Supplemental Digital Content is available in the text
Keywords: atrial fibrillation, chronic heart failure, dyslipidemia
Abstract
In chronic heart failure (CHF), new-onset atrial fibrillation (AF) is associated with increased morbidity and mortality. We aimed to evaluate the influence of dyslipidemia on the incidence of new-onset AF in patients with CHF.
In this single-center observational study, 308 patients with CHF and no history of AF were followed-up for 3 years. Of the 291 patients who attended the 1-year follow-up, 78 had developed AF (AF group; 10 deaths), while 213 had not (sinus rhythm [SR] group). Changes in lipid profile (ΔTC for total cholesterol and ΔLDLc for low-density lipoprotein cholesterol) were analyzed.
The groups differed significantly regarding the decrease in lipid levels from baseline to the 1-year follow-up (AF vs SR: for ΔLDLc, 23.35 vs 7.80 mg/dL, P = .02; for ΔTC, 23.95 vs −2.76 mg/dL, P = .001). At the 3-year follow-up, new-onset AF was noted in 21 of the 188 living patients in the SR group. Cox proportional hazards analysis revealed ΔLDLc and ΔTC as independent risk factors for new-onset AF (hazard ratio, 1.018 and 1.013, respectively, per standard deviation increment), with higher incidence of new-onset AF for ΔTC > 9.65 mg/dL (P = .02) and for ΔLDLc > 9.73 mg/dL (P = .005).
In CHF, pronounced decrease in LDLc and TC is associated with new-onset AF.
1. Introduction
Atrial fibrillation (AF) is a common complication in patients with chronic heart failure (CHF). Patients with AF and CHF have poorer prognosis, higher socioeconomic burden, and higher mortality.[1–4] The order in which CHF and AF develop may also influence prognosis.[5,6] Growing evidence indicates that, in CHF, mortality is higher among patients with new-onset AF than among those with preexisting AF.[4,7,8] Therefore, it is important to identify the risk factors for new-onset AF in patients with CHF.
Many factors have been reported to affect the development of AF, including age, hypertension, coronary artery disease, obesity, and diabetes.[9–12] A recent epidemiological investigation reported that higher levels of low-density lipoprotein cholesterol (LDLc) and total cholesterol (TC) were associated with a lower incidence of AF.[13–15] However, some studies yielded paradoxical results in that the risk of developing AF was associated with the levels of high-density lipoprotein cholesterol (HDLc) and triglycerides (TG) but not LDLc or TC.[16] Moreover, it is unknown whether lipid profile disorders affect the risk of new-onset AF in CHF patients. Accordingly, the purpose of the present study was to explore the correlation between lipid profile disorders and the risk of new-onset AF in CHF patients.
2. Methods
2.1. Patients and study design
This observational study included patients diagnosed with CHF and admitted to Yangzhou First People's Hospital in 2013. The inclusion criteria were as follows: available data regarding lipid levels and electrocardiography findings on admission; New York Heart Association (NYHA) class III or IV; and age, 18–80 years. The exclusion criteria were as follows: history of AF or atrial flutter, including paroxysmal, persistent, or permanent AF; valvular heart disease; permanent pacemaker; expected lifetime below 3 years; atheroma, severe infection, or another condition requiring heart transplant; thyroid dysfunction; and pregnancy or lactation. All patients provided informed consent for undergoing the necessary procedures and investigations, and the study was approved by the institutional ethics committee of Yangzhou First People's Hospital (number of the approval: 2016003).
2.2. Baseline assessments
At each visit, the medical history (including use of medications) was reviewed and physical examination was performed. Serum lipid levels were evaluated as follows. TC and TG levels were assessed using automated enzymatic assays. HDLc levels were measured using the chemical precipitation method. LDLc levels were calculated using the Friedewald formula[17] in patients with serum TG levels ≤400 mg/dL, and obtained by direct measurement using the immunoseparation method in patients with serum TG levels >400 mg/dL. Other serum parameters including creatinine, uric acid, thyrotropin, free thyroxine, free triiodothyronine, and glycated hemoglobin levels were also measured. Echocardiography was used to determine left atrial dimension (LAD), left ventricular end-diastolic dimension, and left ventricular ejection fraction.
