Abstract
To evaluate the prevalence of sarcopenia in patients undergoing pancreatic surgery and to examine its impact on the surgical outcomes and survival of patients.
Skeletal muscle index (SMI) was measured on preoperative CT. A patient was considered sarcopenic if SMI was <38.5 cm2/m2 for a female or <52.4 cm2/m2 for a male. Postoperative pancreatic fistula (POPF) and severe morbidity (Clavien≥3) were analyzed. Survival of patients with cancer was calculated using the Kaplan–Meier method.
In total, 107 consecutive patients were included. Among them, 50 (47%) patients were sarcopenic and 65 (60%) were undernourished. The rates of severe morbidity and mortality were comparable between sarcopenic and nonsarcopenic groups. However, all POPF grade B or C and deaths occurred in the sarcopenic or nonsarcopenic overweight group (BMI > 25) with significantly lengthened hospital stays (P = .003). After pancreatectomy for cancer, 31 (40.2%) patients showed postoperative recurrence and 23 (29.9%) died after a median follow-up of 15 ± 13.5 months. Despite comparable histological types and stages, the median overall and disease-free survivals were lower in sarcopenic patients (16 months vs not reached, P = .02 and 11.1 months vs 22.5 months; P = .04, respectively). The multivariate analysis revealed that, sarcopenia trended to increase the risk of death (HR = 2.04, P = .07).
Sarcopenia negatively impacted short- and long-term outcomes in patients undergoing pancreatectomy.
Keywords: complications, pancreatectomy, sarcopenia, survival
1. Introduction
Pancreatic surgery is a complex procedure that requires a high level of expertise.[1] Improvements in perioperative management have successfully reduced the mortality rates associated with pancreatic surgery, but morbidity remains high.[2] Up to 40% of patients develop complications following pancreatic resection. Postoperative complications not only impact the patient's quality of life, but can also delay adjuvant therapy and adversely impact survival. It is therefore crucial to identify high-risk patients who are likely to develop postoperative complications.
Pancreatic pathologies are often characterized by a marked nutritional imbalance, ranging from undernutrition to cachexia.[3] This imbalance results from a combination of decreased caloric intake and increased catabolism caused by pancreatic diseases. Undernutrition has recently been proposed as a robust predictor of postoperative complications and poor prognosis after pancreatic resection.[4,5] Undernutrition implies a loss of fat mass that is frequently associated with muscle decline or sarcopenia. Sarcopenia was described in 1989 by Rosenberg as a decrease in skeletal muscle mass occurring with aging[6] and was described as a qualitative alteration in muscle function.[7]
Sarcopenia appears to have a negative impact on surgical outcomes after hepatectomy,[8,9] colorectal resection,[10–12] gynaecological surgery,[13] and cardiac surgery.[14] It was also reported as a predictor of poor prognosis in colorectal cancer,[11,15] oesophago-gastric cancer,[16,17] hepatocellular carcinoma,[18,19] and pancreatic adenocarcinoma (PDAC).[20–23]
Nevertheless, data regarding the impact of sarcopenia on outcomes following pancreatic surgery are scarce, with only a few studies focusing on patients with PDAC. Therefore, the purpose of this study was to evaluate the prevalence of sarcopenia in patients undergoing pancreatic surgery for benign or malignant diseases and to examine its impact on the postoperative course and patient survival.
2. Materials and methods
2.1. Patients
We retrospectively reviewed all consecutive patients who underwent pancreatic resection (including pancreaticoduodenectomy [PD], distal pancreatectomy [DP], and total pancreatectomy [TP]) for a benign or malignant disease in our department between May 2011 and July 2015. In order to determine the impact of sarcopenia on postoperative outcome and survival, we excluded patients who had: undergone preoperative abdominal computed tomography (CT) (within 6 weeks prior to surgery) at outside institutions; undergone another surgical procedure (such as enucleation, central pancreatectomy, or Wirsung derivation); no dosing of drain amylase or hyperamylasaemia on postoperative day (POD) 3 for the diagnosis of postoperative pancreatic fistula (POPF); and (iv) a surgical emergency. This study was approved by our institution's ethics committee.
2.2. Data collection
For each patient, the following data were collected from a computerized database: age, sex, date of birth, medical and surgical history, American Society of Anesthesiologists (ASA) score, weight, height body mass index (BMI), percentage of body weight loss, and preoperative biological tests (including aspartate aminotransferase, AST; alanine aminotransferase, ALT; total and combined bilirubin; and creatinine and albumin levels).
A patient was considered as undernourished if one of the following criteria was present: estimated weight loss was ≥5%, BMI was <21 kg/m2, and albumin level was <35 mg/L. Preoperative nutrition support was performed for patients who met the undernutrition criteria.
Intraoperative data, including operative time, estimated blood loss (EBL), intraoperative blood transfusion, pancreatic texture, surgical approach, vascular resection, drainage, and main pancreatic duct diameter were extracted from the pancreas database.
Complications at 3 months postoperatively were rated according to the Clavien–Dindo classification,[24] where severe complications were defined by a grade ≥3. POPF was defined according to the International Study Group of Pancreatic Fistula (ISGPF) and classified into grades A, B, or C.[25]
Bleeding and postoperative gastroparesis were graded according to the international consensus.[26,27] Length of stay (LOS) was also assessed. Operative mortality was defined as death within 90 days after surgery or before discharge from the hospital. For the purposes of survival analysis, only patients with cancer diseases were analysed.
