Abstract
This study was aimed to reveal the changes in survival rates and prognostic factors to survival of chondroblastic osteosarcoma (COS).
Patients from the Surveillance, Epidemiology, and End Results (SEER) database were retrieved. Kaplan–Meier survival analysis and Cox proportional hazard model were used during analysis.
There were significant differences on overall survival between subtypes of osteosarcoma (P < .001∗). Overall survival of COS did not change significantly during last forty years (P = .610), and cancer-specific survival increased to a plateau in 1980s and then remained stable (P = .058). Younger onset age, patients of white race, well and moderately differentiated tumors, and surgery independently predicted better overall (Hazard ratio [HR]: 1.034, P < .001∗; HR: 0.538, P = .004∗; HR: 0.240, P = .020∗ and HR: 0.350, P < .001∗, respectively) and cancer-specific (HR: 1.031, P = .002∗; HR: 0.592, P = .036∗; HR: 0.098, P = .027∗ and HR: 0.253, P < .001∗, respectively) survival. Metastasis at diagnosis independently predicted worse overall (HR: 3.108, P < .001∗) and cancer-specific (HR: 4.26, P < .001∗) survival compared to no metastasis.
Younger onset age, white race, well and moderately differentiated tumors, no metastasis at diagnosis and surgical resection can independently predict better overall and cancer-specific survival of COS.
Keywords: cancer-specific survival, chondroblastic osteosarcoma, overall survival, prognostic factors
1. Introduction
Chondroblastic osteosarcoma (COS) is a rare tumor, and is a common subtype of osteosarcoma with percentage of 11% to 50%. The age at diagnosis of COS appears to be variable in various studies, mainly with a peak at about twenty years old.[1–6] It is characterized by predominant presence of chondroid matrix, which tends to exhibit a high degree of hyaline cartilage and is intimately associated with the non-chondroid element (osteoid or bone matrix).[7]
It is important to distinguish the COS from other subtypes, as gene expression, chemotherapy response and prognosis of the subtypes of osteosarcoma are different from each other.[3,8–11] There have been rare case reports describing diagnosis, treatment strategies and prognosis of COS.[3,7,12–16] However, its clinical characteristics have never been described due to low incidence. Although features of osteosarcoma have been frequently reported, there is controversial evidence as to whether there is prognostic differentiation among its subtypes. One study revealed that patients with COS had a higher survival rate when compared to patients with the osteoblastic osteosarcoma,[17] while another study declared that the 5-year overall survival was significantly higher in patients with fibroblastic and telangiectatic tumors than in those with osteoblastic tumors and COS.[9] Surprisingly, Mirabello et al reported that patients with COS presented similar 5-year survival rates than patients with fibroblastic and telangiectatic tumors in age group of 0 to 24, while they presented lower 5-year survival rates than patients with fibroblastic and telangiectatic tumors in age group of 25 to 59 and higher 5-year survival rates than patients with fibroblastic tumors in age group of 60+.[6] Besides, the subtypes of osteosarcoma presented different signal distributions on MRI.[18] These findings indicate that significant heterogeneity exists among the subtypes of osteosarcoma, thereby COS should be investigated separately.
However, it is difficult to study this disease by 1 single institute due to the very low incidence. In the present study, we revealed the changes in survival and prognostic factors to survival rates of such distal disease in patients from the Surveillance, Epidemiology, and End Results (SEER) database.
2. Methods
2.1. The SEER database and related codes
The SEER database contains information on cancer epidemiology among the United States population.[19] All patients with osteosarcoma listed in the SEER database from 1973 to 2014 were identified. We used code number “011” under the North American Association of Central Cancer Registries item “CS Schema v0204+” as the first filter, which helped find all bone malignancies. Subtypes of osteosarcoma were coded as 9180 to 9187 and 9192 to 9194, and COS was coded as 9181 according to the International Classification of Diseases for Oncology (ICD-O-3 edition). Clinical factors, including age, gender, race, marital status, year of diagnosis, primary site, histology grade, metastasis at diagnosis, tumor size (largest dimension), treatment strategies, follow-up time and survival status were retrieved for this research. All data were extracted using Perl language. We signed the Data-Use Agreement for the SEER 1973 to 2014 Research Data File and had the permission to use the database for research.
