Abstract
Many pyogenic liver abscess (PLA) patients underwent abdominal surgery before. However, little is known about the impact of previous abdominal surgery on the clinical characteristics and prognosis of PLA.
The clinical data of 392 adult PLA patients who received treatment at our hospital from January 1, 2007 to December 31, 2016 were collected. The demographic data, cause, comorbidities, surgery history, clinical features, laboratory results, imaging findings, microbiological characteristics, choices of treatment, and clinical outcomes were analyzed.
In all, 177 PLA patients (45.2%) underwent abdominal surgery before. The median time for the occurrence of PLA after the most recent abdominal surgery was 2.0 (interquartile range 0.25, 6.0) years. PLA patients with a previous abdominal surgery history were more likely to have underlying diseases and presented with more abnormal laboratory values. Klebsiella pneumonia and Escherichia coli were the most common pathogens. Previous abdominal surgery appeared to increase the incidence of E coli. More PLA patients without a previous abdominal surgery history required surgical drainage. However, there were no differences in PLA-related complications, days required for temperature normalization, and length of hospital stay between the 2 groups.
Because a large number of PLA patients had a history of abdominal surgery, and proper screening should be performed for patients with any suspicion of a liver abscess after abdominal surgery. Despite the differences in the coexisting conditions, clinical and microbiological characteristics between PLA patients with and without a previous abdominal surgery history, the overall short-term outcomes were comparable.
Keywords: abdominal surgery, cause, prognosis, pyogenic liver abscess, treatment
1. Introduction
Pyogenic liver abscess (PLA) is the most common type of visceral abscess. Due to an increasing prevalence of diabetes mellitus, more and more aggressive surgical management of hepatic, biliary, and pancreatic disorders, aging population, and wide use of immunosuppressive agents in transplant and cancer patients, the incidence of PLA has increased steadily in recent years.[1–3] In the meantime, the cause, diagnosis, management, and prognosis of PLA underwent significant changes.[4] A few population-based studies have shown that a history of abdominal surgery was associated with an increased risk of PLA.[5–10] Also, many PLA patients underwent abdominal surgery before.[11,12] However, it remained unknown whether there are any differences in the clinical characteristics and prognosis between PLA patients with and without a previous surgery history. The purpose of this study, therefore, was to investigate the impact of previous surgery on the clinical characteristics and prognosis of PLA.
2. Patients and methods
2.1. Study population
In all, 438 liver abscess patients were admitted to the first affiliated hospital of Xi’an Jiaotong University from January, 2007 to December, 2016. Patients who had incomplete medical records (n = 39), amebic liver abscess (n = 1), tuberculous liver abscess (n = 3), or under the age of 18 (n = 3) were excluded from this study. In the remaining 392 patients, 177 had previous abdominal surgery history. The previous abdominal surgery was defined as any abdominal surgery before the occurrence of PLA. All the PLA patients were treated with a combination of a second or third-generation cephalosporin and metronidazole empirically or based on antimicrobial susceptibility testing if available. Depending on the number and size of abscesses, degree of abscess liquefaction, separation of abscess cavity, patient response to antibiotics, and personal experience of the physicians, PLA patients were treated with antibiotics alone or antibiotics plus percutaneous drainage or surgical drainage. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (IRB No: XJTU1AF2015LSL-057). The patients’ informed written consent was waived due to the retrospective nature of this study.
2.2. Data collection
The medical records of all patients were reviewed retrospectively. Clinical data included patients’ age, sex, comorbidities, surgery history, symptoms and body temperatures at admission, and changes during hospitalization. Laboratory data included blood routine test, liver function test, and renal function test, which were documented at admission. Blood cultures were drawn from all patients within the first 24 hours after admission. Aspiration of the abscess was performed under ultrasound or computed tomography (CT) guidance, and aspirated pus was sent for gram stain and culture. The number and size of abscesses were determined based on ultrasound and/or CT images. Clinical outcomes included spontaneous rupture of PLA, portal vein thrombosis, sepsis, septic shock, acute kidney injury, pleural effusion, acute respiratory distress syndrome (ARDS), and in-hospital death.
2.3. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables as absolute numbers and percent frequencies. Differences between continuous data were analyzed using Student t test. Differences between categorical data were analyzed with the chi-square test or Fisher exact test, as appropriate. Univariate and multivariate analyses of risk factors were performed using the Cox proportional-hazards model. All statistical analyses were performed with SPSS version 22.0 (IBM, Armonk, NY). A 2-tailed P value <.05 was considered statistically significant.
