Abstract
Background:
A Chinese herb formula Yufeining (YFN) has showed promise in the treatment of stable chronic obstructive pulmonary disease (COPD), less is known that the impact of YFN in combination with standard Western treatments on lung inflammation. This study evaluated the safety and efficacy of YFN as a treatment for stable COPD and as an anti-inflammatory agent.
Methods:
Sixty patients with stable COPD were randomly assigned to two treatment groups (YFN treatment, N = 30; placebo treatment, N = 30). Both groups received inhaled steroids and bronchodilators during an 8-week intervention, and patient status was assessed at 8 weeks later and 4 months after treatment. The primary outcome included clinical efficacy. The secondary outcomes involved CAT score, mMRC grade, six-minute walking distance (6MWD). IL-8, TNF-α, IL-17A, LTB4, TGF-β1 and CRP were also detection in peripheral serum, as well as adverse reaction conditions.
Results:
The YFN group demonstrated a significant improvement in clinical efficacy (compare 89.3% to 63.3% in the placebo group; P < 0.05). CAT scores and mMRC grades significantly decreased (P < 0.05, P < 0.01), and 6MWD significantly increased (P<0.05), after YFN treatment. The levels of IL-8, TNF-α, LTB4 and CRP decreased significantly after 8 weeks of treatment compared to baseline levels in both groups. Only in the YFN treatment group, the levels of IL-17A decreased significantly after treatment compared to baseline levels (P < 0.05). No changes were observed inTGF-β1 from pre-to post-treatment in either group (P > 0.05). Serum levels of IL-8, TNF-α, IL-17A, LTB4 and CRP decreased significantly after YFN treatment compared to the placebo group (P < 0.05).
Conclusion:
A combinatorial treatment approach with YFN, inhaled steroids and bronchodilators produced a clinically effective treatment for stable COPD, leading to a significant decrease in circulating inflammatory mediators. The study appeared YFN was safety.
Clinical trial registration number:
No. ChiCTR-IOR-17013577.
Keywords: chronic obstructive pulmonary disease, clinical trials, traditional Chinese medicine, Yufeining
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent and limited airflow, leading to progressive dyspnea, coughing, and expectoration.[1] This progressive disease is associated with an enhanced chronic inflammatory response to noxious particles or gases. The mortality rate for patients with COPD is high, and treatment costs can place a significant economic burden on patients and their caregivers.[2,3]
Airway cells respond to environmental triggers by releasing cytokines and inducing an inflammatory response, but the reason why some environmental triggers are more potent at inducing inflammation compared with others is not fully understood. Inhaled particles or gases may cause lung inflammation through the activation of alveolar macrophages, neutrophils, and CD8+ T cells. Activated inflammatory cells release a variety of inflammatory mediators, including leukotriene B4 (LTB4), interleukin-8 (IL-8), IL-17, and tumor necrosis factor alpha (TNF-α). These inflammatory mediators may damage airways and induce the activation of neutrophils, which can lead to further lung damage.[4,5]
Current treatments[6,7] for COPD include inhaled corticosteroids (ICS) and bronchodilators. Although effective at alleviating symptoms, these treatment methods do not alter disease progression.[1] One meta-analysis of treatment efficacy and safety found that a combination of traditional Chinese medicine (TCM) and western medicine produced a positive treatment outcome in COPD.[8] The meta-analysis showed that a combined treatment approach reduced the risk for severe exacerbations, improved lung function, positively impacted quality of life, and improved exercise capacity in stabilized patients with COPD. Patients who received the combined drug treatments did not report any obvious adverse reactions, which indicated that combination treatment could be a safe and effective therapy for COPD.[8]
Yufeining (YFN) is a Chinese herbal formula developed by Dai-Shunzhen, a famous physician in TCM. Preliminary clinical data demonstrated that YFN could improve the symptoms of COPD patients by reducing coughing, sputum production, chest tightness, and asthma.[9,10] In addition, YFN improved pulmonary function, reduced sputum leukocyte and neutrophil counts, and reduced sputum IL-8 levels.[9,10]
Although the use of YFN as a single agent shows promise in the treatment of stable COPD, less is known regarding the impact of YFN in combination with standard western treatments on lung inflammation. This study aims to evaluate the efficacy of YFN in combination with inhaled steroids and bronchodilators on patients with stable COPD. Using a randomized, double-blind, placebo-controlled clinical trial, COPD patients were evaluated for quality of life and exercise tolerance while receiving YFN in combination with western treatment regimens. In addition, the study measured the effects of YFN on the concentration of circulating inflammatory mediators.
2. Methods
2.1. Study design
The randomized, double-blind, placebo-controlled clinical study was conducted in Fujian Province Zhangzhou Municipal TCM Hospital, Affiliated Hospital of Fujian University of TCM from December 2012 to February 2015.