2.3. Follow-up assessments
The patients were followed up for 3 years on an outpatient basis. The present analysis used data from the 1-year and 3-year follow-up visits. Lipid levels, including TC, LDLc, HDLc, and TG levels were measured at the end of the 1-year follow-up visit. The patients were stratified into 2 groups based on whether new-onset AF was noted at the 1-year follow-up (AF group vs sinus rhythm [SR] group). For the purpose of the present study, only patients without new-onset AF at the 1-year follow-up (i.e., from the SR group) were included in the analysis of data from the 3-year follow-up visit. Patients included in the 3-year follow-up analysis were further stratified into subgroups according to the change in lipid levels (Δlipid) from baseline to the 1-year follow-up (high vs low Δlipid subgroups). The primary outcome of this study was the incidence of AF at the 3-year follow-up (i.e., in patients who did not develop AF within the first year of follow-up). AF was defined based on the findings of 12-lead electrocardiography and 24-hour Holter monitoring findings. AF cases were confirmed by consensus among 2 trained cardiologists.
2.4. Data analysis
As the study used data from the 1-year and 3-year follow-up visits, data regarding patients lost to follow-up were excluded. Only data regarding living patients were used in the analysis of SR subgroups. All data were reviewed by 2 experts with statistical background to reduce bias. All data were summarized and recorded as mean ± standard deviation for normally distributed, continuous variables, and as median and interquartile range for non-normally distributed continuous variables. Between-group differences in baseline and follow-up characteristics were evaluated using the unpaired t-test for continuous variables and the chi-square test for categorical variables. Within-group differences reflecting changes from baseline to the 1-year follow-up were evaluated using the paired t-test for continuous variables and the paired chi-square test (McNemar test) for categorical variables. Cox proportional hazards models were used to analyze the relationship of lipid profiles with the incidence of new-onset AF in CHF patients. The analysis was repeated after adjusting the models for the following variables: age, sex, statin use, amiodarone use, NYHA functional class, CHF duration, and LAD. All statistical analyses were performed using SPSS version 12.0 (SPSS, Chicago, IL). Two-sided P-values < .05 were considered to indicate statistical significance.
3. Results
A total of 308 CHF patients without AF or history of AF were enrolled in this study (Fig. 1). Of these, 17 patients were lost to follow-up and 35 patients died (10 with AF and 25 with SR) by the 1-year follow-up visit. Among the remaining 256 patients, 68 patients had new-onset AF. Thus, 78 patients (68 living, 10 dead) were included in the AF group and 213 patients (188 living, 25 dead) were included in the SR group. Of the 188 living patients in the SR group who were included in the 3-year follow-up analysis, 21 had new-onset AF.
Figure 1.
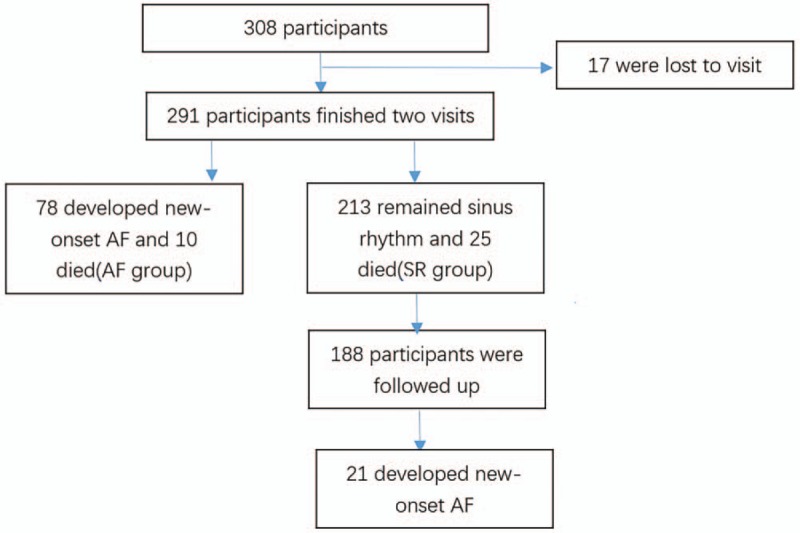
Flow chart of study enrollment and analysis. Patients were followed-up for 3 years and stratified according to whether or not new-onset AF was noted at the 1-year follow-up (AF group vs SR group). AF = atrial fibrillation, SR = sinus rhythm.