2.3. Sarcopenia measurement
Sarcopenia was assessed by measuring the total cross-sectional muscle area (including of the psoas muscles; paraspinal, external, and internal oblique muscles; and the transverse and rectus abdominal muscles). The total muscle area was measured semiautomatically by manually outlining them on preoperative plain CT at the third lumbar vertebra (L3) and setting the density at a threshold of −29 to +150 (Fig. 1). All of the measurements and calculations described above were performed by the same examiner (MV), who was blinded to the surgical outcome at the time of quantification. We used the sarcopenia definition proposed by Prado et al.[28] According to this definition, sarcopenia was defined using the following criteria: skeletal muscle index (SMI) <38.5 cm2/m2 for women or <52.4 cm2/m2 for men (SMI [cm2/m2] = [total muscle area at L3]/[height]2).
Figure 1.
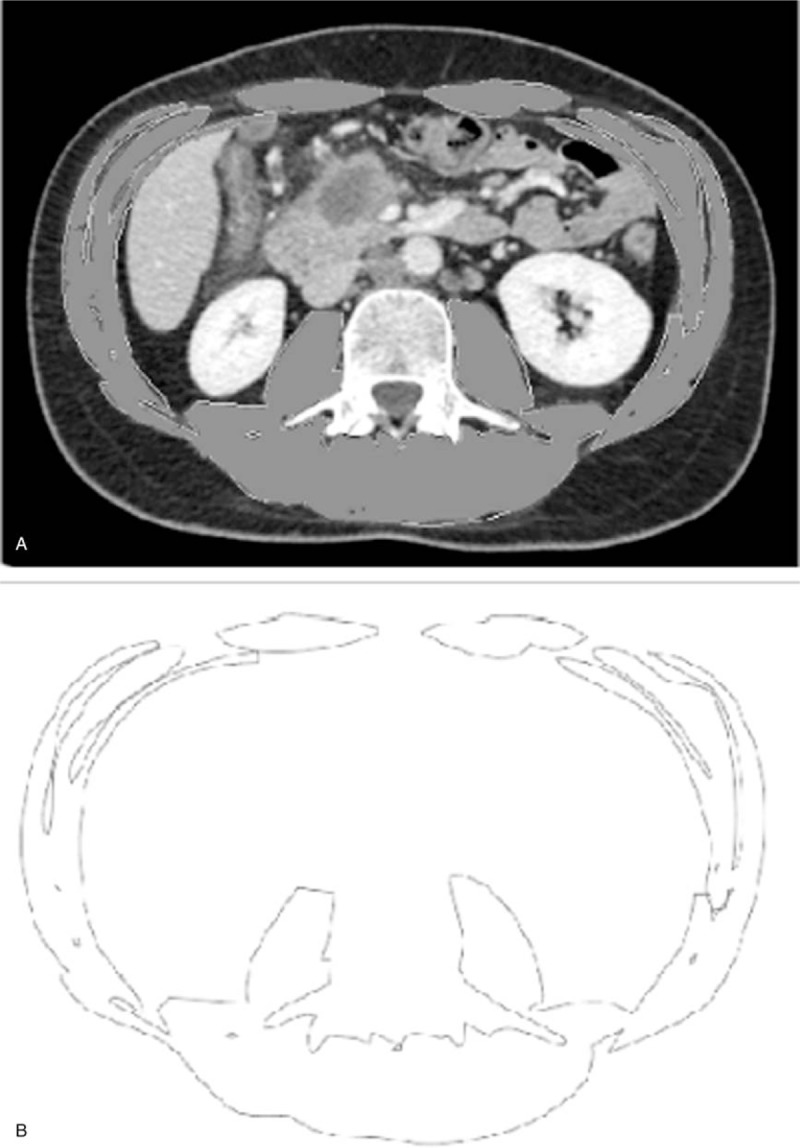
Skeletal muscle index was measured at the level of L3 by measuring a total muscle area. Measurements were performed semi-automatically with manual outlining of abdominal muscles.
2.4. Statistical analysis
Statistical analyses were implemented with SPSS software, version 21.0 (SPSS, Chicago, IL). Continuous variables, expressed as means ± standard deviations, were compared using the Mann–Whitney U test. Binary and categorical variables were compared with either the chi-squared test or Fisher's exact test. Cumulative overall survival (OS) and disease-free survival rates were calculated using the Kaplan–Meier method, and the differences between the curves were evaluated using a log-rank test. Comparisons were performed between sarcopenic and nonsarcopenic patients. Nonsarcopenic patients were stratified by BMI with the aim of considering the impact of being overweight on outcomes following pancreatic surgery.
To assess the independent contribution of each variable, a Cox proportional hazards regression model was utilized. Significance was set at P < .05.
3. Results
3.1. Demographic characteristics
The clinical and pathological characteristics of the 107 patients included in the study are outlined in Table 1. The mean age of the study sample overall was 61 ± 12 years. There were 51 (48%) men and 56 (52%) women. Pancreatic surgery was performed for malignant disease in 77 cases (72%), of which PDAC was the predominant aetiology (53%). Of the 107 patients, 82 (77%) underwent PD and 65 (60%) met the criteria of undernutrition. The prevalence of sarcopenia was 47% in our cohort. There was a similar rate of sarcopenia in patients with malignant or benign disease (44% vs 53%, respectively). Compared to the nonsarcopenic patients, the sarcopenic group was characterized by a higher proportion of men (Table 2, Fig. 2) and a lower BMI. Groups were comparable regarding other pre- and intraoperative parameters (Table 2).
Table 1.
