Abstract
To develop and validate the prognosis model of hypertensive intracerebral hemorrhage based on admission characteristics, which would be applied to predict the 3-month outcome.
For developing the prognosis models, we studied data from 325 patients with retrospectively consecutive hypertensive intracerebral hemorrhage admitted between 2012 and 2016. The predictive value of admission characteristics was tested in logistic regression models, presenting 3-month outcome as the primary outcome. The performance of the models was tested by discrimination and calibration. After development, internal and external validations were used to test the function.
The multivariate analysis of logistic regression indicated that age, Glasgow coma scale score, pupillary light reflex, hypoxemia, intracerebral hemorrhage volume, blood glucose, and D-dimer level were independent factors of the hypertensive intracerebral hemorrhage prognosis model. The prognosis model based on those admission risk factors worked well. The receiver operating characteristic curve was used to analyze the discriminant ability of model A, model A + B, and model A + B + C. Specifically, the area under the receiver operating characteristic curve increased from 0.816 (model A; 95% CI, 0.760–0.872) to 0.913 (model A + B + C; 95% CI, 0.881–0.946), and the models were not overoptimistic and were applicably confirmed by internal and external validations respectively.
This prognosis model could be used to predict the prognosis of patients with hypertensive intracerebral hemorrhage early, simply and accurately, contributing to the clinical treatment eventually.
Keywords: high blood pressure, intracerebral hemorrhage, prediction
1. Introduction
Intracerebral hemorrhage (ICH) is the deadliest form of stroke that accounts for approximately 10% to 30% of all strokes.[1] And the reported 30-day case fatality rate of ICH was around 40% to 50%.[2,3] As we all known, high blood pressure (BP) can lead to spontaneous intracerebral hemorrhage which is also known as hypertensive intracerebral hemorrhage (HICH). The best clinical management of HICH remains unclear due to unproven therapies, such as craniotomy, craniectomy, and hyperosmolar agents. Therefore, HICH is still short of effective treatments. In this respect, it can be a serious public health affair which would increase the burden of social economy.[2]
Therefore, it is of great importance to explore and identify the prognostic risk factors of HICH and establish a HICH prognosis model with excellent performance, which can assist clinicians to make the accurate diagnosis as well as further treatments for HICH patients. Various and complex prognosis models have been reported depending on different parameters,[4–7] but the results were not satisfactory. In this study, we tried to develop a suitable prognosis model of HICH, based on the established ICH treatment database of Shaoxing Central Hospital from 2012 to 2016 and relevant clinical data. The data of patients with HICH in Shaoxing Second Hospital from 2016 to 2017 were used as the external validation to test the predictive performance of this prognosis model.
2. Methods
2.1. Patient selection and study design
In this retrospective cohort study, we reviewed medical records of all spontaneous ICH patients admitted to Shaoxing Central Hospital between January 1, 2012 and December 31, 2016 to develop the prognosis model. Patients were eligible for the study if the baseline nonenhanced computed tomography (CT) scan was performed within 6 hours after symptoms onset. A follow-up CT scan was performed within 30 h after the initial CT scan. The exclusion criteria were: patients who had secondary ICH due to arteriovenous malformation, intracranial aneurysm rupture, traumatic brain injury, brain tumor, or hemorrhagic infarction; patients who had primary intraventricular hemorrhage (IVH); patients with anticoagulant associated ICH; and patients who refused to follow-up clinical assessment after being discharged from hospital. Collected data also included demographic information, medical history, initial evaluation (including vital signs, laboratory data, and radiographic findings) and hospital course.
The primary outcome was assessed by the modified Rankin scale (mRS) for 3 months. The outcome was dichotomized as favorable and poor outcomes from the 3-month mRS score. And the poor outcome was defined by an mRS score of 4 to 6 according to previous definitions.[8,9]
Hypertension was defined by systolic BP ≥ 140 mm Hg, or diastolic BP ≥90 mm Hg.[10] Admission BP was modulated according to the guideline recommendations.[11,12] Also hypoxemia was defined by PaO2 < 60 mm Hg. ICH was diagnosed with noncontrast CT examinations with 5-mm sections. Besides, hematoma volume was measured by the ABC/2 formula, where A, B, and C represent the dimensions of the maximum level of hematoma in 3 perpendicular axes.[13]
In total, 325 patients were enrolled into the database in this study, providing information and data to develop the prognosis model. In addition, we reviewed medical records of patients by using the same selected criteria, who were treated at the Shaoxing Second Hospital for the external validation between January 2015 and October 2017.
