Abstract
Rationale:
Immunoglobulin G4-related disease (IgG4-RD) is an inflammatory condition of unknown cause. Cancer might be related to the development of certain IgG4-RD but to date, little literature documents it.
Patient concerns:
A 78-year old man presented with unilateral proptosis responsive to steroids, initially attributed to nonspecific orbital inflammation.
Diagnosis:
Right hemicolectomy was performed because of a suspicious lesion which turned out to be tubulovillous adenoma on histological analysis. Eight months after the surgery, a mass infiltrating the mesentery was found and biopsy revealed IgG4-RD.
Interventions:
Both the orbital inflammation and abdominal mass infiltrating the mesentery were responsive to steroids and rituximab administered to treat IgG4-RD.
Outcomes:
In the course of IgG4-RD, the patient developed bilateral optic perineuritis, causing bilateral visual loss. Colon cancer with synchronous multiple liver metastases was found 1 year after rituximab treatment.
Lessons:
This case raises the possibility of IgG4-RD being a paraneoplastic syndrome in some patients. Cancer screening should probably be performed in some elderly patients diagnosed with IgG4-RD.
Keywords: colon cancer, IgG4-related disease, optic perineuritis, orbital inflammation, paraneoplastic syndrome
1. Introduction
Immunoglobulin G4-related disease (IgG4-RD), an inflammatory condition initially described in 2001 as autoimmune pancreatitis,[1] is characterized by tumor-like lesions in various organ systems with abundant lymphoplasmocytic and IgG4-positive plasma cell tissue infiltration. The orbit is involved in 23% of patients with IgG4-RD:[2] lacrimal gland is the most commonly involved site, followed by orbital nerves (usually the infraorbital nerve), extraocular muscles and orbital fat,[3] while optic neuropathy is rare. The etiology of IgG4-RD remains obscure, but history of malignancy in a subset of patients suggests that cancer might have a role in IgG4-RD development.[4] On the other hand, malignancies (especially lymphoma) could also occur in IgG4-RD as complications of chronic inflammation.[5,6]
Herein, we present an unusual case of IgG4-RD with bilateral orbital involvement in a patient who subsequently developed colon cancer and optic perineuritis.
2. Case report
A 78-year old Caucasian man with history of chronic obstructive pulmonary disease presented with a subacute progressive right proptosis. Previous ophthalmic history included bilateral glaucoma on treatment with monoprost. Visual acuity was 20/20 in the right eye (RE) and 20/40 in the left eye (LE). Eye fundus examination showed bilateral optic nerve cupping and visual field testing found superior arcuate scotoma in the RE (Fig. 1) Computed tomography (CT) scans revealed inflammatory infiltration of the right orbital fat, the lateral and medial rectus muscles, as well as pansinusitis (Fig. 2). Antineutrophil cytoplasmic bodies (ANCA) serology was negative and serum angiotensin-converting enzyme level and thyroid function tests were normal making granulomatosis and thyroid eye disease unlikely. Initial diagnosis was bilateral nonspecific orbital inflammation associated to pansinusitis treated with oral steroids (1 mg per kg body weight daily for 15 days followed by tapering), which led to a decrease in the right exophthalmos.
Figure 1.
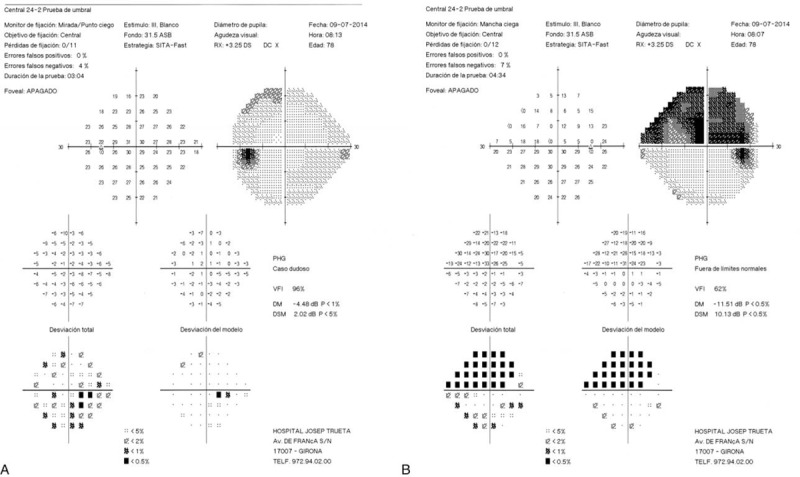
24–2 Humphrey visual field test. (A) Left visual field test is unremarkable. (B) Right visual field test showing right superior arcuate scotoma.
Figure 2.