Characteristics of the population study (n = 107).
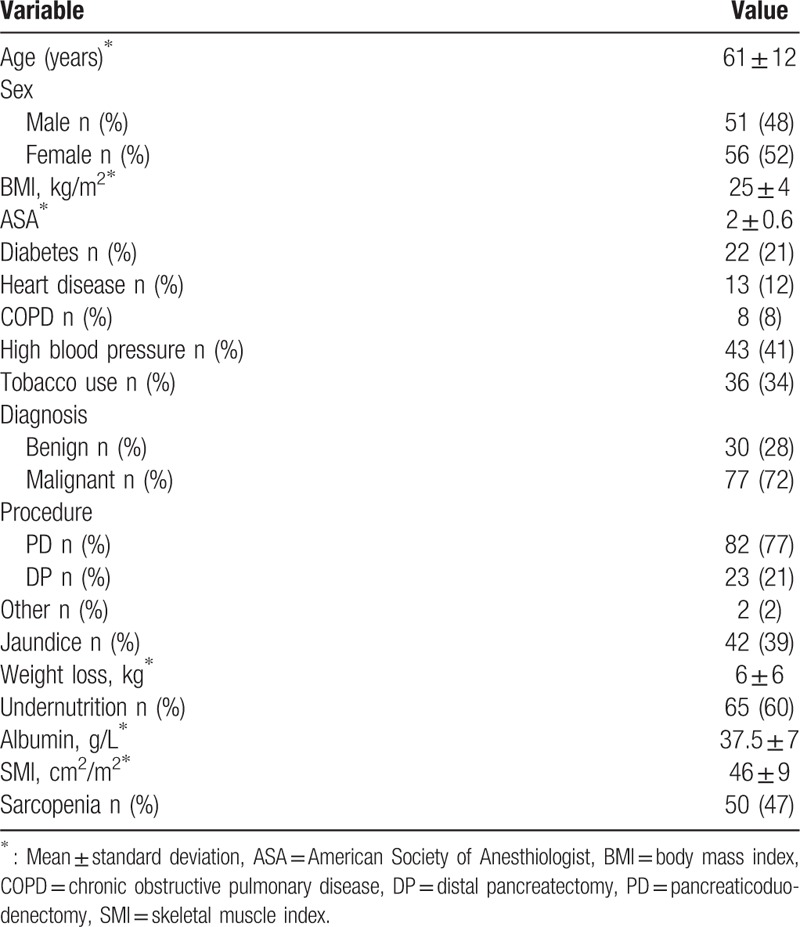
Table 2.
Clinical characteristics of sarcopenic and nonsarcopenic patients.
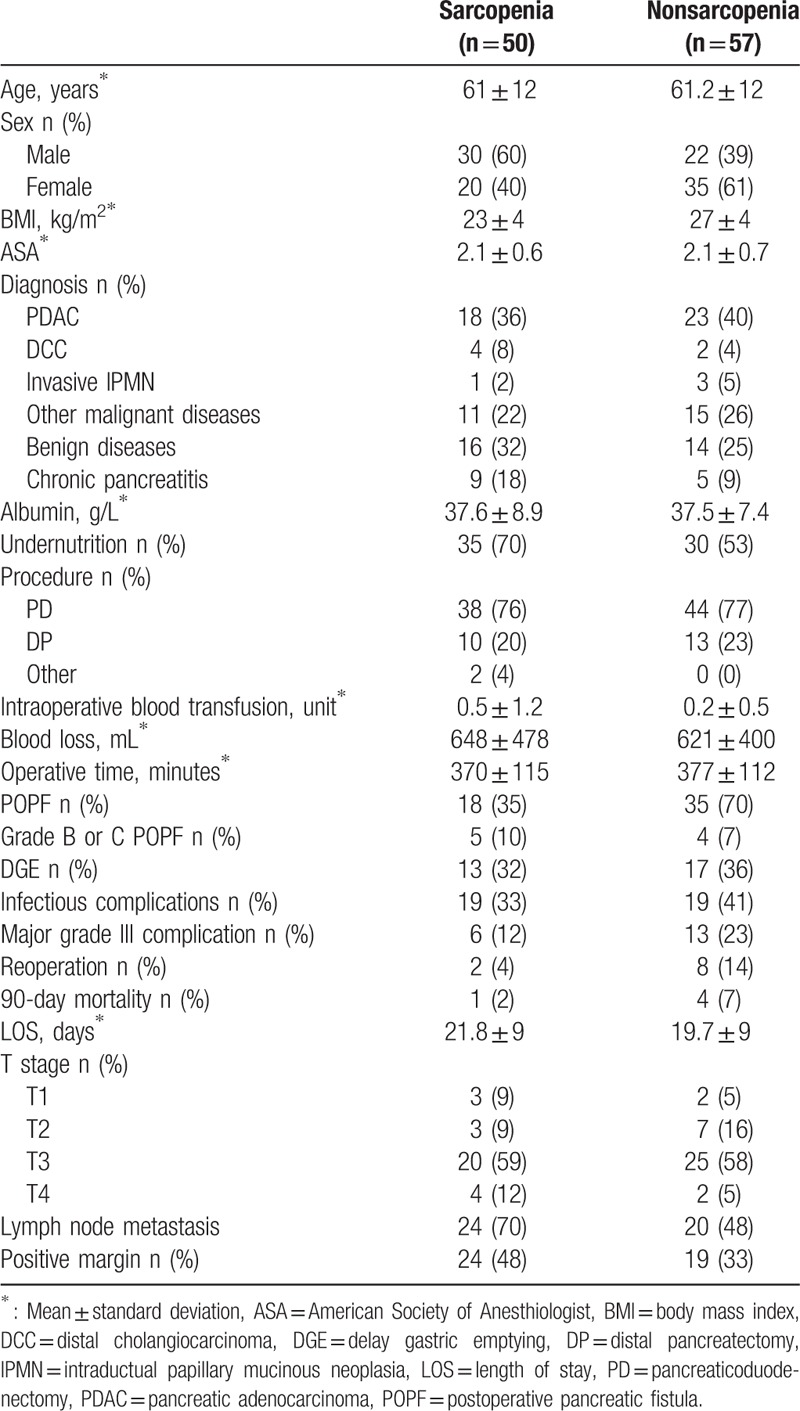
Figure 2.