All aspects of this study were approved by Institutional Review Board. Direct patient identifiers were not collected as part of the data setting due to privacy considerations. Because our study did not address patient care intervention, it was not necessary to obtain written informed consents.
2.2. Model development and validation
The initial evaluated data were used to develop our prognosis models for the outcome prediction of HICH. Three prognosis models were developed based on different independent risk factors. Model A was based on the clinical condition on admission (GCS score and pupil light reflex) together with patient age. Model B was built based on the amount of extravasated blood from CT scan. Model C was built based on the result of laboratory data.
The discriminative performance was described by an area under the receiver operating characteristic curve (AUC) with a corresponding 95% confidence interval (CI). The calibration of the models was evaluated by using the Hosmer–Lemeshow (H-L) goodness-of-fit test, which was considered as reliable if P > .2.
Meanwhile, we internally validated our models with bootstrapping techniques, that is, in each bootstrap sample, the entire modeling process was repeated to correct the overestimation.[14] In addition, we evaluated both discrimination and calibration of the risk chart in the validation cohort.
2.3. Statistical analysis
The categorical variables were divided into 2 groups (mRS > 3 and mRS ≤ 3), and the baseline characteristics were summarized appropriately as mean ± SD or as median and interquartile ranges (IQRs). Continuous variables were compared by using Student's t-test, and categorical variables were compared by using Pearson's chi-squared test. The variables that were significantly related to 3-month outcome in univariate analysis were entered within the logistic regression model by using a forward stepwise selection. The maximum like hood method was used after adjusting for confounding factors and identifying independent predictors of 3-month outcome. All statistical analyses were performed by using the SPSS software package version 19.0 (IBM Corporation, Armonk, NY), and P < .05 was considered to be statistically significant.
3. Results
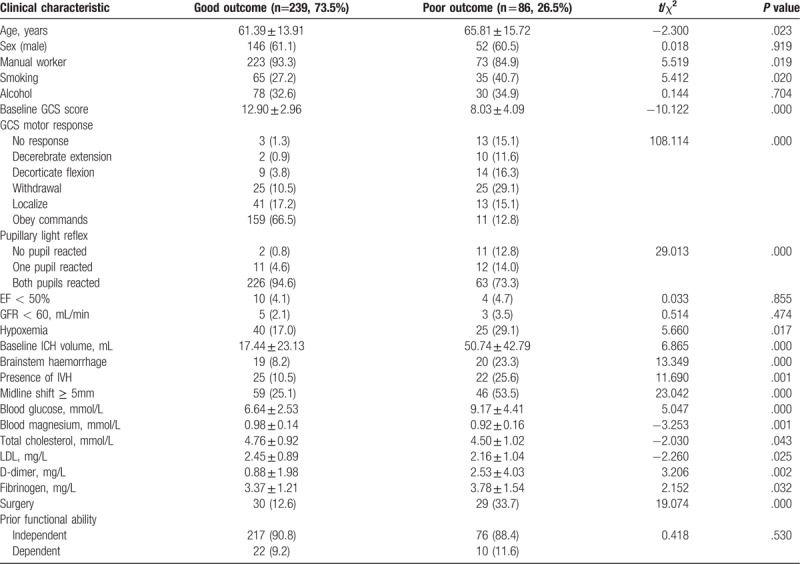
A total of 325 patients, including 198 males (60.9%) with mean age of 60.9, were recruited in the developmental cohort. All patients had a history of high blood pressure, the mean systolic BP on arrival at hospital was 203 ± 45 mm Hg (range, 55–364 mm Hg) and the mean heart rate was 98 ± 24 bpm (range, 52–180 bpm). Depending on the mRS score, 239 (73.5%) patients had the good outcome, 86 (26.5%) patients had the poor outcome. Main characteristics of the cohort were summarized in Table 1. In general, the average age, ICH volume, blood glucose, D-dimer, and fibrinogen level in the poor prognosis group were higher than those in the good prognosis group (P < .05), whereas the average scores of GCS, the level of blood magnesium, total cholesterol, and low-density lipoprotein (LDL) in the poor prognosis group were lower than those in the good prognosis group (P < .05). Furthermore, the proportions of manual worker, smoking, hypoxemia, brainstem hemorrhage, IVH, and midline shift ≥ 5 mm in the poor prognosis group were higher than those in the good prognosis group (P < .05). And surgery was considered as a risk factor for the poor prognosis.
