Abstract
Background:
Several epidemiological studies had been carried out in different population cohorts to estimate the relationship between the shortened telomere length and stroke. However, the results still remained dispute. Consequently, we conducted this meta-analysis to estimate the relationship between them.
Methods:
PubMed, EMBASE, and Web of Science were systematically searched for related articles to evaluate the association between “stroke” and “telomere length. STATA 12.0 software was used to perform the meta-analysis. The Cochran Q test and inconsistency index (I2) were used to assess the heterogeneity. Begg funnel plot and Egger test were used to assess publication bias.
Results:
The meta-analysis was composed of 11 studies, consisting of 25,340 participants. We found a significant relationship between shortened telomere length and stroke (OR: 1.50, 95% CI: 1.13–2.0; P = .005); however, in the prospective and retrospective study subgroup, we did not find a statistical significant relationship between shortened telomere length and stroke (the prospective subgroup: OR: 1.41, 95% CI: 1–1.98; P = .051) (the retrospective subgroup: OR: 1.89, 95% CI: 0.96–3.72; P = .067).
Keywords: a meta-analysis, stroke, telomere length
1. Introduction
Stroke is a global health problem, consisting the major reason of disability and death all over the world. It is well known that the older age, sex, and genetics are risk factors of stroke[1] however; according to Global Burden of Disease (GDB) 2013 STUDY, the burden of global stroke continues to increase globally.[2] Consequently, it is essential to find out other important genetic factors related to the mechanisms of the disease.
Telomeres are the DNA–protein structures located at the end of chromosomes[3]; in regularly, the telomere length of normal somatic cells is gradually shortened with the cell division. Several studies had proved that telomere length may be a biomarker for biological age and age-related diseases,[4–6] including some cancers, diabetes, chronic kidney diseases, Alzheimer's disease, hypertension, cardiovascular diseases.[7–13] Besides, study has showed that short telomere length is also closely related with early-onset and delayed carotid atherosclerosis.[14] Telomeres also has close relationship with the risk factors of cardiovascular diseases, such as smoking, age, sex, mental stress, excessive alcohol intake, and so on.[15] Stroke also has a close relationship with age, cardiovascular diseases, and carotid atherosclerosis; consequently, it may be associated with TL. Besides, previous studies had revealed that inflammation factors play an important role in stroke pathogenesis, and inflammation exerts a strong influence on immune aging and is closely correlated with telomere length.[16–18] To further access the relationship between telomere length and stroke, several studies had been conducted in different population cohorts, including African Americans, Chinese, and Europeans.[3,7,19] However, the result still remained dispute, some studies reported that shortened length of telomere was an independent risk biomarker for stroke,[7,20–22] whereas the other studies did not get the same conclusion.[21,23] Consequently, we conducted the meta-analysis to give a more accurate estimate of the relationship between telomere length and stroke.
2. Methods
2.1. Ethics statement
As all analyses were based on previously published studies, no ethical approval or patient consent was required.
2.2. Literature search
Pubmed, EMBASE, and Web of Science were systematically searched for related articles published before January 2018 to evaluate the association between “stroke” and “telomere length”. The following MeSH terms were used: (stroke or cerebrovascular accident cerebrovascular or Accidents or cerebrovascular accident, cerebrovascular accident) and (telomere or telomere length). Only articles published in English were considered to be included. Besides, to find the potential literatures, hand searching was also performed. Our study was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).[24]
2.3. Selection criteria and exclusion criteria
Studies which met the following criteria were considered to be included: study evaluated the association between short TL and stroke with a prospective or retrospective design. The study provided odds ratios (OR), relative risks (RRs), or hazard ratios (HRs) with 95% confidence intervals (CIs) for shorter (or shortest) TL, or the studies provided sufficient information to estimate the above effect sizes. Besides, there were no restrictions on the type of stroke, the measurement of TL and DNA source. Studies published in English.
2.4. Exclusion criteria
The exclusion criteria were as follows: studies did not provide the necessary data information, such as ORs, RRs, or HRs with 95% CIs for shorter (or shortest) TL; studies such as case reports, review articles, commentaries, and editorials. Studies did not estimate the relationship between the short TL and stroke.
