Abstract
Dexmedetomidine is a highly selective α2 receptor agonist, this study aimed to investigate the effects of different doses of intranasal dexmedetomidine on the preoperative sedation and postoperative agitation in pediatric with total intravenous anesthesia (TIVA) for adenoidectomy with or without tonsillectomy.
This is a double-blind placebo-controlled randomized trial. Pediatric were randomly divided into the D1, D2, and S groups, each group contained 30 patients. Twenty-five to 40 minutes before surgery, the D1 and D2 groups received intranasally dexmedetomidine 1 μg kg−1 or 2 μg kg−1, respectively, while the S group received saline of the same volume. A unified protocol of TIVA induction and maintenance was used for the three groups. The preoperative sedation, behavior of separation from parents, postoperative agitation, and postoperative pain of the children were evaluated.
The proportions of satisfactory sedation in the D1, D2, and S groups were 63.3%, 76.7%, and 0%, respectively. There was a statistically significant difference between D1 and S groups (P = .000) and D2 versus S groups (P = .000), while there was no statistically significant difference between D1 and D2 groups (P = .399). As for scale on the behavior of separation from parents, there was a statistically significant difference between D1 and S groups (P = .009) and D2 versus S groups (P = .009), whereas there was no significant difference between D1 and D2 groups (P = 1). The incidence of postoperative agitation in the D1, D2, and S groups was 43.3%, 30.0%, and 63.3%, respectively, and there was a statistical difference between D2 and S groups (P = .010). There was a significant difference in the Pediatric Anesthesia Emergence Delirium (PAED) scale between D2 and S groups (P = .029). The Children's Hospital of Eastern Ontario Pain Scale (CHEOPS) in the D2 group was significantly lower than the S group (P = .013).
The intranasal dexmedetomidine of 1 or 2 μg kg−1 25 to 40 minute before induction of anesthesia both could deliver effective preoperative sedation, reducing the children's distress of separation from parents. Moreover, intranasal dexmedetomidine of 2 μg kg−1 could deliver more effective postoperative analgesia and reduce postoperative agitation, without prolonging postoperative recovery or causing severe adverse events.
Keywords: adenotonsillectomy, dexmedetomidine, intravenous anesthesia, pediatrics, postoperative emergence agitation, premedication, sedation
1. Introduction
Adenoidectomy and tonsillectomy are the most frequently performed surgeries in children. Children undergoing surgeries often experience anxiety, stress, fear, and reluctance, due to fear of pain, unfamiliar operating room setting, and fasting, especially during time of separation from parents. The reluctant children who are transferred from the wards to the operating room may suffer from long-term psychological trauma.[1] Children who are about to undergo surgery often show extreme stress or fear in the waiting area or during anesthesia induction.[2] Studies have shown that children's preoperative anxiety is associated with the increased use of postoperative analgesics, postoperative agitation, and postoperative behavioral changes (e.g., eating problems, sleep disorders, anxiety of separating from parents).[3] Therefore, it is necessary to manage the children's perioperative anxiety from the perspectives of both medical ethics and clinical practice.
Otorhinolaryngology procedure is 1 of the risk factors of postoperative agitation for pediatric, as about 26% of children show such condition after these surgeries. The common manifestations are thrashing (86%) and kicking (64%), whereas only a small proportion (14%) show restless and incoherent.[4] Eckenhoff, et al believed that suffocation may lead to the high incidence of postoperative agitation in the otorhinolaryngology procedures.[5] After adenoidectomy or tonsillectomy, edema of the surgical site may resuit in short-term airway obstruction and hypoxemia. In addition, postoperative agitation increases the difficulty of mask ventilation and increasing bleeding from the surgical site. The children may pull out the drainage tubes or intravenous catheters, increase injury to themselves or the nurses.[4] Although medications that lessen postoperative agitation can prolong the extubation time and the length of stay in post-anesthesia care unit (PACU), it is inevitable to use them for children with severe postoperative agitation. Therefore, it is of great significance to explore drugs that can prevent postoperative agitation without prolonging postoperative recovery.
Dexmedetomidine is a highly selective α2 receptor agonist featuring sedative, hypnotic, antianxiety, and analgesic properties. With a short half-life, it can deliver an awakable sedation without causing respiratory depression.[6] Nasal administration is a convenient, simple, non-invasive approach of administration that reduces first-pass effects and has been successfully applied to midazolam, ketamine, and sufentanil.[7–9] Studies have shown that the bioavailability of a single dose of intranasal dexmedetomidine by 84 μg in healthy adults is 65%, and the its peak plasma concentration lasts for 38 minutes. Compared with intravenous administration, nasal administration takes a longer time to take effect.[10] Yuen, et al intranasally administrated dexmedetomidine of 1 μg kg−1 in children, proved that the time for onset of sedation is 25 (25–30) minutes, which would last for 85 (35–100) minutes.[11] Based on these features, it could be speculated that intranasal dexmedetomidine 25 to 40 minutes before surgery could provide desirable sedative and antianxiety effects in children. In addition, it could prevent postoperative agitation after a minor surgery (e.g., adenoidectomy and tonsillectomy) without delaying the postoperative recovery. Although it has been demonstrated in inhaled anesthesia that intranasal dexmedetomidine can alleviate postoperative agitation,[12] its effectiveness in intravenous anesthesia has not been studied. Therefore, this study was intended to observe the effects of intranasal dexmedetomidine on the preoperative sedation and postoperative agitation of children who underwent total intravenous anesthesia (TIVA) for adenoidectomy with or without tonsillectomy.