2.2. Statistics
Demographic characteristics of patients and tumors were described with number and percentage. Changes in survival of COS were analyzed using Kaplan–Meier survival analysis for all cohort. To avoid bias produced by incomplete data over such a long period (1973–2014), only patients diagnosed between 2004 and 2014 were further enrolled to determine the prognostic factors to survival via Cox proportional hazard model. Patients with unknown treatment strategies (code number 8 and 9) were excluded when investigating the influence of clinical factors on survival. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. The significance level for all tests was 2-sided at 5%. All data were analyzed using SPSS Statistics software (V.24; IBM Corporation). Survival time for patients who died during follow up was calculated from the date of diagnosis to the date of death and survival time for patients who were still alive at last follow up was calculated from the date of diagnosis to the date of the last follow-up.
3. Results
3.1. Demographic characteristics
Totally 5950 patients were diagnosed with osteosarcoma in SEER database between 1973 and 2014. Pathology of osteosarcoma was classified as “NOS” (not otherwise specified) for 70.1% (n = 4172) of cases; otherwise, COS was the most common subtypes (12.2%, n = 724), followed by fibroblastic (5.1%, n = 304), parosteal (4.7%, n = 277), telangiectatic (2.7%, n = 162), central (1.7%, n = 102), osteosarcoma in Paget disease (1.4%, n = 86), periosteal (0.9%, n = 54), small cell (0.8%, n = 46), high grade surface (0.3%, n = 16) and instrosseous well differentiated (0.1%, n = 7). The incidence rates of osteosarcoma and COS were 0.3 and 0.03 per 100,000 and was age-adjusted to the 2000 US Standard Population, respectively. The mean follows up time was 83.8 months. There were significant differences in overall survival among different subtypes of osteosarcoma (P < .001∗, Fig. 1).
Figure 1.
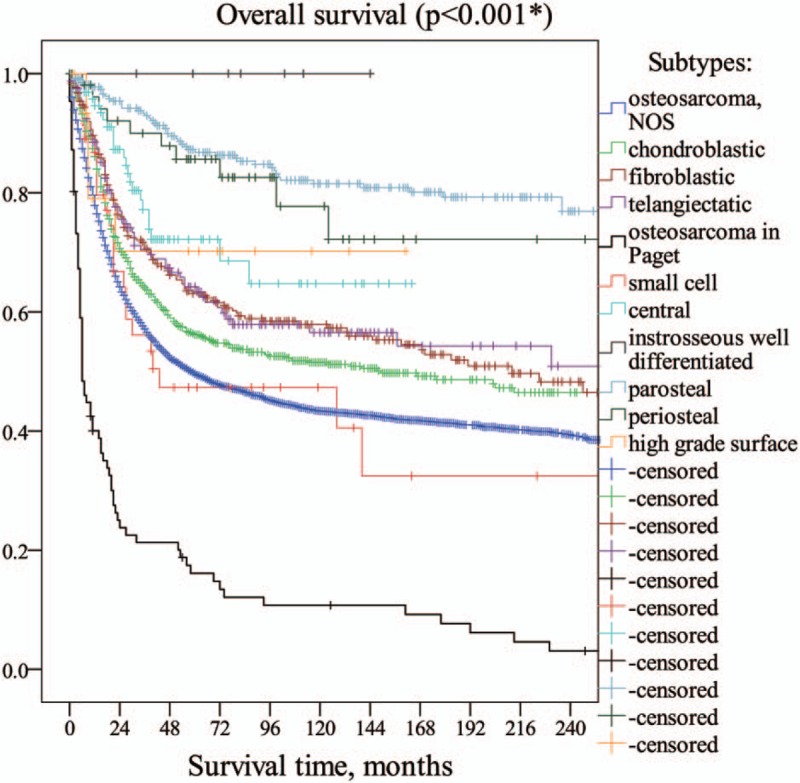
There were significant differences between subtypes of osteosarcoma (P < .001∗).
Totally 724 patients were diagnosed with COS in SEER database from 1973 to 2014. Characteristics of the enrolled cases were summarized in Table 1.
Table 1.
Characteristics of patients with COS.

3.2. Overall survival during last forty years
1-year, 3-year, 5-year, and 10-year overall survival of all cohort were 86.1%, 64.1%, 56.2%, and 51.5%, respectively. The overall survival didn’t change significantly over last 40 years (P = .610, Fig. 2A).
Figure 2.

A: The overall survival did not change significantly over last 40 years (P = .610). B: Cancer-specific survival increased to a plateau in 1980 s and then remained stable (P = .058).
2772 patients were diagnosed with osteosarcoma between 2004 and 2014, 14.1% (n = 391) patients were COS. 140 (35.8%) patients with COS were dead at last follow up, and 251 (64.2%) were still alive at last follow up.
