Abstract
Dezocine is proposed as an adjunctive analgesic for postoperative pain control. This randomized, double-blind, controlled study aimed to investigate the effect of preoperative Dezocine therapy on postoperative pain following laparoscopic cholecystectomy as well as the underlying mechanisms.
Eighty patients scheduled for laparoscopic cholecystectomy were randomly allocated into 2 groups as follows: patients in Group D received Dezocine 0.15 mg/kg before anesthesia induction and patients in Group S received same volume of saline. The pain intensity, sedation score, sufentanil-based patient-controlled analgesia (PCA) consumption were recorded for 24 hours after surgery. Plasma concentrations of norepinephrine and serotonin were also measured.
During the first 24 hours after surgery, the patients in Group D experienced lower pain score assessed by numerical rating scale (NRS) at 3 hours (rest: P = .038; movement: P = .036), 6 hours (rest: P = .038; movement: P = .036), 12 hours (rest: P = .038; movement: P = .036), and 24 hours (rest: P = .038; movement: P = .036). Dezocine also decreased the sedation levels at 5 minutes (P = .031) after arrival at the PACU. Sufentanil-based PCA consumption in Group D was decreased when compared with Group S in the second to fourth phase after surgery (6–12 hours: P = .017; 12–18 hours: P = .003; 18–24 hours: P = .039). Plasma norepinephrine and serotonin concentrations were higher in the Group D at 24 hours after surgery (norepinephrine: P = .009, serotonin: P = .042). In addition, Group D showed less incidence of nausea/vomiting (P = .032) as well as a higher postoperative satisfaction score after surgery (P = .017).
In conclusion, preemptive Dezocine administration is suggested to be useful for the management of postoperative pain in short-lasting surgery such as laparoscopic cholecystectomy.
Keywords: Dezocine, ERAS, norepinephrine, postoperative analgesia, serotonin
1. Introduction
With the development of the enhanced recovery after surgery (ERAS) concept, there is an urgent need for a more satisfying postoperative pain control and reducing hospital length of stay following short-lasting surgery. Laparoscopic cholecystectomy is one of the most common short-lasting surgery in China; however, the postoperative pain management of such patients did not get enough attention in clinical practice. Therefore, postoperative acute pain has become the most common adverse effect and the primary reason for prolonged convalescence after laparoscopic cholecystectomy.[1,2]
Dezocine has been proven to be a partial μ-receptor agonist, a κ-receptor antagonist, and a norepinephrine and serotonin reuptake inhibitor (via norepinephrine transporter and serotonin transporter).[3] In neuropathic pain mouse models, Dezocine has been shown to attenuate thermal and mechanical pain hypersensitivity through spinal μ-receptor agonist activation and norepinephrine reuptake inhibition.[4,5] Besides, it is now widely used in the clinical as a good adjunctive analgesic with minimum side effects and low dependence liability.[6,7] Recent clinical trials have found that Dezocine can enhance the analgesic effect of morphine and reduce the opioids consumption and opioid-related side effects in patients undergoing open abdominal surgery and thoracotomy.[8,9] However, whether preoperative administration of Dezocine could provide a beneficial effect of postoperative pain management after laparoscopic cholecystectomy is largely unknown.
To address this question, we investigated the analgesic effect of preemptive Dezocine before general anesthesia in patients undergoing laparoscopic cholecystectomy. In addition, we also measured the levels of norepinephrine and serotonin in the plasm of patients to determine if they are involved in Dezocine-mediated clinical pain modulation. This study will provide useful information for guiding future use of Dezocine under general anesthesia, especially in short-lasting surgery.
2. Materials and methods
2.1. Subjects
The study was approved by the Ethic Committee of Zhejiang Armed Police Corps Hospital and was conducted in accordance with the Declaration of Helsinki. All the subjects gave an informed consent.
Patients aged 40 to 70 years of both genders, with American Society of Anesthesiologists (ASA) physical status I–II and scheduled for laparoscopic cholecystectomy under general anesthesia, were recruited. The exclusion criteria were body mass index (BMI) > 30 kg/m2, medication allergy, opioid used for more than 1 month, bronchial asthma, coronary heart disease, severe hypertension, and diabetes mellitus. Patients were also excluded if laparoscopic surgery was converted to open surgery.