2. Patients and methods
2.1. Patients
This double-blind randomized controlled trial was conducted at the Second Hospital of Jilin University and was approved by the Medical Ethics Committee of the Second Hospital of Jilin University. After obtaining the informed consent from their parents or proxies, the children aged 2 to 7 years, scheduled for elective adenoidectomy with or without tonsillectomy, of American Society of Anesthesiologists (ASA) I or II were enrolled in this study. Exclusion criteria: Congenital heart diseases, congenital dysplasia, obstructive sleep apnea syndrome, upper respiratory tract infection, body mass index (BMI) ≥25, allergy to dexmedetomidine, and severe liver or kidney disease.
2.2. Random grouping and sample size
The sample size of this study was calculated based on the fact that, in the pilot experiment, 50% of children in the control group showed agitation after adenoidectomy with or without tonsillectomy under TIVA. It was assumed that α = 0.05 and β = 0.2, then the calculated sample size of 30 in each group could detect a decrease in the incidence of postoperative agitation to 15%. The 90 children who met the enrolled criteria were numbered according to the time of surgery. After that, they were randomly divided into three groups (n = 30) according to the random number table generated by SPSS-21.0 for windows version (SPSS Inc, Chicago, IL). The D1 group was administered with intranasal dexmedetomidine of 1 μg kg−1, the D2 group of 2 μg kg−1, and the S group was intranasally administered with 0.9% normal saline. The grouping result was put into a sealed, opaque envelope. Children who had not been successfully administered before surgery due to fierce resistance were removed from the study. Then extra children were added to the same group for substitution, so that the number of children in each group remained equal.
2.3. Protocol for the preoperative holding area
The undiluted dexmedetomidine (Jiangsu Hengrui Pharmaceutical Co., Ltd., Jiangsu Province, China) was prepared at a concentration of 100 μg mL−1 and then drawn up into a 1 ml syringe. For the D1 group, the same volume of normal saline was added to make the dexmedetomidine concentration of 50 μg mL−1. The D2 group was not diluted. For the S group, the 1 ml syringe was added with 0.9% normal saline. The final amount of titration for each group was 0.02 mL kg−1. All drugs prepared by a researcher who was not involved in the observation or anesthesia.
The establishment of venous access could affect the preoperative anxiety or postoperative agitation of the children. For this reason, venous access had been established for all the children in the wards. After they had been fasted for six hours in accordance with our protocol, the children were taken to the preoperative holding area 25 to 45 minutes before surgeries. Accompany by a parent or proxies were allowed. Then their vital signs (heart rate, pulse, oxygen saturation, and non-invasive arterial blood pressure) were measured. A nurse who is mask of the research asked the children to lie on their parent's lap according to the random grouping. Each nostril was intranasally administered with the same volume of liquid, and the children were kept recumbent position for another 3 minutes to ensure that the drug was fully absorbed.
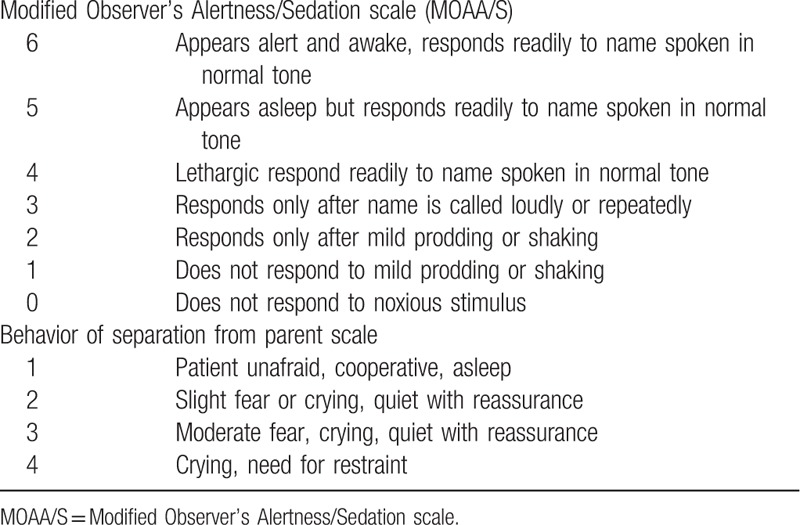
After administration, another nurse who was blind of the grouping used the Modified Observer's Alertness/Sedation scale (MOAA/S) to appraise the children's sedation every 5 minutes (Table 1). For statistical analysis, MOAA/S ≤3 was considered as satisfactory sedation. Non-invasive blood pressure was measured every 10 minutes, while heart rate, pulse, and oxygen saturation were continuously monitored and recorded every 5 minutes, until the children were taken into the operating room. When a child was taken to the operating room with separating from parent, the same nurse used a 4-point scale to evaluate the child's behavior of separation from parent (Table 1). If the child showed fierce resistance, ketamine could be injected intravenously by 1 mg kg−1. The preoperative administration duration (from the intranasal administration of drug to the induction of anesthesia) was recorded. Low heart rate was defined as a heart rate 30% lower than the baseline, hypotension was defined as a mean arterial press (MAP) 30% lower than the baseline, and hypoxemia was defined as pulse oxygen saturation lower than 93%.
Table 1.
Sedation and behavior of separation from parent scale.
