Supplemental Digital Content is available in the text
Keywords: fever, non-steroidal anti-inflammatory drugs, non-small cell lung cancer, overall survival, progression-free survival, survival analysis
Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used to relieve postoperative fever, surgery pain, and inflammation. In addition, NSAIDs have anticancer activity and may reduce the risk and mortality of several cancers. However, the association between postoperative NSAIDs and the clinical outcome of non-small cell lung cancer (NSCLC) patients with fever after surgery is not fully understood. We performed a retrospective study of NSCLC patients who underwent surgery between July 2011 and June 2012, aiming to evaluate the effect of postoperative NSAIDs on overall survival (OS) and progression-free survival (PFS). Differences in clinical data between the postoperative NSAIDs group and non-NSAIDs groups were analyzed by Chi-square tests. Kaplan-Meier curves method and Cox regression analysis were conducted for survival analysis. The primary and secondary endpoints were OS and PFS, respectively. This retrospective study included 347 NSCLC patients. There were no significant differences in the clinical characteristics between the NSAIDs group and non-NSAIDs group except for age (P = .024) and differential degree (P = .040). Administration of postoperative NSAIDs was related to longer OS (hazards ratio [HR] 0.528, 95% confidence interval [CI] 0.278–0.884, P = .006) and longer PFS (HR 0.557, 95% CI 0.317–0.841, P = .002) in the multivariate Cox regression model. Subgroup analysis showed statistically significant differences in elderly individuals, male subjects, low smoking index, poor differentiation, and non-adenocarcinoma subgroups, respectively. In conclusion, the administration of postoperative NSAIDs was related to longer OS and PFS in NSCLC patients with postoperative fever.
1. Introduction
Despite recent progress in the treatment, lung cancer remains 1 of the most common types of malignancies,[1] both in terms of new cases (1.8 million, 12.9% of total) and deaths (1.6 million, 19.4% of total) because of its high case f mortality rate.[2] Therefore, lung cancer remains an intractable problem that notably affects the health and survival of the humans worldwide and poses significant clinical, societal and health-payer burdens., societal and health payer perspective. Lung cancer is broadly divided into small cell (SCLC) and non-small cell lung cancer (NSCLC). For NSCLC patients with early-stage disease, surgery is the most effective treatment and offers more clinical benefits.[3]
Surgeries are often accompanied by complications. The most common complications include postoperative fever and surgery pain, which are mainly caused by inflammation. The potential relationship between inflammation and cancer has been widely studied; findings suggest that an inflammatory microenvironment promotes tumor growth, progression, and immunosuppression.[4,5] Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit cyclooxygenase (COX)-2 enzymes, which are important in the inflammatory process and in the pathogenesis of lung cancer, particularly adenocarcinoma.[6–8] Accordingly, NSAIDs are widely used for the treatment of postoperative fever and surgery pain. In addition, there is growing evidence that NSAIDs have anticancer activity and could decrease cancer risk.[9,10] However, the association between postoperative NSAIDs use and the clinical outcome (long-term survival) in lung cancer has not been definitely investigated and remains unclear.
We performed a retrospective study of NSCLC patients who underwent surgery between July 2011 and June 2012 to evaluate the effect of postoperative NSAIDs use on overall survival (OS) and progression-free survival (PFS).
2. Patients and methods
2.1. Ethics permission
This study acquired permission from the Ethical Committee of Provincial Hospital Affiliated with Shandong University. All included patients provided a signed consent for study participation. Written informed consent for the use of clinical data was obtained at the time of surgery.
2.2. Patients
We performed a retrospective study of NSCLC patients who underwent surgery at the Department of Thoracic Surgery, Provincial Hospital Affiliated to Shandong University. These lung cancer cases were identified and selected from the Bio-Bank of Shandong Provincial Hospital. Surgeries were performed between July 2011 and June 2012. Patients with postoperative fever within 48 hours after surgery were the objects of our study.
According to their postoperative medication status, the patients were divided into 2 groups (NSAIDs and non-NSAIDs groups). The NSAIDs in this study included indomethacin (rectal suppositories or oral tablets) and ibuprofen (oral tablets). The usages were as follows:
-
1)
indomethacin suppositories, 75 mg (1 piece), q.d;
-
2)
indomethacin tablets, 25 mg, t.i.d; or
-
3)
ibuprofen tablets, 200 mg, t.i.d. The drugs were administrated continuously until the postoperative fever had completely subsided. The median time NSAIDs use was 6 days, varying from 2 to 10 days. The patients in the non-NSAIDs group were treated with physical cooling (ice bag with 10% ice saline).