2.2. Ethics
The study was approved by the Ethical Research Committee of Fujian Province Zhangzhou Municipal TCM Hospital, Affiliated Hospital of Fujian University of TCM (No. 2012-006). The study adhered to the principles of the Declaration of Helsinki. All subjects provided informed consent before their enrollment in the study.
2.3. Study participants
2.3.1. Diagnostic criteria for COPD
The diagnostic criteria for this study were established according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD).[1] In addition, diagnosis and treatment plans adhered to the Chinese Treatment Guidelines for COPD (2007), established by the COPD Study Group of the Chinese Society for Respiratory Diseases.[11]
2.3.2. Traditional Chinese medicine (TCM) syndrome differentiation criteria
In TCM syndrome, differentiation criteria for lung, spleen, and kidney deficiencies associated with retention of phlegm and blood stasis were referred to a previous study[10] and TCM diagnosis and treatment guidelines of COPD established by the lung disease professional committee internal medicine branch of China association of Chinese medicine (2011 edition).[12] The details were as follows: cough and white phlegm; shortness of breath, especially movement; fatigue or spontaneous perspiration; susceptible to cold; inappetence; stomach turgor or loose stool; soreness and weakness of waist and knees or dizziness and tinnitus; edema of face or lower limbs; frequent urination especially at night; gloomy complexion or dark lip; dark or dark red tongue or with stasis or sublingual varicose. Having 2 of 3 items in (1)-(4), 1 of 2 items in (5)- (6), 1 of 3 items in (7)-(9), 1 of 2 items in (10)- (11), diagnosis can be made with these items.
2.3.3. Inclusion criteria
Inclusion criteria for the study included adult patients (age range: 40–80 years; both genders were accepted), with a confirmed diagnosis of COPD based on the GOLD diagnostic criteria. Patients also had to exhibit the COPD diagnostic criteria used in TCM syndrome differentiation criteria.[12] The COPD status had to be stable, with pulmonary function at grade II-IV. Patients had to receive routine COPD medications for at least 6 weeks before enrollment in the study. By signing an informed consent form, patients agreed to voluntarily receive all treatments during the study.
2.3.4. Exclusion criteria
Patients were excluded from the study if any of the following criteria were met: patients were diagnosed with acute upper respiratory tract infections in the past 2 weeks; systemic corticosteroids were used in the past month; patients were enrolled in other clinical trials during the previous 3 months; patients were diagnosed with serious cardiovascular, liver, kidney, or hematopoietic diseases; and patients were diagnosed with psychiatric disorders.
2.3.5. Randomization and masking
The randomized, double-blind, placebo-controlled clinical study was performed by the SAS 9.0 system (Crumlin, Co. Antrim, United Kingdom), according to a predetermined proportion of 1:1. The 60 enrolled patients were randomized to the treatment group (YFN group) or the placebo control group (placebo group), with 30 patients in each group. Personnel not involved in the clinical study supervised the randomization process. After enrollment in the trial, the participants were given a fixed prescription and received study medications from pharmacy staff according to the enrollment sequence. Both YFN granule and placebo granule had the same appearance, shape, color, and packaging, so the research physicians and participants did not know the differences. The research physicians and participants were not informed about individual treatment details until the end of the treatment course and after receiving laboratory test results.
2.3.6. Medications
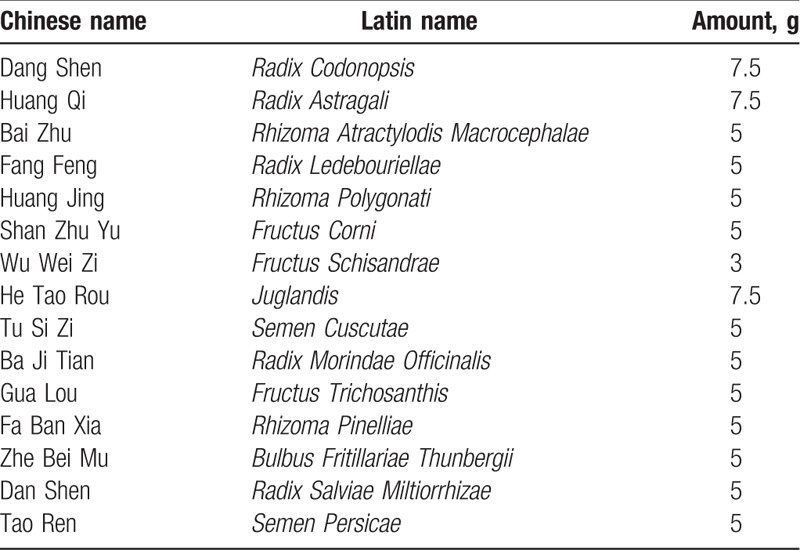
Pharmaceutical-grade YFN was provided by Fujian Province Zhangzhou Municipal TCM Hospital, Affiliated Hospital of Fujian University of TCM. The main composition and dosage of YFN are summarized in Table 1. YFN granules were produced by the Peili (Nanning) Pharmaceutical Co., Ltd (batch 3180, specifications: 10 g/package). The placebo contained soluble starch and dextrin was manufactured by the Peili (Nanning) Pharmaceutical Co. Ltd. The Peili (Nanning) Pharmaceutical Co. Ltd is certified by the national Good Manufacturing Practice (GMP) and is the enterprise approved by the Chinese food and drug administration, which can produce pilot production of TCM formula particles in China [national food drug safety note (2004) 579 file]. It also got the China national accreditation service for conformity assessment (CNAS) national laboratory accreditation and Australian therapeutic goods administration (TGA) certification.