2.4. Protocol for anesthesia
All children were induced by intravenous injection of 1 to 2 mg kg−1 ketamine (not if already injected at separation from parents), 0.15 mg kg−1 cisatracurium besilate, and 2 mg kg−1 propofol. The proper endotracheal tube was chosen for insertion and using volume-controlled ventilation, the partial pressure of carbon dioxide at the end of expiration was maintained at 35 to 40 mmHg. Anesthesia was maintained by pump injection of 6 to 8 mg kg−1 h−1 propofol and 2 to 3 μg kg−1 h−1 remifentanil. Non-invasive blood pressure, heart rate, pulse, and oxygen saturation were routinely monitored during surgery. 5% glucose was intraoperatively infused of 6 mL kg−1h−1. If the intraoperative heart rate was 30% lower than the baseline, atropine could be injected intravenously. Pump injection of propofol and remifentanil were stopped immediately after surgery. The patients were sent to the PACU (in 1 min), and the surgery time and anesthesia time (from the induction of anesthesia to the end of pumping anesthetics) were recorded. The physicians who performed the anesthesia and surgeon were mask of the grouping result.
2.5. Protocol for PACU
Once arrival at the PACU, the children were connected to anesthetic machine and ventilated immediately, and their non-invasive blood pressure, heart rate, pulse, and oxygen saduration were monitored. When the children showed stable breathing patterns, purposeful movements, and cough reflexes, the endotracheal tube was removed and the removal time (from the end of surgery to the removal of the endotracheal tube) was recorded. A nurse who was blind of preoperative administration used a 5-point scale to continuously evaluate the children's postoperative agitation as follows: sleepy; awake and quiet; irritated and crying; inconsolably crying; fiercely reluctant and disoriented. The highest score was recorded. A child scored ≥3 was considered as postoperative agitation.
Meanwhile, the Pediatric Anesthesia Emergence Delirium (PAED) scale was used to assess the severity of postoperative agitation.[13] A child scored ≥15 was considered as serious postoperative agitation, and propofol was injected intravenously of 1 mg·kg−1. Postoperative pain was evaluated using Children's Hospital of Eastern Ontario Pain Scale (CHEOPS), which constituted a comprehensive assessment of the children's pain based on their crying, facial expressions, verbal exprassions, and movements.[14] When the CHEOPS score was ≥8, dezocine was injected intravenously of 0.1 mg·kg−1. The children's recovery was assessed by the modified Aldrete scoring system,[15] the children would be discharged from the PACU when the score was ≥9, and the time was recorded. The incidence of adverse events such as vomiting, shivering, low heart rate (heart rate 30% lower than the baseline), hypotension (blood pressure 30% lower than the baseline), and hypoxemia (pulse oxygen saturation below 93%) were recorded. The nurses who participated in the children's care and was mask of grouping used a numerical analog scale (with 0 points the most dissatisfactory and 10 the most satisfactory) to evaluate the anesthesia. Two hours after discharging from the PACU, the same method was used to investigate the satisfaction of the children's families for this anesthesia.
3. Statistical analysis
The scores on sedation and response of separation from parents were analyzed by the Kruskal–Wallis test. If the results were statistically significant, comparisons between groups were made using Mann–Whitney U test. The χ2 test was applied for the categorical data. If there was a significant difference, the P value should be adjusted for comparison between the groups, the P value was adjusted to .017 at a significance level of .05. The categorical data was represented by the number of patients or percentage. Continuous data (PAED score, CHEOPS score, and demographic data except for gender and ASA rating) was first tested using the Kolmogorov–Smirnov test to verify normal distribution. If the data was in normal distribution, then one-way analysis of variance (ANOVA) was adopted, and then the Tukey test was applied to the post hoc pairwise comparisons. Continuous data was reported as mean ± SD. The data analysis software used was SPSS-21.0 (SPSS Inc, Chicago, IL). P < .05 was considered statistically significant.
4. Results
The 102 children who had undergone elective surgery were identified as possible candidates. Twelve of them were excluded according to the exclusion criteria, including 10 cases of upper respiratory tract infection, one of congenital heart disease, and 1 of congenital dysplasia. The remaining 90 children were divided into 3 groups (n = 30). Seven (8%) children failed to take intranasal premedication due to fierce resistance, including 2 (7%) in the D1 group, 3 (10%) in the D2 group, and 2 (7%) in the S group. Another seven children who met the inclusion criteria were added to keep the number equal in each group (Fig. 1). There was no statistically significant difference in the demographic data between the children excluded due to fierce resistance and the others enrolled. Eventually, there was no significant difference in age, gender, weight, ASA rating, preoperative administration duration (from intranasal administration to anesthesia induction), anesthesia time, surgery time, and surgical type between the groups of the 90 children enrolled (Table 2). During preoperative administration, the proportions of achieving satisfactory sedation in the D1, D2, and S groups were 63.3%, 76.7%, and 0%, respectively. There was a statistically significant difference between the D1 and S groups (P = .000) and the D2 versus S groups (P = .000), while there was no statistically significant difference between the D1 and D2 groups (P = .399). The changes in the proportions of achieving satisfactory sedation in the D1 and D2 groups were shown in Figure 2. As for behavior of separation from parents, there was a statistically significant difference between the D1 and S groups (P = .009), as well as between the D2 and S groups (P = .009), while there was no significant difference between the D1 and D2 groups (P = 1). No low heart rate (Fig. 3), hypotension (Fig. 4), or hypoxemia occurred in all groups during preoperative administration.