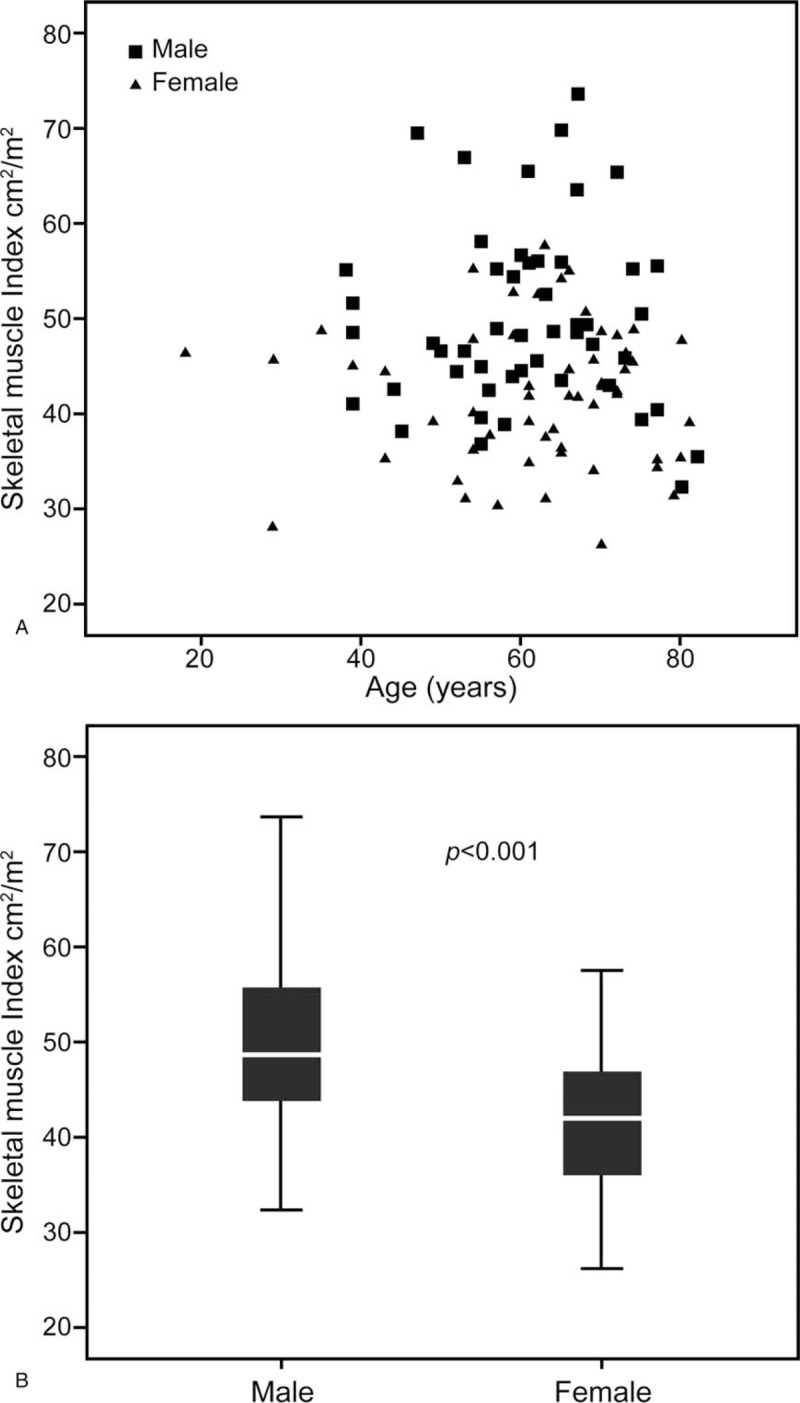
Distribution of skeletal muscle index according to age (A) and gender (B).
3.2. Impact of sarcopenia on 3-month mortality and the postoperative course
The 3-month mortality was comparable between groups. Severe complications were reported in 6 (12%) sarcopenic and 13 (22%) nonsarcopenic patients. Unexpectedly, POPF occurred more frequently in the nonsarcopenic group (70% vs 36% in sarcopenic patients), although the rates of severe POPF (i.e., grades B and C) were comparable between groups. Regarding other complications, the rates of gastroparesis, infectious complications, and reoperation were similar between groups. LOS was also similar between sarcopenic and nonsarcopenic patients.