Table 1.
Main composition of YFN.
2.4. Intervention
During the trial, patients in the YFN group took oral YFN granule (2 bags per dose, twice daily). Patients in the placebo group received placebo granule and followed the same dosing schedule. The intervention plan was based on conventional therapies, containing ICS with a long-acting β2-agonist (LABA) and low-dose theophylline.[11] The intervention treatment lasted for 8 weeks from the date of enrollment. During this time, all patients were not permitted to receive other medications for their COPD. After the 8-week intervention, all patients received a follow-up assessment at 4 months post-treatment.
2.5. Outcome measures and follows up
Outcome measures compared baseline data from the start of the trial to data obtained immediately after treatment ended and at 4 months post-treatment.
2.5.1. Primary outcome
The primary outcome included clinical efficacy. The efficacy evaluating was referred to guiding principle of clinical research on new drugs of TCM.[13] The efficacy scores are evaluated based on the nimodipine method as follows: significantly effective (I), indicating a significant reduction of clinical symptoms and a decrease in the TCM syndrome total score of no less than 70%; effective (II), indicating some of the clinical symptoms have improved and a decrease in the TCM syndrome total score of no less than 30%; ineffective (III), indicating no improvement in clinical symptoms and a decrease in the TCM syndrome total score of less than 30%.
2.5.2. Secondary outcomes
The secondary outcomes measures included the following: COPD assessment test (CAT)[14]; the Dyspnea Scale Questionnaire, which was first developed by the British Medical Research Council (mMRC)[15]; and the 6-minute walking distance (6MWD).[16]
And circulating inflammatory factors such as IL-8, TNF-α, IL-17A, LTB4, TGF-β1, and C-reactive protein (CRP) were measured in patient serum. IL-8 and TNF-α were measured by ELISA, according to the manufacturer's instructions (BD Biosciences, Singapore, Singapore). The concentration of IL-17A, LTB4, and TGF-β1 were measured by enzyme-linked immunosorbent assay (ELISA) (Ebioscience, IN). The serum CRP concentration was determined by immunoturbidimetry (Randox Laboratories, Antrim, UK).
2.6. Safety
The routine blood and urine tests, liver and kidney function tests, and ECG were assessed before and after treatment in both study groups. Any adverse reactions were recorded in detail, including frequency of occurrence, time following treatment, clinical symptoms, duration, follow-up treatment measures, and time until symptoms disappeared.
2.7. Statistical analysis
Data were analyzed using the SPSS 20.0 statistical software package. The pp analysis was performed. The continuous variables were expressed as the mean ± standard deviation (SD). Paired t test was used for before and after treatment comparisons within the YFN group and placebo groups. The independent-sample t test was used for data comparisons between the 2 groups. The Chi-squared test was applied to compare qualitative data. Ranked data were assessed by Ridit analysis. P < .05 was considered statistically significant.
3. Results
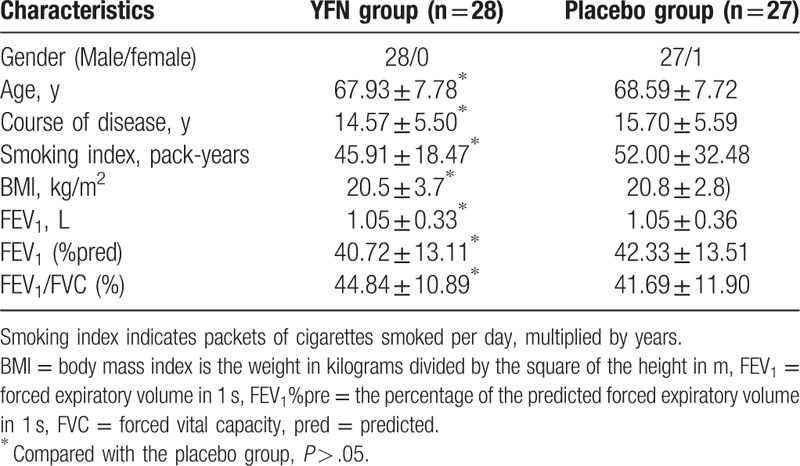
A total of 60 patients with stable COPD were eligible for the study (Fig. 1). A total of 30 patients were assigned to receive the YFN granule, and 30 patients were assigned to receive the placebo granule. One case was excluded, and 1 case was lost during the initial YFN treatment. No patients were lost to follow-up after treatment. Twenty-eight of the 30 patients enrolled in the YFN group completed the study. In the placebo group (N = 30), 3 cases were lost during the treatment. No patients were lost to follow-up, and 27 of 30 patients completed the study. The gender, age, course of disease, smoking index, body mass index (BMI), forced expiratory volume in 1 second (FEV1), FEV1%pred, and FEV1/forced vital capacity (FVC) were compared between the 2 groups. No statistical significance was found among these patient demographics (P > .05; Table 2).