Table 1.
Baseline characteristics of patients with or without poor outcome in the developmental cohort.
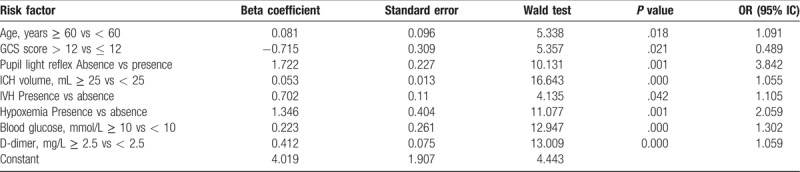
Significant risk factors selected by univariate analyses were further evaluated by logistic regression analyses with forward, stepwise selection procedures. Only 8 predictors, including age ≥ 60, GCS score ≤ 12, the absence of pupillary light reflex, ICH volume ≥ 25 ml, the presence of IVH, hypoxemia, higher blood glucose (≥ 10 mmol/L), and D-dimer (≥ 2.5 mg/L) were identified as significant and independent predictors of the 3-month poor outcome (Table 2).
Table 2.
Multivariate analysis of predictors for the poor outcome in 3 months.
3.1. Prognostic models
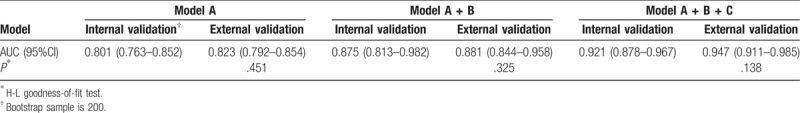
As described above, model A was based on 3 predictors of age, GCS score and pupillary light reflex on admission. Model B was built based on amount of extravasated blood from CT scan result including ICH volume and the presence of IVH. Model C was built based on the independent risk facts of laboratory data, such as hypoxemia, blood glucose, and D-dimer. The receiver operating characteristic curve was used to analyze the discriminant ability of model A, model A + B, and model A + B + C. With the increase of risk factors evolved in the analysis, the discriminant ability of the prognosis model was enhanced (Table 3 and Fig. 1). Concretely, the AUC increased from 0.816 (model A; 95% CI, 0.760–0.872) to 0.913 (model A + B + C; 95% CI, 0.881–0.946).
Table 3.
The discriminant ability of each combinatorial prognosis model.
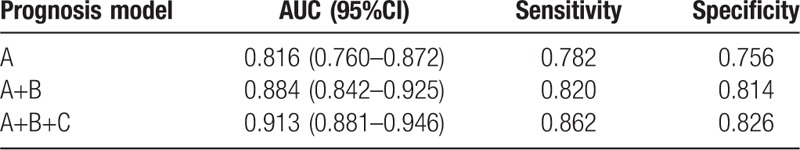
Figure 1.