2.5. Data extraction
The following information were extracted from the included studies: the name of the first author, the publication year, the country, the number of the patients with stroke and the participants in the studies, study design, the method of measuring telomere length, the average age, the stroke type, unadjusted odds ratios (ORs) of shorter telomeres compared with those in the longer and the 95% CI of the ORs. Data were extracted by 2 reviewers (XJ and XJD) independently from the included studies, with regard to the conflicts or different opinions during the process of data extraction, the consensus was resolved by a third reviewer (DPX and HLW).
2.6. Statistical analysis
STATA 12.0 software (StataCorp, College Station, TX) was used to perform the meta-analysis. The unadjusted ORs or HRs for individuals in the 50th percentile of shorter telomeres compared with those in the longer telomeres and their 95% CI of the studies were selected in the analysis. The Cochran Q test and inconsistency index (I2) were used to assess the heterogeneity. The percentages of I2 around 25%, 50%, and 75% indicate low, medium, and high heterogeneity, respectively.[25] In this meta-analysis, the Begg funnel plot and Egger test were also used to assess publication bias. If the P < .05, it indicates the possibility of publication bias, and vice versa. Sensitivity analysis was also conducted to investigate the effect of each study on the overall result.
3. Results
3.1. Literature search
On the initial search, 492 studies were identified by the MeSH terms mentioned in the search strategy through the literature search (Fig. 1). After removing the duplicates, 107 articles were left. Abstracts or articles were screened for eligibility and 26 articles remained. By carefully reviewing these articles, we found that 9 articles did not report the data of the relationship between short telomeres and stroke. Besides, telomere length in 6 articles was not used as a category variable was also excluded. At last, 11 articles which met the inclusion criterion were included in this meta-analysis.
Figure 1.
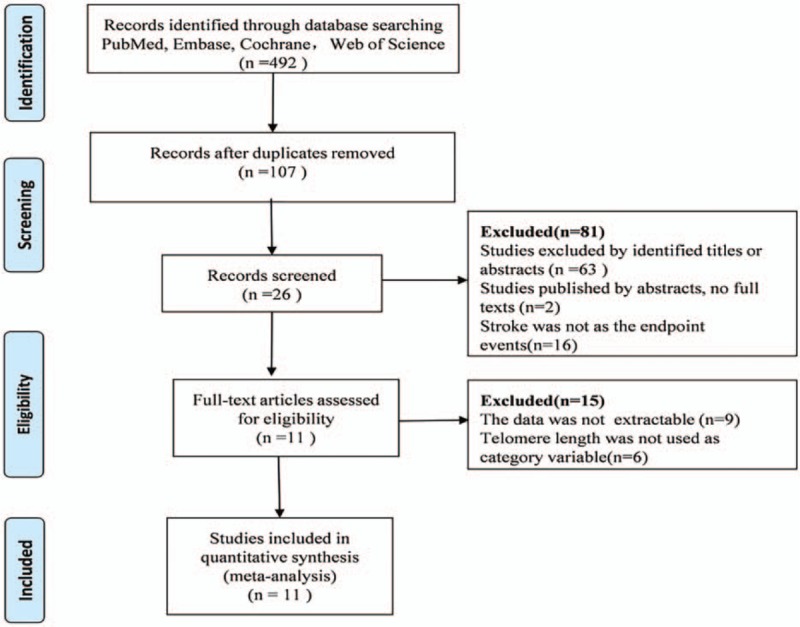
Flow chart for the process of selecting eligible publications.
3.2. Characteristics of the selected studies
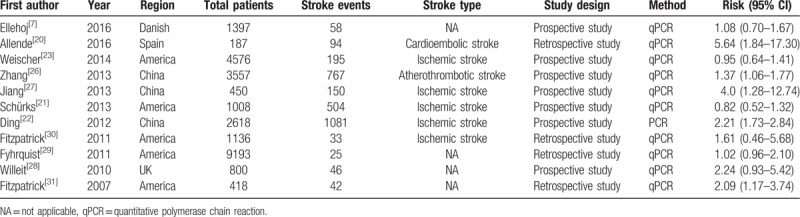
The final analysis included 11 studies, consisting of 25,340 participants. Among the included studies, 7[7,21–23,26–28] were prospective studies, 4[20,29–31] were retrospective studies. Five[21,23,29–31] studies were carried out in America and 3[22,26,27] in China, the other 3[7,20,28] studies were implemented in the United Kingdom, Spain, and Danish, respectively. Five[21–23,27,30] studies reported the ischemic stroke as the endpoint event; the other 2[20,26] studies reported the atherothrombotic stroke and cardioembolic as the endpoint event respectively, whereas the remained 4[7,28,29,31] studies did not report the stroke type (Table 1).