Figure 1.
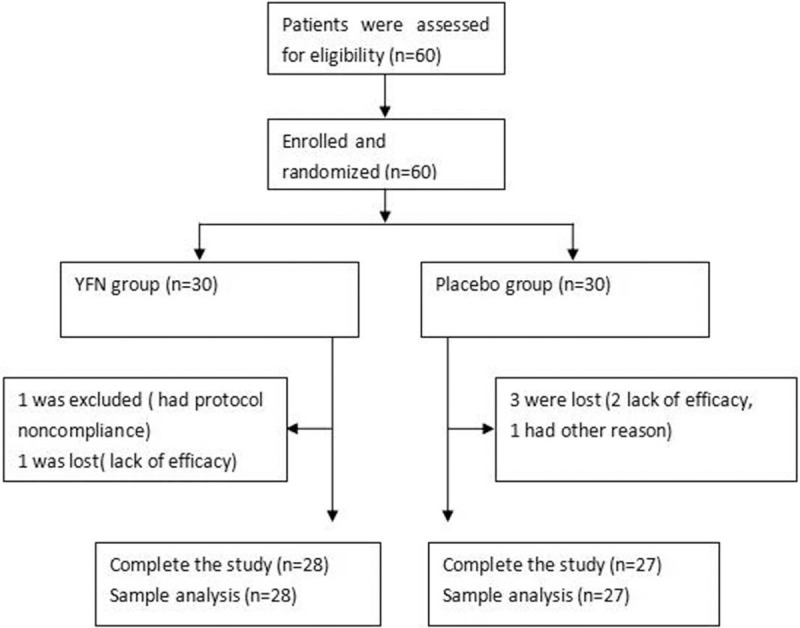
The CONSORT flow chart.
Table 2.
Baseline characteristics of patients in 2 groups.
3.1. Quality control of YFN
The major compounds of YFN were carefully analyzed and quality controlled as described in Chinese Pharmacopoeia (2015 Edition). Phytochemical screening showed that the aqueous extract contained glycoside, alkaloids, and terpenoids. The main contents of lobetyolin, astragaloside, loganin, hyperoside were calculated, and compounds present in the extract were lobetyolin (100 mg/g), astragaloside (0.25 mg/g), loganin (4 mg/g), and hyperoside (0.4 mg/g).
3.2. Primary outcome
3.2.1. Comparison of clinical efficacy between 2 groups after treatment
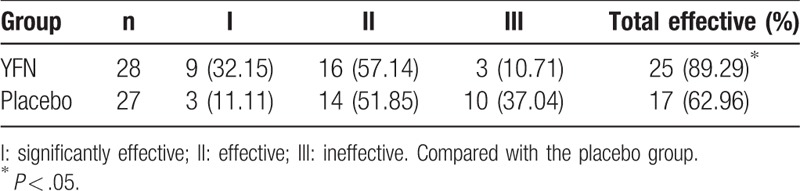
The clinical efficacy in the YFN group was 89.3%, which was significantly higher than the placebo group (63.3%; P < .05; Table 3).
Table 3.
Comparison of clinical efficacy [n (%)].
3.3. Secondary outcomes
3.3.1. CAT, mMRC, and 6MWD
There were no significant differences between the baseline CAT, mMRC, and 6MWD scores for the 2 study groups (P > 0.05; Fig. 2). After 8 weeks of YFN treatment, there were significant decreases in the CAT score, mMRC grade, and 6MWD compared with baseline scores (P < .05, P < .01, P < .05, respectively). The CAT score also significantly decreased between the pre- and post-treatment assessments in the placebo group (P < .05; Fig. 2A). Although there was post-treatment reduction in CAT scores for the YFN group, the lower scores were not significant compared with the placebo group (P = .057). At the 4-month follow-up visit, the CAT scores and mMRC grades in the YFN group continued to remain significantly less than the scores recorded at baseline (P < .05, respectively; Fig. 2A, B). The 6MWD failed to improve in the YFN group and the results were similar to those obtained at the start of the trial (P > .05; Fig. 2C). In the placebo group, the CAT score at the 4-month follow-up showed an increase (Fig. 2A), but there was no significant difference between the scores at the follow-up time compared with baseline (P > .05).