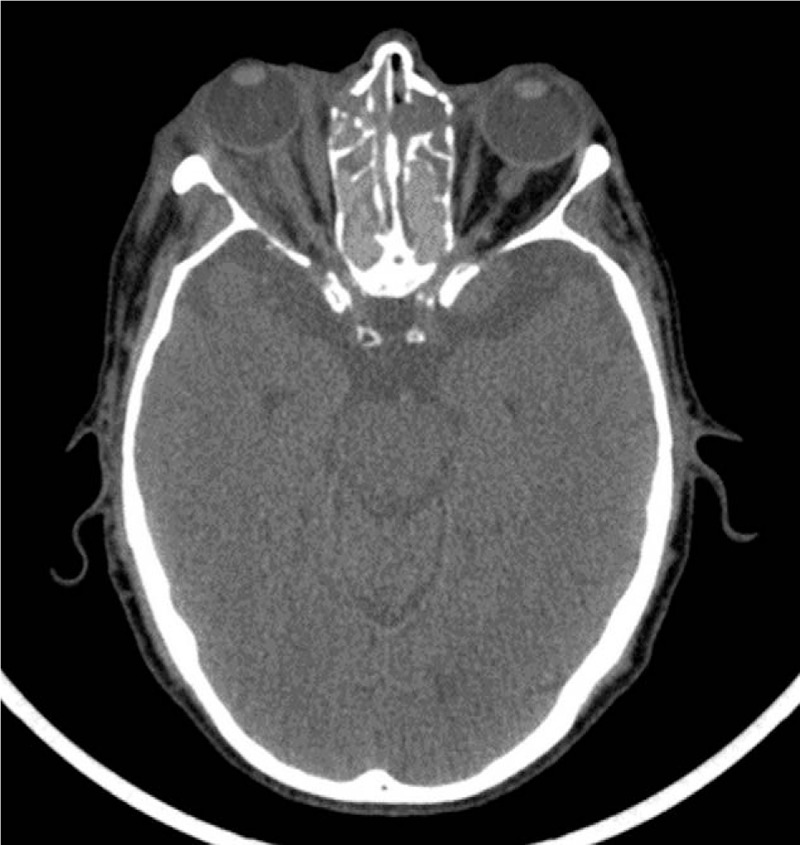
Axial CT scan showing right proptosis. There is an inflammatory infiltration of the right orbital fat as well as an enlargement of the lateral and medial right rectus muscles. CT = computed tomography.
Three months after the initial presentation, right orbital inflammation recurred with increase in the right proptosis and the patient was referred to the oculoplastics department. Magnetic resonance imaging (MRI) of the right orbit showed proptosis associated to muscle and orbital fat inflammatory infiltration (Fig. 3A and B). Proptosis regressed after a new course of oral corticosteroids at the same dose as before.
Figure 3.
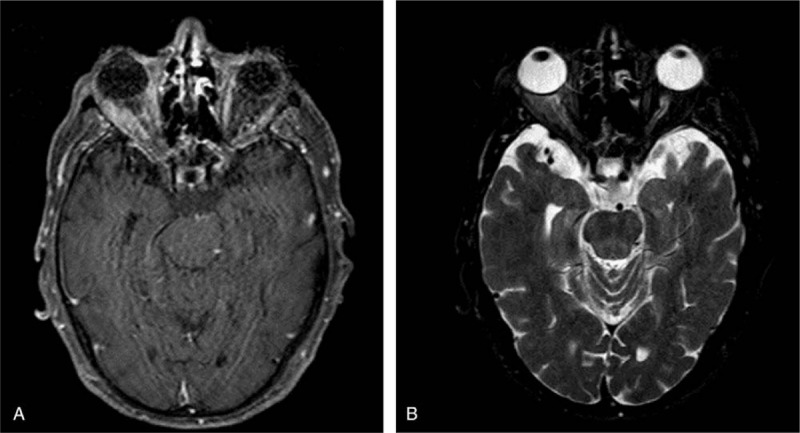
(A-B) Axial MRI scan showing right proptosis as well as extraocular muscle and orbital fat inflammatory infiltration.
Two months later, the patient presented with rectal bleeding and colonoscopy found a suspicious sessile lesion measuring 4 cm in diameter which was not resecable endoscopically. Right hemicolectomy was therefore performed and histological analysis showed complete excision of a tubulovillous adenoma. A K-RAS activating mutation was found on the tumor (mutation in exon 2, codon 12, G12D); no mutation was found on the NRAS gene. Eight months after surgery, follow-up CT imaging showed an abdominal mass measuring 17 × 10 cm and infiltrating the mesentery, the mesocolon, the transverse colon, the duodenal frame, the mesenteric vein and the surrounding fat. Histological analysis of multiple biopsies from the lesion and lymph nodes revealed a lymphoplasmocytic and IgG4-positive plasma cell tissue infiltration; storiform fibrosis and obliterative phlebitis were present in the mass and IgG4/IgG ratio was 42.2% (Fig. 4A–E). Serum IgG4 was within normal limits (71 mg/dL, normal values 9–104 mg/dL) with an IgG4/IgG ratio of 7.4%. Oral corticotherapy at the initial dose of 60 mg daily was reinitiated and maintained at the dose of 5 mg daily after tapering. The abdominal infiltration progressively decreased under treatment over a 4-month period, reaching dimensions of 9 × 5 cm (Fig. 5). Right exophthalmos further decreased under corticosteroid treatment. In this context, the bilateral orbital adnexal condition was considered to be IgG4-related ophthalmic disease (IgG4-ROD). However, visual acuity that had progressively altered over the course of the 5 months prior to the diagnosis of IgG4-ROD (initially attributed to evolutionary corticosteroid-induced glaucoma despite normal intraocular pressure with antiglaucoma drugs) continued to alter after corticotherapy. During corticotherapy, visual acuity went down to 20/50 in the RE and counting fingers in the LE and visual field presented significant alterations both eyes (Fig. 6). Orbital MRI revealed bilateral optic perineuritis (Fig. 7); visual evoked potentials (VEP) showed no response in the LE and normal amplitude with delayed P100 latency in the RE (Fig. 8).