Table 1.
Characteristics of the included studies.
3.3. The main outcomes and subgroup analysis
The raw data were extracted from 11 included studies, when the eligible studies were pooled into the meta-analysis, we found a significant relationship between shortened telomere length and stroke (OR: 1.50, 95% CI: 1.13–2.0; P = .005), subgroup analysis was performed among the included studies by study design, however, in the prospective and retrospective study subgroup, we did not find a statistically significant association between shortened telomere length and stroke (the prospective subgroup: OR: 1.41, 95% CI: 1–1.98; P = .051); (the retrospective subgroup: OR: 1.89, 95% CI: 0.96–3.72; P = .067) (Fig. 2).
Figure 2.
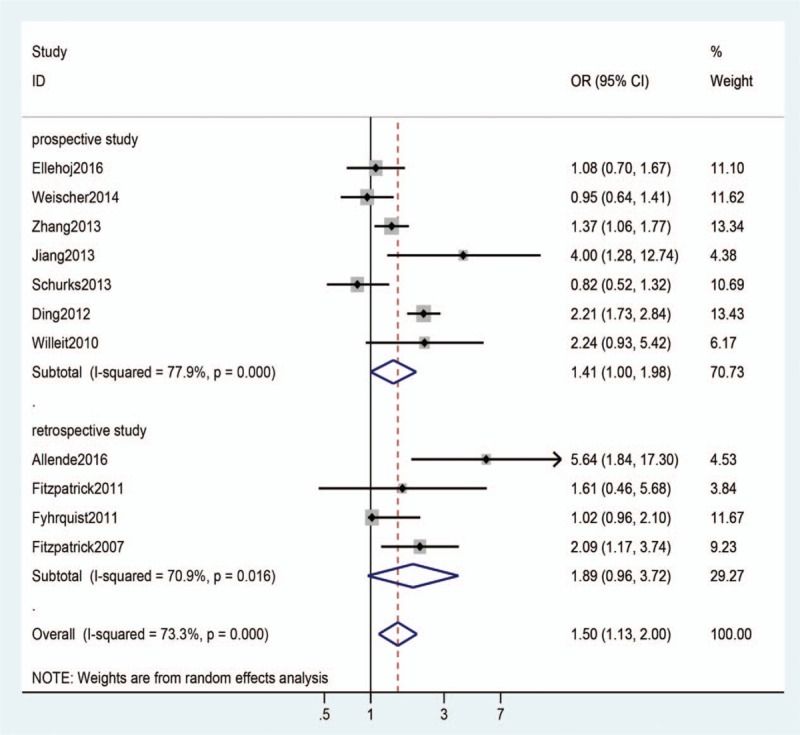
Meta-analysis of the association between telomere length and stroke.
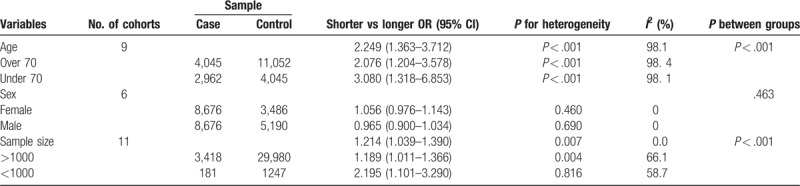
Besides, subgroup analysis was also performed among the included studies by age, sex, sample size (Table 2). From the subgroup analysis, we can conclude that the pooled ORs were increased in several subgroups. The pooled OR of younger participants was higher than the pooled OR for older participants, and was statistically significant (OR: 2.249, 95% CI: 1.363–3.712, P < .001). Besides, studies with the sample size under 1000 have a statistical significance increased risk compared with smaller sample size (OR: 1.214, 95% CI: 1.039–1.390, P < .001). However, in the sex subgroup, we could not find a statistical significance between female and male (Table 2).
Table 2.
Subgroup analysis of the meta-analysis.