Figure 2.
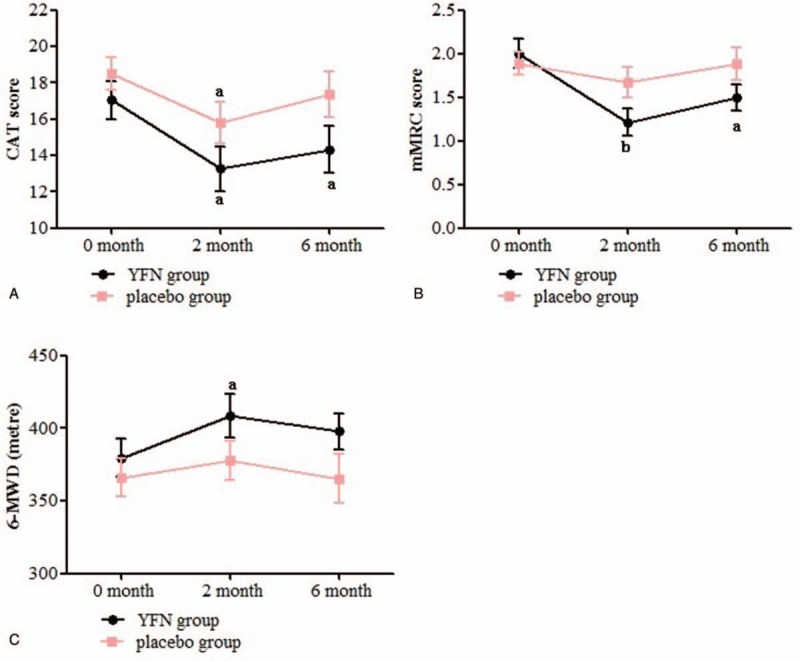
Comparison of CAT, mMRC and 6MWD between two treatment groups over time. Abbreviations: CAT (COPD assessment test); mMRC (the modified British medical research council questionnaire for assessing the severity of breathlessness); 6MWD (6-minute walking distance).Statistical significance compared either 2-month or 4-month follow-up data to baseline values within each group: aP < .05, bP < .01.
3.3.2. Inflammatory factors
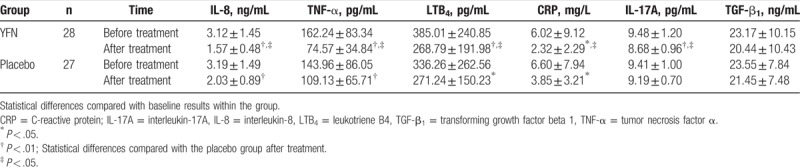
There were no significant differences between the baseline serum levels of IL-8, TNF-α, IL-17A, LTB4, TGF-β1, and CRP in either treatment group (P > .05, respectively; Table 4). After 8 weeks of YFN treatment, the levels of IL-17A, IL-8, TNF-α, and LTB4 significantly decreased compared with the baseline values (P < .01). A significant decrease in CRP was also observed after YFN treatment compared with baseline levels (P < .05). The placebo group demonstrated a significant decrease in levels of IL-8 and TNF-α after 8 weeks of treatment compared with baseline levels (P < .01). LTB4 and CRP levels in the placebo group also decreased significantly after treatment compared with baseline levels (P < .05). No changes in TGF-β1 were observed after treatment in either study group (P > .05). After treatment, the serum levels of IL-8, TNF-α, IL-17A, LTB4, and CRP from the YFN group dropped significantly compared with those in the placebo group (P < .05).
Table 4.
Comparison of circulating inflammatory factors from 2 treatment groups.
3.4. Adverse reactions
There were no significant changes in blood routine, liver and kidney function, and electrocardiogram before and after treatment. In YFN group, phlegm was transferred from white thick to pale yellow in 1 case but did not affect treatment. In placebo group, epigastric discomfort occurred in 2 cases and diarrhea in 1 case. But the symptoms were mild.
4. Discussion
This study was conducted to evaluate the efficacy and safety of YFN using a randomized double-blind, placebo-controlled trial. The effects of YFN on quality of life and exercise tolerance were observed in patients with COPD. The circulating inflammatory factors of COPD patients were also evaluated in order to further explore the mechanism of action of YFN on the treatment of COPD. As the fall off patients in the 2 groups have not been evaluated and analyzed, at the same time, there were no reports of adverse reaction during the phone visit, and PP analysis was performed instead of ITT analysis.
