Abstract
The clinical significance of palliative interventional therapy in the management of patients with advanced hilar cholangiocarcinoma (HCCA; stages III–IV) has yet to be studied. The present work was aimed to compare the clinical outcomes of the patients treated with surgery or interventional therapy.
A total of 90 patients with advanced HCCA, who admitted Fuyang First People's Hospital from May 2015 to February 2016, were enrolled. Forty-five of them were assigned to the experimental group receiving biliary drainage as the interventional therapy, and the remaining 45 patients were designated as the conventional group receiving radical/palliative surgery. Before and after the treatment total bilirubin from blood was measured. The length of treatment and medical cost were also examined. All patients were followed up for at least 1 year after the treatment.
For both the experimental and conventional groups, the serum bilirubin levels after treatment were significantly lower than those before treatment (P < .05); however, no significant differences between groups were seen. There were no significant differences between experimental and conventional groups in the incidence of postoperative complications and survival outcomes. Of note, the length of treatment of the experimental group was substantially shorter than that of the conventional group (P < .05). The medical expense of the experimental group was only about one-third of that of the conventional group (P < .05).
Although the interventional therapy does not improve patients’ survivals and reduce the incidence of complications, it significantly shortens the treatment length, reducing substantially the medical expense. This finding provides new insights into the treatment strategy for patients with advanced HCCA.
Keywords: biliary drainage, hilar cholangiocarcinoma, interventional therapy
1. Introduction
Hilar cholangiocarcinoma (HCCA), also named as Klatskin tumor,[1] is the biliary tract cancer arising from the proximal biliary tree.[2] It represents the major type of the bile duct malignancy, accounting for approximately 58% to 66% of the cases. The incidence of HCCA in Western countries is relatively low, but is more commonly seen in Asian countries with incidences as high as 113 per 100,000 men and 50 per 100,000 women.[3] The etiology of HCCA is thus far unclear. A variety of risk factors have, however, been associated with the risk of HCCA including old age, male sex, cirrhosis, chronic pancreatitis, and inflammatory bowel disease.[4] Primary sclerosing cholangitis and congenital biliary cystic disease have been identified as factors conferring an increased risk.[5]
Radical resection with a microscopically negative margin is the only potentially curative way for the treatment of HCCA. However, due to the anatomical location of biliary system, HCCA is asymptomatic. Most patients presented to the clinics because of the complaint of jaundice are diagnosed with advanced HCCA. Radical resection of such advanced tumors is technically challenging.[6] More unfortunately, for patients with advanced cholangiocarcinoma, they are at an elevated risk of tumor recurrence after surgery.[7] In this context, most patients with high operative risk can only be offered palliative drainage of interventional therapy. Percutaneous transhepatic cholangio-drainage and endoscopic retrograde cholangiopancreatography are among the draining techniques being used for the palliation of jaundice in patients with advanced tumors that are not resectable.[8] The biliary drainage has been shown to reduce liver bilirubin, improving patients’ quality of life and survival outcomes.[9,10]
Despite the clinical benefits of interventional therapy in ameliorating jaundice has been shown, the significance of the therapy regarding patients’ survival, treatment length, and medical expense has also yet to be established. As such, the present study conducted a comprehensive analysis comparing the clinical outcomes of patients with advanced HCCA receiving conventional surgery or interventional therapy. The findings would be valuable to the refinement of the clinical management of advanced HCCA.
2. Patients and methods
2.1. Patient population
The present study enrolled 90 patients with advanced HCCA, who were admitted in Fuyang First People's Hospital (Hangzhou, Zhejiang, China) from May 2015 to February 2016. The diagnosis of advanced HCCA (i.e., stages III and IV) was done following international criteria. The staging of tumors was performed per the standard of the American Joint Committee on Cancer.[11] Patients not eligible to surgical treatment were considered for interventional therapy. These patients were complicated with systemic diseases and intrahepatic and/or distinct metastasis. Informed consents were obtained from the recruited patients. The background, aim, significance, and methodology of the present study were explained to the participants.
Ninety patients were randomly assigned into conventional group receiving surgical treatment (n = 45), and experimental group receiving interventional therapy (n = 45). For the conventional group, there were 25 men and 20 women, of whom the average age was 45.17 ± 3.26 years from a range between 22 and 68 years. For the experimental group, there were 26 men and 19 women, of whom the average age was 45.20 ± 3.44 years from a range between 21 and 69 years. No significant differences (P > .05) between 2 groups in patients’ demographic data were seen.