3.4. Heterogeneity and sensitivity analysis
A fixed effects meta-analytic estimate was calculated first, however, the overall I2-statistic was 74% (P = .000), which meant the heterogeneity was relatively high; consequently, the random-effect model was conducted in the meta-analysis. According to the sensitivity analysis (exclusion one of the included studies at a time), we did not find any single study can change the pooled ORs qualitatively; consequently, we can conclude that the results of the meta-analysis was stable (Fig. 3).
Figure 3.
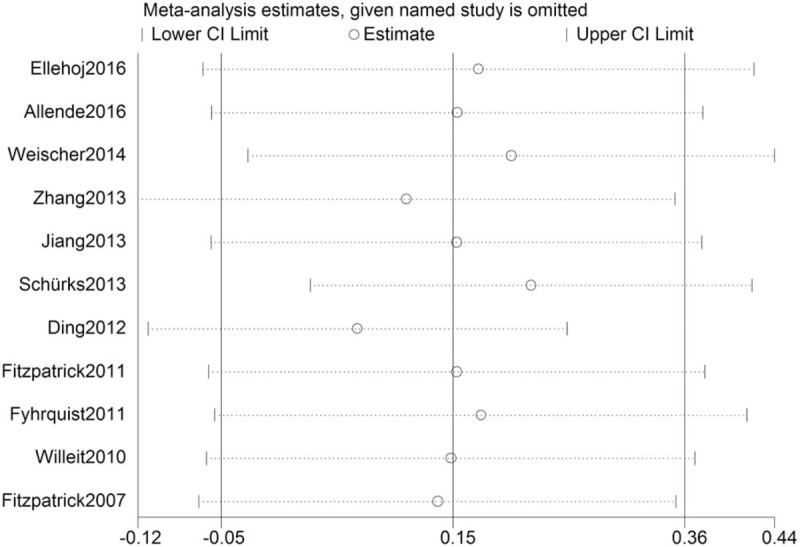
Sensitivity analyses of selected studies.
3.5. Publication bias
The potential publication bias was estimated by Begg funnel plot with pseudo 95% CIs, according to the Begg test, the result suggests no obvious publication bias (Pr > |z| = 0.697, P > .05) (Fig. 4).
Figure 4.
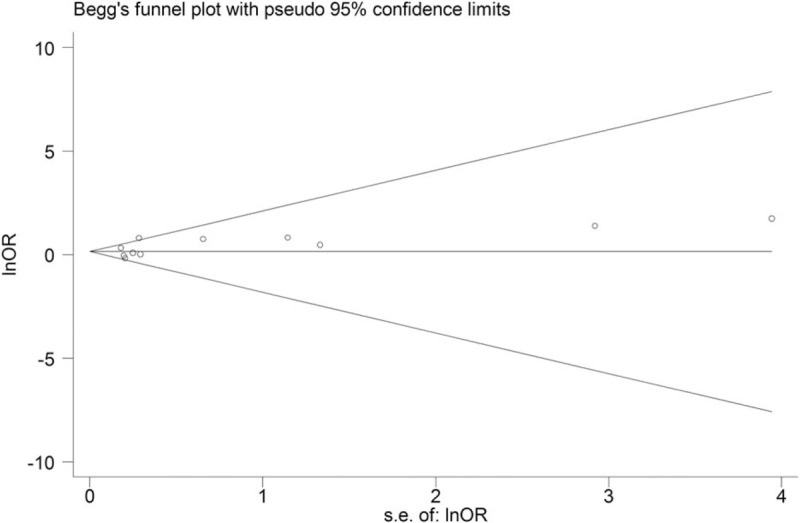
Begg funnel plot with pseudo 95% CIs.
4. Discussion
In this meta-analysis, 11 cohort studies consisting of 25,340 participants were used to evaluate the relationship between shortened telomere length and the risk of stroke. According to the result, the shortened telomere length is significantly related with stroke.
Previous studies have proved that oxidative stress is an early response to cerebral ischemia and is likely to play an important role in the pathogenesis of cerebral ischemic injury and neuronal damage.[32,33] Besides, oxidative stress is also regarded as the important contributor to pathophysiologic sequelae of stroke and is progressive after stroke.[34] Antioxidants have also been treated as the treatment of stroke.[35,36] In brief, oxidative stress level is increased in patients with stroke and plays an important role in the mechanism of stroke. Although the role of oxidative stress in telomere attrition in vivo is still unclear, the association between telomere attrition and stroke has been proved by experimental and clinical studies.[37,38] The increased concentrations of iron in the body can cause oxidative stress and consequently can lead to shortening of telomeres. Besides, the site-specific DNA damage at the GGG sequence by oxidative stress may play an important role in increasing the rate of telomere shortening with aging. Consequently, the shortening of telomere length in stroke patients may be caused by elevated oxidative stress; however, the mechanisms still need to be further explored.