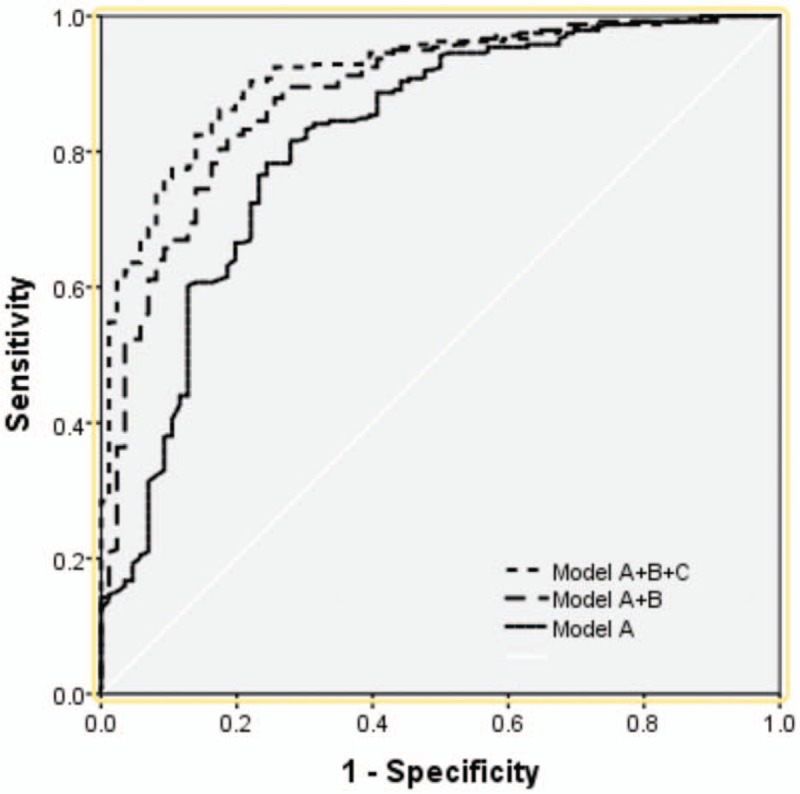
Receiver operating characteristic curves of each combinatorial prognosis model for predicting 3-month outcome.
Table 4 showed that old age, GCS score, pupil light reflex, ICH volume, IVH, hypoxemia, blood glucose, and D-dimer were risk facts of the poor outcome in the validation cohort. Moreover, Table 5 showed that the internal validity of these models was high, and there was no over optimism. Meanwhile, when applying the prognostic models to the validation cohort, the external validity of model A, model A+B, and model A+B+C was very high. Besides, the H-L goodness-of-fit test, that was used to test the calibration ability, indicated that each prognosis model had a good performance and a high applicability. Thus, these prognosis models could be clinically applied to accurately and effectively predict the prognosis of patients with ICH.
Table 4.
Baseline characteristics of patients with or without a poor outcome in the validation cohort.
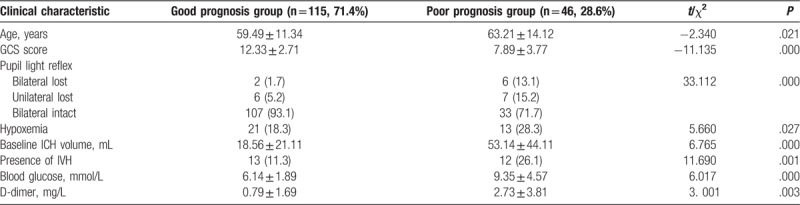
Table 5.
The performance of prognosis models.
4. Discussion
The prognosis model is a statistical model combined with various risk factors to predict, assist, and improve the prognosis of patients, which would be more accurate and effective than clinicians’ own experience. Recently, prognosis models based on statistical methods have been developed rapidly; several scholars have put forward their prognosis models about predicting the death and short-term outcome of ICH patients. In 2001, Hemphill raised a 6-point scoring system that included the factors of GCS score, age, infratentorial origin of ICH, ICH volume, and presence of IVH, showing the 30-day mortality increasing from 13% (1 point) to 97% (5 point).[4] Furthermore, other studies introduced new parameters such as NIHSS score, IVH score, history of hypertension, subarachnoid extension and serum glucose into the model, which could improve the predictive performance of the new models.[15–17] These models presented a good predictive performance during the internal and external validations. At present, there are plenty of researches focusing on the prognostic risk factors of spontaneous HICH. However, suitable prognosis models that can be widely applied for clinical prognosis have not been studied clearly to date. In this study, we systematically analyzed the risk factors of admission, and selected significant risk factors to develop different prognosis models. After development, we performed internal and external validations to test their function, and eventually confirmed that the prognosis models were applicable to the clinical practice.