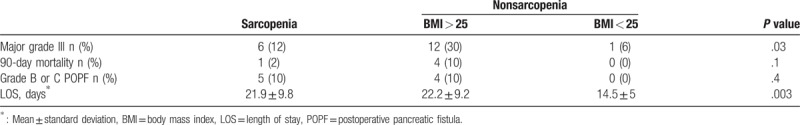
To identify potential confounding factors for postoperative morbidity and mortality, we further stratified the patients according to their BMI (<25 kg/m2 or ≥25 kg/m2). Overall, major complications primarily occurred among sarcopenic (12%) or overweight nonsarcopenic patients (30%) rather than among nonoverweight, nonsarcopenic patients (6%, P = .03). Similarly, POPF grades B or C and death occurred exclusively among sarcopenic or overweight nonsarcopenic patients. In addition, the LOS was significantly longer among sarcopenic patients (21.9 days) and overweight nonsarcopenic patients (22.2 days), compared to 14.5 days for the nonoverweight, nonsarcopenic patients (P = .003) (Table 3).
Table 3.
Hospital stay and mobimortality of sarcopenic and overweight no sarcopenic patients.
3.3. Impact of sarcopenia on survival
PDAC was the most frequent indication for pancreatic surgery in our study. Among cancer patients (n = 77) treated with pancreatectomy, 31 (40%) experienced disease recurrence, and 23 (30%) died after 15 ± 13.5 months. The median disease-free survival was 11.1 ± 2.6 months in the sarcopenic group compared to 22.5 ± 15 months in the nonsarcopenic group (P = .04). OS was also lower in the sarcopenic group (16 ± 3.6 months in the sarcopenic group vs not achieved in the nonsarcopenic group, P = .02) (Fig. 3). We also analysed the 1- and 2-year OS rates between sarcopenic and nonsarcopenic patients. Survival within 1 to 3 years postoperatively were higher among the nonsarcopenic than among the sarcopenic patients (66% vs 45%, P = .02, and 58% vs 38%, P = .02, respectively).
Figure 3.
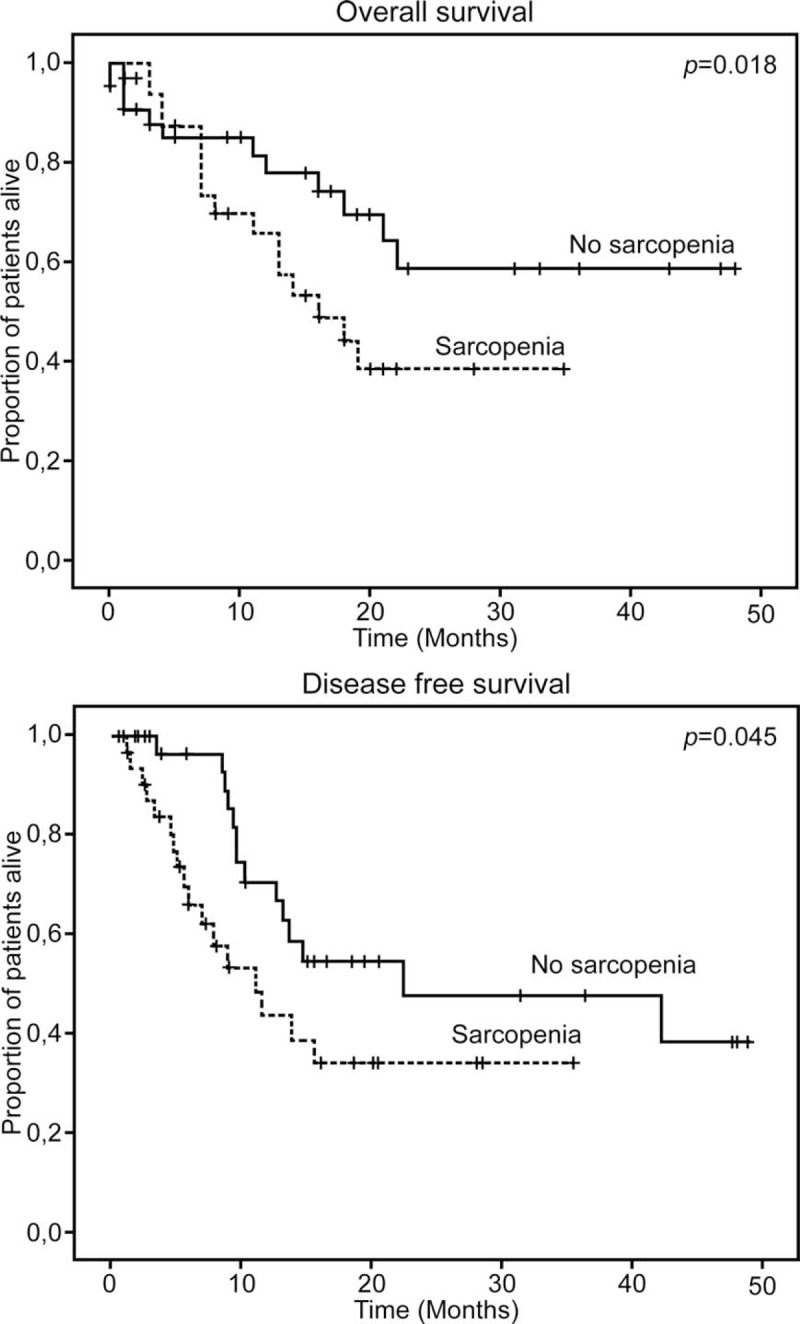
Overall and disease free survival according to sarcopenic status in patients with malignant diseases.
We also examined the impact of being overweight on OS and disease-free survival (Fig. 4). Interestingly, overweight patients (BMI > 25 kg/m2) had poorer long-term prognoses, as shown by the overall disease-free survival curves in Figure 4.