Studies have proved that telomere length acts as an independent predictor of atherosclerosis, which is an early marker of vascular burden.[39] Other studies have suggested that there is a close relationship between carotid atherosclerosis and the occurrence of progressive ischemic stroke.[40] Besides, telomere length plays an important role in different stages of atherosclerosis, which is likely to cause stroke.[28] Consequently, the relationship between telomere length shortening and stroke patients may also be induced by atherosclerosis. Besides, the mutations in genes can lead to telomerase failure and telomere shortening, some recessive inherited disease can involve multiple systems, such as the Anderson–Fabry disease can impair the central nervous system, the manifestation is cerebral infarction and transient ischemic attack, which is closely related with stroke.[41–44]
The overall results of this meta-analysis showed a significant relationship between shortened telomere length and stroke, whereas the results of the subgroups did not show the same conclusion. However, we should still focus on the result of the overall result. Relevant study indicated that debates about subgroup effects may be framed as absolute acceptance or rejection, yes or no with nothing in between, which is undesirable and destructive.[45] Besides, other studies revealed that subgroup analysis and interaction analysis are exploratory in most cases, consequently, the results of subgroup analysis should be treated objectively, and the focus should always be on the overall effects instead the results of subgroup analysis and subgroup effects.[46,47] In this meta-analysis, we performed the subgroup analyses by the type of study instead of the intervention, and the subgroup analyses were conducted for purpose of exploring the publication bias. That the outcomes of prospective and retrospective subgroups were different from the overall result may be caused by the number of the studies or other factors. Many studies have reported that there exists difference of telomere length between sex; however, we did not find the same result in the subgroup meta-analysis, because of insufficient information, we did not make further analysis.[48] Besides, there were different stroke subtypes in the included studies, because of insufficient information, we also did not make the subgroup analysis.
Several limitations of the current meta-analysis should be noted. First, publication bias may exist in this meta-analysis, studies included in this meta-analysis were selected, whereas the studies reported the negative were less likely to be published. Besides, this study only estimate the studies published in English, which can also lead to publication bias. Thirdly, although the quantitative PCR based method were used to measure the telomere length in the included studies, the telomere length was reported in different ways, including T/S ratio and KB, which can also induce less accurate conclusions.
In conclusion, we can conclude that shortened telomere length is related to the risk of stroke. In this meta-analysis, however, because there are still some potential confounders including the stroke subtypes, sex, we did not make analysis. Consequently, further studies with larger sample size and more detailed information are needed to clarify whether telomere shortening is a cause or a consequence of stroke.
Author contributions
Conceptualization: xiao Jin, Xiaojing Dang.
Data curation: xiao Jin, Biqi Pan.
Formal analysis: Xiaojing Dang.
Funding acquisition: Xiaojing Dang, Danping Xu.
Writing—original draft: Huanlin Wu, Danping Xu.
Xiao Jin orcid: 0000-0002-4676-1062
Footnotes
Abbreviations: GDB = Global Burden of Disease, TL = telomere length.
HW and DX equally contributed to this work.
This project was funded by the National Natural Science Foundation Project (General Program, No. 81774219), Guangzhou Science and Technology Plan project (No. 201710010107), Guangdong Provincial Science and Technology Plan (No. 2016A020226011), Joint Innovation Specific Project in Key Areas from Guangdong Branch Institute of China Academy of Chinese Medical Sciences (No. ZZ0908065), Guangdong Medical Science and Technology Research Foundation Project (No. A2017215).
The authors have no conflicts of interest to disclose.
Authorship: Conceived of and designed the experiments: XJ and DPX. Performed the experiments: XJ, XJD, and DPX. Analyzed the data: XJD and XJ. Contributed reagents/materials/analysis tools: JX, DPX, and HLW. Wrote the paper: XJ and BQP. Revised the paper: HLW.