Figure 4.
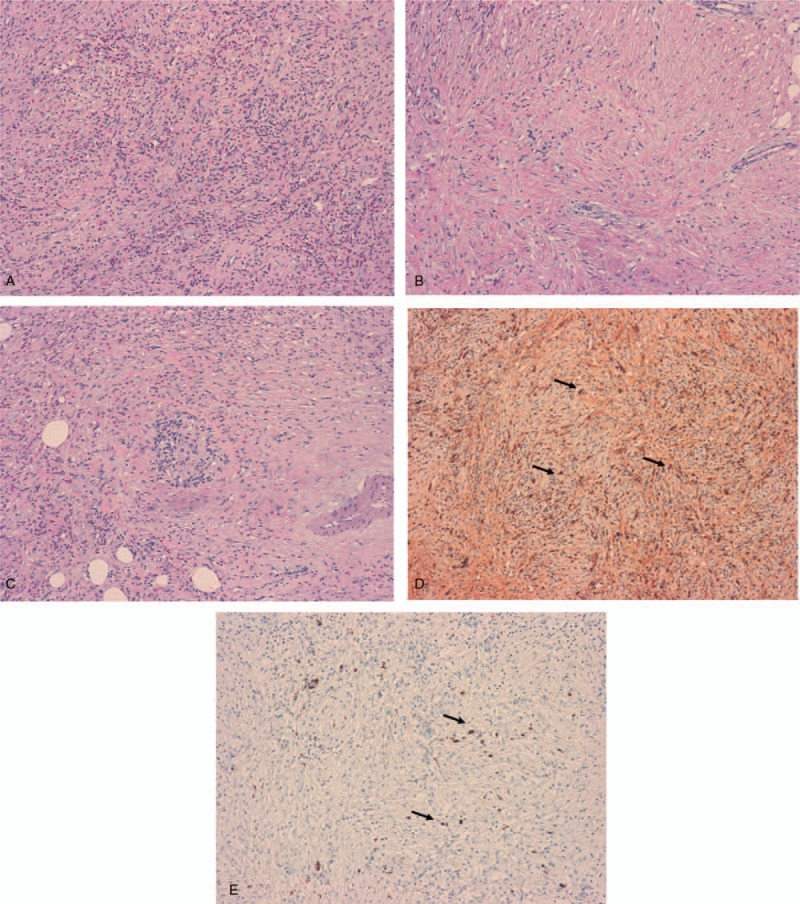
Histological and immunochemical analysis of the biopsies from the abdominal mass. (A) Severe inflammatory infiltration of the mesentery made of eosinophils, lymphocytes and plasma cells (hematoxylin eosin staining, H&E, ×10 magnification). (B) Histological section (H&E, × 10 magnification) showing a storiform pattern of fibrosis. (C) The vein in the center of the image is obliterated by the infiltration of inflammatory cells (obliterative phlebitis). (D) IgG-immunostaining (×10 magnification) showing IgG positive plasma cells (arrows). (E) IgG4-immunostaining (×10 magnification) showing IgG4 positive plasma cells (arrows).
Figure 5.
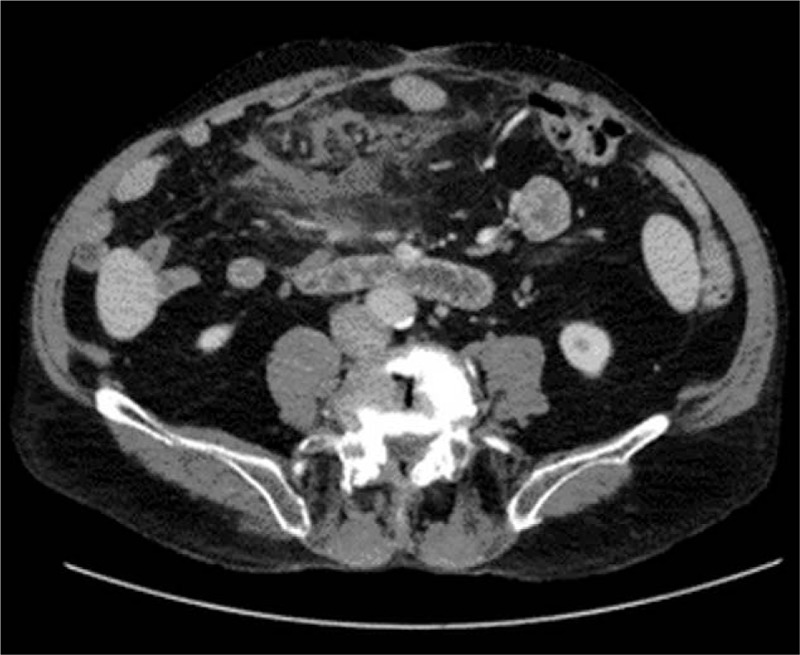
Axial abdominal CT scan showing a mesenteric mass with poorly defined limits, measuring approximately 9 × 5 cm. CT = computed tomography.