Figure 4.
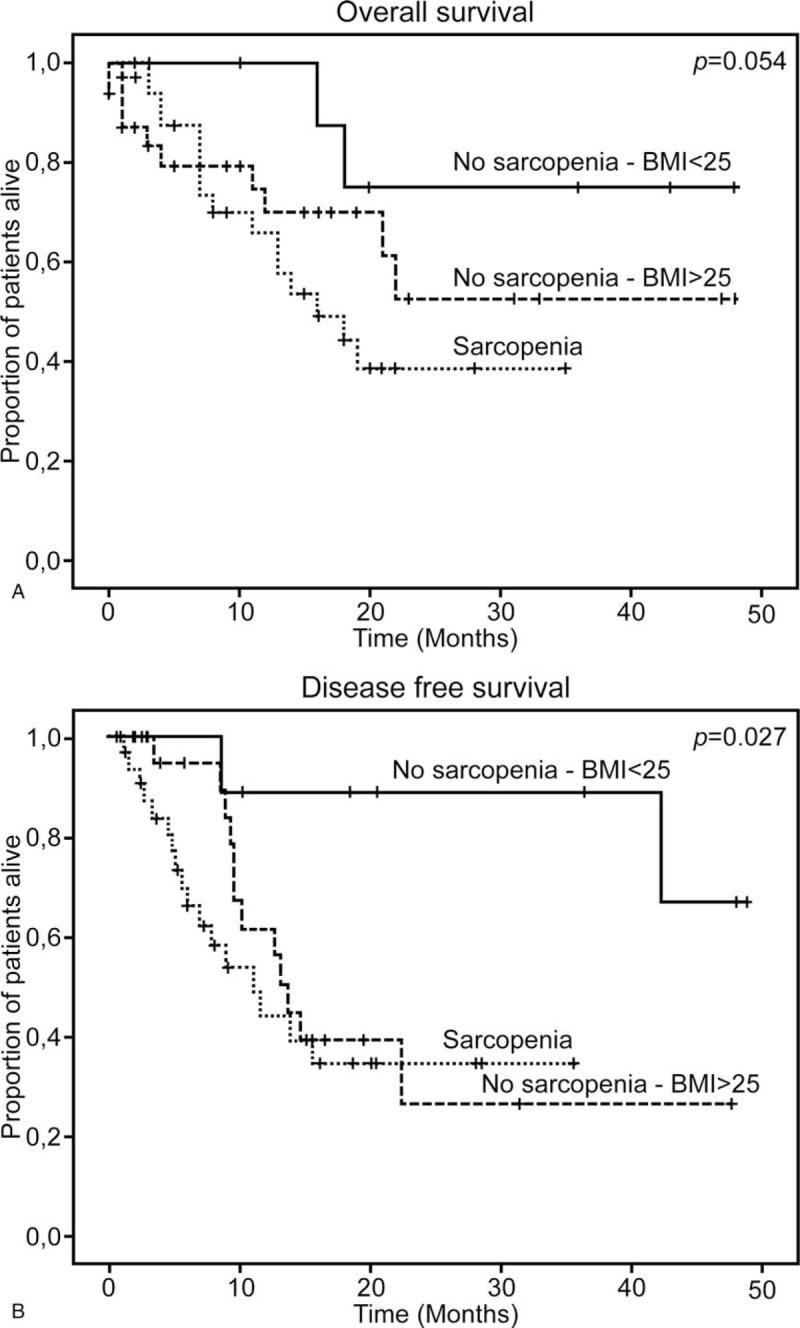
Overall and disease free survival of sarcopenic and nonsarcopenic patients stratified by BMI.
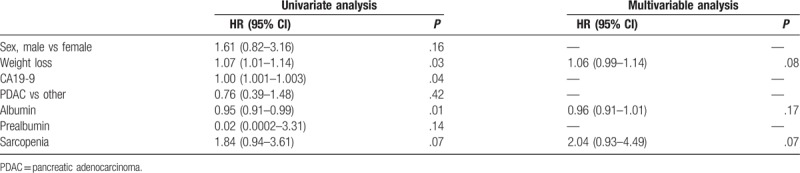
The multivariate analysis showed that sarcopenia tended to increase the risk of death (hazard ratio, 2.04; 95% confidence interval, 0.93–4.49; P = .07; Table 4).
Table 4.
Univariable and multivariable cox proportional hazards regression analysis of overall survival.
4. Discussion
Pancreatic surgery is a complex procedure with a high rate of morbidity. It is challenging to identify preoperatively the patients at risk of experiencing complications in order to improve their postoperative outcomes. Preoperative evaluation of nutritional status can predict these complications, as it is a significant factor that affects postoperative outcomes.[29] However, nutritional assessment requires clinical and biological data whose relevance and reproducibility remain insufficient. Sarcopenia, on the other hand, is an assessment of muscle reserve that reflects the nutritional status. Here, we analysed the impact of sarcopenia on postoperative outcomes and survival in a series of 107 patients. Even though there was no significant difference between the 2 groups with respect to overall morbidity and death rates, POPF grades B or C and deaths occurred exclusively in the sarcopenic patients or overweight nonsarcopenic patients, who had significantly longer LOSs. These results are consistent with data reported in literature. Joglekar et al,[30] showed in 118 patients that sarcopenia was an independent predictor of major grade III complications; LOS; intensive care unit admission; delayed gastric emptying; and infectious, gastrointestinal, pulmonary, and cardiac complications. Similarly, Amini et al[23] reported a correlation between sarcopenia and postoperative morbidity in a series of 763 patients. A few studies have attempted to examine the impact of sarcopenia on the POPF rate and its severity. Joglekar et al[30] reported no correlation between sarcopenia and pancreatic fistula, but they did not analyse grades of severity. However, Nishida et al[31] highlighted that preoperative sarcopenia was a strong and independent risk factor for clinically relevant POPF formation after PD. Despite the large numbers of patients included in these studies, their definition of sarcopenia remains unclear. In these studies, sarcopenia was not defined according to the international consensus, which was used in the present study. This limitation should be considered when interpreting the data.