According to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0,[11] a body temperature ≥38.0C°C was defined as a fever and fever was divided into 5 grades: fever ≥38.0°C and <39.0°C was defined as grade 1; ≥39.0°C and <40.0°C as grade 2; ≥40.0°C for ≤24 hours as grade 3; ≥40.0°C for >24 hours as grade 4; and death due to fever as grade 5. For safety, patients with grade 4 or 5 fever were sent to the Intensive Care Unit (ICU) immediately. These patients received comprehensive and emergency treatment rather than postoperative NSAIDs alone.
In this study, the exclusion criteria were as follows:
-
1)
prior diagnosis of cancer or multiple primary tumors;
-
2)
incomplete clinical data;
-
3)
other histological type such as SCLC;
-
4)
postoperative death in hospital;
-
5)
advanced stage or unclear pathologic stage; or
-
6)
grade 4 or 5 postoperative fever. In view of these criteria, 221 of 568 patients were excluded and 347 patients were ultimately selected in our study.
Patients were followed-up every 3 months for the first year post-surgery and then every 6 months. We contacted the patients or their family members by phone to collect follow-up data. The incidence of loss to follow-up was 18.7% (65 patients). Fifty-two of 65 patients were out of touch owing to moving, personal information changes, and so forth. A further 13 of 65 cases refused to participate in the present study. OS was defined as the time from operation to death for any reason. For the patients who were alive, OS was defined as the time from operation to the date of the last follow-up. PFS was defined as the time from operation to recurrence (local recurrence or distant metastasis). For patients without recurrence, PFS was defined as the time from operation to the date of the last follow-up or death. The histology stage was determined according to the classification criteria for lung tumors from the World Health Organization and International Association for the Study of Lung Cancer (WHO/IASLC).
2.3. Statistical analysis
The relationship between OS/PFS and postoperative NSAIDs use was assessed with Kaplan–Meier survival estimates. Univariate and multivariate Cox proportional hazards regression analysis (hazards ratio [HR], 95% confidence intervals [CIs], and P-values) were calculated to evaluate the correlations between OS/PFS and multiple variables. Differences in clinical features between the NSAIDs and no-NSAIDs groups were analyzed by Chi-square tests.
Regarding the clinical data, categorical variables were represented as counts and continuous variables as medians. We transformed each continuous variable into dichotomous values for further statistical analysis. The optimal cut-off value was defined as the maximum log-rank statistical value identified by receiver operating characteristic (ROC) curves. Two-tailed P-values were calculated and differences were considered statistically significant if the P-value was less than .05. All the statistical analyses were calculated using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY).
3. Results
3.1. Baseline clinical data summary
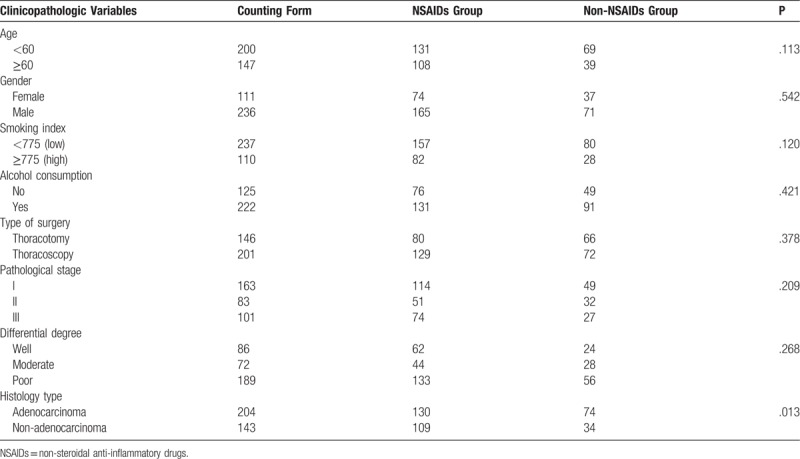
The features of the included patients are presented in Table 1. This study included 111 women (32.0%) and 236 men (68.0%). Their ages varied from 21 to 83 years, with a median of 60 years. The follow-up duration varied from 1 to 60 months (median: 32 months). The NSAIDs and non-NSAIDs groups included 239 (68.9%) and 108 (31.1%) patients, respectively. Table 1 showed that there were no significant differences between the 2 groups in gender, smoking index, pathological stage, and histology type except for age (P = .024) and differential degree (P = .040).