In multivariate model (n = 391, Table 2), younger onset age (HR: 1.034, 95% CI: 1.019–1.049, P < .001∗), patients of white race (HR: 0.538, 95% CI: 0.354–0.818, P = .004∗, Fig. 3A), well and moderately differentiated tumors (HR: 0.240, 95% CI: 0.072–0.799, P = .020∗, Fig. 3B), and surgery (HR: 0.350, 95% CI: 0.207–0.592, P < .001∗, Fig. 3C) independently predicted better overall survival, while metastasis at diagnosis independently predicted a worse overall survival compared to no metastasis (HR: 3.108, 95% CI: 1.972–4.898, P < .001∗, Fig. 3D).
Table 2.
Prognostic factors to overall survival of COS: Cox proportional hazards analysis.

Figure 3.

Overall survival curves for prognostic factors of race, tumor grade, metastasis at diagnosis and surgery in multivariate analysis.
3.3. Cancer-specific survival analysis during last forty years
Totally 127 patients died due to other reasons instead of COS, thus the remaining 597 cases were included to analyze cancer-specific survival. 1-year, 3-year, 5-year and 10 year cancer-specific survival were 90.2%, 68.8%, 61.8%, and 58.2%, respectively. Cancer-specific survival increased to a plateau in 1980s and then remained stable (P = .058, Fig. 2B).
In multivariate model (n = 332, Table 3), younger onset age (HR: 1.031, 95% CI: 1.011–1.051, P = .002∗), patients of white race (HR: 0.592, 95% CI: 0.363–0.966, P = .036∗, Fig. 4A), well and moderately differentiated tumors (HR: 0.098, 95% CI: 0.013–0.770, P = .027∗, Fig. 4B), and surgery (HR: 0.253 95% CI: 0.128–0.500, P < .001∗, Fig. 4C) independently predicted better cancer-specific survival, while metastasis at diagnosis independently predicted a worse cancer-specific survival compared to no metastasis (HR: 4.26, 95% CI: 2.640–7.421, P < .001∗, Fig. 4D).
Table 3.
Prognostic factors to cancer-specific survival of COS: Cox proportional hazards analysis.

Figure 4.

Cancer-specific survival curves for prognostic factors of race, tumor grade, metastasis at diagnosis and surgery in multivariate analysis.
3.4. Variations in survival in different age groups
Patients were divided into 4 subgroups based on the age at diagnosis, which varied between 3 to 86 years old: 3 to 19, 20 to 39, 40 to 59 and 60 to 86. Almost half of the total patients were diagnosed between 3 to 19 years old. Patients older than 60 had lowest overall and cancer-specific survival rates than other age groups (Table 4, Fig. 5).
Table 4.
Survival rates in different age subgroups.

Figure 5.

Comparison of survival rates in different age groups.
4. Discussion
As 1 of the most common subtypes of osteosarcoma, COS has never been well described due to low incidence. In the present study, all patients with osteosarcoma were reviewed from SEER database. We found that overall survival differed among different subtypes of osteosarcoma. Overall survival of COS did not change significantly during last forty years, and cancer-specific survival increased to a plateau in 1980s and then remained stable. Younger age and no metastasis at diagnosis, white race, well and moderately differentiated tumors, as well as surgical resection, can independently predict better overall and cancer-specific survival of COS.
Some studies described a second peak of incidence in patients older than 60 years old. However, we did not observe a second peak for the COS. An institution reported that the 5-year overall survival rate of COS was 60% (n = 132).[9] In the present study, the 5-year overall survival rates were 56.2%, which was lower than the previous outcomes but still comparable.
Prognostic factors to survival of osteosarcoma varied in many studies. One study reported that tumor volume, age and histological subtypes were associated with disease free survival via multivariate analyses,[11] while another study revealed that primary metastasis, tumor necrosis after chemotherapy, tumor site, surgical margins, extremity tumor, tumor volume, and location within the limb had prognostic significance via multivariate analyses.[20] In the present study, we demonstrated that different subtypes of osteosarcoma had different overall survival, supporting our decision to investigate the survival of COS separately, and it is consistent with the previously reported results.[11] Moreover, we found that onset age and no metastasis at diagnosis, white race, tumor grade as well as surgical resection were associated with survival of COS, while tumor size and tumor location of long bones cannot independently predict survival rates.
Although different gene expression features and prognosis have been reported among subtypes of osteosarcoma, currently, there is no difference on the treatment strategies between COS and the other subtypes. The resection with tumor-free margins and systemic chemotherapy are the standard treatment option.[3,7,21,22] Osteosarcoma is quite resistant to radiotherapy. However, in special locations where surgery is not feasible or for cases of bone metastases or in multifocal osteosarcoma, radiotherapy can prolong survival, and decrease pain intensity.[23] However, radiotherapy predicted worse survival rates in our study. This has to be interpreted with caution because only unresectable tumors were included in their study, while both resectable and unresectable tumors were included in our study. Many patients did not receive radiation but underwent tumor resection, thereby presenting better overall survival in this study. Our outcomes may be interpreted as those patients eligible for radiation presented relatively worse overall survival in all patients with COS.