3. Results
3.1. Prevalence of a previous surgery history in PLA patients
There were 392 PLA patients in this retrospective study. Of the 177 patients (45.2%) with a previous abdominal surgery history, 38 (21.5%) had cholecystectomy, 18 (10.2%) had biliary tract surgery, 13 (7.3%) had gastrointestinal surgery, 10 (5.6%) had hepatectomy, 3 (1.7%) had pancreatic surgery, 3 (1.7%) had gynecologic surgery, 19 (10.7%) had other surgery, and 73 (41.2%) had more than 1 surgery (Fig. 1). The median time for the occurrence of PLA after the most recent abdominal surgery was 2.0 (interquartile range [IQR] 0.25, 6.0) years.
Figure 1.
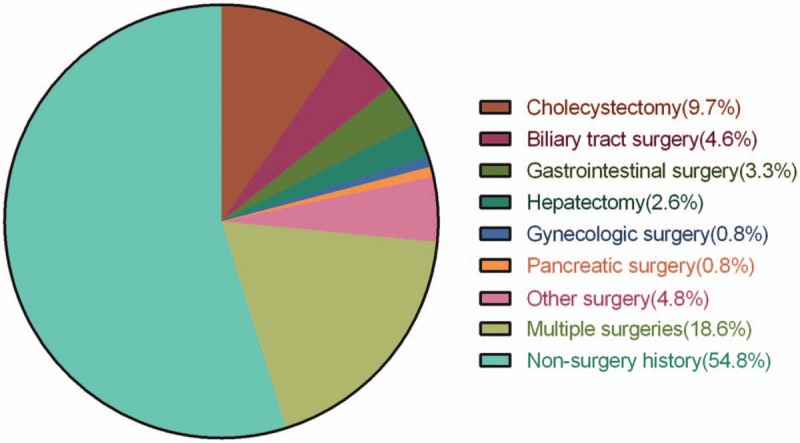
Types of previous abdominal surgery in PLA patients. PLA = pyogenic liver abscess.
3.2. Demographic data and coexisting conditions
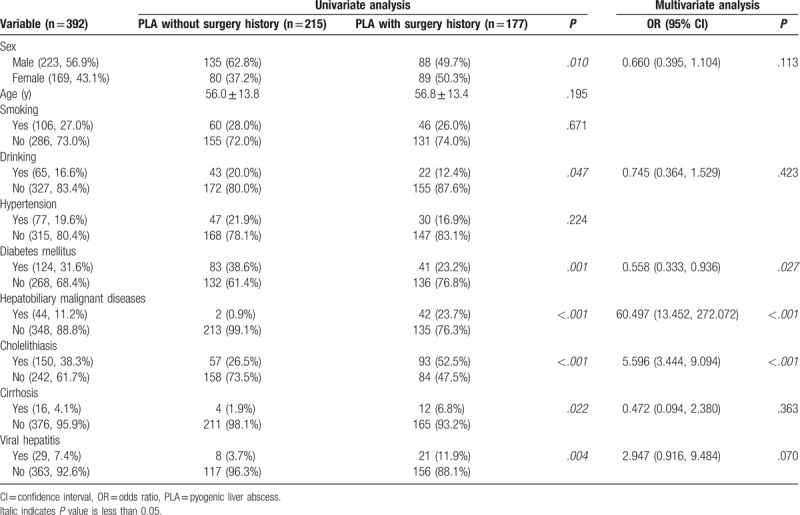
As shown in Table 1, PLA patients without a previous abdominal surgery history were more likely to be male (62.8% vs 49.7%; P = .009), have a drinking history (20.0% vs 12.4%; P = .045), and suffer diabetes mellitus (38.6% vs 23.2%; P = .001) (Table 1) than those with a previous abdominal surgery history. On the contrary, PLA patients with a previous abdominal surgery history were more likely to have underlying hepatobiliary malignant diseases (23.7% vs 0.9%; P < .001), cholelithiasis (52.5% vs 26.5%; P < .001), cirrhosis (6.8% vs 1.9%; P = .014), and viral hepatitis (11.9% vs 3.7%; P = .002) than those without a previous abdominal surgery history (Table 1). Multivariate analysis showed that diabetes mellitus (odds ratio [OR] 0.558, 95% confidence interval [CI] 0.333–0.936, P = .027), hepatobiliary malignant diseases (OR 60.497, 95% CI 13.452–272.072, P < .001), and cholelithiasis (OR 5.596, 95% CI 3.444–9.094, P < .001) were independent factors associated with previous abdominal surgery history in PLA patients (Table 1).