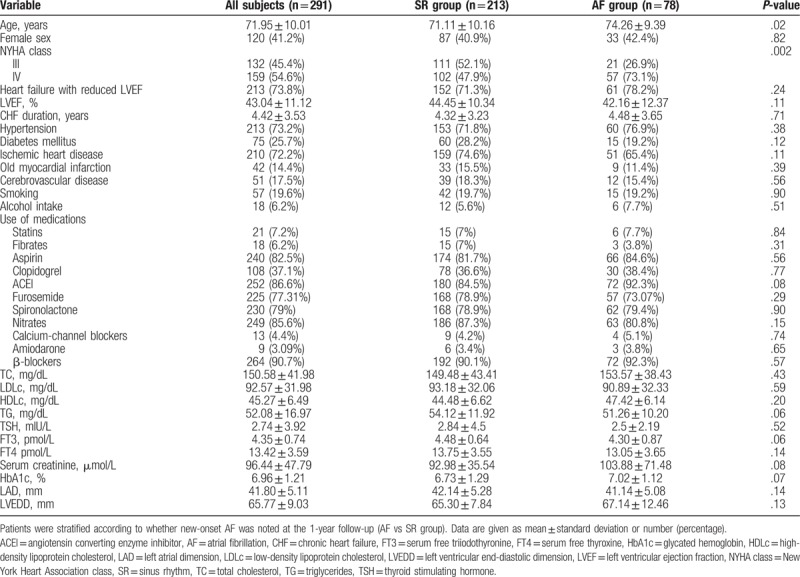
The mean age of the 291 patients included in the analysis was 71.95 ± 10.01 years, and 41.2% of patients were female (Table 1). Patients in the AF group were older than those in the SR group (74.26 ± 9.39 vs 71.11 ± 10.16 years, P = .02). At baseline, the percentage of patients with NYHA class IV was significantly higher in the AF group than in the SR group (73.1% vs 47.9%, P = .002). No significant between-group differences were found regarding other baseline parameters including blood lipid levels. However, at the 1-year follow-up, TC and LDLc levels were lower in the AF group than in the SR group (129.62 ± 30.14 vs 152.24 ± 38.99 mg/dL, P < .01; 67.53 ± 25.68 vs 85.38 ± 32.38 mg/dL, P < .01; respectively) (Table S1, Supplemental Content, which provides an overview of the clinical parameters at baseline and at the 1-year follow-up).
Table 1.
Baseline characteristics of patients with chronic heart failure.
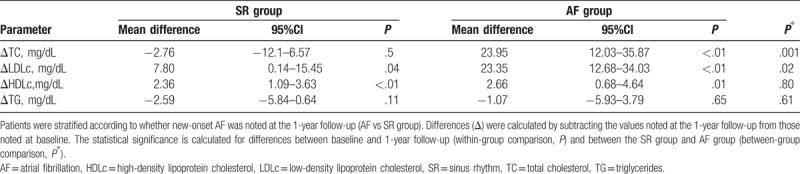
To explore the relationship between the occurrence of new-onset AF and the changes in lipid profiles, we examined Δlipid from baseline to the 1-year follow-up (Table 2). The LDLc and HDLc levels in both groups decreased significantly from baseline to the 1-year follow-up, while the TC levels decreased in the AF group but not in the SR group. Furthermore, ΔTC and ΔLDLc were significantly higher in the AF group than in the SR group (mean ΔTC: 23.95 vs −2.76 mg/dL, P = .001; mean ΔLDLc: 23.35 vs 7.8 mg/dL, P = .02), while ΔHDLc was comparable between the 2 groups.
Table 2.
Change in lipid profile between baseline and the 1-year follow-up.