Table 1.
Baseline characteristics of patients and the association with postoperative NSAIDs.
3.2. Survival analysis
The OS and PFS Kaplan–Meier survival curves of the NSAIDs and non-NSAIDs groups are shown in Figures 1 and 2, respectively. There was a significant separation in the survival curves between the 2 groups for both OS (P = .015) and PFS (P = .018). This finding suggests that the administration of postoperative NSAIDs was related to longer OS and PFS in NSCLC patients with postoperative fever after surgery.
Figure 1.
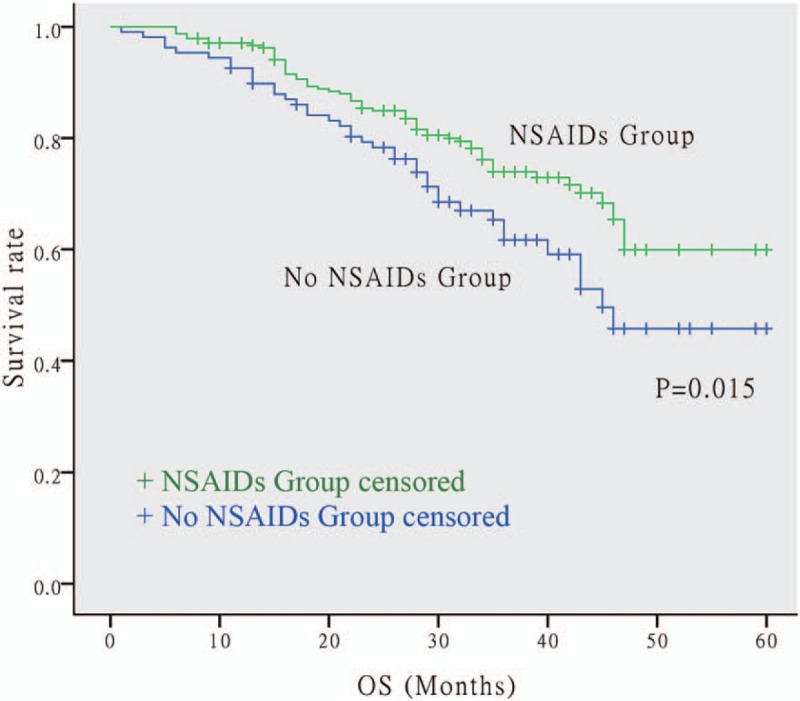
Kaplan–Meier survival curve for OS between NSAIDs group and none NSAIDs group. The NSCLC patients using postoperative NSAIDs had longer OS. The P value is .015. NSAIDs = non-steroidal anti-inflammatory drugs, NSCLC = non-small cell lung cancer, OS = overall survival.
Figure 2.
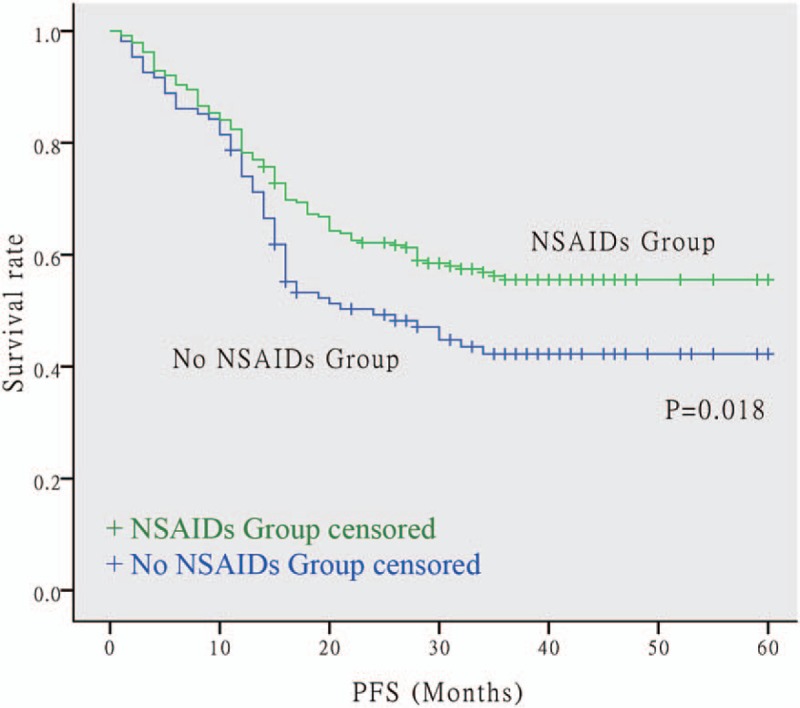
Kaplan–Meier survival curve for PFS between NSAIDs group and none NSAIDs group. The NSCLC patients using postoperative NSAIDs had longer PFS. The P value is .018. NSAIDs = non-steroidal anti-inflammatory drugs, NSCLC = non-small cell lung cancer, PFS = progression-free survival.