Figure 6.
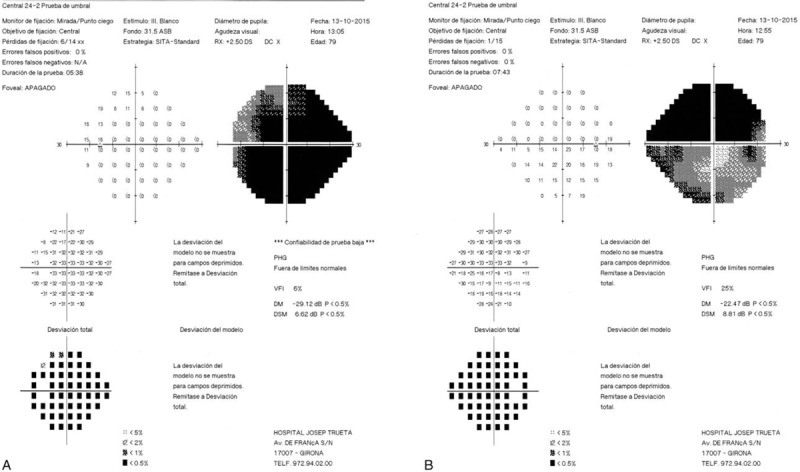
(A-B) 24–2 Humphrey visual field test showing significant alterations in both eyes, compared to the initial examination in Fig. 1.
Figure 7.
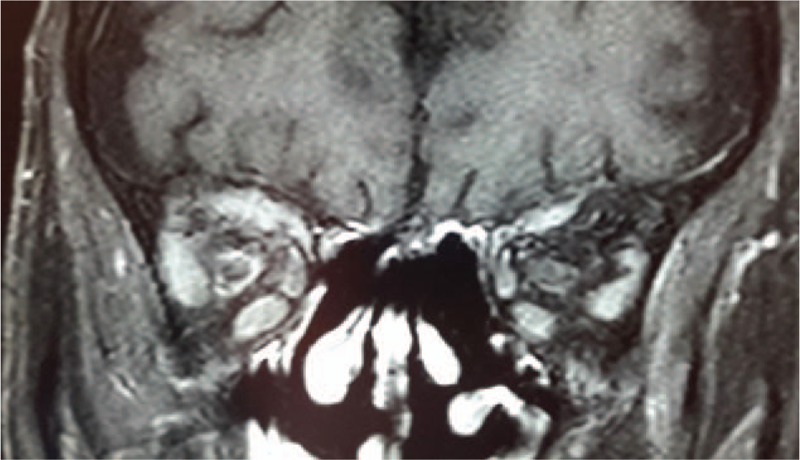
Coronal MRI scan showing bilateral optic perineuritis visible as a peripheral enhancement of both optic nerves (enhancement is more important on the right optic nerve). MRI = magnetic resonance imaging.
Figure 8.
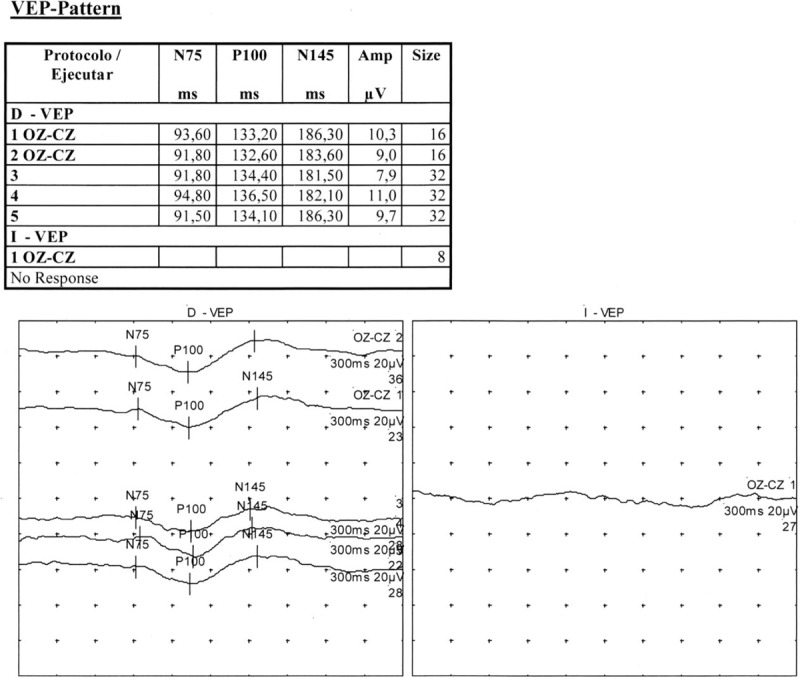
Visual evoked potentials (VEP) showing no response in the left eye and normal amplitude with delayed p100 latency in the right eye. VEP= visual evoked potentials.