Table 1.
Demographic data and coexisting conditions.
3.3. Clinical features, laboratory results, and imaging findings
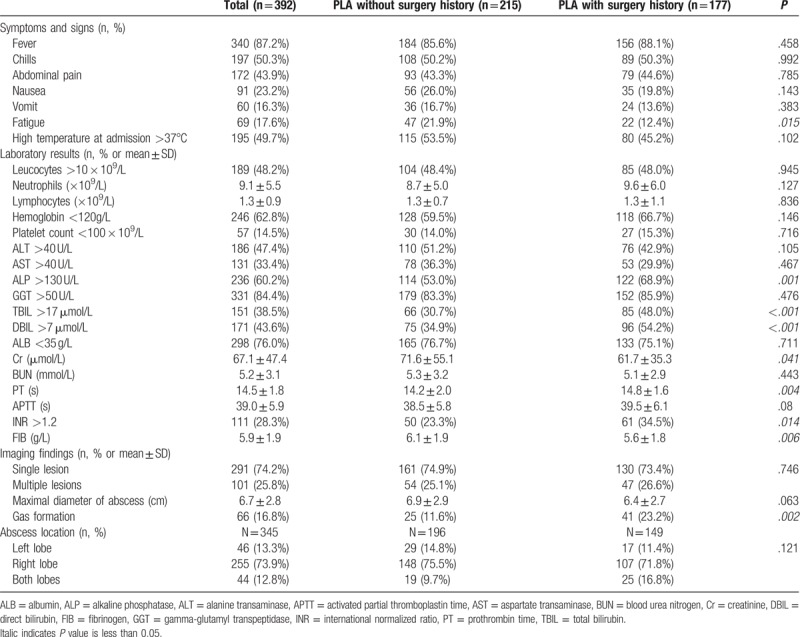
Other than more patients in the PLA without a previous abdominal surgery group presented with fatigue at admission than those with a previous abdominal surgery history, most of the symptoms and signs were similar between the 2 groups (Table 2). However, patients with a previous abdominal surgery had significantly higher levels of alkaline phosphatase (ALP), total and direct bilirubin, prothrombin time (PT), international normalized ratio (INR), and fibrinogen (FIB) than those without a previous abdominal surgery history (P < .05 for all; Table 2). In terms of the imaging findings, there were no significant differences in the size and location of the liver abscesses between the 2 groups (Table 2). However, PLA patients with a previous surgery history were more likely to have gas-forming abscesses than those without a previous surgery history (23.2% vs 11.6%; P = .002, Table 2).
Table 2.
Clinical features, laboratory results and imaging findings, and abscess location.
3.4. Microbiological characteristics
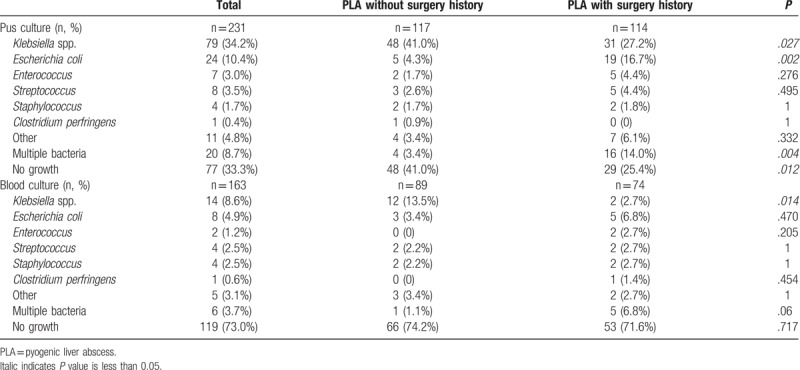
The pus culture result was available in 231 (58.9%) patients in this cohort (Table 3). Among them, 154 (66.7%) patients had an identifiable organism. Patients with a previous abdominal surgery had a significantly higher positive rate on pus culture than those without a previous abdominal surgery history (74.6% vs 59.0%; P = .012). Although Klebsiella pneumonia and Escherichia coli were the 2 most common pathogens on pus culture in both groups, the K pneumonia-positive rate was lower and the E coli-positive rate was higher in patients with a previous abdominal surgery than those without a previous abdominal surgery (P = .027 and .002, respectively; Table 3). Among pus culture-positive patients, 13.0% were polymicrobial. Patients with a previous abdominal surgery history were more likely to be polymicrobial than those without a previous abdominal surgery (18.8% vs 5.8%; P = .017). The blood culture result was available in 163 (41.6%) patients (Table 3). Among them, 44 (27.0%) showed positive bacterial culture. K pneumonia remained the most common pathogen in patients without previous abdominal surgery, whereas E coli were the most common pathogen in patients with previous abdominal surgery on blood culture.