Predictive risk factors were the basis of prognosis models. In the multivariate analysis, we found that old age, lower GCS score, the absence of pupillary light reflex, ICH volume ≥ 25 mL, the presence of IVH, hypoxemia, higher blood glucose, and D-dimer level were independent factors of the patient prognosis. Specifically, age ≥60, GCS score ≤ 12 and the absence of pupillary light reflex indicated a poor prognosis. Moreover, CT examination was usually used as an important assistant examination for diagnosis within HICH patients. In addition to ICH volume and IVH, subarachnoid dilatation, cisterna ambiens compression and midline shift were also defined as significant risk factors of the poor prognosis.
Likewise, laboratory examination also provided the clinical data in the early stage, and clinicians could correct the abnormal values during the laboratory examination in order to improve the patient prognosis. However, it had been controversial to consider blood glucose as a prognostic factor for a long time. Some studies showed that high blood glucose could have a biologically plausible association with poor outcomes in ICH patients, while other study showed patients with or without HE had similar blood glucose (179 ± 68 mg/dL vs 153 ± 71 mg/dL) and diabetes incidence (14% vs 25%).[18] Some studies showed the deleterious effect of hyperglycemia was attributed to its secondary promotive effects of acidosis, free radical formation, and inflammatory cytokines release. Those secondary effects accelerated the BBB breakdown, impaired the integrity of adjacent vessels surrounding the initial bleeding sites, and promoted emerging or continuous bleeding.[19–21] Previous studies had demonstrated that blood glucose levels between 3.7 and 7.3 mmol/L were significantly associated with favorable functional outcomes in acute ischemic stroke,[22] and higher blood glucose variability was reported to be significantly associated with poor outcomes in subarachnoid hemorrhage.[23] However, the pool of available evidence pertaining to blood glucose and ICH was still limited. Thus, further studies should investigate the association between blood glucose levels and functional outcomes with multiple time points and variability of blood glucose. Importantly, our study also proved that D-dimer level was responsible for the prognosis of HICH patients. Among ICH patients, the high level of D-dimer in the early phase was related with the progressive hemorrhagic injury, and patients with progressive hemorrhage had a poor prognosis.[24,25] While many studies had examined the influence of various patient characteristics (cardiac ejection fraction and renal function) on D-dimer concentration,[26,27] it was necessary to exclude confounding factors to verify the effect of D-dimer on prognosis. In this study, there was no significant difference in renal function and cardiac ejection fraction between the 2 groups, the prognosis utility of an elevated D-dimer level with cerebral hemorrhage was clear.
After the development of prognosis models, the internal and external validations as well as the model performance were identified as important basis for supporting the prognosis models to be applied to the accurate clinical prediction. The internal validity presented the ability of predicting the new data which were similar to the original data used for establishment. Also the external validity, also known as external applicability, showed the ability of predicting new data that went beyond the limit of original database. In this study, we established different prognosis models in terms of different risk factors. With the increase of risk factors evolved into the analysis, the prognosis model was developed with a higher discriminant ability, and in the same time, there was no decrease of calibration ability or loss of statistical significance. Therefore, the prognosis models were verified to obtain a good discriminant ability and a well-overall performance. Specifically, according to the data of external validation, the discriminant ability of these prognosis models has been improved because of the increased AUC. Similarly, there was no loss of calibration significance with the change of external data, no statistically significant difference between prediction and observation outcomes, which meant the prognosis models had a well-overall performance and could be applied for prognosis with the data from new patients.
The prognosis model of HICH could improve the accuracy of the clinical prediction and provides the assistance for further treatment decisions, in favor of the effective use of limited medical resources. Notably, although the prognosis model can assist the clinician to accurately and effectively predict the prognosis, it actually cannot completely replace the judgment and diagnosis made by clinicians. During the usage of the prognosis model, the accurate diagnosis should be made with the specific characteristics of patients. The performance of prognosis models can be optimized when introducing more risk factors into the analysis, such as intracranial pressure[28] and motor-evoked potential.[29] In recent years, some researchers have found that molecular markers such as serum sCD163 protein,[30] S100,[31,32] CRP,[33] BNP,[31] may also have correlations with prognosis, so they can also be tested as new HICH risk factors to improve the accuracy of prognosis. In this study, we focused on the establishment of prognosis models based on risk factors on admission to make a prediction in the early stage. Through the validations, the established models were confirmed to have a good performance and a wide applicability so that they could be widely facilitated into the clinical applications as a predictive tool.