Figure 1.
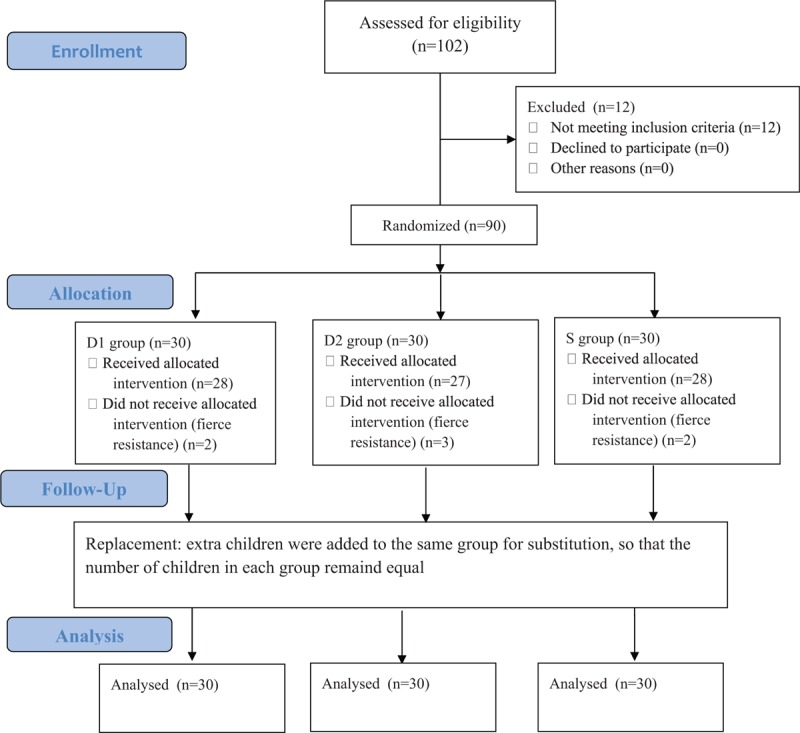
Flow diagram.
Table 2.
Demographic, surgery, and anesthesia data.
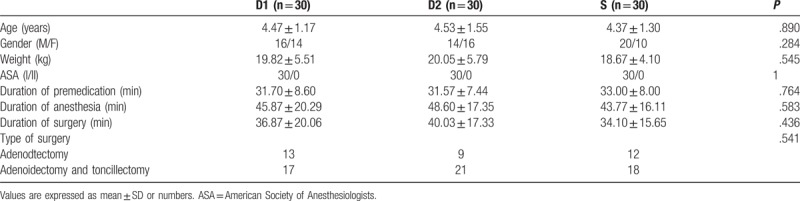
Figure 2.
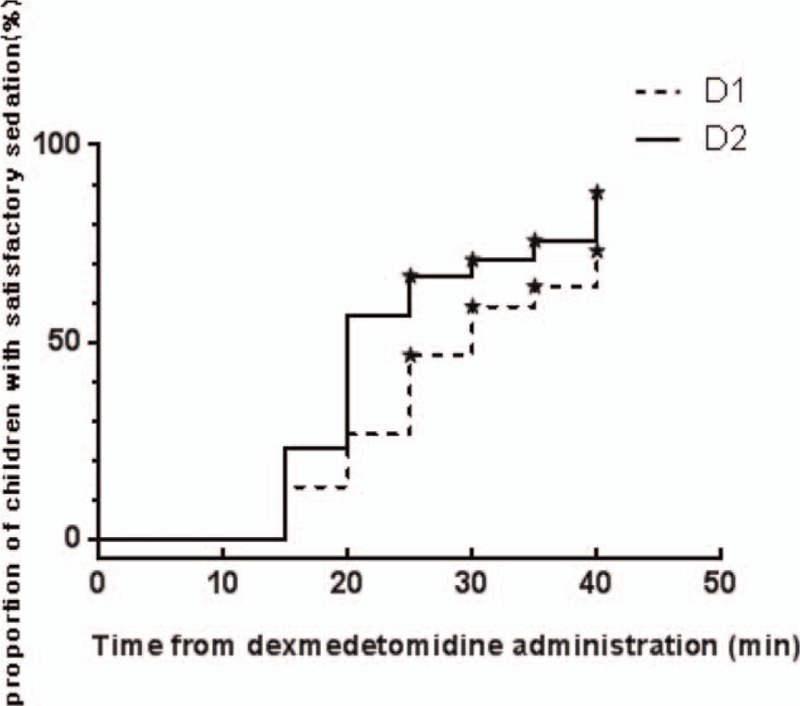
Time after intranasal dexmedetomidine administration and the proportion of children with satisfactory sedation. Each “★” indicates a child was not yet sedated at the duration of premedication.
Figure 3.