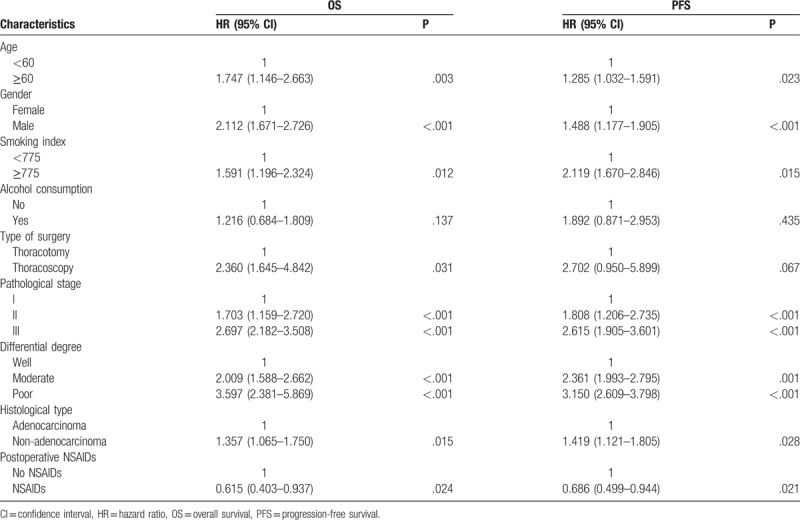
3.3. Univariate Cox regression analysis
The univariate Cox regression analyses of the prognostic factors for OS and PFS are shown in Table 2. After testing the proportional hazards assumption, variables including age, gender, smoking index, pathological stage, differential degree, histological type, and postoperative NSAIDs use met the inclusion criteria for multivariate Cox analysis for both OS and PFS. The results showed that elder age, male sex, higher smoking index, worse pathological stage, poorer differential degree, and non-adenocarcinoma predicted shorter OS and PFS. Only postoperative NSAIDs use predicted longer OS (HR 0.615, 95% CI 0.403–0.937, P = .024) and PFS (HR 0.686, 95% CI 0.499–0.944, P = .021).
Table 2.
Univariate Cox regression analysis of prognostic factors for OS and PFS.
3.4. Multivariate Cox regression analysis
Multivariate Cox regression was performed to examine the independent prognostic factors for OS and PFS. The results are shown in Table 3. For both OS and PFS, age, gender, pathological stage, differential degree, and postoperative NSAIDs use were all significant independent prognostic factors (all P < .05). In addition, the smoking index was also a significant independent prognostic factor for PFS (P < .05). In summary, the administration of postoperative NSAIDs was associated with a decreased risk of recurrence (P = .002) and lower mortality (P = .006).
Table 3.
Multivariate Cox regression analysis of prognostic factors for OS and PFS.