However, this study still has several limitations. First, this study was a single-center retrospective cohort study. Many of our patients were lost to follow up or missing information, both of which resulted in a smaller sample size. Although the derivation cohort and, particularly, the validation cohort were relatively small, the derivation cohort sample size was large enough to develop the prognosis model, and validation cohort sample size was large enough to validate the prognosis model. The study with a larger sample size needs to be conducted in the future to make sure the prognosis model has a good performance in patients with HICH. Second, the evaluation of the functional outcome was conducted via phone during the follow-ups. The lack of on-site supervision by medical professionals might have some negative impacts on the accuracy of the mRS score because some of the interviewees might not be able to answer the questions accurately.
5. Conclusions
The prognosis models based on the risk factors on admission can predict the prognosis early, simply and accurately, which is convenient for clinical practice. Clinicians can operate this predictive tool to make a prognosis of patients with HICH, lead to the correct clinical decision, make a good use of medical resources, and eventually provide the best treatment for patients.
Author contributions
Conceptualization: Gang Zhen.
Data curation: Wu Ding, Gang Zhen.
Formal analysis: Chuanjian Tu.
Funding acquisition: Chuanjian Tu, Dagang Song.
Investigation: Dagang Song.
Methodology: Wu Ding, Chuanjian Tu, Zhiwei Gu.
Project administration: Chuanjian Tu, Zhiwei Gu.
Resources: Chuanjian Tu, Zhiwei Gu.
Software: Wu Ding, Jiansheng Liu.
Supervision: Jiansheng Liu.
Validation: Wu Ding, Zhiwei Gu.
Visualization: Dagang Song.
Writing – original draft: Wu Ding.
Writing – review & editing: Chuanjian Tu, Zhiwei Gu.
Footnotes
Abbreviations: AUC = area under the receiver operating characteristic curve, BP = blood pressure, CI = confidence interval, CT = computed tomography, EF = ejection fraction, GCS = Glasgow coma scale, GFR = glomerular filtration rate, HE = hematoma expansion, HICH = hypertensive intracerebral hemorrhage, H-L = Hosmer–Lemeshow, ICH = intracerebral hemorrhage, IQRs = interquartile ranges, IVH = intraventricular hemorrhage, LDL = low-density lipoprotein, mRS = modified Rankin scale.
The authors have no conflicts of interest to disclose.
References
- [1].Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;373:1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zia E, Engström G, Svensson PJ. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke 2009;40:3567–73. [DOI] [PubMed] [Google Scholar]
- [3].van Asch CJ, Luitse MJ, Rinkel GJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–76. [DOI] [PubMed] [Google Scholar]
- [4].Hemphill JC, Bonovich DC, Bessmertis L. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–7. [DOI] [PubMed] [Google Scholar]
- [5].Godoy DA, Boccio A. ICH score in a rural village in the republic of Argentina. Stroke 2003;33:1455–6. [DOI] [PubMed] [Google Scholar]
- [6].Godoy DA, Pinero G, Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage can modify to the original score improve the prediction? Stroke 2006;37:1038–44. [DOI] [PubMed] [Google Scholar]
- [7].Ruiz-Sandoval JL, Chiquette E, Romero-Vargas V. Grading scale for prediction of outcome in primary intracerebral hemorrhage. Stroke 2007;38:1641–4. [DOI] [PubMed] [Google Scholar]
- [8].Qureshi AI, Palesch YY, Barsan WG. ATACH-2 trial investigators and the neurological emergency treatment trials network: intensive blood pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 2016;375:1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boulouis G, Morotti A, Brouwers HB. Noncontrast computed tomography hypodensities predict poor outcome in intracerebral hemorrhage patients. Stroke 2016;47:2511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chobanian AV, Bakris GL, Black HR. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- [11].Morgenstern LB, Hemphill JC, 3rd, Anderson C. American Heart Association Stroke Council and Council on Cardiovascular Nursing: Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hemphill JC, 3rd, Greenberg SM, Anderson CS. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology: Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]