Therefore, bilateral optic perineuritis and the associated visual loss as well as the incomplete response of the abdominal mass to first-line corticosteroid treatment justified a second line treatment with rituximab (1000 mg administered twice, 2 weeks apart). Three months after the end of rituximab treatment, visual function remained stable, orbital MRI showed an almost complete disappearance of muscle and fat infiltration with no proptosis, CT scans revealed a marked decrease in the size of the abdominal infiltration (Fig. 9) and serum IgG4 levels were low (6 mg/dL), with a IgG4/IgG ratio in the serum of 0.7% and no peripheral CD19+ plasmablasts, suggesting a good IgG4-RD control.
Figure 9.
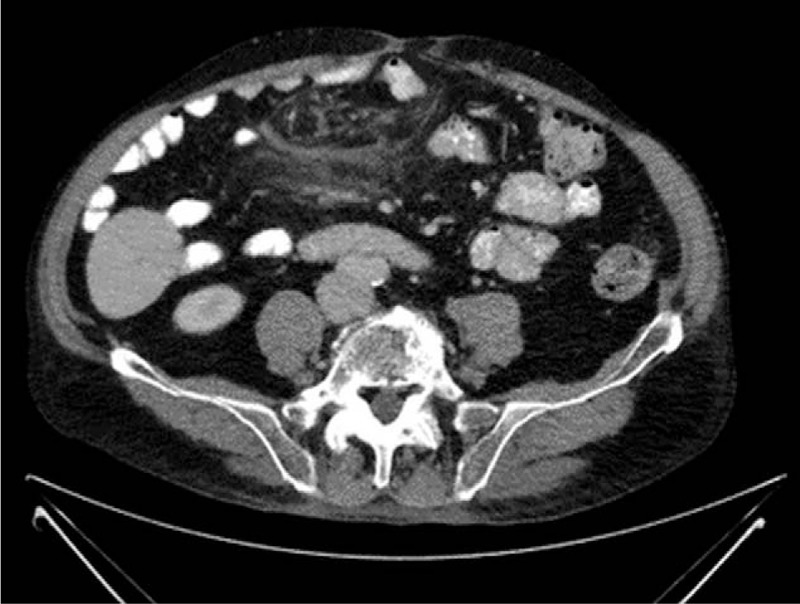
Axial abdominal CT scan showing a marked decrease in the size of the abdominal mass in Fig. 5. CT = computed tomography.
One year after rituximab treatment (and 2 years after the surgery for tubulovillous adenoma), CT scans revealed thickening of the ileo-caecal valve and multiple hepatic lesions of metastatic origin, as revealed by biopsy. Stage IV colonic adenocarcinoma was diagnosed and the patient subsequently died one year later.
Ethics committee approval was not necessary for the publication of this case report. Patient consent was unavailable because he was deceased at the time this case report was written. Informed consent was obtained from the patient's family for publication of this case report details.
3. Discussion
3.1. Diagnosis of IgG4-RD
IgG4-RD induces either concurrent or metachronous tumor-like lesions in single or multiple organs. Histology is the gold standard for the diagnosis,[7,8] in addition to compatible organ involvement. Elevated serum IgG4 levels are also characteristic of IgG4-RD,[9] but they are neither sensitive nor specific;[10] more particularly, serum IgG4 levels are within normal limits in up to 30% to 40% of patients with IgG4-RD.[11] In a series of patients with IgG4-ROD, 55.3% had elevated serum IgG4 levels.[12] In some cases, the levels of IgG4 may be falsely low due to the prozone effect.[7,13]
In our patient, the association of bilateral orbital involvement with an abdominal mass, the histological analysis of the latter and low IgG4 levels makes the diagnosis of IgG4-RD probable according to the diagnostic criteria of this disease.[8,9] The serum IgG4 levels were low possibly due to a prozone effect that could not be ruled out by making a new IgG4 dosage (serum samples had been disposed). The abdominal and orbital infiltrations responded to IgG4-RD treatment consisting of first-line corticosteroids followed by rituximab, which is also in favor of the positive diagnosis of IgG4-RD (by analogy with one of the diagnostic criteria of autoimmune pancreatitis which is response to corticosteroid therapy).[14]
3.2. Relationship between IgG4-RD and cancer
The etiology of IgG4-RD is unknown, but considering that a history of malignancy is 2.5 times higher in patients with IgG4-RD than in the general population, cancer may represent a trigger for IgG4-RD in certain patients.[4] Prostate cancer and lymphoma are the most common malignancies diagnosed prior to IgG4-RD and, interestingly, no cases of IgG4-RD involved the organ previously affected by cancer.[4] We report a rare association of IgG4-RD with colon cancer. To our knowledge, there are only 4 case reports of rectal cancer associated to IgG4-RD.[15–18] All were elderly Asian patients (aged between 66 and 72 years) with high serum IgG4 levels. None of these cases had orbital involvement of IgG4-RD, which makes the present case exceptional.