Chemotherapeutic options depend on the institutions. The European Osteosarcoma Intergroup established a protocol which consists of cycles of cisplatin and doxorubicin, and the Brazilian Protocol for Metastatic and No Metastatic Osteosarcoma also made a protocol with cycles of cisplatin, ifosfamide and doxorubicin.[24] The Cooperative Osteosarcoma Study group of Germany, Austria, and Switzerland reported a combination regimen of Methotrexate (12 g/m2 per course), Adriamycin (60–90 mg/m2 per course), Cisplatin (90–150 mg/m2 per course), Ifosfamide (6–10 g/m2 per course), and Bleomycin, Cyclophosphamide and Dactinomycin used in varying combinations.[20] However, whether a combination chemotherapy may prolong survival remains controversial. There was no survival benefit with a multi-agent regimen compared with a 2-drug regimen.[25,26] Some other studies also revealed that intensification of chemotherapy did not improve the overall survival.[27–30] Unfortunately, only the information of whether the patients were treated by chemotherapy or not was recorded, there was no information about which chemotherapeutic regimens were used in the SEER database (incomplete data).
5. Conclusion
In this retrospective study, we found that there were significant differences in overall survival between subtypes of osteosarcoma, and the overall and cancer-specific survival did not change significantly during last few decades. Younger onset age, white race, well and moderately differentiated tumors, no metastasis at diagnosis and surgical resection can independently predict better overall and cancer-specific survival of COS.
We acknowledge some limitations existing in this study. First of all, the retrieved data partly relies on accuracy of written records of individuals. Second, although chemotherapy plays an important role in managing patients with COS, the chemotherapeutic regimens were unknown in SEER database. Third, the surgical margin status was unknown in this study. Despite these deficiencies, to the best of our knowledge, this is the largest scale study investigating survival of COS.
Author contributions
Conceptualization: Kai-Jin Guo.
Data curation: Hui-Hui Sun, Xiang-Yang Chen.
Formal analysis: Hui-Hui Sun, Xiang-Yang Chen, Jia-Qu Cui.
Methodology: Xiang-Yang Chen, Zhao-Ming Zhou.
Resources: Hui-Hui Sun, Xiang-Yang Chen.
Software: Hui-Hui Sun, Xiang-Yang Chen, Zhao-Ming Zhou.
Supervision: Kai-Jin Guo.
Validation: Hui-Hui Sun, Xiang-Yang Chen, Jia-Qu Cui, Zhao-Ming Zhou, Kai-Jin Guo.
Visualization: Jia-Qu Cui, Zhao-Ming Zhou.
Writing – original draft: Hui-Hui Sun.
Writing – review & editing: Kai-Jin Guo.
Kai-Jin Guo orcid: 0000-0002-8689-7423.
Footnotes
Abbreviations: CI = confidence interval, COS = chondroblastic osteosarcoma, HR = hazard ratio, SEER = the Surveillance, Epidemiology, and End Results.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We signed the Data-Use Agreement for the SEER 1973 to 2014 Research Data File and had the permission to use the database for research.
We signed the Data-Use Agreement for the SEER 1973 to 2014 Research Data File and had the permission to use the database for research.
The authors have no conflicts of interest to disclose.