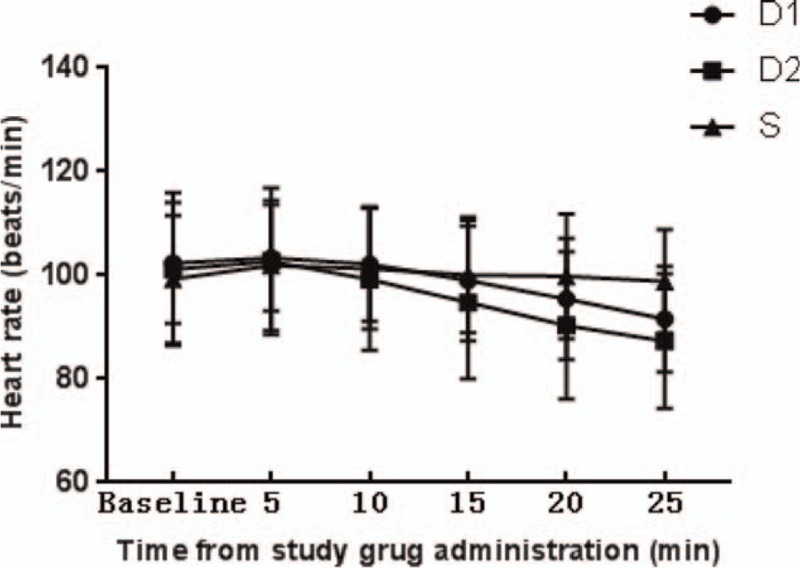
Changes in heart rates after drug administration.
Figure 4.
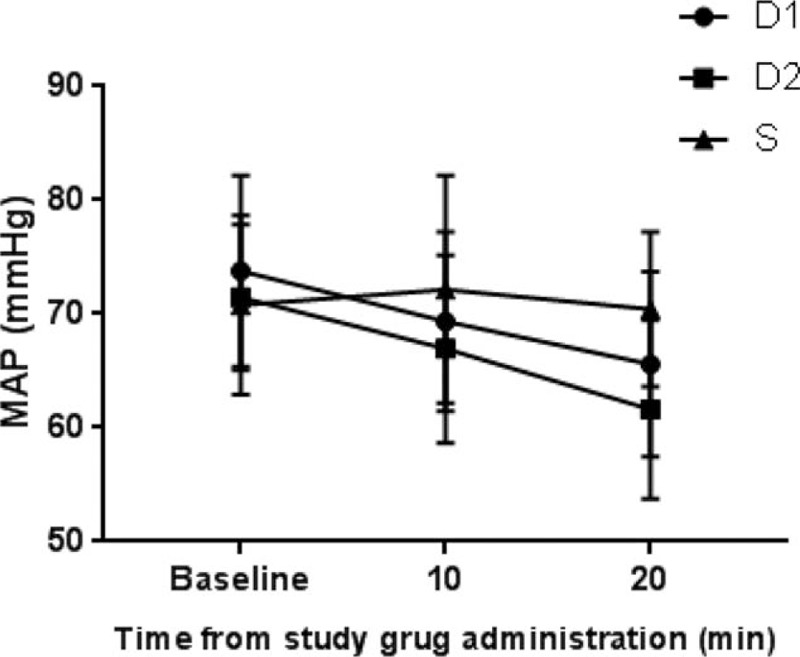
Changes in MAP after study drug administration. MAP = mean blood pressure.
During postoperative recovery, the proportions of postoperative agitation in the D1, D2, and S groups were 43.3%, 30.0%, and 63.3%, respectively. There was a statistical difference between the D2 and S groups (P = .010), while there was no significant difference between the D1 and S groups (P = .099) and the D1 versus D2 groups (P = .211). As for the PAED scale scores, there was a significant difference between the D2 and S groups (P = .029), while there was no significant difference between the D1 and S groups (P = .087), or between the D1 and D2 groups (P = .890) (Fig. 5). There was a significant difference of CHEOPS scores between the D2 and S groups (P = .013), while there was no significant difference between the D1 and S groups (P = .483), or between the D1 and D2 groups (P = .199) (Fig. 6). None of children in the three groups was found with serious postoperative agitation and postoperative pain requiring intravenous propofol or dezocine.
Figure 5.
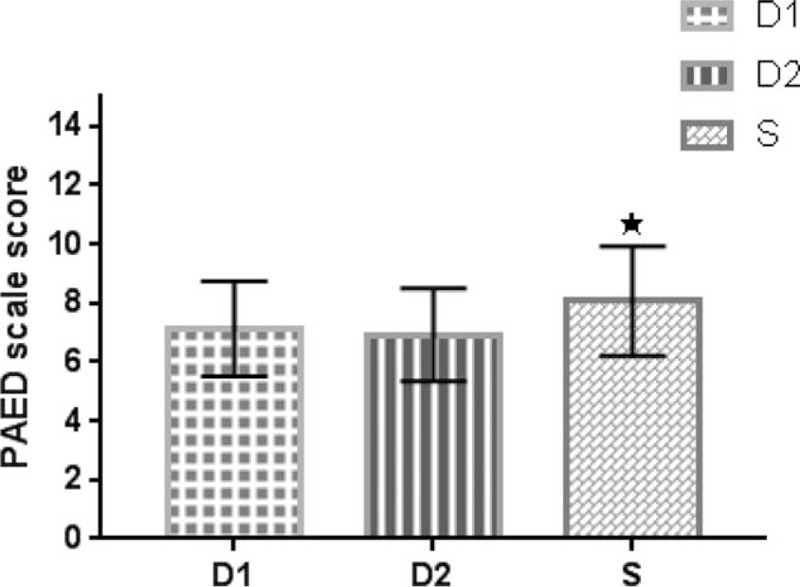
PAED scale score in 3 groups. Values are expressed as mean ± SD. “★” indicates P < .05 between group D2 and group S. PAED = Pediatric Anesthesia Emergence Delirium, SD = standard deviation.