The mechanism by which cancer could lead to IgG4-RD is not known. It has been hypothesized that IgG4-RD and cancer could have some shared risk factors, that cancer could trigger autoantigen expression leading to IgG4-RD and that there could be an increased risk of IgG4-RD after some anti-cancer therapies like radiation and chemotherapy.[4] Also, IgG4 antibodies seem to be directly implicated in tumor-immune escape[19] but a possible link with the subsequent occurrence of IgG4-RD remains to be established. So far, it has only been shown that melanoma cells initiate tumor-specific IgG4 production, which leads to high IgG4 serum levels,[20] correlated negatively to patient survival rate.[21] In regards to colorectal cancer, a phenomenon similar to that observed in melanoma could take place, but this has not yet been demonstrated. In the particular case of our patient, the chronology of IgG4-RD and colon cancer evokes the possibility of IgG4-RD being a paraneoplastic syndrome rather than an independent disease, as has been suggested previously.[22–24]
IgG4-RD is in itself a predisposing condition to cancer, since malignancies are observed in 10.4% of the IgG4-RD patients.[5,6] Malignancies complicating IgG4-RD are classified as lymphoma (Mucosa-associated lymphoid tissue [MALT] and non-Hodgkin lymphoma) and non-lymphoid tumors. Lymphoma could be a consequence of the chronic inflammation present in IgG4-RD.[25] Other cancers that can be diagnosed at the same time as IgG4-RD or during its subsequent follow-up include lung cancer and colon cancer.[6] Cancer screening should therefore be performed in some patients with IgG4-RD, especially if they are over 60 at the time of IgG4-RD diagnosis.[6] In this context, PET (positron emission tomography) could be useful for both systemic evaluation in IgG4-RD and cancer detection.[26,27] PET was not performed in our patient. However, given that the prevalence of certain cancers is high in the elderly population (in particular colon cancer), a chance association between IgG4-RD and cancer remains possible and more epidemiological studies are needed to study the relationship between the two.
3.3. IgG4-RD and optic nerve involvement
Enlarged orbital nerves are a common feature of IgG4-ROD; bilateral infraorbital nerve enlargement is almost pathognomonic of this disease.[3] Histological analysis of the branches of the trigeminal nerve in IgG4-ROD shows inflammatory infiltration of the epineurium, whereas the endoneurium and perineurium are unaffected.[28] On the contrary, optic nerve involvement is uncommon in IgG4-RD.[29–34] It is most often unilateral[35] and due to compression of the optic nerve by enlarged orbital structures affected by IgG4-ROD;[36–38] in a series of patients with IgG4-ROD, compressive optic neuropathy was found on orbital imaging in 9.2% of the cases.[35] In some cases a meningeal infiltration of the optic nerve is found on imaging, suggesting perineuritis.[30,31,33,39] Only one case of unilateral IgG4-related optic neuropathy occurred in the absence of any infiltrating mass.[32] In view of this only case, it seems that IgG4-related optic neuropathy may rarely be due to direct infiltration of the optic nerve by IgG4-related inflammation (by analogy with what is found in the biopsies of the branches of the trigeminal nerve, but this hypothesis is subject to confirmation by an actual optic nerve biopsy).[36]
Our patient developed bilateral optic perineuritis, visible on orbital MRI as a circumferential optic nerve sheath enhancement in the coronal plane likely due to optic nerve sheath infiltration by IgG4-related inflammation. Optic perineuritis is defined as the inflammation of the optic nerve sheath[40,41] and only 3 cases of IgG4-related optic perineuritis have been reported so far.[22,42] A dural biopsy was performed in one case,[42] showing an infiltration of lymphocytes and plasma cells, with IgG4-positive cells representing over 40% of the total plasma cell population.[42]
4. Conclusion
In summary, we report a unique case of IgG4-RD preceding the diagnosis of colon adenocarcinoma, which, in addition to the existing medical literature, further highlights a possible causal or consequential relationship between these 2 entities. More particularly, this case raises the possibility that IgG4-RD could be a paraneoplastic syndrome in some patients. Cancer screening should therefore be performed in some elderly patients diagnosed with IgG4-RD. A rare bilateral optic perineuritis occurring in the course of IgG4-ROD is also described, possibly caused by direct IgG4-related inflammation of the optic nerve sheath. To our knowledge, this is the fourth reported case of optic perineuritis secondary to IgG4-RD.
Author contributions
Conceptualization: Miguel González-Candial, Stephanie Lemaitre.
Data curation: Stephanie Lemaitre, Gemma Mateu Esquerda, Antoni Castro Guardiola, Jordi Teruel Agustin, Miguel González-Candial.