Eighty patients were randomized randomly assigned into 2 groups of 40 patients each. Randomization was achieved using computer generated random codes, which was concealed by opaque envelope method. Drugs were prepared in unlabeled 10 mL syringes outside the operating room by an anesthesiologist who was not involved in the induction of anesthesia.
2.2. Anesthesia
None of the patients received any premedication. After the patients arrived at the operating room, standard monitors, including noninvasive arterial pressure, heart rate, electrocardiography, and peripheral capillary oxygen saturation were applied. Oxygen gas flow at 2 L/min was given via a facial mask and ringer lactate was infused at a rate of 4–6 mL/min. After preoxygenation for 2 minutes, the pretreatment drug Dezocine (Yangtze River Pharmaceutical Group, Taizhou, Jiangsu, China) or saline was given. Group D received Dezocine 0.15 mg/kg before induction, while Group S received similar volume of saline. Two minutes after infusion, general anesthesia was induced with midazolam 0.05 mg/kg, sufentanil 0.2 to 0.5 μg/kg, and propofol 2 to 2.5 mg/kg. Tracheal intubation was facilitated with rocuronium 0.7 mg/kg, and then maintained with continuous infusion of 0.3 μg/kg/min remifentanil, sevoflurane [1–3%, according to bispectral index (BIS) and hemodynamics]. Ephedrine was used to maintain blood pressure within 70% of the baseline and atropine was used when the heart rate was < 50 beats/min. The patients were mechanically ventilated through a tracheal intubation to maintain an end-tidal CO2 between 35 and 45 mm Hg. The depth of anesthesia was adjusted on the basis of targeting BIS (40–60) and hemodynamic changes. When the BIS value reached 80, response to oral command was observed, followed by eye opening and spontaneous breathing rate exceeding 10 bpm; the patient was extubated and moved to the postanesthetic care unit (PACU) for at least 1 hour. Each patient was administered analgesics using a patient control patient-controlled analgesia (PCA) pump containing sufentanil (2 μg/kg) in normal saline in a total volume of 100 mL after discharge from the PACU. The device was set to deliver a basal infusion of 2 mL/h and bolus doses of 1 mL with a 15-minute lockout period.
2.3. Data collection
Patient's demographic information was collected on admission. Hemodynamic indexes were monitored every 5 minutes during operation. Postoperative pain at rest and on movement states were evaluated on a 10-point numerical rating scale (NRS): 0 = no pain; and 10 = worst pain imaginable. Ramsay scale was used to get sedation scores: 1 = anxious and agitated, 2 = cooperative and tranquil, 3 = responds to command only, 4 = asleep, but has a brisk response to light tactile stimulus or a simple verbal command, 5 = asleep, but arousable only by strong physical stimulus; and 6 = asleep, unarousable. Sedation scores were obtained at 5, 10, 15, 30, and 60 minutes after arrival at the PACU. The sufentanil consumption was recorded at 6, 12, 18, and 24 hours after leaving the PACU. Related side effects were also recorded for 24 hours after surgery. In addition, postoperative comfort and satisfaction scale (0 = pretty dissatisfied; 3 = highly satisfactory) was documented through assessment of pain. Blood samples for plasma norepinephrine and serotonin concentration analysis were taken before induction and at 24 hours postoperatively. Norepinephrine and serotonin concentrations were measured using high performance liquid chromatography.
2.4. Statistical analysis
The results of this study were evaluated using the Graphpad Prism 5.0 (GraphPad Software Inc., San Diego, CA). Continuous variables were described as mean ± standard deviation (SD) and differences between groups were analyzed by using unpaired t test for normally distributed data. Categorical variables were described as number (%) and analyzed by Fisher exact test. Data from the Ramsay scores, NRS scores, and concentration of serotonin and norepinephrine according to the time points were analyzed by 2-way analysis of variance (ANOVA) followed by Bonferroni correction. P value < .05 was considered to be statistically significant.
3. Results
3.1. Patient recruitment
Patient recruitment took place from March 1, 2017, to August 1, 2017. A total of 84 patients scheduled to undergo laparoscopic cholecystectomy were assessed for eligibility, with 80 patients enrolled and allocated randomly (Fig. 1). Four of these patients were excluded because of meeting exclusion criteria (Fig. 1).