Being overweight is a determinant of postoperative complications following gastrointestinal surgery.[32] We observed that overweight patients had an equivalent behaviour to sarcopenic patients. We showed a similar rate of grades B and C POPF in sarcopenic and overweight nonsarcopenic patients. These patients also had significantly more postoperative complications and significantly longer LOSs. We could not analyse the impact of sarcopenia for overweight patients due to an insufficient number of patients. Recent data suggest, however, that this combination could influence postoperative outcomes and prognosis.[33,34]
In our series, sarcopenia was a major prognostic factor among patients who underwent surgery for malignant diseases. The overall and disease-free survivals were significantly shorter in sarcopenic patients. The association between sarcopenia and survival rates has been previously studied in pancreatic disease. Peng et al reported an increased risk of death after 3 years in a large series of 557 patients undergoing pancreatectomy for PDAC.[20,22]
The oncological impact of sarcopenia is multifactorial. The skeletal muscles play a fundamental role in protein synthesis, and sarcopenia might interfere with immune defence.[35] A large number of postoperative complications in sarcopenic patients is likely to delay the time to administration of adjuvant therapy and its subsequent impact on prognosis. Moreover, the hypertoxicity of drugs in sarcopenic patients has been studied. Prado et al[36] investigated 62 patients treated surgically for colorectal cancer and reported their tolerance towards 5-FU adjuvant chemotherapy.
Sarcopenia can also affect patients with benign disease. Sixteen patients who underwent pancreatic surgery for benign diseases were sarcopenic, and the rate of sarcopenia was comparable to that of patients treated surgically for malignant diseases. Chronic pancreatitis was the most common aetiology for pancreatectomy in our study. This entity is commonly associated with undernutrition and cachexia. To our knowledge, there are no studies on the impact of sarcopenia in patients with chronic pancreatitis. Thus, studies are needed to show whether there is sarcopenia affects these patients.
Some measures that have been proposed to treat sarcopenia have not been supported by evidence. Optimization of the patient's nutritional status should be achieved by adopting a program with nutritional objectives. However, no study has shown an increase in lean mass following the usual nutritional treatments. It has been suggested that physical activity may have a beneficial effect on the surgical outcomes of patients undergoing surgery for malignant disease; an American study of 252,925 patients showed that postoperative mortality was lower as the patient's normal physical activity levels increased.[37] Physical activity may also improve the quality of life or even the prognosis of patients treated surgically for malignancies.[38] A few encouraging results in the elderly show that physical activity might improve lean mass and strength.[39]
Our study has some limitations. First, our definition of sarcopenia is very restrictive. Measurement of muscle strength and grip is possible in current practice, but it requires a prospective assessment. Second, our definition of sarcopenia according to Prado's cut-off is based on a Canadian cohort of obese sarcopenic patients with respiratory or gastrointestinal tumours.[28] However, this definition was the most commonly used in the literature. Moreover, Mourtzakis et al demonstrated the validity of Prado's cut-off in cancer patients with a strong correlation to appendicular skeletal muscle mass obtained with dual energy x-ray absorptiometry (which is the gold-standard method). Finally, our study contains some potential bias because of its retrospective design. However, only patients who had CT performed in our institution were included, allowing a homogeneous assessment of sarcopenia. Despite the study limitations, our data clearly show the clinical impact of sarcopenia in pancreatic surgery.
5. Conclusion
Sarcopenia negatively impacts short- and long-term outcome in patients undergoing pancreatectomy. The measurement of skeletal muscle mass with a CT is simple and enables the selection of patients for pancreatectomy. The identification of sarcopenic patients before pancreatectomy allows the implementation of early strategies to improve muscle mass in order to improve prognosis and patient selection.
Author contributions
Conceptualization: Mehdi El Amrani, Maxence Fulbert, François-René Pruvot.
Data curation: Mehdi El Amrani, Maxence Fulbert, Katia Lecolle, François-René Pruvot.
Formal analysis: Mehdi El Amrani, Mathilde Vermersch, Maxence Fulbert, François-René Pruvot.
Funding acquisition: Mehdi El Amrani.
Investigation: Mehdi El Amrani, Mathilde Vermersch, Olivier Ernst.
Methodology: Mehdi El Amrani, Mathieu Prodeau, Olivier Ernst.
Project administration: Mehdi El Amrani.
Resources: Mehdi El Amrani, Mathieu Prodeau, Stephanie Truant.
Software: Mehdi El Amrani, Mathieu Prodeau.
Supervision: Mehdi El Amrani, Mohamed Hebbar, Stephanie Truant.
Validation: Mehdi El Amrani, Mohamed Hebbar, Stephanie Truant.
Visualization: Mehdi El Amrani, Mathilde Vermersch, Mohamed Hebbar, Stephanie Truant.
Writing – original draft: Mehdi El Amrani, Maxence Fulbert, Mohamed Hebbar, François-René Pruvot, Stephanie Truant.