Table 3.
Microbiological characteristics.
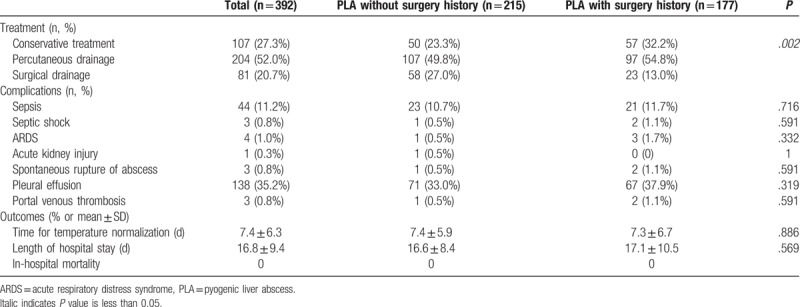
3.5. Treatment and outcomes
As shown in Table 4, in patients with previous abdominal surgery, 32.2% were managed with conservative treatment alone, 54.8% required percutaneous drainage, and 13.0%required surgical drainage. In patients without previous abdominal surgery, on the contrary, 23.3% were managed with conservative treatment alone, 49.8% required percutaneous drainage, and 27% required surgical drainage. No patients required surgical resection for PLA in this cohort. The differences in the treatment methods were statistically significant (P = .002; Table 4). However, there were no differences in PLA-related complications, days required for temperature normalization, and length of inhospital stay between the 2 groups. In this cohort, no patients died during their stay in the hospital (Table 4).
Table 4.
Treatment, complications, and outcomes.
4. Discussion
This was the first study to systemically investigate the impact of previous abdominal surgery on the clinical characteristics and prognosis of PLA. Here, we found that there were significant differences in the coexisting conditions, and clinical and microbiological characteristics between PLA patients with and without a previous abdominal surgery history. However, likely due to improvements in the diagnosis and management of PLA patients, there were no major differences in the overall short-term outcomes between the 2 groups.
Various abdominal surgical procedures including splenectomy, appendectomy, pancreaticoduodenectomy, and gastrectomy have been shown to increase the risk of PLA.[5–8] Consistent with these findings, almost half of PLA patients in our current study have undergone abdominal surgery before. Although our data were incomplete to identify previous abdominal surgery as an independent risk factor for PLA, such a high percentage of PLA patients had abdominal surgery history suggests that abdominal surgery history may be a major risk factor for PLA. Most of the patients undergoing abdominal surgery suffer from malnutrition during the perioperative period and their immune function could be compromised.[13] In addition, patients who underwent abdominal surgery for underlying malignant diseases might also receive chemotherapy, which would further suppress their immune function.[14] All these factors contribute to their increased susceptibility to PLA after abdominal surgery. However, future studies will be needed to explore the detailed mechanisms responsible for the increased risk of developing PLA in patients who underwent abdominal surgery. Because PLA is a serious postoperative complication, proper screening should be performed for those who present with any signs of PLA after abdominal surgery.
It is well-known that diabetic patients are more vulnerable to PLA.[15–17] In the current cohort, we also found that 31.6% of all PLA patients had underlying diabetes mellitus. An interesting finding of this study was that less patients in the PLA with previous surgery group suffered from diabetes mellitus than those in the PLA without previous surgery group. Because it is unlikely that a previous abdominal surgery history would reduce the chance of developing PLA in diabetic patients, the possible explanation for this result is that the combined effect of abdominal surgery and diabetes mellitus overshadowed the effect of diabetes mellitus on the development of PLA in these patients. However, a large population-based study is required to either confirm or deny this finding. As to other comorbidities, more patients in the PLA with previous surgery group suffered from various underlying diseases than those in the PLA without surgery group. This is not surprising as the previous abdominal surgery was most likely the treatment for the underlying diseases of these patients.