Figure 1.
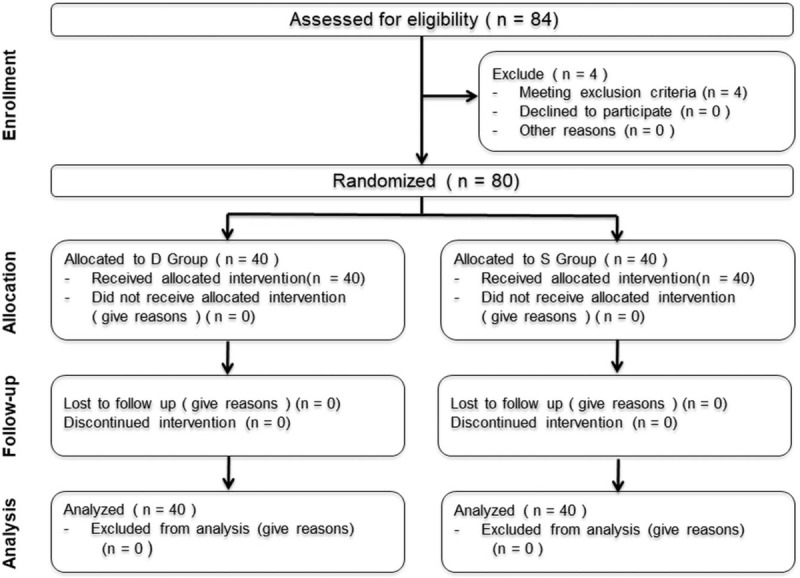
Flowchart showing details of clinical procedures throughout the study.
3.2. Demographic data and surgery/anesthesia-related information
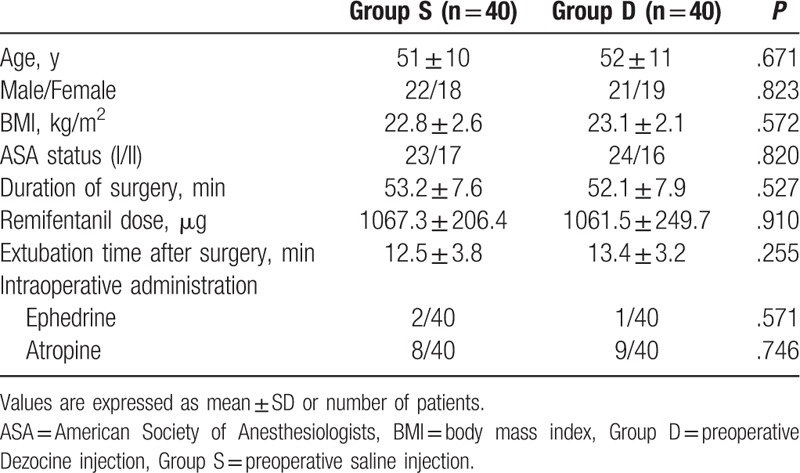
Patient characteristics are presented in Table 1. The 2 groups were comparable in terms of age, gender, BMI, ASA status, duration of surgery, remifentanil dose, extubation time after surgery, and intraoperative ephedrine and atropine injection.
Table 1.
Demographic date and surgery/anesthesia-related information.
3.3. Postoperative pain intensity and sedation levels
Compared with Group S, Group D showed lower NRS scores at both resting and movement state at 3, 6, 12, and 24 hours after surgery. Ramsay score at 5 minutes after arrival at the PACU was significantly lower in Group D than in Group S (Fig. 2).
Figure 2.