Writing – review & editing: Mehdi El Amrani, Mathilde Vermersch, Maxence Fulbert, Mathieu Prodeau, Olivier Ernst, François-René Pruvot, Stephanie Truant.
Footnotes
Abbreviations: ALT = alanine aminotransferase, ASA = American Society of Anesthesiologists score, AST = aspartate aminotransferase, BMI = body mass index, CT = computed tomography, DP = distal pancreatectomy, EBL = estimated blood loss, ISGPF = International Study Group of Pancreatic Fistula, PD = pancreaticoduodenectomy, PDAC = pancreatic adenocarcinoma, POD = post-operative day, POPF = postoperative pancreatic fistula, SMI = skeletal muscle index, TP = total pancreatectomy.
The authors have no conflicts of interest to disclose.
References
- [1].Amini N, Spolverato G, Kim Y, et al. Trends in hospital volume and failure to rescue for pancreatic surgery. J Gastrointest Surg 2015;19:1581–92. [DOI] [PubMed] [Google Scholar]
- [2].Büchler MW, Wagner M, Schmied BM, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg 2003;138:1310–4. [DOI] [PubMed] [Google Scholar]
- [3].Bachmann J, Heiligensetzer M, Krakowski-Roosen H, et al. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193–201. [DOI] [PubMed] [Google Scholar]
- [4].Mourão F, Amado D, Ravasco P, et al. Nutritional risk and status assessment in surgical patients: a challenge amidst plenty. Nutr Hosp 2004;19:83–8. http://www.ncbi.nlm.nih.gov/pubmed/15049409 [PubMed] [Google Scholar]
- [5].La Torre M, Ziparo V, Nigri G, et al. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol 2013;107:702–8. [DOI] [PubMed] [Google Scholar]
- [6].Rosenberg IH. Summary comments. Am J Clin Nutr 1989;50:1231–3. [Google Scholar]
- [7].Cooper C, Dere W, Evans W, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int 2012;23:1839–48. [DOI] [PubMed] [Google Scholar]
- [8].Otsuji H, Yokoyama Y, Ebata T, et al. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg 2015;39:1494–500. [DOI] [PubMed] [Google Scholar]
- [9].Valero V, Amini N, Spolverato G, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg 2015;19:272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reisinger KW, van Vugt JL a, Tegels JJW, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2014;0:1–8. [DOI] [PubMed] [Google Scholar]
- [11].Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang D-D, Wang S-L, Zhuang C-L, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after colorectal cancer surgery. Color Dis 2015;17:O256–64. [DOI] [PubMed] [Google Scholar]
- [13].Kuroki LM, Mangano M, Allsworth JE, et al. Pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol 2015;22:972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Visser M, van Venrooij LMW, Vulperhorst L, et al. Sarcopenic obesity is associated with adverse clinical outcome after cardiac surgery. Nutr Metab Cardiovasc Dis 2013;23:511–8. [DOI] [PubMed] [Google Scholar]
- [15].Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol 2015;22:2663–8. [DOI] [PubMed] [Google Scholar]
- [16].Tamandl D, Paireder M, Asari R, et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol 2016;26:1359–67. [DOI] [PubMed] [Google Scholar]
- [17].Harada K, Ida S, Baba Y, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus 2016;29:627–33. [DOI] [PubMed] [Google Scholar]
- [18].Harimoto N, Shirabe K, Yamashita YI, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 2013;100:1523–30. [DOI] [PubMed] [Google Scholar]
- [19].Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg 2014;0:1–1. [DOI] [PubMed] [Google Scholar]
- [20].Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tan BHL, Birdsell L a, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973–9. [DOI] [PubMed] [Google Scholar]
- [22].Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery 2015;157:1088–98. [DOI] [PubMed] [Google Scholar]
- [23].Amini N, Spolverato G, Gupta R, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg 2015;19:1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- [26].Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–8. [DOI] [PubMed] [Google Scholar]
- [27].Grützmann R, Rückert F, Hippe-Davies N, et al. Evaluation of the International Study Group of Pancreatic Surgery definition of post-pancreatectomy hemorrhage in a high-volume center. Surgery 2012;151:612–20. [DOI] [PubMed] [Google Scholar]
- [28].Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- [29].Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 2011;98:268–74. [DOI] [PubMed] [Google Scholar]
- [30].Joglekar S, Asghar A, Mott SL, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 2015;111:771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nishida Y, Kato Y, Kudo M, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg 2016;20:1586–94. [DOI] [PubMed] [Google Scholar]
- [32].Mullen JT, Davenport DL, Hutter MM, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol 2008;15:2164–72. [DOI] [PubMed] [Google Scholar]
- [33].Tan BHL, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol 2014;41:333–8. [DOI] [PubMed] [Google Scholar]
- [34].Lodewick TM, Van Nijnatten TJA, Van Dam RM, et al. Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB 2015;17:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012;4:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Prado CMM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 2007;13:3264–8. [DOI] [PubMed] [Google Scholar]
- [37].Leitzmann MF, Park Y, Blair a, et al. Physical activity recommendations and decreased risk of mortality. Arch Intern Med 2007;167:2453–60. [DOI] [PubMed] [Google Scholar]
- [38].Speck RM, Courneya KS, Masse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 2010;4:87–100. [DOI] [PubMed] [Google Scholar]
- [39].Phu S, Boersma D, Duque G. Exercise and Sarcopenia. J Clin Densitom 2015;18:488–92. [DOI] [PubMed] [Google Scholar]