2.2. Surgery treatment and interventional therapy
For patients of the conventional group, 30 of them received radical resection of HCCA per the standard procedure that consisted of resection of the caudate lobe and part of segment IV, combined with a right or left hepatectomy, bile duct resection, and lymphadenectomy of the hepatic hilum. The remaining 15 patients of the same group received palliative resection of the malignant tumors together with the obstructed biliary duct. For all patients of the interventional group, biliary drainage was applied through PTBD approach. The procedures were performed with the ultrasound-guided angiography. The most appropriate liver segments for drainage was selected, and after that, a metallic stent was inserted alongside the guidewire into the segment.[8,12] After surgery or interventional therapy, patients were monitored closely for their vital signs, liver and kidney functions, and oxygen saturation. Abnormal enlargement of abdomen and wound infection was also under surveillance.
2.3. Clinical measurement and follow-up
For both the conventional and experimental groups, total bilirubin levels before and after the treatment were measured. In the first postoperative year, every patient was routinely followed up. Data on patients’ survivals, postoperative complications, length of treatment, and medical expense were collected for statistical analysis.
2.4. Statistical analysis
All statistical tests were performed using SPSS version 18.0. Count data were presented as percentage and were analyzed using chi-square test. Measurement data were presented as average ± standard derivation and were compared using t test. P value <.05 indicated the difference was statistical significance.
3. Results
3.1. Total bilirubin of patients with hilar cholangiocarcinoma
Representative radiological images of HCCA are shown in Figure 1. The pre- and post-treatment total bilirubin levels of the conventional and experimental groups were determined (Table 1). Before treatment the total bilirubin of both groups was measured comparable to each other. In the conventional group, the level dropped from 272.32 ± 98.25 to 88.52 ± 45.20 μmol/L (P < .05) upon treatment, whereas in the experimental group, the level decreased from 272.64 ± 98.45 to 88.23 ± 45.14 μmol/L (P < .05) upon treatment. The post-treatment total bilirubin levels of both groups were not significantly different from each other. For a more detailed analysis patients of the conventional and experimental groups were stratified into subgroups according to their genders and ages (Table 2). In each of the subgroups, the total bilirubin level after the treatment was significantly reduced compared to that before treatment (P < .05).
Figure 1.
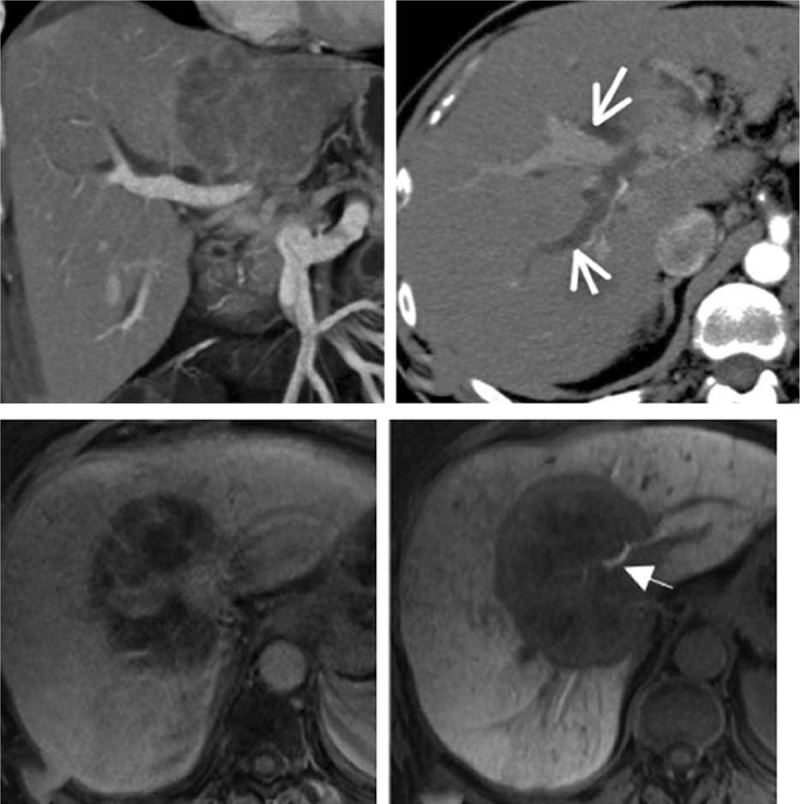
Representative computed tomography (CT) scans of 2 patients with hilar cholangiocarcinoma (arrowed).
Table 1.
Serum bilirubin of the 2 patient groups before and after treatment.