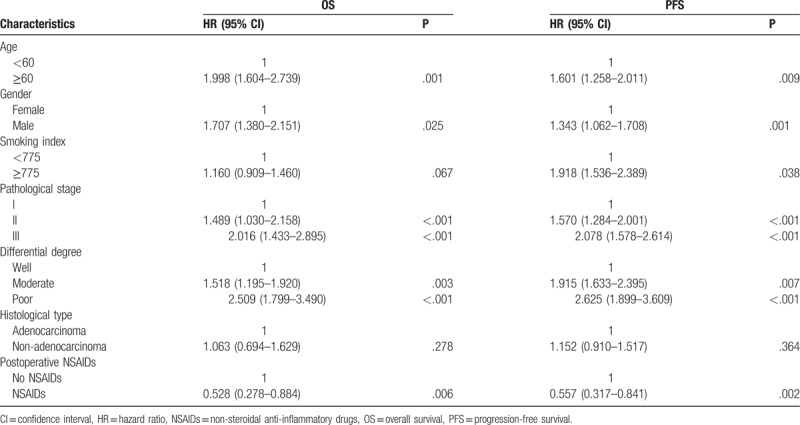
3.5. Subgroup analysis
The results of subgroup analyses are shown in Figure 3. There were statistically significant differences in the following subgroups: elderly (age ≥60 years, P = .030), male (P = .037), low smoking index (P = .010), non-adenocarcinoma (P = .032), and poor differentiation (P = .003). In these subgroups, patients who used postoperative NSAIDs presented a longer survival than those in patients who did not. In the other subgroups, no statistically significant differences between the NSAIDs and non-NSAIDs groups were observed.
Figure 3.
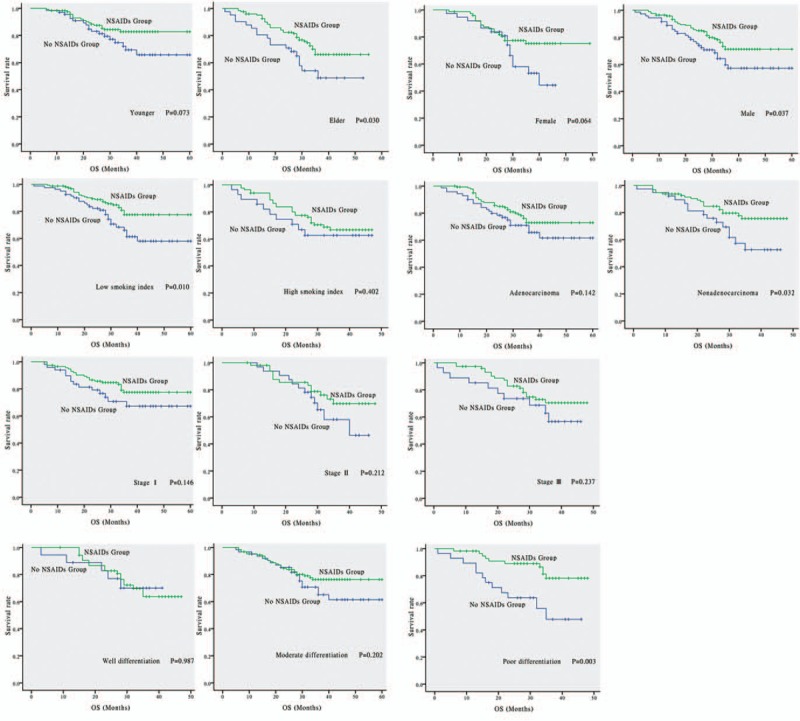
Subgroups analysis of OS between NSAIDs group and none NSAIDs group. There are statistically significant differences in following subgroups: elders (age ≥ 60, P = .030), males (P = .037), low smoking index (P = .010), nonadenocarcinoma (P = .032) and poor differentiation (P = .003). There are no statistically differences in following subgroups: youngsters (age < 60, P = .073), females (P = .064), high smoking index (P = .402), adenocarcinoma (P = .142), stage I (P = .146), stage II (P = .212), stage III (P = .237), well differentiation (P = .987) and moderate differentiation (P = .202). NSAIDs = non-steroidal anti-inflammatory drugs, OS = overall survival.
To verify our results, we collected and analyzed data from Eastern Hospital of Shandong Provincial Hospital as Center 2. These data are shown in Supplementary Tables 1, 2, and 3. The results were comparable to those of the previous data.
4. Discussion
In our study, the results of survival analysis suggested that patients who used postoperative NSAIDs had longer OS and lower recurrence than those in patients who did not use postoperative NSAIDs. In addition, the administration of postoperative NSAIDs may extend the OS and PFS in early-stage NSCLC patients with postoperative fever. This phenomenon may be related to the anticarcinogenic activity of NSAIDs. Trial evidence and epidemiologic data have supported the anticarcinogenic effects of NSAIDs, which could be used as a common means of chemoprevention for cancers.[12–14] However, the anticancer mechanisms of NSAIDs are not yet well understood so far.
