.Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990;85:64; discussion 75. [DOI] [PubMed] [Google Scholar]
- 27.Campisi C, Bellini C, Campisi C, et al. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery. 2010;30:256. [DOI] [PubMed] [Google Scholar]
- 28.Maegawa J, Hosono M, Tomoeda H, et al. Net effect of lymphaticovenous anastomosis on volume reduction of peripheral lymphoedema after complex decongestive physiotherapy. Eur J Vasc Endovasc Surg. 2012;43:602. [DOI] [PubMed] [Google Scholar]
- 29.Ewert A, Folse D. Animal model of human disease. Lymphatic filariasis. Am J Pathol. 1984;115:135. [PMC free article] [PubMed] [Google Scholar]
- 30.Ogata F, Fujiu K, Koshima I, et al. Phenotypic modulation of smooth muscle cells in lymphoedema. Br J Dermatol. 2015;172:1286. [DOI] [PubMed] [Google Scholar]
- 31.Brorson H. Adipose tissue in lymphedema: the ignorance of adipose tissue in lymphedema. Lymphology. 2004;37:175. [PubMed] [Google Scholar]
- 32.Brorson H, Svensson H. Complete reduction of lymphoedema of the arm by liposuction after breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1997;31:137. [DOI] [PubMed] [Google Scholar]
- 33.Brorson H, Svensson H. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg. 1998;102:1058; discussion 1068. [PubMed] [Google Scholar]
- 34.Brorson H. Liposuction normalizes lymphedema induced adipose tissue hypertrophy in elephantiasis of the leg—a prospective study with a ten-year follow-up. Plast Reconstr Surg. 2015;136(Suppl 4):133. [Google Scholar]
- 35.Brorson H. Complete reduction of arm lymphedema following breast cancer—a prospective twenty-one years’ study. Plast Reconstr Surg. 2015;136(Suppl 4):134. [Google Scholar]
- 36.Brorson H. Liposuction in lymphedema treatment. J Reconstr Microsurg. 2016;32:56. [DOI] [PubMed] [Google Scholar]
- 37.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208. [DOI] [PubMed] [Google Scholar]
- 38.Bagheri S, Ohlin K, Olsson G, et al. Tissue tonometry before and after liposuction of arm lymphedema following breast cancer. Lymphat Res Biol. 2005;3:66. [DOI] [PubMed] [Google Scholar]
- 39.Sanderson J, Tuttle N, Box R, et al. The pitting test; an investigation of an unstandardized assessment of lymphedema. Lymphology. 2015;48:175. [PubMed] [Google Scholar]
- 40.Swedborg I. Voluminometric estimation of the degree of lymphedema and its therapy by pneumatic compression. Scand J Rehabil Med. 1977;9:131. [PubMed] [Google Scholar]
- 41.Brorson H, Höijer P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J Plast Surg Hand Surg. 2012;46:410. [DOI] [PubMed] [Google Scholar]
- 42.Klein JA. The tumescent technique for lipo-suction surgery. Am J Cosmetic Surg. 1987;4:263. [Google Scholar]
- 43.Wojnikow S, Malm J, Brorson H. Use of a tourniquet with and without adrenaline reduces blood loss during liposuction for lymphoedema of the arm. Scand J Plast Reconstr Surg Hand Surg. 2007;41:243. [DOI] [PubMed] [Google Scholar]
- 44.Damstra RJ, Voesten HG, Klinkert P, et al. Circumferential suction-assisted lipectomy for lymphoedema after surgery for breast cancer. Br J Surg. 2009;96:859. [DOI] [PubMed] [Google Scholar]
- 45.Schaverien MV, Munro KJ, Baker PA, et al. Liposuction for chronic lymphoedema of the upper limb: 5 years of experience. J Plast Reconstr Aesthet Surg. 2012;65:935. [DOI] [PubMed] [Google Scholar]
- 46.Boyages J, Kastanias K, Koelmeyer LA, et al. Liposuction for advanced lymphedema: a multidisciplinary approach for complete reduction of arm and leg swelling. Ann Surg Oncol. 2015;22:S1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brorson H, Ohlin K, Olsson G, et al. Quality of life following liposuction and conservative treatment of arm lymphedema. Lymphology. 2006;39:8. [PubMed] [Google Scholar]
- 48.Brorson H, Svensson H, Norrgren K, et al. Liposuction reduces arm lymphedema without significantly altering the already impaired lymph transport. Lymphology. 1998;31:156. [PubMed] [Google Scholar]
- 49.Hoffmann JN, Fertmann JP, Baumeister RG, et al. Tumescent and dry liposuction of lower extremities: differences in lymph vessel injury. Plast Reconstr Surg. 2004;113:718; discussion 725–726. [DOI] [PubMed] [Google Scholar]
- 50.Greene AK, Voss SD, Maclellan RA. Liposuction for swelling in patients with lymphedema. N Engl J Med. 2017;377:1788. [DOI] [PubMed] [Google Scholar]
- 51.Lee D, Piller N, Hoffner M, et al. Liposuction of postmastectomy arm lymphedema decreases the incidence of erysipelas. Lymphology. 2016;49:85. [PubMed] [Google Scholar]