The most common pathogens identified in the current study were K pneumonia and E coli. Previous studies in Asian countries also demonstrated the predominance of these 2 microorganisms.[12,18–20] However, this is in contrast to studies in the United States, Canada, Australia, and European countries, which have consistently reported a high prevalence of Gram-positive organisms such as Streptococcus species in PLA patients.[2,21–23] In the current study, we also found that previous abdominal surgery appeared to increase the incidence of E coli. And on blood culture, E coli surpassed K pneumonia as the most predominant organism in PLA patients with a previous abdominal surgery history. In addition, PLA patients with a previous abdominal surgery history were more likely to be involved with multiple types of bacteria, emphasizing the need for either a broad-spectrum antibiotic or combination antibiotic therapy in the initial management of these patients.
There were no major differences in the clinical symptoms, signs, and also the size and location of the liver abscesses between the 2 groups. PLA patients with a previous abdominal surgery history appeared to have more abnormal laboratory values than those without a previous abdominal surgery history. However, this does not necessarily mean patients with a previous abdominal surgery history had more serious PLA than other PLA patients, as the differences can be explained by the underlying diseases. Furthermore, PLA patients without previous abdominal surgery are more likely to require surgical drainage than those with previous abdominal surgery. In terms of the outcome of PLA, we did not observe any significant differences between the 2 groups, suggesting that the prognosis of PLA is more closely related to the management than cause of PLA.
These findings, however, should be interpreted with caution, as there are several limitations of this study. First, this is a retrospective study. The results, therefore, are subject to a selection bias and some residual confounding. Second, only a single center's data were included in the analysis. Most of the patients in this cohort came from Shaanxi Province of China. Recent studies from different regions of the world have demonstrated considerable differences in cause and treatment of PLA.[24–26] Therefore, our findings need to be validated by multicenter studies. Lastly, we only focused on the short-term outcomes of PLA in this study. This is due to the consideration that the underlying disease would significantly influence the long-term outcomes of patients with previous abdominal surgery. To evaluate the impact of previous abdominal surgery on the long-term outcomes of PLA, a prospective propensity score-matched study is warranted in the future.
5. Conclusions
In conclusion, since a large number of PLA patients had a history of abdominal surgery, proper screening should be performed for patients who present with any signs of PLA after abdominal surgery. Despite the differences in the coexisting conditions, and clinical and microbiological characteristics between PLA patients with and without a previous abdominal surgery history, the overall short-term outcomes were comparable.
Author contributions
Wu R and Zhang X designed the research; Wu Z and Lv Y supported the research; Zhang J, Du Z and Bi J collected the data; Zhang J, Du Z and Wu R analyzed the data; Wu R and Zhang J wrote the manuscript; Wu R supervised the whole research; all authors have read and agreed with the final manuscript.
Conceptualization: Xufeng Zhang, Rongqian Wu.
Data curation: Jia Zhang, Zhaoqing Du, Jianbin Bi.
Formal analysis: Jia Zhang, Zhaoqing Du, Rongqian Wu.
Project administration: Zheng Wu, Yi Lv.
Supervision: Rongqian Wu.
Writing – original draft: Jia Zhang, Rongqian Wu.
Writing – review & editing: Rongqian Wu.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, APTT = activated partial thromboplastin time, ARDS = acute respiratory distress syndrome, AST = aspartate transaminase, BUN = blood urea nitrogen, Cr = creatinine, CT = computed tomography, DBIL = direct bilirubin, FIB = fibrinogen, GGT = gamma-glutamyl transferase, INR = international normalized ratio, IQR = interquartile range, PLA = pyogenic liver abscess, PT = prothrombin time, SD = standard deviation, TBIL = total bilirubin.
JZ and ZD contributed equally to this work.
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81770491), Ministry of Education Innovation Team Development Program of China (No. IRT16R57), and a research fund for Young Talent Recruiting Plans of Xi’an Jiaotong University (RW).
The authors report no conflicts of interest.