COX is a family of isozymes responsible for the formation of prostanoids, including thromboxane and prostaglandins (PGs) such as PGE2.[15] They catalyze the initial step in the production of PGs in a variety of physiological processes such as inflammatory reactions. Additionally, COX is broadly involved in the process of tumorigenesis. However, their precise role and causality in tumorigenesis remain controversial. According to their association with COX, the relevant mechanisms may be divided into COX-dependent and COX-independent mechanism.
COX-dependent mechanism is associated with the activity and function of COX (mainly referred to COX-2) and PGs (mainly referred to PGE2). Although lots of questions remain elusive, we can still partly explain how COX-2/PGE2 contributes to cancers.[16] PGE2 is the only prostanoid demonstrated to play a predominant role in promoting tumor formation, progression, and metastasis by acting directly on tumor cells. The PGE2 concentration is elevated in cells with COX-2 overexpression and PGE2 is considered as the most important downstream effector of COX-2.[17] The possible downstream signaling pathways include
-
1)
Phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B (PI3K/Akt) pathway. PGE2 can induce apoptosis inhibition by stimulation of the PI3K/Akt pathway.[18]
-
2)
Epidermal growth factor receptor (EGFR) pathway. The EGFR pathway can be transactivated by PGE2 and thus induce or promote tumor growth.[19]
-
3)
Vascular endothelial growth factor (VEGF) pathway. PGE2 could enhance the production of VEGFs through hypoxia-inducible factor 1-alpha (HIF1α) nuclear translocation and then promote angiogenesis.[20] In addition, PGE2 can promote the activation of Rac1 (a small G-protein that can drive actin formation of lamellipodia and promote cell-cell adhesion and migration of different tumor cells) and induce the expression of matrix metalloproteinases (MMPs).[21] COX-2/PGE2 overexpression can inhibit tumor cell apoptosis; promote tumor growth and angiogenesis; and favor tumor cell adhesion, motility, and invasion. NSAIDs can reduce COX-2/PGE2 concentration by inhibiting the activity of COX-2, exerting antitumor activity.
There is evidence that NSAIDs exhibit COX-independent antineoplastic activity.[22] The possible mechanisms include:
-
1)
NSAIDs can affect the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway. NF-κB activation is associated with the increased survival of tumor cells and resistance to chemotherapy. Aspirin and some other NSAIDs can inhibit this pathway by interacting with IκB kinase (IKK).[23]
-
2)
NSAIDs can affect transforming growth factor beta 1 (TGF-β) signaling. Nonsteroidal anti-inflammatory drug-activated gene-1 (NAG-1), a member of the TGF-β superfamily of proteins, is highly induced by most conventional NSAIDs.[24]
-
3)
NSAIDs such as indomethacin can selectively activate the double-stranded RNA-dependent protein kinase (PKR). The activation of PKR can cause rapid phosphorylation of eukaryotic initiation factor 2-alpha (eIF2α) and inhibit protein synthesis in cancer cells. The PKR-mediated translational block is followed by the inhibition of cancer cell proliferation and induction of apoptosis.[25]
In summary, both COX-dependent and COX-independent mechanisms of NSAIDs play vital roles in anti-tumorigenesis. However, the precise mechanisms remain a matter of debate. Further studies are required to elucidate these complicated mechanisms.
In subgroup analysis, patients using postoperative NSAIDs presented a better OS, especially in the elderly, male, low smoking index, poor differentiation, and non-adenocarcinoma subgroups, respectively. This finding indicated that the use of postoperative NSAIDs could provide additional clinical benefits to patients in these subgroups.