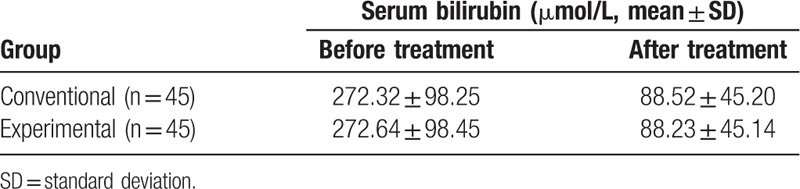
Table 2.
The relationship between age, sex, and serum bilirubin of the 2 patient groups (μmol/L, mean ± standard deviation).

3.2. Survival outcome within the first postoperative year
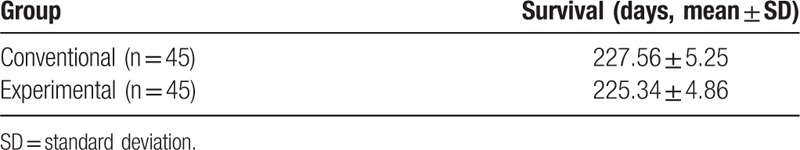
Within the first year after the treatment patients of both groups were continuously followed up. For the conventional group, the average survival length after surgery was 227.56 ± 5.25 days (Table 3). That for the experimental group was 225.43 ± 4.86 days. The difference in survival length between 2 groups was statistically not significant (P > .05).
Table 3.
Postoperative survivals of the 2 patient groups within the 1-year follow-up.
3.3. Postoperative complications
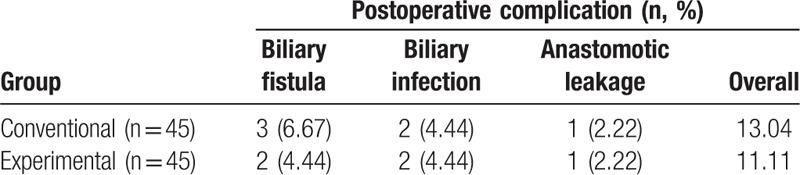
The complications after treatment of 2 groups were also studied (Table 4). In both groups, postoperative complications including biliary fistula, biliary infection, and anastomotic leakage were observed. The incidence rate of the conventional and experimental group was 13.04% and 11.11%, respectively. Although the rate of experimental group was slightly lower than that of the conventional group, the difference again was not statistically significant.
Table 4.
The incidence of postoperative complications of the 2 patient groups.
3.4. Length and cost of treatment
How much time and money the conventional and experiment treatment cost was also addressed (Table 5). For the conventional group, the average length of treatment was 27.16 ± 3.22 days, spending 89,066.6 ± 17,458.6 USD, whereas for the experimental group, the average length was 13.26 ± 2.11 days costing 32,431.0 ± 5220.6 USD. Between 2 groups, the differences in length and cost of treatment were statistically significant (P < .05).
Table 5.
Treatment length and medical expense of the 2 different groups.
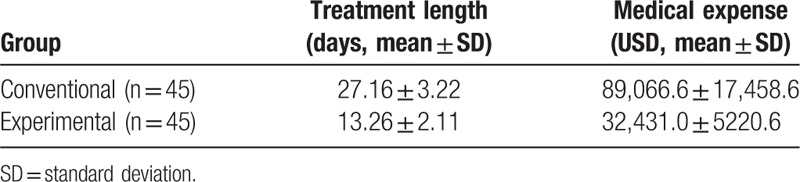
4. Discussion
Early HCCA is asymptomatic, and so early detection of the malignant very often not possible. Most patients complaining severe jaundice are diagnosed advanced cholangiocarcinoma at their first presentations at the clinics. Prognosis of patients with advanced HCCA is generally dismal because of the significant liver injury resulted from the jaundice, and because the treatment options have remained very limited, with surgery as the primary potentially curative treatment.[13] Resection of bile duct together with liver lobectomy has yielded a successful rate of 50% to 80% with a relatively low mortality rate of approximately 6% to 12%, achieving a 5-year survival rate of approximately 30% to 50%.[14–16] This surgical approach is technically challenging, and in China and some countries the successful rate was reported only approximately 27.5% with a 3-year survival rate of 15%. Moreover, many patients with advanced HCCA are not eligible to surgery but interventional therapy as a palliative treatment. Clinical guidelines for the decision of treatment strategy for patients with advanced HCCA are yet to be established. The present study was therefore aimed to explore the clinical significance of surgical interventional therapy in the management of patients with advanced HCCA.