Formal analysis: Stephanie Lemaitre, Gemma Mateu Esquerda, Antoni Castro Guardiola, Jordi Teruel Agustin.
Investigation: Stephanie Lemaitre, Nicolae Sanda.
Methodology: Stephanie Lemaitre.
Resources: Miguel González-Candial.
Supervision: Miguel González-Candial.
Validation: Antoni Castro Guardiola, Miguel González-Candial.
Writing – original draft: Stephanie Lemaitre, Nicolae Sanda.
Writing – review & editing: Antoni Castro Guardiola, Miguel González-Candial, Stephanie Lemaitre.
Footnotes
Abbreviations: ANCA= antineutrophil cytoplasmic bodies, CT= computed tomography, IgG4-RD = Immunoglobulin G4-related disease, IgG4-ROD= IgG4-related ophthalmic disease, LE= left eye, MALT= mucosa-associated lymphoid tissue, MRI= magnetic resonance imaging, PET= positron emission tomography, RE= right eye, VEP= visual evoked potentials.
The authors have no conflicts of interest to disclose.
References
- [1].Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732–8. [DOI] [PubMed] [Google Scholar]
- [2].Wallace ZS, Deshpande V, Stone JH. Ophthalmic manifestations of IgG4-related disease: single-center experience and literature review. Semin Arthritis Rheum 2014;43:806–17. [DOI] [PubMed] [Google Scholar]
- [3].McNab AA, McKelvie P. IgG4-related ophthalmic disease. Part II: clinical aspects. Ophthal Plast Reconstr Surg 2015;31:167–78. [DOI] [PubMed] [Google Scholar]
- [4].Wallace ZS, Wallace CJ, Lu N, et al. Association of IgG4-related disease with history of malignancy. Arthritis Rheumatol (Hoboken, NJ) 2016;68:2283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ahn S S, Song F, Park Y -B, et al. Malignancies in Korean patients with immunoglobulin G4-related disease. Int J Rheum Dis 2017;20:1028–35. [DOI] [PubMed] [Google Scholar]
- [6].Yamamoto M, Takahashi H, Tabeya T, et al. Risk of malignancies in IgG4-related disease. Mod Rheumatol 2012;22:414–8. [DOI] [PubMed] [Google Scholar]
- [7].Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet 2015;385:1460–71. [DOI] [PubMed] [Google Scholar]
- [8].Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181–92. [DOI] [PubMed] [Google Scholar]
- [9].Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012;22:21–30. [DOI] [PubMed] [Google Scholar]
- [10].Ngwa TN, Law R, Murray D, et al. Serum immunoglobulin G4 level is a poor predictor of immunoglobulin G4-related disease. Pancreas 2014;43:704–7. [DOI] [PubMed] [Google Scholar]
- [11].McNab AA, McKelvie P. IgG4-related ophthalmic disease. Part I: background and pathology. Ophthal Plast Reconstr Surg 2015;31:83–8. [DOI] [PubMed] [Google Scholar]
- [12].Park J, Lee MJ, Kim N, et al. Risk factors for extraophthalmic involvement and treatment outcomes in patients with IgG4-related ophthalmic disease. Br J Ophthalmol 2017;102:736–41. [DOI] [PubMed] [Google Scholar]
- [13].Khosroshahi A, Cheryk LA, Carruthers MN, et al. Brief report: spuriously low serum IgG4 concentrations caused by the prozone phenomenon in patients with IgG4-related disease. Arthritis Rheumatol (Hoboken, NJ) 2014;66:213–7. [DOI] [PubMed] [Google Scholar]
- [14].Grados A, Ebbo M, Jean E, et al. IgG4-related disease treatment in 2014: Update and literature review. Rev Med Interne 2015;36:395–404. [DOI] [PubMed] [Google Scholar]
- [15].Liu Y, Chen L, Li F. Predominant IgG4 disease and concurrent early-stage rectal cancer. Clin Nucl Med 2011;36:1135–6. [DOI] [PubMed] [Google Scholar]
- [16].Itokawa N, Atsukawa M, Nishino T, et al. A case of IgG4-related disease with rectal cancer. Clin J Gastroenterol 2011;4:374–80. [DOI] [PubMed] [Google Scholar]
- [17].Tsuchiya T, Yagi T, Tsukamoto M, et al. A case of IgG4-related disease coexisted with rectal cancer. Surg Case Rep 2015;1:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Koumo T, Maeda Y, Nagatani S, et al. A case of immunoglobulin G4-related lymphadenopathy associated with rectal cancer. Nihon Rinsho Geka Gakkai Zasshi (J Jpn Surg Assoc) 2013;74:2817–23. [Google Scholar]
- [19].Trampert DC, Hubers LM, van de Graaf SFJ, et al. On the role of IgG4 in inflammatory conditions: lessons for IgG4-related disease. Biochim Biophys Acta 2018;1864(4 pt B):1401–9. [DOI] [PubMed] [Google Scholar]