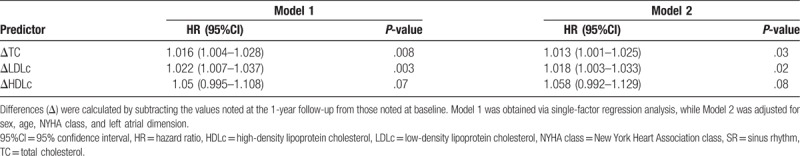
To study the value of Δlipid for predicting new-onset AF in CHF patients, the 188 living patients in the SR group were stratified into subgroups according to dichotomized values of ΔTC, ΔLDLc, and ΔHDLc, and the differences in patient characteristics at the 1-year follow-up were analyzed (Tables S2 to S4, Supplemental Content, which provide subgroup comparisons according to ΔTC, ΔLDLc, and ΔHDLc, respectively). The corresponding subgroups (i.e., high vs low Δlipid) were similar in terms of the prevalence of comorbidities (diabetes, hypertension, and coronary artery disease), use of lipid-lowering therapy, and CHF duration. Between the 1-year and 3-year follow-up visits, 21 of the 188 patients analyzed developed new-onset AF, 6 of whom died. We then used the Kaplan–Meier survival curves to illustrate the 2-year AF-free probability in the subgroups defined based on dichotomized values of ΔTC, ΔLDLc, and ΔHDLc (Fig. 2). High ΔLDLc (>9.73 mg/dL) and ΔTC (>9.65 mg/dL) were associated with increased risk of new-onset AF (P = .005 and P = .02, respectively). High ΔHDLc (>3.72 mg/dL) tended to be associated with increased occurrence of new-onset AF, but this trend was not significant. Finally, we used Cox proportional hazards models to assess the impact of changes in lipid levels on the incidence of new-onset AF over the course of 2 years (between the 1-year and 3-year follow-up visits). The hazard ratio (HR) with 95% confidence interval (95%CI), which reflect the risk of new-onset AF, were 1.022 (1.007–1.037) and 1.016 (1.004–1.028) for each standard deviation increment in ΔLDLc and ΔTC, respectively; when adjusting the models for age, sex, use of medications, CHF duration, NYHA class, and LAD, these values were 1.018 (1.003–1.033) and 1.013 (1.001–1.025), respectively (Table 3).
Figure 2.
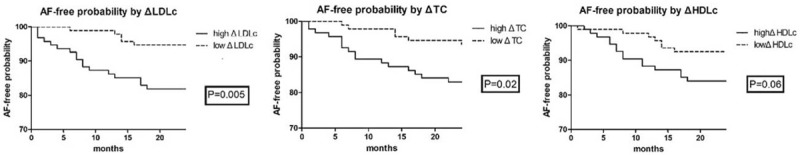
Kaplan–Meier curves describing AF-free probability as a function of lipid profiles. The levels of various indicators were dichotomized (high vs low). Differences (Δ) were calculated by subtracting the values noted at the 1-year follow-up from those noted at baseline. AF = atrial fibrillation, HDLc = high-density lipoprotein cholesterol, LDLc = low-density lipoprotein cholesterol, TC = total cholesterol.
Table 3.
Risk of new-onset atrial fibrillation in patients with chronic heart failure.
4. Discussion
Lipid disorders, especially those affecting LDLc levels, are strongly associated with an increased risk of cardiovascular disease. Statins are commonly used to lower LDLc levels and thus reduce the risk of coronary heart disease.[18] However, the exact relationship between lipid profiles and the incidence of arrhythmia remains unclear. AF is a common arrhythmia that usually induces thrombotic complications, leading to increased mortality and economic burden.[19] Many AF risk factors have been reported, including age, hypertension, diabetes, atrial dilation, and heart failure.[20] Some clinical studies have examined the relationship between dyslipidemia and AF but reported contradictory conclusions.[14–16] Specifically, some studies found the risk of new-onset AF to be increased by low HDLc levels, while other studies reported the same for low LDLc levels but not HDLc levels.[13,16] AF often occurs in patients with CHF partly because these conditions share the same etiology.[1] Moreover, new-onset AF was found to increase mortality in patients with CHF.[17,21] Dyslipidemia is typically noted in patients with CHF, and lower baseline TC levels are associated with poor prognosis of heart failure.[22] However, whether dyslipidemia contributes to new-onset AF in CHF has not been studied.