References
- [1].Chen YC, Lin CH, Chang SN, et al. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000–2011. J Microbiol Immunol Infect 2016;49:646–53. [DOI] [PubMed] [Google Scholar]
- [2].Meddings L, Myers RP, Hubbard J, et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol 2010;105:117–24. [DOI] [PubMed] [Google Scholar]
- [3].Tsai FC, Huang YT, Chang LY, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 2008;14:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Malik AA, Bari SU, Rouf KA, et al. Pyogenic liver abscess: changing patterns in approach. World J Gastrointest Surg 2010;2:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lai SW, Lai HC, Lin CL, et al. Splenectomy correlates with increased risk of pyogenic liver abscess: a nationwide cohort study in Taiwan. J Epidemiol 2015;25:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liao KF, Lai SW, Lin CL, et al. Appendectomy correlates with increased risk of pyogenic liver abscess: a population-based cohort study in Taiwan. Medicine (Baltimore) 2016;95:e4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tsai MS, Lin CL, Jeng LB. Gastrectomy is associated with an increased risk of pyogenic liver abscess: a 13-year nationwide cohort study. Sci Rep 2016;6:33788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Njoku VC, Howard TJ, Shen C, et al. Pyogenic liver abscess following pancreaticoduodenectomy: risk factors, treatment, and long-term outcome. J Gastrointest Surg 2014;18:922–8. [DOI] [PubMed] [Google Scholar]
- [9].Hsu YN, Liu CJ, Lin JK, et al. Liver abscess after liver metastasectomy is a poor prognostic factor in patients with colorectal cancer. J Gastrointest Surg 2011;15:1798–806. [DOI] [PubMed] [Google Scholar]
- [10].Furugaki K, Chijiiwa K, Ogawa T, et al. Pyogenic liver abscess after hepatobiliary and pancreatic surgery. Int Surg 2001;86:67–71. [PubMed] [Google Scholar]
- [11].Shi SH, Feng XN, Lai MC, et al. Biliary diseases as main causes of pyogenic liver abscess caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Liver Int 2017;37:727–34. [DOI] [PubMed] [Google Scholar]
- [12].Du ZQ, Zhang LN, Lu Q, et al. Clinical charateristics and outcome of pyogenic liver abscess with different size: 15-year experience from a single center. Sci Rep 2016;6:35890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sungurtekin H, Sungurtekin U, Balci C, et al. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr 2004;23:227–32. [DOI] [PubMed] [Google Scholar]
- [14].Steele TA. Chemotherapy-induced immunosuppression and reconstitution of immune function. Leuk Res 2002;26:411–4. [DOI] [PubMed] [Google Scholar]
- [15].Tian LT, Yao K, Zhang XY, et al. Liver abscesses in adult patients with and without diabetes mellitus: an analysis of the clinical characteristics, features of the causative pathogens, outcomes and predictors of fatality: a report based on a large population, retrospective study in China. Clin Microbiol Infect 2012;18:E314–30. [DOI] [PubMed] [Google Scholar]
- [16].Foo NP, Chen KT, Lin HJ, et al. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol 2010;105:328–35. [DOI] [PubMed] [Google Scholar]
- [17].Keynan Y, Rubinstein E. Diabetes mellitus and pyogenic liver abscess: risk and prognosis. Clin Infect Dis 2007;45:801. [DOI] [PubMed] [Google Scholar]
- [18].Keller JJ, Tsai MC, Lin CC, et al. Risk of infections subsequent to pyogenic liver abscess: a nationwide population-based study. Clin Microbiol Infect 2013;19:717–22. [DOI] [PubMed] [Google Scholar]
- [19].Luo M, Yang XX, Tan B, et al. Distribution of common pathogens in patients with pyogenic liver abscess in China: a meta-analysis. Eur J Clin Microbiol Infect Dis 2016;35:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qian Y, Wong CC, Lai S, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep 2016;6:38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol 2004;2:1032–8. [DOI] [PubMed] [Google Scholar]
- [22].Barakate MS, Stephen MS, Waugh RC, et al. Pyogenic liver abscess: a review of 10 years’ experience in management. Aust N Z J Surg 1999;69:205–9. [DOI] [PubMed] [Google Scholar]
- [23].Hansen PS, Schonheyder HC. Pyogenic hepatic abscess. A 10-year population-based retrospective study. APMIS 1998;106:396–402. [DOI] [PubMed] [Google Scholar]
- [24].Cerwenka H. Pyogenic liver abscess: differences in etiology and treatment in Southeast Asia and Central Europe. World J Gastroenterol 2010;16:2458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nah BK, Kim YS, Moon HS, et al. [Recent changes of organism and treatment in pyogenic liver abscess]. Taehan Kan Hakhoe Chi 2003;9:275–83. [PubMed] [Google Scholar]
- [26].Mezhir JJ, Fong Y, Jacks LM, et al. Current management of pyogenic liver abscess: surgery is now second-line treatment. J Am Coll Surg 2010;210:975–83. [DOI] [PubMed] [Google Scholar]