Figure 6.
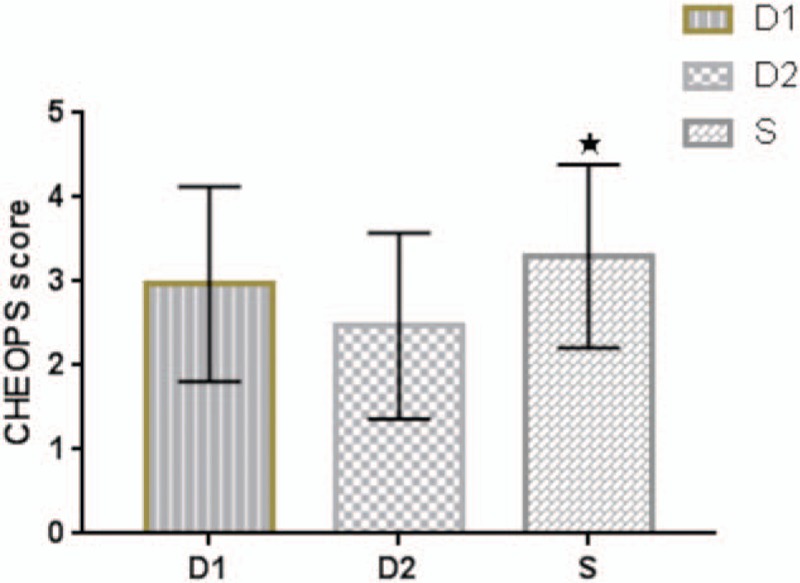
(CHEOPS) score in three groups. Values are expressed as mean ± SD. “★” indicates P < .05 between group D2 and group S. CHEOPS = Children's Hospital of Eastern Ontario Pain Scale, SD = standard deviation.
There was no statistically significant difference in the extubation time (P = .465) or in the PACU time (P = .626) between the three groups. There was no difference in the nurses’ satisfaction (P = .147) and the parents’ satisfaction (P = .438) with the anesthesia. After surgery, there was 1 child with shivering in the S group and 1 with vomiting in the D1 group, and no other adverse events were observed.
5. Discussion
This study showed that intranasal dexmedetomidine by 1 μg kg−1 or 2 μg kg−1 25 to 40 minutes before surgery could achieve satisfactory sedation in most children compared with the control group. In addition, the children were less distressful at separation from parents, and both their heart rate and blood pressure were within 30% of the baseline and no hypoxemia occurred. After adenoidectomy with or without tonsillectomy with TIVA, compared with the dose of 1 μg/kg, intranasal dexmedetomidine of 2 μg·kg−1 was more effective in reducing postoperative agitation and postoperative pain, without prolonging the postoperative recovery time or causing adverse events.
Dexmedetomidine is a highly selective α2 adrenergic receptor agonist featuring sedative, antianxiety, and anagesia properties.[6] Unlike other sedative drugs that act on the gamma-aminobutyric acid system, dexmedetomidine induces hypnosis through an endogenous hypnotic promoting pathway, which delivers awakable sedation without causing respiratory depression.[16] Nasal administration is a convenient, non-invasive, effective approach of administration that avoids the first-pass effects. Many studies have confirmed that intranasal dexmedetomidine can enable children to achieve satisfactory sedation during imaging scan or anesthesia induction. Yuen, et al administered the children aged 1 to 8 years old with intranasal dexmedetomidine of 1 μg kg−1 and 2 μg kg−1 about 30 minutes before surgery, and the proportions of achieving satisfactory sedation during anesthesia induction were 53% and 66%, respectively.[17] However, in this study, such proportions in the D1 and D2 groups were 63.3% and 76.7%, respectively. The underlying reason for this difference may be the different criteria for the study population and satisfactory sedation. The time of intranasal administration was also an important factor affecting preoperative sedation. In another study on the optimal time of intranasal dexmedetomidine before surgery, Yuen et al found that when the preoperative administration was extended to 45 minutes, 91% of the children undergoing intranasal dexmedetomidine of 1 μg kg−1 achieved satisfactory sedation during venipuncture.[11] In this study, the time of preoperative administration was 25 to 40 minutes before induction. When the duration was extended, the proportion of children achieving satisfactory sedation would increase. In the actual clinical practice, however, the duration of preoperative administration was not fixed but depended on the arrangement of the operating room. The subjects in this study were preschool children aged 2 to 7 years old, and the criteria for satisfactory sedation were a MOAA/S score ≥3. In practice, the requirements for the sedation of children at different ages may differ. Children aged 5 to 7 years old can remain cooperative and calm at a lighter deep level of sedation, while those aged 2 to 4 years old need a deeper level of sedation, especially at separation from parents. However, the sample size in this study was underpowered to allow a hierarchical study of children at different ages. For the proportion of satisfactory sedation and score of movement at separation from parents, there was no significant difference between the D1 and D2 groups. This might be due to the fact that the sample size in this study was underpowered to detect the difference between the 2 groups, or that for the preoperative sedation, the dose of 1 μg kg−1 might achive the ceiling effect.