We designed the present study to investigate the association between dyslipidemia and new-onset AF in patients with CHF. In our cohort of approximately 300 CHF patients without AF at baseline, 26.8% (78/291) were diagnosed with new-onset AF within the first year, which is higher than the AF incidence in the general population[16] and thus confirms that patients with CHF have higher risk of AF. In our study, age was higher and baseline heart function was poorer in the AF group than in the SR group, which is likely related to the fact that advanced age and more severe heart dysfunction are usually associated with more complications, and which is consistent with previous observations that the prevalence of AF in the general population increases with age.[23] Importantly, the 2 groups did not differ regarding baseline characteristics including use of medications, LAD, left ventricular ejection fraction, or blood lipid levels (TC, HDLc, LDLc, and TG). However, at the 1-year follow-up, LDLc and HDLc decreased significantly in both groups, whereas TC decreased only in the AF group; moreover, LDLc and TC were lower in the AF group. These results suggest that, in CHF, the risk of new-onset AF is affected more by dynamic changes in blood lipid levels than by baseline levels. However, it is inappropriate to conclude that dyslipidemia leads to AF since AF itself may account for abnormal lipid metabolism. Hence, to investigate the value of dynamic changes in lipid levels for predicting new-onset AF in patients with CHF, we further examined the occurrence of new-onset AF only in patients with SR at 1 year. Upon adjustment for age, sex, use of medications, CHF duration, NYHA class, and LAD, the decreases in LDLc and TC levels were independent risk factors for new-onset AF, while the decrease in HDLc levels was a less significant factor. Subgroup analysis using the dichotomized values of the change in lipid levels (high vs low Δlipid) revealed that subgroups with high ΔLDLc and those with high ΔTC had higher incidence of new-onset AF, suggesting that the continuing decreases in LDLc and TC are associated with new-onset AF in CHF. Cholesterol control is one of the most important strategies for prevention and treatment of ischemic heart disease. Statins are widely used in atherosclerotic disease. In this study, 72.2% of the enrolled subjects had ischemic heart disease, which did not affect AF incidence as blood lipid levels decreased. Although we did not find a correlation between the use of drugs (including statins) and AF incidence, use of statins in CHF should be cautious.
Our present findings may be explained in terms of the relationship between lipid metabolism and the stability of ion channels in the membrane of cardiac myocytes. Enhanced ectopic rhythmicity, multiple wavelet re-entrant, atrial fibrosis, atrial remodeling, inflammatory response, and oxidative stress are known contributors to AF.[24–27] Electrical and structural remodeling of atrial cardiomyocytes is regarded as the pathologic foundation of AF.[28] Abnormal lipid metabolism may detrimentally influence the electrical stability of membrane ion channels in atrial myocytes, since such membranes consist of lipid bilayers.[29] Patients with end-stage heart failure often show extreme emaciation.[30] Gastrointestinal congestion and restriction is the main cause of malnutrition in patients with CHF. Malnutrition is usually associated with abnormal lipid metabolism, and decreased serum LDLc levels indicate advanced metabolic imbalance.[31] Structural abnormalities of the cell membrane, induced by abnormal lipid metabolism, result in abnormal electrical activity across the membrane. Indeed, in vitro studies have shown that cholesterol modulates the distribution and function of some ion channels potentially involved in AF, such as the Kv1.5 potassium channel.[29] Dyslipidemia was found to be associated with subclinical hyperthyroidism in the elderly.[32] AF was determined to be an independent predictor of intensive care unit mortality among severely ill patients.[33] In patients with CHF, decreased blood lipid levels may reflect a higher risk of complications, including AF. Therefore, it may be harmful to reduce serum LDLc levels excessively in patients with heart failure.
Several limitations of this study must be acknowledged. First, this was a single-center study with a limited sample size (approximately 300 patients). Because our data pertains to patients from a single geographical area in China, the findings may not be generalizable to all patients with CHF. We measured the left atrial diameter rather than the atrium volumes by echocardiography. However, atrial volumes may be a more sensitive indicator of atrial remodeling. Finally, we analyzed blood lipid levels at only 2 time points, which may not reflect the characteristics of the entire curve of dynamic change in lipid levels.
Despite the study limitations, we found compelling evidence that decreasing serum levels of LDLc and TC are associated with higher incidence of new-onset AF in patients with CHF, independently of other risk factors, suggesting that aggressive LDLc and TC lowering may not be appropriate in this patient population, regardless of CHF etiology.
Author contributions
Data curation: Chen Liu, Aihua Li.
Formal analysis: Xiaochen Yuan.
Investigation: Chen Liu, Jin Geng, Xiao Ye, Xiaochen Yuan.
Project administration: Zhengang Zhang, Biao Xu, Yingwei Wang.
Writing – original draft: Chen Liu.
Writing – review & editing: Chen Liu.
Supplementary Material
Footnotes
Abbreviations: 95%CI = 95% confidence interval, ACEI = angiotensin converting enzyme inhibitor, AF = atrial fibrillation, CHF = chronic heart failure, FT3 = serum free triiodothyronine, FT4 = serum free thyroxine, HbA1c = glycated hemoglobin, HDLc = high-density lipoprotein cholesterol, HR = hazard ratio, LAD = left atrial dimension, LDLc = low-density lipoprotein cholesterol, LVEDD = left ventricular end-diastolic dimension, LVEF = left ventricular ejection fraction, NYHA class = New York Heart Association class, SR = sinus rhythm, TC = total cholesterol, TG = triglycerides, TSH = thyroid stimulating hormone.