Dai-Shunzhen is a second batch of famous veteran experts in TCM of China who identified several TCM pathogenic characteristics of COPD. She established that the main pathogenesis of COPD was a deficiency of the lung, spleen, and kidney accompanied with retention of phlegm and blood stagnation. On the basis of her clinical experience, Dai-Shunzhen created the YFN formula, which included several herbal ingredients. Components of Dangshen (Radix Codonopsis) and Huangqi (Radix Astragali) in YFN formulate as monarch drugs possess the effects of invigorating the lung and strengthening the spleen, replenishing qi to strengthen exterior defense. Shanzhuyu (FructusCorni) and TuSiZi (Semen Cuscutae) as minister drugs may replenish kidney to receive qi. Gua Lou (FructusTrichosanthis), ZheBei Mu (BulbusFritillariaeThunbergii), Dan Shen (Radix SalviaeMiltiorrhizae), and Tao Ren (Semen Persicae) as adjuvant drugs and guiding drugs have the actions of resolving phlegm and eliminating stasis. The combination of ingredients brings about the effects of reinforcing lung and strengthening spleen, replenishing kidney and holding qi, resolving phlegm and removing stasis, relieving cough and asthma. The combination of ingredients has proven to be an effective treatment for COPD.[9,10]
Treatment with YFN led to a significant improvement in clinical efficacy (89.3%) compared with the placebo group (63.3%; Table 3). Clinical studies have shown that combining TCM with western pharmaceutical agents leads to higher clinical efficacy than treatment with the pharmaceutical agents alone.[17,18] The results from this study support the hypothesis that TCM treatment for COPD could improve clinical efficacy and relieve COPD symptoms when used in combination with ICS and bronchodilators.
The CAT score, mMRC grade, and 6MWD are main outcome measures that reflect health status in COPD and can evaluate the efficacy of TCM interventions.[19,20] After YFN treatment, both the CAT scores and mMRC grades were reduced and the 6MWDscore improved (Fig. 2). In contrast, only the CAT scores decreased in COPD patients after placebo treatment, and there was no statistical significance between the post-treatment CAT scores in either treatment group (P = .057). The small sample size and short observation time may have contributed to this observation, and it would be necessary to expand the sample size and prolong the course of treatment to determine if CAT scores are significantly reduced with YFN treatment compared with a placebo. The CAT scores and mMRC grades in the YFN group rebounded slightly during the 4-month follow-up but were still lower than the baseline scores (P < .05, respectively). The results indicated that YFN could improve the quality of life, relieve dyspnea symptoms, and improve exercise tolerance in patients with COPD, as well as produce some long-term benefits.
The chronic inflammation of airway, lung parenchyma, and pulmonary vasculature are the pathological changes characteristic of COPD.[1] Neutrophils, macrophages, and other inflammatory cells are involved in the pathogenesis of COPD release of LTB4, TNF-α, IL-8, and other inflammatory mediators. This leads to the destruction of lung tissue and chronic respiratory inflammation. It is increasingly recognized that COPD is not only a chronic inflammation of the respiratory tract, but it is also the pathological state of systemic inflammation.[21] CRP is a circulating pentraxin that is a part of the acute inflammatory response. It induces the expression of proinflammatory cytokines and chemokines and upregulates adhesion molecules that promote lung inflammation.[22] This study showed that YFN significantly lowered serum levels of IL-8, TNF-a, LTB4, and CRP in patients with COPD (Table 4). These data are consistent with other clinical reports,[23–26] suggesting that YFN could inhibit systemic inflammatory by reducing the release of inflammatory mediators.
T lymphocytes are one of the key components of inflammation in COPD. T helper cell 17 (Th17 cells)are effector T cells characterized by the production of IL-17, which is a pro-inflammatory cytokine that plays an important role in the neutrophil response to lung.[5,27] Our previous study found that when peripheral blood mononuclear cells (PBMCs) from patients with stable COPD were stimulated by phytohemagglutinin (PHA), these cells secreted IL-17A at levels significantly higher than mononuclear cells from the healthy control group.[28] Furthermore, injection of the TCM compound Chuankezhi could inhibit IL-17A secretion from PBMCs.[28] The study suggested that TCM influences downregulation of IL-17A. Serum levels of IL-17A in patients with COPD decreased significantly after YFN treatment (Table 4), which indicated that YFN could decrease inflammatory reactions by reducing the release of the proinflammatory cytokine IL-17A.
TGF-β1 plays an important role in airway remodeling in COPD. TGF-β1 can induce the proliferation of fibroblasts and airway smooth muscle cells, deposition of extracellular matrix, and the repair of epithelial cells.[4] Guan et al[29] showed that the expression of TGF-β1 significantly increased in the peripheral lung tissue of COPD. Some studies have reported that TCM could decrease the level of TGF-β1 in COPD treatment.[23,30] Our results show that the serum level of TGF-β1 slightly declined after YFN treatment in patients with COPD and warrants further study.