- [20].Karagiannis P, Gilbert AE, Josephs DH, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest 2013;123:1457–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Karagiannis P, Villanova F, Josephs DH, et al. Elevated IgG4 in patient circulation is associated with the risk of disease progression in melanoma. Oncoimmunology 2015;4:e1032492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Asano J, Watanabe T, Oguchi T, et al. Association between immunoglobulin G4-related disease and malignancy within 12 years after diagnosis: an analysis after longterm followup. J Rheumatol 2015;42:2135–42. [DOI] [PubMed] [Google Scholar]
- [23].Shiokawa M, Kodama Y, Yoshimura K, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol 2013;108:610–7. [DOI] [PubMed] [Google Scholar]
- [24].Wang Y, Zhu J. Need for caution in diagnosing IgG4-related disease: a possible paraneoplastic syndrome? Comment on the article by Wallace et al. Arthritis Rheumatol (Hoboken, NJ) 2017;69:681–2. [DOI] [PubMed] [Google Scholar]
- [25].Uehara T, Ikeda S, Hamano H, et al. A case of Mikulicz's disease complicated by malignant lymphoma: a postmortem histopathological finding. Intern Med 2012;51:419–23. [DOI] [PubMed] [Google Scholar]
- [26].Yamamoto M, Takahashi H, Shinomura Y. IgG4-related disease and malignancy. Intern Med 2012;51:349–50. [DOI] [PubMed] [Google Scholar]
- [27].Zhao Z, Wang Y, Guan Z, et al. Utility of FDG-PET/CT in the diagnosis of IgG4-related diseases. Clin Exp Rheumatol 2016;34:119–25. [PubMed] [Google Scholar]
- [28].Hardy TG, McNab AA, Rose GE. Enlargement of the infraorbital nerve: an important sign associated with orbital reactive lymphoid hyperplasia or immunoglobulin g4-related disease. Ophthalmology 2014;121:1297–303. [DOI] [PubMed] [Google Scholar]
- [29].Behbehani RS, Al-Nomas HS, Al-Herz AA, et al. Bilateral intracranial optic nerve and chiasmal involvement in IgG4-related disease. J Neuroophthalmol 2015;35:229–31. [DOI] [PubMed] [Google Scholar]
- [30].Soussan M, Medjoul A, Badelon I, et al. IgG4-related diffuse perineural disease. Neurology 2014;83:1877–8. [DOI] [PubMed] [Google Scholar]
- [31].Hwang G, Jin S-Y, Kim H-S. IgG4-related disease presenting as hypertrophic pachymeningitis and compressive optic neuropathy. Joint Bone Spine 2016;83:601–2. [DOI] [PubMed] [Google Scholar]
- [32].Zhang W, Luo J, Jiao J. Optic nerve involvement in immunoglobulin G4-related disease: a case report. Exp Ther Med 2016;12:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Noshiro S, Wanibuchi M, Akiyama Y, et al. IgG4-related disease initially presented as an orbital mass lesion mimicking optic nerve sheath meningioma. Brain Tumor Pathol 2015;32:286–90. [DOI] [PubMed] [Google Scholar]
- [34].Takahashi Y, Kitamura A, Kakizaki H. Bilateral optic nerve involvement in immunoglobulin G4-related ophthalmic disease. J Neuroophthalmol 2014;34:16–9. [DOI] [PubMed] [Google Scholar]
- [35].Sogabe Y, Ohshima K, Azumi A, et al. Location and frequency of lesions in patients with IgG4-related ophthalmic diseases. Graefes Arch Clin Exp Ophthalmol 2014;252:531–8. [DOI] [PubMed] [Google Scholar]
- [36].Kashii S. IgG4-related disease: a neuro-ophthalmological perspective. J Neuroophthalmol 2014;34:400–7. [DOI] [PubMed] [Google Scholar]
- [37].Tomio R, Ohira T, Wenlin D, et al. Immunoglobulin G4-related intracranial inflammatory pseudotumours along both the oculomotor nerves. BMJ Case Rep 2013;2013:bcr2012007320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu P-C, Tien P-T, Li Y-H, et al. IgG4-related cerebral pseudotumor with perineural spreading along branches of the trigeminal nerves causing compressive optic neuropathy: a case report. Medicine (Baltimore) 2017;96:e8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].AbdelRazek M, Stone JH. Neurologic features of immunoglobulin G4-related disease. Rheum Dis Clin North Am 2017;43:621–31. [DOI] [PubMed] [Google Scholar]
- [40].Hickman SJ. Optic perineuritis. Curr Neurol Neurosci Rep 2016;16:16. [DOI] [PubMed] [Google Scholar]
- [41].Purvin V, Kawasaki A, Jacobson DM. Optic perineuritis: clinical and radiographic features. Arch Ophthalmol 2001;119:1299–306. [DOI] [PubMed] [Google Scholar]
- [42].Lee CS, Harocopos GJ, Kraus CL, et al. IgG4-associated orbital and ocular inflammation. J Ophthalmic Inflamm Infect 2015;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]