References
- [1].Gueniat J, Breniere C, Graber M, et al. Increasing burden of stroke: the Dijon Stroke Registry (1987–2012). Neuroepidemiology 2018;50:47–56. [DOI] [PubMed] [Google Scholar]
- [2].Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 Study. Neuroepidemiology 2015;45:161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mwasongwe S, Gao Y, Griswold M, et al. Leukocyte telomere length and cardiovascular disease in African Americans: the Jackson Heart Study. Atherosclerosis 2017;266:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zgheib NK, Sleiman F, Nasreddine L, et al. short telomere length is associated with aging, central obesity, poor sleep and hypertension in Lebanese individuals. Aging Dis 2018;9:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao Y, Li S, Liu H. Estimating the survival advantage based on telomere length and serum biomarkers of aging. J Transl Med 2017;15:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang WG, Jia LP, Ma J, et al. Peripheral blood leukocyte telomere length is associated with age but not renal function: a cross-sectional follow-up study. J Nutr Health Aging 2018;22:276–81. [DOI] [PubMed] [Google Scholar]
- [7].Ellehoj H, Bendix L, Osler M. Leucocyte telomere length and risk of cardiovascular disease in a cohort of 1,397 Danish men and women. Cardiology 2016;133:173–7. [DOI] [PubMed] [Google Scholar]
- [8].Zhang C, Chen X, Li L, et al. The association between telomere length and cancer prognosis: evidence from a meta-analysis. PLoS One 2015;10:e0133174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Testa R, Olivieri F, Sirolla C, et al. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabetic Med 2011;28:1388–94. [DOI] [PubMed] [Google Scholar]
- [10].Tellechea ML, Pirola CJ. The impact of hypertension on leukocyte telomere length: a systematic review and meta-analysis of human studies. J Hum Hypertens 2017;31:99–105. [DOI] [PubMed] [Google Scholar]
- [11].Karimi B, Yunesian M, Nabizadeh R, et al. Is leukocyte telomere length related with lung cancer risk? A meta-analysis. Iran Biomed J 2017;21:142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ameh OI, Okpechi IG, Dandara C, et al. Association between telomere length, chronic kidney disease, and renal traits: a systematic review. OMICS 2017;21:143–55. [DOI] [PubMed] [Google Scholar]
- [13].Forero DA, Gonzalez-Giraldo Y, Lopez-Quintero C, et al. Meta-analysis of telomere length in Alzheimer's disease. J Gerontol A Biol Sci Med Sci 2016;71:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Toupance S, Labat C, Temmar M, et al. Short telomeres, but not telomere attrition rates, are associated with carotid atherosclerosis. Hypertension 2017;70:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fyhrquist F, Saijonmaa O. Telomere length and cardiovascular aging. Ann Med 2012;44Suppl.:S138–42. [DOI] [PubMed] [Google Scholar]
- [16].Idicula TT, Brogger J, Naess H, et al. Admission C-reactive protein after acute ischemic stroke is associated with stroke severity and mortality: the ’Bergen stroke study’. BMC Neurol 2009;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kwan J, Horsfield G, Bryant T, et al. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp Gerontol 2013;48:960–5. [DOI] [PubMed] [Google Scholar]
- [18].Jose SS, Bendickova K, Kepak T, et al. Chronic inflammation in immune aging: role of pattern recognition receptor crosstalk with the telomere complex? Front Immunol 2017;8:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang S, Ji G, Liang Y, et al. Polymorphisms in telomere length associated TERC and TERT predispose for ischemic stroke in a Chinese Han population. Sci Rep 2017;7:40151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Allende M, Molina E, González-Porras JR, et al. Short leukocyte telomere length is associated with cardioembolic stroke risk in patients with atrial fibrillation. Stroke 2016;47:863–5. [DOI] [PubMed] [Google Scholar]
- [21].Schürks M, Prescott J, Dushkes R, et al. Telomere length and ischaemic stroke in women: a nested case-control study. Eur J Neurol 2013;20:1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ding H, Chen C, Shaffer JR, et al. Telomere length and risk of stroke in Chinese. Stroke 2012;43:658–63. [DOI] [PubMed] [Google Scholar]