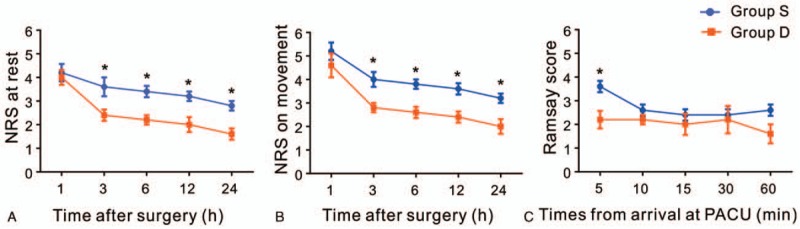
Postoperative pain intensity and sedation levels. NRS score for pain at rest (A) and on movement (B) was assessed at 1 h (rest: P = .092; movement: P = .594), 3 h (rest: P = .038; movement: P = .036), 6 h (rest: P = .038; movement: P = 0.036), 12 h (rest: P = .038; movement: P = .036), and 24 h (rest: P = .038; movement: P = .036) after surgery in Group S (blue line) and Group D (red line). (C) Sedation levels were assessed using Ramsay scores at 5 min (P = .031), 10 min (P = .932), 15 min (P = .932), 30 min (P = .997), and 60 min (P = .210) after arrival at the PACU. NRS = numerical rating scale, PACU = postanesthetic care unit. ∗P < .05 versus Group S.
3.4. Postoperative PCA consumption
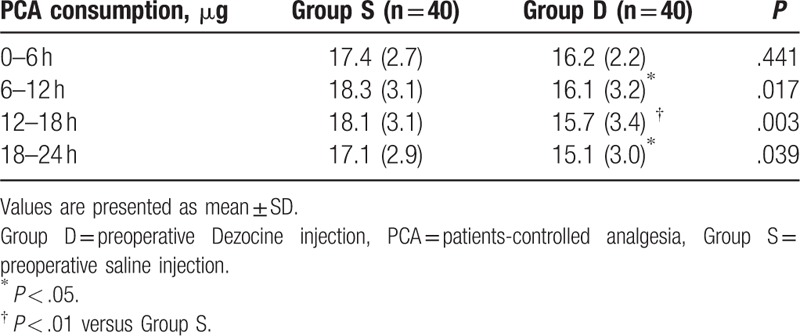
With respect to PCA consumption, preoperative Dezocine administration (Group D) significantly reduced sufentanil consumption during 6 to 12, 12 to 18, and 18 to 24 hours after surgery when compared with that of saline injection group (Group S) (Table 2).
Table 2.
Postoperative sufentanil consumption.
3.5. Plasma concentrations of norepinephrine and serotonin
As shown in Fig. 3, the concentrations of serotonin and norepinephrine in the plasma were significantly increased after preoperative Dezocine administration at 24 hours after surgery.
Figure 3.
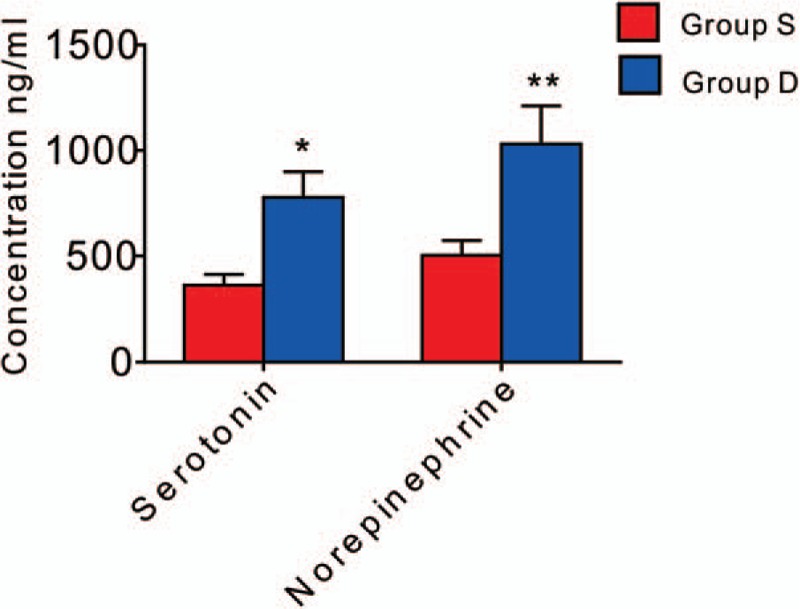
Levels of serotonin and norepinephrine in the plasma. Serotonin and norepinephrine levels were increased in Group D than in Group S at 24 h after surgery (Serotonin: P = .042; norepinephrine: P = .009). ∗P < .05, †P < .01 versus Group S.