Hemodynamic changes should be noted when using dexmedetomidine. When healthy adult volunteers were injected intravenously with dexmedetomidine of 2 μg kg−1 within 5 minutes, the mean arterial pressure increased by 22% and heart rate decreased by 27%.[18] In another study, Petroz et al found that after pumping dexmedetomidine to children of 2 μg kg−1h−1, 4 μg kg−1h−1, and 6 μg kg−1h−1 for 10 minutes, heart rate and blood pressure decreased gradually with increasing does of dexmedetomidine.[19] In our study, after dexmedetomidine was administered in D1 and D2 groups, the mean arterial pressure and heart rate decreased during preoperative administration, but the decreases were acceptable (within 30% of the baseline) and no extra intervention was required. This may be related to the speculation that intranasal administration slows down the drug absorption and thus avoids sharp fluctuations in hemodynamics.
As 1 of the common postoperative complications of children, postoperative agitation is mainly described as purposeless restlessness, kicking, crying, moaning, and disorientation.[13] The etiology of postoperative agitation has not yet been fully understood. Postoperative pain may be one of its predisposing factors. This also explains why fentanyl can reduce the occurrence of postoperative agitation.[20] However, fentanyl may increase the risks of postoperative nausea and vomiting. Moreover, if respiratory depression caused by opioids is handled improperly, which will bring serious consequence.[21] The sedative and analgesic effects of dexmedetomidine make us expect its perioperative use. Cao et al observed the effects of intraoperative pump injection of dexmedetomidine on the children's recovery, their results showed that intraoperative pump injection of dexmedetomidine could relieve postoperative pain and postoperative agitation.[22] Peng et al. also demonstrated that intraoperative pump injection of dexmedetomidine could decrease postoperative agitation in infants and young children with intravenous-inhalation combined anesthesia to 15% compared with the control group (90%).[23] It has been shown that intranasal dexmedetomidine can reduce children's postoperative agitation in inhaled anesthesia.[12] However, the effects of intranasal dexmedetomidine before surgery on the postoperative agitation of children with TIVA have not been studied. This study demonstrated that intranasal dexmedetomidine of 2 μg kg−1 before surgery could reduce the postoperative delirium and lessen postoperative pain in children with TIVA, without causing obvious adverse events or prolonging the recovery time. Compared with the control group, intranasal dexmedetomidine of 1 μg kg−1 tended to blunt postoperative delirium and pain, but there was no statistically significant difference. The possible reason might be that the drug concentration was too low to alleviate postoperative delirium and pain, or that the sample size of this study was underpowered to detect the difference between the two groups.
Factors such as volatile anaesthetics, type of surgery, patient age, parental and patient anxiety, patient pre-existing behaviour, parent and patient interaction with hearth-care providers have been associated with emergency delirium.[24] Although studies have shown that TIVA can reduce postoperative agitation compared with inhalational anesthesia, postoperative agitation remains a inevitable issue with TIVA.[25] Tsiotou et al observed that intravenous dexmedetomidine 1 μg kg−1 significantly reduced the incidence of postoperative agitation to 16.0% and 12.9% respectively (in comparison with 48.3% and 41.4% respectively in the control group) after 20 and 30 minutes arriving at the PACU in children undergoing tonsillectomy with TIVA, without prolonging the extubation time.[26] The incidence of postoperative agitation in D1, D2, and S groups of our study was 43.3%, 30.0%, and 63.3%, respectively. These differences primarily due to the different pathway and time of dexmedetomidine administration, and the assessment criteria for postoperative agitation.
There were some deficiencies in our study. First of all, because of the unique nature of clinical research, the children were numbered and randomized according to the sequence of the surgery time, instead of the children's substantial characteristics. Second, the children who had failed in preoperative administration due to fierce resistance were excluded from our study, but statistical analysis showed no significant difference in the demographic characteristics between them and the ultimately enrolled ones. Another deficiency is that this study adopted the MOAA/S to evaluate the children's preoperative sedation. Although the observers involved in the assessment were mask of the grouping result, it could be inevitably inferred from the children's behaviors (children undergoing intranasal dexmedetomidine were more sedated), making the assessment not entirely unbiased. In addition, the duration of preoperative administration were not fixed in this study, which could coincide with the actual clinical practices. Furthermore, statistical analysis showed that the duration of preoperative administration was not significantly different between the 3 groups. Finally, the children's preoperative anxiety was associated with postoperative behavioral changes.[2] This study did not track the effects of intranasal dexmedetomidine on the children's postoperative behaviors, which might be further investigated in future.
In summary, the intranasal dexmedetomidine of 1 μg kg−1 or 2 μg kg−1 25–40 minutes before induction of anesthesia both could deliver effective preoperative sedation, reducing the children's distress on separation from parents. Moreover, intranasal dexmedetomidine of 2 μg kg−1 could deliver more effective postoperative analgesia and reduce postoperative agitation, without prolonging postoperative recovery or causing severse adverse events.
Acknowledgments
We thank for assistans of the nurses in the Department of Anesthesiology, Jilin University Second Hospital.
Author contributions
Conceptualization: Li-Qin Li, Hou-Zhong Zhang.
Data curation: Hong-Liu Lu.
Formal analysis: Li-Qin Li, Hong-Liu Lu.
Investigation: Cong Wang.
Methodology: Li-Qin Li, Cong Wang.
Software: Cong Wang, Hong-Yu Xu, Hong-Liu Lu.
Supervision: Hou-Zhong Zhang.
Writing – original draft: Li-Qin Li, Hong-Yu Xu.
Writing – review & editing: Hou-Zhong Zhang.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, CHEOPS = Children's Hospital of Eastern Ontario Pain Scale, PACU = post-anesthesia care unit, PAED = Pediatric Anesthesia Emergence Delirium, TIVA = total intravenous anesthesia.