- [23].Weischer MBSE, Nordestgaard BG. Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet 2014;10:e1004191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91–2. [DOI] [PubMed] [Google Scholar]
- [25].Higgins JPTS, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang W, Chen Y, Wang Y, et al. Short telomere length in blood leucocytes contributes to the presence of atherothrombotic stroke and haemorrhagic stroke and risk of post-stroke death. Clin Sci (Lond) 2013;125:27–43. [DOI] [PubMed] [Google Scholar]
- [27].Jiang X, Dong M, Cheng J, et al. Decreased leukocyte telomere length (LTL) is associated with stroke but unlikely to be causative. PLoS One 2013;8:e68254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Willeit P, Willeit J, Brandstatter A, et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol 2010;30:1649–56. [DOI] [PubMed] [Google Scholar]
- [29].Fyhrquist F, Silventoinen K, Saijonmaa O, et al. Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. J Hum Hypertens 2011;25:711–8. [DOI] [PubMed] [Google Scholar]
- [30].Fitzpatrick AL, Kronmal RA, Kimura M, et al. Leukocyte telomere length and mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci 2011;66A:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 2007;165:14–21. [DOI] [PubMed] [Google Scholar]
- [32].Radak D, Resanovic I, Isenovic ER. Link between oxidative stress and acute brain ischemia. Angiology 2014;65:667–76. [DOI] [PubMed] [Google Scholar]
- [33].Lorenzano S, Rost NS, Khan M, et al. Oxidative stress biomarkers of brain damage: hyperacute plasma F2-isoprostane predicts infarct growth in stroke. Stroke 2018;49:630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tsai NW, Chang YT, Huang CR, et al. Association between oxidative stress and outcome in different subtypes of acute ischemic stroke. Biomed Res Int 2014;2014:256879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Slemmer JE, Shacka JJ, Sweeney MI, et al. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem 2008;15:404–14. [DOI] [PubMed] [Google Scholar]
- [36].Bahonar A, Saadatnia M, Khorvash F, et al. Carotenoids as potential antioxidant agents in stroke prevention: a systematic review. Int J Prev Med 2017;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zglinicki TV. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci 2000;908:99–110. [DOI] [PubMed] [Google Scholar]
- [38].Jelle J, Boonekamp CB. Ellis Mulder and Simon Verhulst. Does oxidative stress shorten telomeres? R Soc 2017;13:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Spigoni V, Aldigeri R, Picconi A, et al. Telomere length is independently associated with subclinical atherosclerosis in subjects with type 2 diabetes: a cross-sectional study. Acta Diabetol 2016;53:661–7. [DOI] [PubMed] [Google Scholar]
- [40].Tei H, Uchiyama S, Ohara K, et al. Deteriorating ischemic stroke in 4 clinical categories classified by the Oxfordshire Community Stroke Project. Stroke 2000;31:2049–54. [DOI] [PubMed] [Google Scholar]
- [41].Feldt-Rasmussen U. Fabry disease and early stroke. Stroke Res Treat 2011;2011:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tuttolomondo A, Pecoraro R, Simonetta I, et al. Neurological complications of Anderson-Fabry disease. Curr Pharm Des 2013;19:6014–30. [DOI] [PubMed] [Google Scholar]
- [43].Tuttolomondo A, Pecoraro R, Simonetta I, et al. Anderson-Fabry disease: a multiorgan disease. Curr Pharm Des 2013;19:5974–96. [DOI] [PubMed] [Google Scholar]
- [44].Rigoldi M, Concolino D, Morrone A, et al. Intrafamilial phenotypic variability in four families with Anderson-Fabry disease. Clin Genet 2014;86:258–63. [DOI] [PubMed] [Google Scholar]
- [45].Sun X, Ioannidis JP, Agoritsas T, et al. How to use a subgroup analysis: users’ guide to the medical literature. JAMA 2014;311:405–11. [DOI] [PubMed] [Google Scholar]
- [46].Brookes ST, Whitley E, Peters TJ, et al. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5:1–56. [DOI] [PubMed] [Google Scholar]
- [47].Guillemin F. Primer: the fallacy of subgroup analysis. Nat Clin Pract Rheumatol 2007;3:407–13. [DOI] [PubMed] [Google Scholar]
- [48].Harte AL, da Silva NF, Miller MA, et al. Telomere length attrition, a marker of biological senescence, is inversely correlated with triglycerides and cholesterol in South Asian males with type 2 diabetes mellitus. Exp Diabetes Res 2012;2012:895185. [DOI] [PMC free article] [PubMed] [Google Scholar]