In YFN group, phlegm was transferred from white thick to pale yellow in 1 case due to the medicinal property of warn in nature of YFN but did not affect treatment. The gastrointestinal response to placebo may be related to soluble starch and dextrin.
5. Conclusion
The study showed that YFN combined with conventional western pharmaceutical drugs was effective and safe for treatment of COPD patients with some long-term benefits. YFN could also downregulate the levels of inflammatory mediators. But it cannot be denied that this study has limitations: the sample size was small and the course of treatment was too short. Further studies are required. Larger sample sizes, prolonged treatment periods, and additional follow-up assessments will strengthen the associations between YFN treatment and the long-term benefits for COPD patients.
Acknowledgment
The authors would like to thank Dai-Shunzhen, chief physician, who provided the Chinese herb YFN formula and guided TCM syndrome differentiation key points.
Author contributions
Conceptualization: Minli Hong, Candong Li.
Data curation: Minli Hong, Chunlin Hong, Huinuan Chen, Gengshen Ke, Jinrong Huang, Xiaohua Huang, Yanhong Liu, Fengsen Li, Candong Li.
Formal analysis: Minli Hong, Chunlin Hong, Huinuan Chen, Gengshen Ke, Jinrong Huang, Xiaohua Huang, Yanhong Liu, Fengsen Li, Candong Li.
Funding acquisition: Minli Hong, Fengsen Li, Chunlin Hong.
Investigation: Minli Hong, Xiaohua Huang, Yanhong Liu, Fengsen Li, Candong Li.
Methodology: Minli Hong, Huinuan Chen, Gengshen Ke, Jinrong Huang, Xiaohua Huang, Yanhong Liu, Fengsen Li, Candong Li.
Project administration: Minli Hong, Candong Li.
Resources: Minli Hong, Fengsen Li, Chunlin Hong.
Software: Minli Hong, Candong Li.
Supervision: Minli Hong, Candong Li.
Validation: Minli Hong, Chunlin Hong, Huinuan Chen, Candong Li.
Visualization: Minli Hong, Candong Li.
Writing – original draft: Minli Hong, Candong Li.
Writing – review & editing: Minli Hong, Candong Li.
Footnotes
Abbreviations: 6MWD = 6-minute walking distance, BMI = body mass index, CAT = COPD Assessment Test, CNAS = China National Accreditation Service for Conformity Assessment, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, ECG = electrocardiography, ELISA = enzyme-linked immunosorbent assay, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, GMP = Good Manufacturing Practice, GOLD = the Global Initiative for Chronic Obstructive Lung Disease, ICS = inhaled corticosteroids, IL-17A = interleukin-17A, IL-8 = interleukin-8, LABA = long-acting β2-agonist, LTB4 = leukotriene B4, mMRC = Modified British Medical Research Council Questionnaire for Assessing the Severity of Breathlessness, PBMCs = peripheral blood mononuclear cells, PHA = phytohemagglutinin, SD = standard deviation, SPSS = Statistical Package for the Social Sciences, TCM = Traditional Chinese Medicine, TGA = Therapeutic Goods Administration, TGF-β1 = transforming growth factor-β1, Th17 = T helper cell 17, TNF-α = tumor necrosis factor alpha, YFN = Chinese Herbal Formula Yufeining.
Funding/support: Funding was provided by the National Chinese Medicine Clinical Research Institution Business Construction Research Program of State Administration of TCM (No. JDZX2012168); Natural Science Foundation of Fujian, Zhangzhou (No. ZZ2012J38); Key Laboratory Construction Project of Traditional Chinese Medicine of Lung Disease (No. 2013-Internal Medicine-5).
The author(s) of this work have nothing to disclose.
The authors declare no potential conflicts of interest with regard to the authorship and/or the publication of this article.