References
- [1].Ajura AJ, Lau SH. A retrospective clinicopathological study of 59 osteogenic sarcoma of jaw bone archived in a stomatology unit. Malays J Pathol 2010;32:27–34. [PubMed] [Google Scholar]
- [2].Fernandes R, Nikitakis NG, Pazoki A, et al. Osteogenic sarcoma of the jaw: a 10-year experience. J Oral Maxillofac Surg 2007;65:1286–91. [DOI] [PubMed] [Google Scholar]
- [3].Khandekar S, Dive A, Munde P, et al. Chondroblastic osteosarcoma of the left zygomatic bone: rare case report and review of the literature. J Oral Maxillofac Pathol 2014;18:281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ong ST, Shim CK, Ng KH, et al. Osteosarcoma presenting as an aggressive nodular mass in the region of the mandible. J Oral Sci 2004;46:55–9. [DOI] [PubMed] [Google Scholar]
- [5].Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: national cancer data base report. Clin Orthop Relat Res 2007;459:40–7. [DOI] [PubMed] [Google Scholar]
- [6].Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115:1531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Almeida E, Mascarenhas BA, Cerqueira A, et al. Chondroblastic osteosarcoma. J Oral Maxillofac Pathol 2014;18:464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Machado I, Lopez Guerrero JA, Navarro S, et al. Galectin-1 (GAL-1) expression is a useful tool to differentiate between small cell osteosarcoma and Ewing sarcoma. Virchows Arch 2013;462:665–71. [DOI] [PubMed] [Google Scholar]
- [9].Bacci G, Bertoni F, Longhi A, et al. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer 2003;97:3068–75. [DOI] [PubMed] [Google Scholar]
- [10].Nakajima H, Sim FH, Bond JR, et al. Small cell osteosarcoma of bone. Review of 72 cases. Cancer 1997;79:2095–106. [DOI] [PubMed] [Google Scholar]
- [11].Ferrari S, Bertoni F, Mercuri M, et al. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity: an analysis of 300 patients treated at the Rizzoli institute. Ann Oncol 2001;12:1145–50. [DOI] [PubMed] [Google Scholar]
- [12].Zheng YF, Lin J, Yang HL. Chondroblastic osteosarcoma secondary to fibrosarcoma: a case report and literature review. Oncol Lett 2015;10:3573–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ludhani PM, Anathakrishnan R, Chandrasekar P, et al. Unusual case of chondroblastic osteosarcoma of the rib in an adult. Asian Cardiovasc Thorac Ann 2014;22:745–7. [DOI] [PubMed] [Google Scholar]
- [14].Korkmaz O, Goksel S, Egilmez HR, et al. Extraskeletal chondroblastic osteosarcoma in the left atrium. Interact Cardiovasc Thorac Surg 2014;19:1077–9. [DOI] [PubMed] [Google Scholar]
- [15].El Ochi MR, Zouaidia F, Kabaj R, et al. Primary chondroblastic osteosarcoma of the breast. Turk Patoloji Derg 2014;30:225–7. [DOI] [PubMed] [Google Scholar]
- [16].Rasband J, Wenger DE, Bancroft LW. Radiologic case study. Chondroblastic osteosarcoma and osteochondromas after prior total body irradiation. Orthopedics 2013;36: 820, 886–9. [DOI] [PubMed] [Google Scholar]
- [17].Junior AT, de Abreu Alves F, Pinto CA, et al. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol 2003;39:521–30. [DOI] [PubMed] [Google Scholar]
- [18].Zeitoun R, Shokry AM, Ahmed Khaleel S, et al. Osteosarcoma subtypes: magnetic resonance and quantitative diffusion weighted imaging criteria. J Egypt Natl Canc Inst 2018;30:39–44. [DOI] [PubMed] [Google Scholar]
- [19].Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40Suppl 8: IV-3-18. [DOI] [PubMed] [Google Scholar]
- [20].Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776–90. [DOI] [PubMed] [Google Scholar]
- [21].Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 2009;152:3–13. [DOI] [PubMed] [Google Scholar]
- [22].Guerra RB, Tostes MD, da Costa Miranda L, et al. Comparative analysis between osteosarcoma and Ewing's sarcoma: evaluation of the time from onset of signs and symptoms until diagnosis. Clinics (Sao Paulo) 2006;61:99–106. [DOI] [PubMed] [Google Scholar]
- [23].Longhi A, Errani C, De Paolis M, et al. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev 2006;32:423–36. [DOI] [PubMed] [Google Scholar]
- [24].Rech A, Castro CG, Jr, Mattei J, et al. Clinical features in osteosarcoma and prognostic implications. J Pediatr (Rio J) 2004;80:65–70. [PubMed] [Google Scholar]
- [25].Bramwell VH, Burgers M, Sneath R, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup. J Clin Oncol 1992;10:1579–91. [DOI] [PubMed] [Google Scholar]
- [26].Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet 1997;350:911–7. [DOI] [PubMed] [Google Scholar]
- [27].Lewis IJ, Nooij MA, Whelan J, et al. Mrc BO, collaborators E, European Osteosarcoma I. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007;99:112–28. [DOI] [PubMed] [Google Scholar]
- [28].Li X, Ashana AO, Moretti VM, et al. The relation of tumour necrosis and survival in patients with osteosarcoma. Int Orthop 2011;35:1847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bispo Junior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo) 2009;64:1177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bacci G, Mercuri M, Longhi A, et al. Grade of chemotherapy-induced necrosis as a predictor of local and systemic control in 881 patients with non-metastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy in a single institution. Eur J Cancer 2005;41:2079–85. [DOI] [PubMed] [Google Scholar]