3.6. Postoperative characteristics
The patients of Group D experienced less nausea and vomiting compared with the patients of Group S (Table 3). In addition, with respect to comfort and satisfaction score, patients reported greater satisfaction during the first 24 hours after surgery in Group D than in Group S (Fig. 4).
Table 3.
Postoperative characteristics.
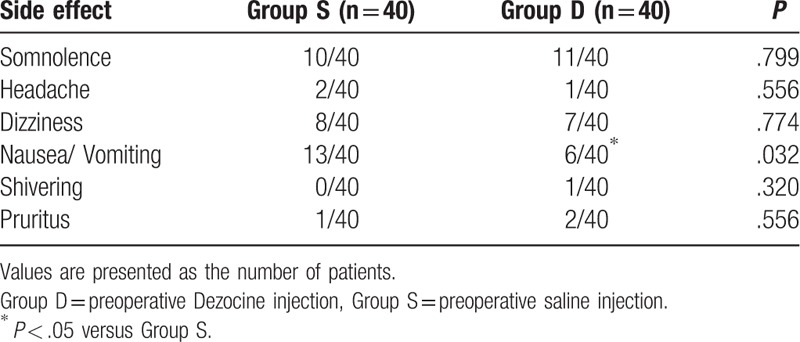
Figure 4.
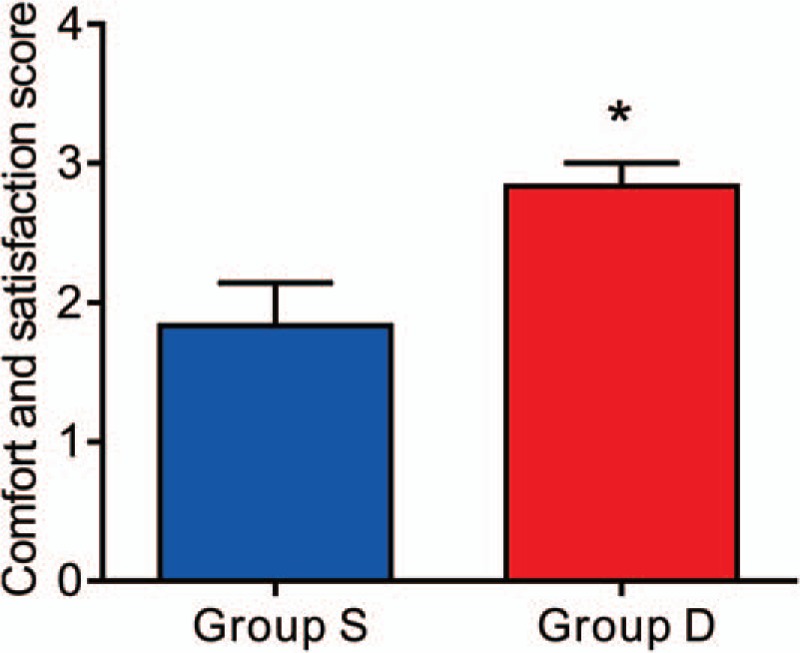
Postoperative satisfaction score. Patients in Group D had a higher satisfaction score than that in Group S (P = .0079). ∗P < .05 versus Group S.
4. Discussion
Fast-track or ERAS has been proposed to improve the quality of perioperative care with the aim of attenuating the loss of functional capacity and accelerating the recovery process. In this concept, postoperative pain management is heavily emphasized.[10,11] Postoperative acute pain after laparoscopic cholecystectomy surgery occurs in the early postoperative period and is not often taken seriously in clinical practice. Poorly managed postoperative pain can lead to complications and prolonged rehabilitation, which is associated with the development of chronic pain and reduction in quality of life.[12,13] Currently, opioids, such as morphine and sufentanil, have been widely used for postoperative analgesia following different kind of surgeries.[14,15] However, addiction and opioid-related side effects such as constipation, nausea, sedation, and pruritus are pushing us to pursuit for novel drugs or combine currently available drugs to reduce the opioids consumption to combat these adverse effects, as well as provide ideal postoperative analgesia.