CL and JG contributed equally to this study and should be regarded as co-first authors.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014;16:103–11. [DOI] [PubMed] [Google Scholar]
- [2].Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation 2012;125:e2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, et al. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines-heart failure. Circ Heart Fail 2012;5:191–201. [DOI] [PubMed] [Google Scholar]
- [4].Chamberlain AM, Redfield MM, Alonso A, et al. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail 2011;4:740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- [6].Smit MD, Moes ML, Maass AH, et al. The importance of whether atrial fibrillation or heart failure develops first. Eur J Heart Fail 2012;14:1030–40. [DOI] [PubMed] [Google Scholar]
- [7].McManus DD, Hsu G, Sung SH, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc 2013;2:e005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rivero-Ayerza M, Scholte Op Reimer W, et al. New-onset atrial fibrillation is an independent predictor of in-hospital mortality in hospitalized heart failure patients: results of the Euro Heart Failure Survey. Eur Heart J 2008;29:1618–24. [DOI] [PubMed] [Google Scholar]
- [9].Mandalenakis Z, Von Koch L, Eriksson H, et al. The risk of atrial fibrillation in the general male population: a lifetime follow-up of 50-year-old men. Europace 2015;17:1018–22. [DOI] [PubMed] [Google Scholar]
- [10].Conen D, Tedrow UB, Koplan BA, et al. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation 2009;119:2146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women's Health Study). J Am Coll Cardiol 2010;55:2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huxley RR, Filion KB, Konety S, et al. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lopez FL, Agarwal SK, MacLehose RF, et al. Blood lipid levels, lipid lowering medications, and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circ Arrhythm Electrophysiol 2012;5:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mora S, Akinkuolie AO, Sandhu RK, et al. Paradoxical association of lipoprotein measures with incident atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Watanabe H, Tanabe N, Yagihara N, et al. Association between lipid profile and risk of atrial fibrillation. Circ J 2011;75:2767–74. [DOI] [PubMed] [Google Scholar]
- [16].Alonso A, Yin X, Roetker NS, et al. Blood lipids and the incidence of atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc 2014;3:e001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- [18].Ford I, Murray H, McCowan C, et al. Long-term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy: 20-year follow-up of West of Scotland Coronary Prevention Study. Circulation 2016;133:1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cotté FE, Chaize G, Gaudin AF, et al. Burden of stroke and other cardiovascular complications in patients with atrial fibrillation hospitalized in France. Europace 2016;18:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ahmed A, Perry GJ. Incident atrial fibrillation and mortality in older adults with heart failure. Eur J Heart Fail 2005;7:1118–21. [DOI] [PubMed] [Google Scholar]
- [22].Greene SJ, Vaduganathan M, Lupi L, et al. Prognostic significance of serum total cholesterol and triglyceride levels in patients hospitalized for heart failure with reduced ejection fraction (from the EVEREST Trial). Am J Cardiol 2013;111:574–81. [DOI] [PubMed] [Google Scholar]
- [23].Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam Study. Eur Heart J 2006;27:949–53. [DOI] [PubMed] [Google Scholar]
- [24].Li J, Solus J, Chen Q, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm 2010;7:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Medi C, Kalman JM, Spence SJ, et al. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:1317–24. [DOI] [PubMed] [Google Scholar]
- [26].Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- [27].Iwasaki YK1, Nishida K, Kato T, et al. Atrial fibrillation pathophysiology: implications for management. Circulation 2011;124:2264–74. [DOI] [PubMed] [Google Scholar]
- [28].Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. [DOI] [PubMed] [Google Scholar]
- [29].Balse E, El-Haou S, Dillanian G, et al. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc Natl Acad Sci USA 2009;106:14681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sze S, Zhang J, Pellicori P, et al. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol 2017;106:533–41. [DOI] [PubMed] [Google Scholar]
- [31].Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 2004;291:451–9. [DOI] [PubMed] [Google Scholar]
- [32].Zhao M, Yang T, Chen L, et al. Subclinical hypothyroidism might worsen the effects of aging on serum lipid profiles: a population-based case-control study. Thyroid 2015;25:485–93. [DOI] [PubMed] [Google Scholar]
- [33].Shaver CM, Chen W, Janz DR, et al. Atrial fibrillation is an independent predictor of mortality in critically ill patients. Crit Care Med 2015;43:2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