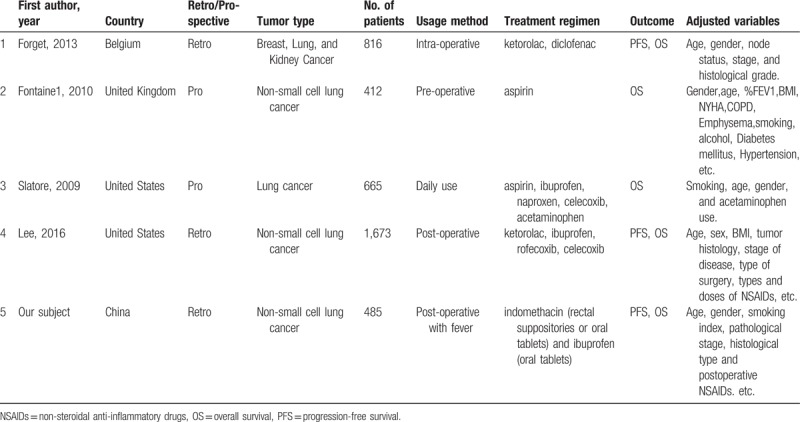
A growing number of studies have assessed the association between NSAIDs use and survival of patients with cancer. The perioperative inflammatory status is associated with survival and the administration of NSAIDs might improve the survival.[14,26,27] However, Lee reported that the perioperative use of NSAIDs was not an independent predictor of survival[28] (Table 4). Controversial findings exist regarding the prognostic value of NSAIDs administration and how to identify individuals most likely to benefit from chemoprevention using NSAIDs. Compared to these studies, we extensively evaluated the indications for NSAID use in patients with postoperative fever within 48 hours after surgery. Fever and pain are the most common indicators of inflammation, which could help to identify the most likely beneficiaries of this therapy.
Table 4.
Baseline characteristics of recent similar studies.
Our study has the inherent limitations of any retrospective study and the results must be interpreted with caution. Confounding variables not included in the statistical analysis could have influenced the survival findings in this study population. For example, we did not account for the impact of immune suppression and inflammation or for the influence of the tumor histological type, recurrence type (locoregional recurrence versus distant metastasis), adjuvant treatment, or intraoperative conditions. Our analysis also did not include variables such as intraoperative versus postoperative use of NSAIDs, class of NSAID, or the total amount of each NSAID. Therefore, we cannot comment on whether the class of NSAID, time, or amount of each NSAID administered might have affected the survival of our population of patients. The statistical power was limited by the number of patients; therefore, type II error was possible.
The National Comprehensive Cancer Network (NCCN) guidelines (version 2, 2017) indicate that aspirin or NSAIDs may decrease the risk of colorectal cancer. Unlike colon cancer, there has not yet been a consensus on the administration of NSAIDs for NSCLC. Based on the results of our study, we suggest that the NSCLC patients with postoperative fever could use NSAIDs as routine medication for their clinical outcome. However, the potential adverse drug reactions of NSAIDs must also be considered. Postoperative NSAIDs should be used under the premise of ensuring patient safety. Moreover, a prospective study is needed to confirm the relationship between postoperative NSAIDs use and long-term outcome of patients. Although there remains controversy, a consensus on the administration of NSAIDs for NSCLC needs to be reached.
Author contributions
Conceptualization: Hongchang Shen, Jiajun Du.
Data curation: Liguang Wang, Wei Dong, Tiehong Zhang.
Formal analysis: Wei Dong, Tiehong Zhang.
Methodology: Liguang Wang, Jiangang Zhang, Kai Wang.
Software: Liguang Wang.
Supervision: Hongchang Shen, Jiajun Du.
Writing – original draft: Jiangang Zhang.
Writing – review & editing: Wensheng Jiang, Liguang Wang, Xiaowei Li, Jiajun Du.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, COX = cyclooxygenase, EGFR = epidermal growth factor receptor, HR = hazards ratio, NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells, NSAIDs = non-steroidal anti-inflammatory drugs, NSCLC = non-small cell lung cancer, OS = overall survival, PFS = progression-free survival, PI3K/Akt = phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B, PKR = double-stranded RNA-dependent protein kinase, TGF-β = transforming growth factor beta 1, VEGF = vascular endothelial growth factor.
WJ and LW contributed equally to this work. JD and HS contributed to the conception and design of the study. LW, JZ, and KW developed the methodology. TZ and WD acquired the data. HS, LW, TZ, and JD analyzed and interpreted the data. LW, WJ, and XL wrote, reviewed, and/ or revised the manuscript. TZ, WD, and HS provided administrative, technical, or material support. JD and HS supervised the study.