Dezocine, a mixed agonist/antagonist of opioid receptors, was first synthesized in the 1970s.[16] It is widely used to manage perioperative pain in China and other Asian countries. Accumulating pieces of evidence have shown that Dezocine can reduce the consumption of opioids and reduce the opioid-related side effects. For example, Wu et al[8] found that low concentration of Dezocine enhance postoperative analgesia and reduce the nausea and pruritus after thoracotomy. Besides, Yu et al[9] demonstrated that Dezocine offers a significant antihyperalgesic and analgesic effect in patients undergoing elective open gastrectomy for up to 48 hours postoperatively. Here, we found that preemptive Dezocine is effective in preventing postoperative pain and significantly reduce the opioids consumption in patients undergoing laparoscopic cholecystectomy. In addition, the preoperative use of Dezocine can also reduce the incidence of nausea/vomiting and make patients more satisfactory.
Initially, Dezocine is believed to act as a partial agonist/antagonist of μ-receptor and an agonist of κ-receptor.[17] The analgesic efficacy of Dezocine is similar to that of morphine and a combined use of Dezocine with morphine can greatly increase the analgesic effects, which indicates that Dezocine may have an additional mechanism of analgesic effect.[18,19] However, a recently published work identified that Dezocine is a κ opioid receptor antagonist and also inhibits norepinephrine and serotonin reuptake in vitro.[3] Therefore, the synergy effect of Dezocine and opioids may due to preventing dynorphin from binding to spinal κ receptor in the central pathway of nociceptive transmission.[20] In addition, serotonin and norepinephrine have been implicated as principal mediators of endogenous analgesic mechanisms in the descending pain pathways.[21] In our study, we measured the norepinephrine and serotonin concentration in the plasma and found that the levels of norepinephrine and serotonin were increased with Dezocine pretreatment, which may have potential interactions with antinociceptive pathways. Besides that, Dezocine plays a role in immunity by regulating IL-12 and IL-10 secretion, and affecting lymphocyte activity in process of tissue injury,[22] which may also contribute to the analgesic effect. To our knowledge, this is the first clinical study that implicated norepinephrine and serotonin in Dezocine-induced postoperative pain modulation.
Visual analogue scale (VAS) and NRS are 2 commonly used scales to evaluate postoperative pain. Here, we used NRS because it was reported to be more reliable than the VAS in some cases.[23] However, 2 limitations of the present study should be highlighted. First, we did not investigate the optimal clinical dose of Dezocine on postoperative hyperalgesia. Whether lower doses of Dezocine can exert the same effect without adverse side effects will be tested in future studies. Second, our study mainly focused on the short-term effect of Dezocine on postoperative pain management. A long-term study about the pain threshold change several weeks later is warranted, as evidence has shown that there might be a link between postoperative acute pain and the risk of chronic pain development.
In conclusion, our study shows that, for patients undergoing laparoscopic cholecystectomy, intravenous injection of 0.15 mg/kg Dezocine before the induction of general anesthesia promotes a better postoperative pain control with less side effects as well as decreases PCA consumption. Therefore, Dezocine is suggested to be a potentially useful adjunctive agent for the management of postoperative pain in short-lasting surgery.
Author contributions
Conceptualization: Guoyong Xu.
Data curation: Haotian Sun.
Investigation: Li Zhou, Youchuan Zhang.
Methodology: Ruchun Hu.
Project administration: Jin Wang.
Software: Jin Wang.
Supervision: Guoyong Xu.
Writing – original draft: Li Zhou.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BIS = bispectral index, ERAS = enhanced recovery after surgery, NRS = numerical rating scale, PACU = postanesthetic care unit, PCA = patient-controlled analgesia, VAS = visual analogue scale.
Funding/support: This work was supported by the Project of Jiaxing Science and Technology Bureau (No. 2017BY18047 to LZ).
The authors have declared that no conflict of interest exists.