This study was supported by the Project of Nature Science Foundation of Jilin Province Science and Technology Department (20160101038JC).
The authors have no conflicts of interest to disclose.
References
- [1].Watson AT, Visram A. Children's preoperative anxiety and postoperative behaviour. Paediatr Anaesth 2003;13:188–204. [DOI] [PubMed] [Google Scholar]
- [2].Kain ZN, Mayes LC, O’Connor TZ, et al. Preoperative anxiety in children. Predictors and outcomes. Archiv Pediatr Adolesc Med 1996;150:1238–45. [DOI] [PubMed] [Google Scholar]
- [3].Kain ZN, Mayes LC, Caldwell-Andrews AA, et al. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics 2006;118:651–8. [DOI] [PubMed] [Google Scholar]
- [4].Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg 2003;96:1625–30. [DOI] [PubMed] [Google Scholar]
- [5].Eckenhoff JE, Kneale DH, Dripps RD. The incidence and etiology of postanesthetic excitment. A clinical survey. Anesthesiology 1961;22:667–73. [DOI] [PubMed] [Google Scholar]
- [6].Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs 2000;59:263–70. [DOI] [PubMed] [Google Scholar]
- [7].Sheta SA, Al-Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr Anaesth 2014;24:181–9. [DOI] [PubMed] [Google Scholar]
- [8].Farnia MR, Jalali A, Vahidi E, et al. Comparison of intranasal ketamine versus IV morphine in reducing pain in patients with renal colic. Am J Emerg Med 2017;35:434–7. [DOI] [PubMed] [Google Scholar]
- [9].Roelofse JA, Shipton EA, De la Harpe CJ, et al. Intranasal sufentanil/midazolam versus ketamine/midazolam for analgesia/sedation in the pediatric population prior to undergoing multiple dental extractions under general anesthesia: a prospective, double-blind, randomized comparison. Anesth Prog 2004;51:114–21. [PMC free article] [PubMed] [Google Scholar]
- [10].Iirola T, Vilo S, Manner T, et al. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol 2011;67:825–31. [DOI] [PubMed] [Google Scholar]
- [11].Yuen VM, Hui TW, Irwin MG, et al. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia 2010;65:922–9. [DOI] [PubMed] [Google Scholar]
- [12].Lin Y, Chen Y, Huang J, et al. Efficacy of premedication with intranasal dexmedetomidine on inhalational induction and postoperative emergence agitation in pediatric undergoing cataract surgery with sevoflurane. J Clin Anesth 2016;33:289–95. [DOI] [PubMed] [Google Scholar]
- [13].Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 2004;100:1138–45. [DOI] [PubMed] [Google Scholar]
- [14].Tyler DC, Tu A, Douthit J, Chapman CR. Toward validation of pain measurement tools for children: a pilot study. Pain 1993;52:301–9. [DOI] [PubMed] [Google Scholar]
- [15].Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth 1995;7:89–91. [DOI] [PubMed] [Google Scholar]
- [16].Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol 2008;21:457–61. [DOI] [PubMed] [Google Scholar]
- [17].Yuen VM, Hui TW, Irwin MG, et al. A randomised comparison of two intranasal dexmedetomidine doses for premedication in children. Anaesthesia 2012;67:1210–6. [DOI] [PubMed] [Google Scholar]
- [18].Dyck JB, Maze M, Haack C, et al. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology 1993;78:813–20. [DOI] [PubMed] [Google Scholar]
- [19].Petroz GC, Sikich N, James M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology 2006;105:1098–110. [DOI] [PubMed] [Google Scholar]
- [20].Kim N, Park JH, Lee JS, et al. Effects of intravenous fentanyl around the end of surgery on emergence agitation in children: Systematic review and meta-analysis. Paediatr Anaesth 2017;27:885–92. [DOI] [PubMed] [Google Scholar]
- [21].Chidambaran V, Olbrecht V, Hossain M, et al. Risk predictors of opioid-induced critical respiratory events in children: naloxone use as a quality measure of opioid safety. Pain Med 2014;15:2139–49. [DOI] [PubMed] [Google Scholar]
- [22].Cao JL, Pei YP, Wei JQ, et al. Effects of intraoperative dexmedetomidine with intravenous anesthesia on postoperative emergence agitation/delirium in pediatric patients undergoing tonsillectomy with or without adenoidectomy: a CONSORT-prospective, randomized, controlled clinical trial. Medicine 2016;95:e5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Peng W, Zhang TJ. Dexmedetomidine decreases the emergence agitation in infant patients undergoing cleft palate repair surgery after general anesthesia. BMC Anesthesiol 2015;15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mason KP. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br J Anaesth 2017;118:335–43. [DOI] [PubMed] [Google Scholar]
- [25].Chandler JR, Myers D, Mehta D, et al. Emergence delirium in children: a randomized trial to compare total intravenous anesthesia with propofol and remifentanil to inhalational sevoflurane anesthesia. Paediatr Anaesth 2013;23:309–15. [DOI] [PubMed] [Google Scholar]
- [26].Tsiotou AG, Malisiova A, Kouptsova E, et al. Dexmedetomidine for the reduction of emergence delirium in children undergoing tonsillectomy with propofol anesthesia: a double-blind, randomized study. Paediatr Anaesth 2018;1–7. [DOI] [PubMed] [Google Scholar]