This study was supported by National Natural Science Foundation of China (project 81602009 and 81672288).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- [3].Flehinger BJ, Kimmel M, Melamed MR. The effect of surgical treatment on survival from early lung cancer. Implications for screening. Chest 1992;101:1013–8. [DOI] [PubMed] [Google Scholar]
- [4].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet (London, England) 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [6].Lee JM, Yanagawa J, Peebles KA, et al. Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol 2008;66:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Sub-cell Biochem 2007;42:93–126. [DOI] [PubMed] [Google Scholar]
- [8].Sandler AB, Dubinett SM. COX-2 inhibition and lung cancer. Sem Oncol 2004;31:45–52. [DOI] [PubMed] [Google Scholar]
- [9].Moody TW, Leyton J, Zakowicz H, et al. Indomethacin reduces lung adenoma number in A/J mice. Anticancer Res 2001;21:1749–55. [PubMed] [Google Scholar]
- [10].Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology (Camb Mass) 1994;5:138–46. [DOI] [PubMed] [Google Scholar]
- [11].Kluetz PG, Chingos DT, Basch EM, et al. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the national cancer institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Am Soc Clin Oncol Edu Book 2016;35:67–73. [DOI] [PubMed] [Google Scholar]
- [12].Harris RE, Beebe-Donk J, Schuller HM. Chemoprevention of lung cancer by non-steroidal anti-inflammatory drugs among cigarette smokers. Oncol Rep 2002;9:693–5. [PubMed] [Google Scholar]
- [13].Chan AT, Giovannucci EL, Meyerhardt JA, et al. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. Jama 2005;294:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Slatore CG, Au DH, Littman AJ, et al. Association of nonsteroidal anti-inflammatory drugs with lung cancer: results from a large cohort study. Cancer Epidemiol Biomarkers Prev 2009;18:1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ghosh N, Chaki R, Mandal V, et al. COX-2 as a target for cancer chemotherapy. Pharmacol Rep: PR 2010;62:233–44. [DOI] [PubMed] [Google Scholar]
- [16].Meric JB, Rottey S, Olaussen K, et al. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol 2006;59:51–64. [DOI] [PubMed] [Google Scholar]
- [17].Wang D, DuBois RN. The Role of Prostaglandin E(2) in Tumor-Associated Immunosuppression. Trends Mol Med 2016;22:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pai R, Soreghan B, Szabo IL, et al. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 2002;8:289–93. [DOI] [PubMed] [Google Scholar]
- [19].Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998;93:705–16. [DOI] [PubMed] [Google Scholar]
- [20].Dormond O, Foletti A, Paroz C, et al. NSAIDs inhibit alpha V beta 3 integrin-mediated and Cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis. Nat Med 2001;7:1041–7. [DOI] [PubMed] [Google Scholar]
- [21].Hull MA, Gardner SH, Hawcroft G. Activity of the non-steroidal anti-inflammatory drug indomethacin against colorectal cancer. Cancer Treat Rev 2003;29:309–20. [DOI] [PubMed] [Google Scholar]
- [22].Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science (NY, NY) 1994;265:956–9. [DOI] [PubMed] [Google Scholar]
- [23].Baek SJ, Okazaki R, Lee SH, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology 2006;131:1553–60. [DOI] [PubMed] [Google Scholar]
- [24].Cekanova M, Lee SH, Donnell RL, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev Res (Philadelphia, Pa) 2009;2:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brunelli C, Amici C, Angelini M, et al. The non-steroidal anti-inflammatory drug indomethacin activates the eIF2alpha kinase PKR, causing a translational block in human colorectal cancer cells. Biochem J 2012;443:379–86. [DOI] [PubMed] [Google Scholar]
- [26].Forget P, Machiels JP, Coulie PG, et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol 2013;20:S650–660. [DOI] [PubMed] [Google Scholar]
- [27].Fontaine E, McShane J, Page R, et al. Aspirin and non-small cell lung cancer resections: effect on long-term survival. Eur J Cardiothoracic Surg 2010;38:21–6. [DOI] [PubMed] [Google Scholar]
- [28].Lee BM, Rodriguez A, Mena G, et al. Platelet-to-lymphocyte ratio and use of NSAIDs during the perioperative period as prognostic indicators in patients with nsclc undergoing surgery. Cancer control J Moffitt Cancer Cent 2016;23:284–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