References
- [1].Kandil TS, El Hefnawy E. Shoulder pain following laparoscopic cholecystectomy: factors affecting the incidence and severity. J Laparoendosc Adv Surg Tech A 2010;20:677–82. [DOI] [PubMed] [Google Scholar]
- [2].Bisgaard T, Kehlet H, Rosenberg J. Pain and convalescence after laparoscopic cholecystectomy. Eur J Surg 2001;167:84–96. [DOI] [PubMed] [Google Scholar]
- [3].Liu R, Huang XP, Yeliseev A, et al. Novel molecular targets of dezocine and their clinical implications. Anesthesiology 2014;120:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu FX, Pan RR, Yu WF, et al. The anti-nociception effect of dezocine in a rat neuropathic pain model. Transl Perioper Pain Med 2014;1:5–8. [PMC free article] [PubMed] [Google Scholar]
- [5].Wang YX, Mao XF, Li TF, et al. Dezocine exhibits antihypersensitivity activities in neuropathy through spinal mu-opioid receptor activation and norepinephrine reuptake inhibition. Sci Rep 2017;7:43137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barrett AC, Smith ES, Picker MJ. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur J Pharmacol 2002;452:163–73. [DOI] [PubMed] [Google Scholar]
- [7].Terner JM, Barrett AC, Grossell E, et al. Influence of gonadectomy on the antinociceptive effects of opioids in male and female rats. Psychopharmacology (Berl) 2002;163:183–93. [DOI] [PubMed] [Google Scholar]
- [8].Wu L, Dong YP, Sun L, et al. Low concentration of dezocine in combination with morphine enhance the postoperative analgesia for thoracotomy. J Cardiothorac Vasc Anesth 2015;29:950–4. [DOI] [PubMed] [Google Scholar]
- [9].Yu F, Zhou J, Xia S, et al. Dezocine prevents postoperative hyperalgesia in patients undergoing open abdominal surgery. Evid Based Complement Alternat Med 2015;2015:946194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chapuis-Roux E, Pellissier L, Browet F, et al. How can recovery be enhanced after single-stage laparoscopic management of CBD stones? Endoscopic treatment versus laparoscopic surgery. Dig Liver Dis 2017;49:773–9. [DOI] [PubMed] [Google Scholar]
- [11].Murphy GS, Szokol JW, Greenberg SB, et al. Preoperative dexamethasone enhances quality of recovery after laparoscopic cholecystectomy: effect on in-hospital and postdischarge recovery outcomes. Anesthesiology 2011;114:882–90. [DOI] [PubMed] [Google Scholar]
- [12].Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth 2001;87:62–72. [DOI] [PubMed] [Google Scholar]
- [13].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [14].Movafegh A, Shoeibi G, Ansari M, et al. Naloxone infusion and post-hysterectomy morphine consumption: a double-blind, placebo-controlled study. Acta Anaesthesiol Scand 2012;56:1241–9. [DOI] [PubMed] [Google Scholar]
- [15].George RB, McKeen DM, Andreou P, et al. A randomized placebo-controlled trial of two doses of pregabalin for postoperative analgesia in patients undergoing abdominal hysterectomy. Can J Anaesth 2014;61:551–7. [DOI] [PubMed] [Google Scholar]
- [16].Fragen RJ, Caldwell N. Comparison of dezocine (WY 16, 225) and meperidine as postoperative analgesics. Anesth Analg 1978;57:563–6. [DOI] [PubMed] [Google Scholar]
- [17].Gharagozlou P, Hashemi E, DeLorey TM, et al. Pharmacological profiles of opioid ligands at kappa opioid receptors. BMC Pharmacol 2006;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gal TJ, DiFazio CA. Ventilatory and analgesic effects of dezocine in humans. Anesthesiology 1984;61:716–22. [DOI] [PubMed] [Google Scholar]
- [19].Downing JW, Brock-Utne JG, Barclay A, et al. WY 16225 (dezocine), a new synthetic opiate agonist-antagonist and potent analgesic: comparison with morphine for relief of pain after lower abdominal surgery. Br J Anaesth 1981;53:59–64. [DOI] [PubMed] [Google Scholar]
- [20].Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008;24:479–96. [DOI] [PubMed] [Google Scholar]
- [21].Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol 2009;7:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Feng C, Feng M, Jiao R, et al. Effect of Dezocine on IL-12 and IL-10 secretion and lymphocyte activation by culturing dendritic cells from human umbilical cord blood. Eur J Pharmacol 2017;796:110–4. [DOI] [PubMed] [Google Scholar]
- [23].Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011;152:2399–404. [DOI] [PubMed] [Google Scholar]