- [13].Kothari RU, Brott T, Broderick JP. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–5. [DOI] [PubMed] [Google Scholar]
- [14].Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: validating a prognostic model. BMJ 2009;338:b605. [DOI] [PubMed] [Google Scholar]
- [15].Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke 2003;34:1717–22. [DOI] [PubMed] [Google Scholar]
- [16].Chuang YC, Chen YM, Peng SK. Risk stratification for predicting 30-day mortality of intracerebral hemorrhage. Int J Qual Health Care 2009;21:441–7. [DOI] [PubMed] [Google Scholar]
- [17].Hallevi H, Dar NS, Barreto AD. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Crit Care Med 2009;37:969–74. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yaghi S, Dibu J, Achi E. Hematoma expansion in spontaneous intracerebral hemorrhage: Predictors and outcome. Int J Neurosci 2014;124:890–3. [DOI] [PubMed] [Google Scholar]
- [19].Qureshi AI, Palesch YY, Martin R. Association of serum glucose concentrations during acute hospitalization with hematoma expansion, perihematomal edema, and three month outcome among patients with intracerebral hemorrhage. Neurocrit Care 2011;15:428–35. [DOI] [PubMed] [Google Scholar]
- [20].Zheng Y, Hu Q, Manaenko A. 17beta-Estradiol attenuates hematoma expansion through estrogen receptor alpha/silent information regulator 1/nuclear factor-kappa b pathway in hyperglycemic intracerebral hemorrhage mice. Stroke 2015;46:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Esposito K, Nappo F, Marfella R. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106:2067–72. [DOI] [PubMed] [Google Scholar]
- [22].Ntaios G, Egli M, Faouzi M. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke 2010;41:2366–70. [DOI] [PubMed] [Google Scholar]
- [23].Kurtz P, Claassen J, Helbok R. Systemic glucose variability predicts cerebral metabolic distress and mortality after subarachnoid hemorrhage: a retrospective observational study. Crit Care (London, England) 2014;18:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hu GW, Lang HL, Guo H. A risk score based on admission characteristics to predict progressive hemorrhagic injury from traumatic brain injury in children. Eur J Pediatr 2017;176:689–96. [DOI] [PubMed] [Google Scholar]
- [25].Chiu CC, Li YN, Lin LJ. Serum D-dimer as a predictor of mortality in patients with acute spontaneous intracerebral hemorrhage. J Clin Neurosci 2012;19:810–3. [DOI] [PubMed] [Google Scholar]
- [26].Spring JL, Winkler A, Levy JH. The influence of various patient characteristics on D-dimer concentration in critically ill patients and its role as a prognostic indicator in the intensive care unit setting. Clin Lab Med 2014;34:675–86. [DOI] [PubMed] [Google Scholar]
- [27].de Boer RA, Nayor M, deFilippi CR. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol 2018;3:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sykora M, Steinmacher S, Steiner T. Association of intracranial pressure with outcome in comatose patients with intracerebral hemorrhage. J Neurol Sci 2014;342:141–5. [DOI] [PubMed] [Google Scholar]
- [29].Ikedo T, Nakamura K, Sano N. Intraoperative motor-evoked potential monitoring as a predictive tool for recovery from spontaneous intracerebral hemorrhage. World Neurosurg 2016;90:518–23. [DOI] [PubMed] [Google Scholar]
- [30].Roy-O’Reilly M, Zhu L, Atadja L. Soluble CD163 in intracerebral hemorrhage: biomarker for perihematomal edema. Ann Clin Transl Neurol 2017;4:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].James ML, Blessing R, Phillips-Bute BG. S100B and brain natriuretic peptide predict functional neurological outcome after intracerebral haemorrhage. Biomarkers 2009;14:388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hu YY, Dong XQ, Yu WH. Change in plasma S100B level after acute spontaneous basal ganglia hemorrhage. Shock 2010;33:134–40. [DOI] [PubMed] [Google Scholar]
- [33].Di Napoli M, Godoy DA, Campi V. C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score. Stroke 2011;42:1230–6. [DOI] [PubMed] [Google Scholar]