References
- [1].Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2017 report. Available at: https://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/. [Google Scholar]
- [2].Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397–412. [DOI] [PubMed] [Google Scholar]
- [4].Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. NovReview 2008;118:3546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol 2010;72:495–516. [DOI] [PubMed] [Google Scholar]
- [6].Kunz LIZ, Postma DS, Klooster K, et al. Relapse in FEV 1 decline after steroid withdrawal in COPD. Chest 2015;148:389–96. [DOI] [PubMed] [Google Scholar]
- [7].Marc M, Anthony DU, Dave S, et al. Pharmacological strategies to reduce exacerbation risk in COPD: a narrative review. Respir Res 2016;17:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang HF, Zhang HL, Li JS, et al. Effectiveness and safety of traditional Chinese medicine on stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Med 2015;23:603–11. [DOI] [PubMed] [Google Scholar]
- [9].Hong ML, Chen WX, Cai SH, et al. Study on the intervention effect of Yufeining on pulmonary function in patients with chronic obstructive pulmonary disease. Chin J Trad Chin Med 2005;20:92–5. [Google Scholar]
- [10].Hong ML, Yang GZ, Chen WX, et al. Effect of Yufeining on induced sputum interleukin-8 in patients with chronic obstructive pulmonary disease at the stable phase. Chin J Integr Med 2005;11:179–82. [DOI] [PubMed] [Google Scholar]
- [11].Chronic obstructive pulmonary disease study group of the Chinese Medical Association of respiratory diseases. Guidelines for the diagnosis and treatment of chronic obstructive pulmonary disease (2007 Edition). Chin J Tuberc Respir Dis 2007;30:8–17. [Google Scholar]
- [12].Department of internal medicine branch of the Chinese Institute of traditional Chinese medicine Specialized Committee. Traditional Chinese medicine diagnosis and treatment guideline for chronic obstructive pulmonary disease (2011 Edition). J Trad Chin Med 2012;53:80–4. [Google Scholar]
- [13].Zheng YY. Guiding Principle of Clinical Research on New Drugs of Traditional Chinese Medicine [M]. 2002;Beijing: China Medical Science and Technology Publishing House, 58–60. [Google Scholar]
- [14].Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir 2009;34:648–54. [DOI] [PubMed] [Google Scholar]
- [15].Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580–6. [DOI] [PubMed] [Google Scholar]
- [16].Gibbons WJ. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- [17].Wang GF, Liu BJ, Cao YX, et al. Effects of two Chinese herbal formulae for the treatment of moderate to severe stable chronic obstructive pulmonary disease: a multicenter, double-blind, randomized controlled trial. PLoS One 2014;8:e103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang ZW, Yang PL, Shen L, et al. Clinical efficacy and mechanism of BaibuYangfei decoction for chronic obstructive pulmonary disease. Jilin J Trad Chin Med 2014;34:899–902. [Google Scholar]
- [19].Li JS, Xie Y, Li SY, et al. Comparison of conventional medicine, TCM treatment, and combination of both conventional medicine and TCM treatment for patients with chronic obstructive pulmonary disease: study protocol of a randomized comparative effectiveness research trial. Trials 2014;15:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhong YQ, Wang XF, Xu GL, et al. Modified Yupingfeng formula for the treatment of stable chronic obstructive pulmonary disease: a systematic review of randomized controlled trials. Afr J Tradit Complement Altern Med 2013;11:1–4. [PMC free article] [PubMed] [Google Scholar]
- [21].Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular disease? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003;25:1514–9. [DOI] [PubMed] [Google Scholar]
- [22].Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guo SJ, Sun ZT, Liu ES, et al. Effect of Bufei granule on stable chronic obstructive pulmonary disease: a randomized, double blinded, placebo-controlled, and multicenter clinical study. J Trad Chin Med 2014;34:437–44. [DOI] [PubMed] [Google Scholar]
- [24].Wang QH, Wang LW, Xu LS, et al. Effect of compound Yifeiduqi capsule on chronic obstructive pulmonary disease and the effect of serum interleukin-8. J TCM Univ Hunan 2011;31:58–60. [Google Scholar]
- [25].Zhang SS, Cheng RJ, Cheng DS, et al. Clinical study on the effect of Jianpibufeiand Gushenyifei on nutritional status, TNF-( and lung function in patients with stable chronic obstructive pulmonary disease. Chin J Trad Med Sci Technol 2010;32:70–1. [Google Scholar]
- [26].Li ZY, Tian CY, Zhi FM. Effect of YiqiBushenHuoxue formula on chronic obstructive pulmonary disease in stable period airway inflammation. J Emerg Trad Chin Med 2015;24:1143–5. [Google Scholar]
- [27].Hong SC, Lee SH. Role of Th17 cell and autoimmunity in chronic obstructive pulmonary disease. Immune Network 2010;10:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hong CL, Hong ML, Chen HN, et al. Effect of Chuankezhi on chronic obstructive pulmonary disease in patients with peripheral blood mononuclear cells secrete interleukin-17A. Chin J Geriatr 2013;32:951–3. [Google Scholar]
- [29].Guan P, Li W, Wu ZY, et al. Expression and clinical significance of transforming growth factor-(1 nuclear transcription factor -κB in chronic obstructive pulmonary disease. Chin J Geriatr 2013;32:733–0. [Google Scholar]
- [30].Feng CL, Zhang Q, Liu ZK, et al. Effect of QizhiYifei Granule on lung tissue of COPD rat model of TGF-β1 expression. J Beijing Univ Trad Chin Med 2013;36:238–41. [Google Scholar]