The analysis of this study showed that although there were no significant differences in serum bilirubin, post-treatment complications, and average survival length between patients receiving conventional surgery and interventional therapy, the treatment length of those treated with interventional therapy was significantly shorter than their counterparts, resulting in a substantial reduction in medical expenses. This was probably because of the minimally invasive nature of the interventional therapy, which allows fast recovery and in turn short hospital stay and medical expense. This finding is valuable to the decision on therapeutic strategy for patients with advanced cholangiocarcinoma, especially for the financially underprivileged. From clinical experiences, we have realized the use of ultrasonography, computed tomography, and magnetic resonance imaging can make the diagnosis more efficient. Their values in interventional therapy warrants further in-depth investigations.
Despite the interventional therapy itself is not a curative treatment, it significantly reduces bilirubin and improves liver function, allowing arterial infusion chemotherapy and radiation therapy against cholangiocarcinoma. Indeed, studies have demonstrated that the combined use of interventional therapy and chemotherapy/radiation is superior to the interventional therapy alone regarding to the improvement in patients’ quality of life and survival. The therapeutic efficacy of the combined use of interventional therapy and chemotherapy or radiation therapy are being examined.
To conclude, the present study conducted a comprehensive analysis on patients with HCCA to show that comparing to the conventional surgery; the interventional therapy did not demonstrate any advantages in patients’ survivals and post-treatment complications. However, of note, the interventional therapy led to shorter treatment time and lower medical expense. In view of the minimally invasive nature of the interventional therapy, the strategic use of interventional therapy combined with chemotherapy or radiotherapy should be further examined.
Author contributions
Conceptualization: Hai-Ping Zhao.
Data curation: Zheng-Hua Zhang.
Formal analysis: Zheng-Hua Zhang.
Methodology: Hai-Ping Zhao.
Project administration: Ai-Hong Zheng.
Resources: Ai-Hong Zheng.
Writing – original draft: Ai-Hong Zheng.
Writing – review and editing: Zheng-Hua Zhang.
Footnotes
Abbreviation: HCCA = hilar cholangiocarcinoma.
H-PZ and Z-HZ contributed equally to this article.
The authors have no conflicts of interest to disclose.
References
- [1].Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med 1965;38:241–56. [DOI] [PubMed] [Google Scholar]
- [2].Nakeeb A, Lipsett PA, Lillemoe KD, et al. Biliary carcinoembryonic antigen levels are a marker for cholangiocarcinoma. Am J Surg 1996;171:147–52. discussion 152–143. [DOI] [PubMed] [Google Scholar]
- [3].Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Suarez-Munoz MA, Fernandez-Aguilar JL, Sanchez-Perez B, et al. Risk factors and classifications of hilar cholangiocarcinoma. World J Gastrointest Oncol 2013;5:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burak K, Angulo P, Pasha TM, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2004;99:523–6. [DOI] [PubMed] [Google Scholar]
- [6].Ramos E. Principles of surgical resection in hilar cholangiocarcinoma. World J Gastrointest Oncol 2013;5:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fairweather M, Balachandran VP, D’Angelica MI. Surgical management of biliary tract cancers. Chin Clin Oncol 2016;5:63. [DOI] [PubMed] [Google Scholar]
- [8].Lee SH, Park JK, Yoon WJ, et al. Optimal biliary drainage for inoperable Klatskin's tumor based on bismuth type. World J Gastroenterol 2007;13:3948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brountzos EN, Ptochis N, Panagiotou I, et al. A survival analysis of patients with malignant biliary strictures treated by percutaneous metallic stenting. Cardiovasc Intervent Radiol 2007;30:66–73. [DOI] [PubMed] [Google Scholar]
- [10].Van Delden OM, Lameris JS. Percutaneous drainage and stenting for palliation of malignant bile duct obstruction. Eur Radiol 2008;18:448–56. [DOI] [PubMed] [Google Scholar]
- [11].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [12].Shao JH, Fang HX, Li GW, et al. Percutaneous transhepatic biliary drainage and stenting for malignant obstructive jaundice: a report of two cases. Exp Ther Med 2015;10:1503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg 1996;224:628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].DeOliveira ML, Kambakamba P, Clavien PA. Advances in liver surgery for cholangiocarcinoma. Curr Opin Gastroenterol 2013;29:293–8. [DOI] [PubMed] [Google Scholar]
- [15].Aljiffry M, Abdulelah A, Walsh M, et al. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg 2009;208:134–47. [DOI] [PubMed] [Google Scholar]
- [16].Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 2007;31:1256–63. [DOI] [PubMed] [Google Scholar]


